Abstract
INTRODUCTION:
Dengue virus (DENV) causes a wide range of diseases in humans, from acute febrile illness Dengue fever (DF) to life-threatening Dengue hemorrhagic fever (DHF) or Dengue shock syndrome (DSS). Factors believed to be responsible for spread of Dengue virus infection include explosive population growth, unplanned urban overpopulation with inadequate public health systems, poor standing water and vector control, climate changes and increased international recreational, business, military travel to endemic areas. All of these factors must be addressed to control the spread of Dengue and other mosquito-borne infections. The detection of Dengue virus RNA by reverse transcriptase PCR (RT-PCR) in human serum or plasma samples is highly indicative of acute Dengue fever. Moreover, the method is able to identify the Dengue virus serotype by demonstrating defined sequence homologies in the viral genomic RNA.
METHODS AND RESULTS:
During the nine year period of this study analysis, 6767 strongly suspected cases were tested by RT-PCR. 1685 (24.9%) were Dengue PCR positive and confirmed as Dengue cases. Observations on the seasonality were based on the nine year's data as the intensity of sampling was at its maximum during monsoon season. Dengue typing was done on 100 positive samples after storage of Dengue RNA at – 80°C. Dengue serotypes were detected in 69 samples of which Dengue 2 was most predominant. 576 samples were processed for NS1 antigen and PCR simultaneously. 19/576 were positive (3.3 %) for NS1 as well as by PCR. 23/576 samples were negative for NS1 antigen, but were positive by RT-PCR. The remaining 534 samples which were negative for NS1 antigen were also negative by Dengue RT-PCR.
CONCLUSION:
In this study we sought to standardize rapid, sensitive, and specific fluorogenic probe-based RT-PCR assay to screen and serotype a representative range of Dengue viruses that are found in and around Mumbai. Qualitative Dengue virus TaqMan assays could have tremendous utility for the epidemiological investigation of Dengue illness and especially for the study of the viremic response with candidate live-attenuated dengue virus vaccines.
Key words: Dengue, Mumbai, polymerase chain reaction
Introduction
Dengue (DEN) infection caused by antigenically distinct, one of four closely related, DEN virus (DENV) serotypes (DEN-1, DEN-2, DEN-3, and DEN-4) is primarily a disease of the tropics. The causative virus is maintained in a cycle that involves humans. DENV is distributed in tropical and subtropical areas of the world and is declared as a major global health threat by the World Health Organization (WHO).[1] DENV causes a wide range of diseases in humans, from Dengue Fever (DF) which is an acute febrile illness to Dengue Hemorrhagic Fever (DHF) or Dengue Shock Syndrome (DSS) which are life-threatening.
The clinical symptoms mimic other febrile infections and may be misdiagnosed as the onset of leptospirosis, malaria, hepatitis A or other viral, bacterial or protozoan infections. As vaccination and selective treatment for DEN infection are not available, rapid and specific diagnosis during early onset of the disease is critical and can help prevent progression of the disease through monitoring and supportive therapy.[2,3] Most importantly, early diagnosis can help prevent the spread of DEN infection through mosquito bites from infected patients during the viremic phase.
Current diagnostic antigen detection based rapid and ELISA assays cannot provide a definitive result before 3-5 days post infection. DENV specific immunoglobulins(Ig), IgM are present in the sera of patients post 5 days, indicative of acute primary infection but the IgM response may be low or sometimes even absent in secondary DF.[4] Both DENV-specific immunoglobulins (Igs) and IgM antibodies are present in the sera of patients post 5 days, indicative of acute primary infection, while the IgM response may be low or sometimes even absent in secondary DF.[4] However, strong antibody cross-reactivity exists among the flavivirus family. Therefore, the antibody response may be difficult to interpret with regard to an acute DF, if other flavivirus infections cannot be excluded by clinical, laboratory, or epidemiological means.
Detection of DENV RNA by reverse transcriptase-polymerase chain reaction (RT-PCR) in human serum or plasma samples is confirmative of acute DF[5] and is positive from day 0 to day 5 of infection. As mentioned in references earlier, this method also identifies the DENV serotype by demonstrating defined sequence homologies in the viral genomic RNA. The information on the distribution of the four DENV serotypes and even of strains or quasispecies in tropical areas is relevant in our understanding of epidemiology and pathogenesis of DENV infection.[4] Thus, PCR has been successfully applied in the diagnosis of DEN in the acute phase of illness.[6] Real-time PCR assays for detection and typing of DEN have been published.[7,8]
Mumbai, the financial capital of India, receives an average rainfall of 2386 mm during monsoon months, from June to September, wherein DEN viral infection wreaks havoc. Every case of acute febrile illness approaching secondary or tertiary care hospitals is advised a screening or confirmatory test depending on the number of days of illness. Literature search has revealed that though there is adequate information about trends in DEN infection, prevalent serotypes from North and South India and also from Pune in West India, there exists a paucity of data from Mumbai, and hence, through this study, we highlight the trends in DEN through 9 years, seasonal variation, prevalent DEN types in Mumbai, and the diagnostic importance of DEN real-time PCR in early diagnosis. This helps us to determine the hotspots to enforce and strengthen vector control measures.
Materials and Methods
In the city of Mumbai, laboratories conducting DEN PCR for early detection are in the private sector, and the cost per test is very high and not affordable by government and municipal hospitals.
Molecular Diagnostic Reference Laboratory at an infectious disease hospital in Mumbai, is the public health laboratory of Municipal Corporation of Greater Mumbai (MCGM) and receives samples from all tertiary and secondary health care facilities under MCGM for DEN PCR in the early phase of illness (0–7 days). MCGM extends financial support for the same.
The study was reviewed and approved by Institutional Review Board of Kasturba Hospital Research Society, Mumbai.
Blood samples from 6767 patients clinically suspected of DEN infection admitted in tertiary and secondary care facilities of Municipal Corporation Hospitals in Mumbai, from 2007 to 2015, were received at the Molecular Diagnostic Reference Laboratory, Kasturba Hospital.
A volume of 4–5 ml blood was collected in plain and EDTA vacutainer under aseptic conditions and transported to the laboratory within 6 hours of collection maintaining the cold chain at 4 °C. Blood samples were collected from day 1–7 of illness only. Clinical and epidemiological information of patients were collected on a standardized questionnaire.
Dengue serology
Serum was separated and tested for DEN-specific IgM and IgG antibodies by capture ELISA using DEN Microlisa IgM and IgG kits from J. Mitra Co. Pvt., Ltd. NS1 antigen was tested in 576 samples using DEN NS1 Ag direct sandwich ELISA from J. Mitra Co. Pvt., Ltd.
Reverse transcriptase-polymerase chain reaction
Dengue RNA was isolated from plasma using the High Pure Viral RNA Acid kit from 03730964001, Roche Diagnostics GmbH, Mannheim, Germany. The purified viral RNA is free of intact virus, nucleases, and all cellular components that interfere with RT-PCR. Selection of primers and probe for TaqMan-based RT-PCR for detection of DEN Primers and 5' nuclease probes were designed using DNA database entries. All sequences available from the European Molecular Biology Laboratory (EMBL), GenBank, and DNA Data Bank of Japan (DDBJ) databases (accessed autumn 2000) were included in the search for possible primer binding regions.[14] The TaqMan RNA Amplification Kit (04331885001, Roche Diagnostics GmbH, Mannheim, Germany) was used for amplification. It contains RNA MIX and Mn2+ reagents designed for reverse transcription and PCR amplification of target RNA in a real-time fluorescent detection assay.
The extension cycle time was added onto annealing cycle time as the same temperature was used for both steps.
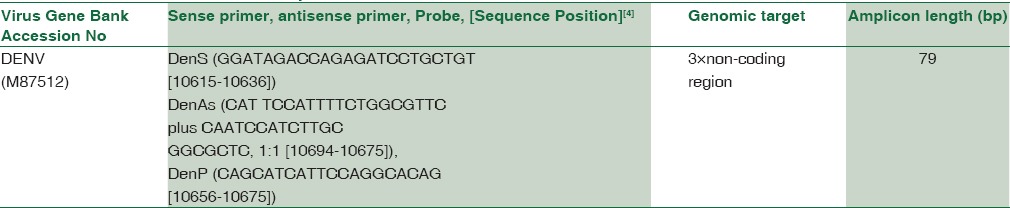
The final sequences of probes and primers are given in Table 1.
Table 1.
Primers and 5×nuclease probes
Dengue serotyping
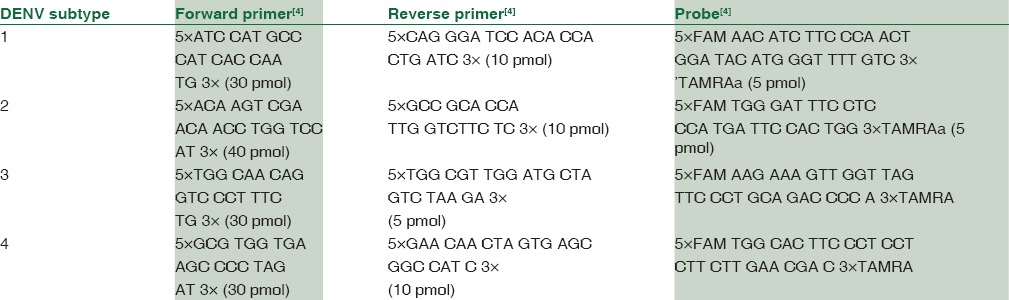
Dengue RNA was stored at -80°C and typing was done in 100 PCR positive samples. Individual primers had to be chosen for the amplification of each serotype. The final sequences of probes and primers are given in Table 2.
Table 2.
Probes and Primers for sub typing of Dengue (Den 1-4)
All controls, probes and primers for detection of Dengue and subtypes were made available from TIB MOLBIOL Syntheselabor GmbH, Eresburgstraebe 22-23. D-12103 Berlin Germany.
Data analysis for dengue typing
The data analysis was done by following the data analysis instructions given in the software supplied with the Cobas TaqMan instrument.
Adaptation of dengue reverse transcriptase-polymerase chain reaction to TaqMan conditions
A highly conserved region of the DENV genome was chosen to allow optimum annealing not only of the primers but also of the labeled probe. The individual primers and probes for DENV 1–4 and for each serotype amplification were run in parallel using identical time and temperature profiles. The concentration of MgSO4 and the amount of primers adapted well to the TaqMan conditions thus giving precise results.
Results
Reverse transcriptase-polymerase chain reaction
A total of 6767 blood samples from strongly suspected cases of DF in the period 2007–2015 were processed for RT-PCR testing. One thousand six hundred and eighty-five, i.e., 24.9% patients were confirmed as DEN cases based on DEN-specific PCR-positive results.
Time trends
The percentage positivity was 12% in 2007, followed by a sharp rise to 32.8% in 2008, 7.2% in 2009, 12.4% in 2010, 17.1% in 2011, 19% in 2012, then a rise to 29.5% in 2013, 25.8% in 2014, and 29.8% in 2015 [Figure 1].
Figure 1.
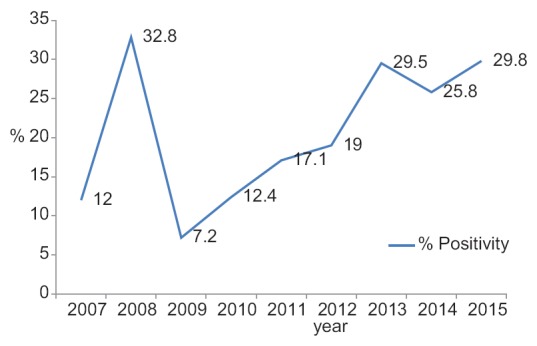
Trends in dengue positivity
Trends in seasonality and climate
According to the intensity of the rainfall, weather data were divided in three periods namely: premonsoon period – from February to May, monsoon period – from June to September, and postmonsoon period – from October to January. Observations on the seasonality were based on the 9 years' data as the intensity of sampling was at its maximum during monsoon season. Of the total of 6767 samples processed for DEN PCR during the 9 year study, 37/312 (11.9%) samples in the premonsoon period were positive, 697/4564 (15.3%) samples in the monsoon period were positive, and 433/1891 (22.9%) samples in the postmonsoon period were positive by RT-PCR. The maximum DEN positive cases coincided mainly with the postmonsoon period.
Age and sex
Of the 1685 confirmed DEN PCR positive cases, 1094 patients were male, and 591 patients were female, and the male to female ratio was 1.9. Age-specific DEN morbidity rates are highest in the young adult population (21–40 years). The mean age of suspected DEN cases was 25.77 years [Figure 2].
Figure 2.
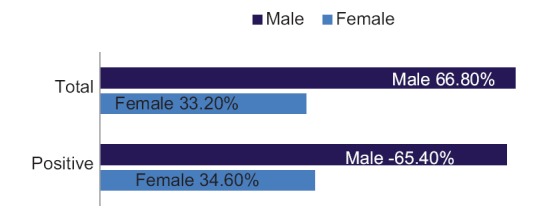
Sex distribution in cases of dengue
Days of illness
Of the 1685 samples positive for RT-PCR, 1343 (79.7%) patients had fever for 1–3 days and 342 (20.3%) had fever for 4–7 days.
Clinical symptoms
Fever, hepatomegaly, vomiting, bleeding tendencies, erythematous rash, thrombocytopenia, elevated liver enzymes, and deranged prothrombin time were the predominant clinical and laboratory features.
Hemorrhagic symptoms
Out of the 1685 DEN RT-PCR positive cases, 667 patients (39.6%) had hemorrhagic symptoms. The corresponding figure was 1782 (35.1%) for the total of 5082 DEN RT-PCR negative cases. Hemorrhagic manifestations included petechiae, purpura, epistaxis, gum bleeding, and rash.
Complications
One hundred and fifty-eight (9.4%) patients out of the total 1685 DEN RT-PCR positive cases and 562 (11.1%) patients out of the total 5082 DEN RT-PCR negative cases had complications such as jaundice, renal failure, ascites, altered sensorium, and acute respiratory distress syndrome.
Dengue serology
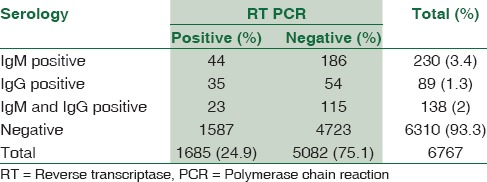
Of the 6767 sera tested, 230 (3.4%) samples were positive for DEN-specific IgM antibody. Forty-four out of 230 (19.1%) were tested positive by RT-PCR. Convalescent blood samples could not be collected from patients whose acute samples were negative for DEN IgM.
DEN-specific IgG antibodies were found in 227/6767 (3.4%) patients enabling categorizing the cases as secondary DEN. Of these, only IgG antibodies were positive in 89 (39.2%) and both IgM and IgG antibodies were found in 138 (60.8%). PCR was positive in 58/227 (25.6%) patients with secondary DEN infection.
One hundred and eighty-six (2.8%) samples out of the total 6767 samples were only IgM positive and RT-PCR negative showing recent DEN infection.
Fifty-four (0.8%) samples out of the total 6767 samples were only IgG positive and RT-PCR negative showing past DEN infection.
One hundred and fifteen (1.7%) cases had both IgG and IgM antibodies and RT-PCR negative results showing secondary infection of DEN [Table 3].
Table 3.
Results of dengue serology and reverse transcriptase-polymerase chain reaction
A total of 576 samples were processed for NS1 antigen and PCR simultaneously in 2010. Out of 576 samples, 19 were positive (3.3%) for NS1 as well as by PCR. Twenty-three samples were negative for NS1 antigen but were positive by RT-PCR. The remaining 534 samples which were negative for NS1 antigen were also negative by DEN RT-PCR [Table 4].
Table 4.
Results of nonstructural protein antigen and dengue polymerase chain reaction

Dengue typing
DEN serotypes were detected in 69 samples out of a total 100 PCR-positive samples from 2007 to 2010.
Fifty Seven of 69 (82.6%) were positive for DEN-2, of which 73.7% were males and 26.3% were females. 35% patients were <18 years of age, 60% were in the age group of 18–45 years, and only 3 patients were 46–60 years old.
Nine of 69 (13%) were positive for DEN-1. 6/9 patients were females in the age group of 18–60 years and 3/9 were males in the age group of 18–45 years.
Two samples (2.9%) were positive for DEN 1 and 2. Both were males in the age group of 5–20 years.
One male child, 12 years of age (1.4%) was positive for DEN-4.
Thirty-one samples were not amplified for any of the four serotypes, namely, DEN-1, DEN-2, DEN-3, and DEN-4. The possible reason may be that as DEN RNA is heat-labile, freezing and thawing must have caused degradation of the RNA [Table 5].
Table 5.
Trends in Typing of Dengue isolates

In the years 2012–2013 and 2014–2015, 50% were DEN-2 and 50% were DEN-3, predominantly males in the age group of 18–45 years.
Discussion
Mumbai experiences torrential rains in July through September. Hence, we can divide the seasons into premonsoon, monsoon, and postmonsoon. This manuscript elucidates role of real-time PCR in the diagnosis of DEN among cases of acute febrile illness and also describes the changing serotypes in the 9-year study.
Molecular tests offer a significant advantage in the diagnosis of DEN during the acute phase of illness. However, these tests depend on preanalytical variables, which include the storage and transport of patient samples, the stability of viral RNA, and the methods of isolating viral RNA of high yield and quality, which impacts the results.[9,10] Hence, the technical challenges associated with these preanalytical issues have been identified and optimized at our laboratory.
Our results highlight the potential of PCR in the early and rapid diagnosis of DF. The DEN TaqMan assay is a rapid, specific, and sensitive tool for diagnosis of DENV. As described in detail previously, these assays have several advantages over traditional identification methods. The TaqMan RT-PCR assays are more rapid than traditional methods. The TaqMan RT-PCR assay can be made highly portable using several commercially available instruments.[7] Of 6767 samples tested by RT-PCR, 1685 (24.9%) were positive for DEN. These results are consistent with the incidence of 18.2% positivity of DEN by RT-PCR in a 4-year study at a tertiary care hospital as reported by Velathanthiri et al. from Sri Lanka.[11]
The trends in DEN viral infection over 9 years with reference to rainfall, seasonal variation, and demography are highlighted in this paper.
Observations on the seasonality were based on the 9 years' data as the intensity of sampling was at its maximum during monsoon season. The maximum DEN-positive cases coincided mainly with the postmonsoon period.
Demography
Age-wise distribution of the DEN cases in all 9 years shows that significant number of cases were in older age group (21–40 years). Our observation is quite in accordance with a previous reported study from a tertiary care hospital in Delhi, by Gupta et al.[13] and with other studies by Chakravarti et al.[14] from Delhi.[14] However, Vijay Kumar et al. in a study from South India[15] found that children were more susceptible to infection than adults. We have reported preponderance among young adults in the age group of 21–40 years. A similar trend was reported in studies from Singapore,[16,17] by Goh and Chan et al. and a study in Malaysia as reported in the WHO weekly bulletin.[18] This may be due to the fact that adults are not immune to all strains of the DENV. In Singapore, where vector control measures are being carried out since 1973, the mean age of occurrence of DF had increased from 14 years in 1973 to 28 years in 1994. In Malaysia also, DF occurs among young adults. Further, increased mortality from this disease has also been reported in young adults in both countries.[16,18,19,20]
Clinical symptoms and days of illness
Fever was seen in 100% of patients in our study. Laboratory-positive DENV infection was defined in patients with a febrile illness consistent with DEN according to WHO criteria.[12] About 79.7% patients had fever for 1–3 days and 20.3% had fever for 4–7 days.
Clinical symptoms in patients were in accordance with that of DEN-like illness. However, no significant correlation was found between the proportion of bleeding manifestation among patients with platelet count in the range of 20,000-50,000 and >50,000/mm3. The same has also been observed by other workers, which possibly indicated the role of factors other than thrombocytopenia in the causation of bleeding manifestation in these patients.[22] Low platelet counts do not predict clinically significant bleeding in DEN. It follows that platelet or blood transfusions should not be administered based on platelet count alone. DHF or DSS cases frequently have compensated consumptive coagulopathy that seldom requires treatment. Bleeding is most likely caused by activated platelets resulting from damaged capillary endothelium. There is no specific treatment for DF. However, careful clinical management, continuous fluid replacement, and monitoring frequently save the lives of DHF patients. With appropriate intensive supportive therapy, mortality may be reduced to <1%. Maintenance of the circulating fluid volume is the central feature of DHF case management.
Dengue serology
A primary DEN infection is defined as the absence of specific anti-DEN IgG antibodies in the first serum sample, with anti-DEN IgM, virus isolation, and/or virus RNA being present, and DENV IgG being detected in the subsequent sample. In contrast, secondary DEN infection is defined by the presence of specific anti-DEN IgG and the absence of anti-DEN IgM in the first sample, together with a positive RT-PCR and/or virus isolation. Defining primary and secondary DEN infections by means of these rigorous criteria is very expensive, and most clinical laboratories in DEN-endemic countries cannot realistically perform all these assays on all of their samples. Moreover, even when all the assays are available, because of the dates of the blood collections and the immunological windows, it is not always possible to unambiguously define primary versus secondary DEN infections. Our observation revealed that 230 out of 6767 samples were positive for DEN-specific IgM antibody, 44 of 230 (19.1%) tested positive by RT-PCR. The presence of DEN-specific IgG antibodies in 89 of 6767 patients further categorized the cases into secondary DEN infection. Thirty-five of these were positive by RT-PCR (40.9%). Both IgG and IgM antibodies were found in 138 of 6767 cases; out of which, 23 (16.7%) were positive by RT-PCR. We could not test for rising titers as consecutive samples were not available for testing of rise in antibody titer as study patients were from all over Mumbai. Hence, it was difficult to follow-up the same. We presume that most adult patients in Mumbai, would present with secondary DEN as they would be exposed to one of the types of circulating DEN strains. The differentiation of primary from secondary infection may be of great prognostic value for DEN patients, particularly children and the elderly; in whom, a secondary DEN infection is more likely to result in DHF.[23,24] Furthermore, for epidemiological purposes, it is important to characterize the DEN serological immune response during DEN outbreaks.[24]
Dengue typing
We report the emergence of DEN-2 as the predominant serotype in Mumbai, in the years 2007 to 2010. In the years 2012-13 and 2014-15, 50% were DEN-2 and 50% were DEN-3. One of the largest outbreaks in North India occurred in Delhi and adjoining areas in the year 1996. The 1996 epidemic in Delhi was mainly due to DEN-2 virus[25,26] as reported by Brooret al. and Dar et al. Following this in the postepidemic period, in 1997, DEN-1 virus activity was seen in Delhi[27] as reported by Vajpayee et al. Thereafter, in the year 2003, another outbreak occurred in Delhi and all four DENV serotypes were found to be cocirculating as reported by Gupta and Dar et al.[28,29] However, DEN-3 was reported to predominate in certain parts of North India in 2003[30] by Dash et al. The need of the hour is to characterize the circulating serotypes of DENV in our community and understand the evolutionary processes influencing the DENV, as this is expected to have an impact on pathogenesis and vaccination strategies in the future. DF and DHF, owing to all four DEN serotypes, have been reported from various parts of India.[31,32] However, among all these, type-2 DEN has been reported most frequently. There is a paucity of reports from Western India, with no reports from Mumbai and only one report from Pune of the detection of DENV-4 in Pune, Maharashtra in 2009–2010.[21]
Therefore, a high index of suspicion of Dengue among cases of acute febrile illness supported by molecular diagnosis and typing of the virus enables patients to receive appropriate management. The knowledge of prevalent subtypes can guide policy makers in forecasting outbreaks, planning prevention and improve vector control strategies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.World Health Organization. Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention and Control. 2nd ed. Geneva, Switzerland: World Health Organization; 1997. pp. 12–47. [Google Scholar]
- 2.Gubler DJ, Kuno G, Sather GE, Velez M, Oliver A. Mosquito cell cultures and specific monoclonal antibodies in surveillance for dengue viruses. Am J Trop Med Hyg. 1984;33:158–65. doi: 10.4269/ajtmh.1984.33.158. [DOI] [PubMed] [Google Scholar]
- 3.Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992;30:545–51. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laue T, Emmerich P, Schmitz H. Detection of dengue virus RNA in patients after primary or secondary dengue infection by using the TaqMan automated amplification system. J Clin Microbiol. 1999;37:2543–7. doi: 10.1128/jcm.37.8.2543-2547.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drosten C, Göttig S, Schilling S, Asper M, Panning M, Schmitz H, et al. Rapid detection and quantification of RNA of Ebola and Marburg viruses, Lassa virus, Crimean-Congo hemorrhagic fever virus, Rift Valley fever virus, dengue virus, and yellow fever virus by real-time reverse transcription-PCR. J Clin Microbiol. 2002;40:2323–30. doi: 10.1128/JCM.40.7.2323-2330.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morita K, Tanaka M, Igarashi A. Rapid identification of dengue virus serotypes by using polymerase chain reaction. J Clin Microbiol. 1991;29:2107–10. doi: 10.1128/jcm.29.10.2107-2110.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callahan JD, Wu SJ, Dion-Schultz A, Mangold BE, Peruski LF, Watts DM, et al. Development and evaluation of serotype- and group-specific fluorogenic reverse transcriptase PCR (TaqMan) assays for dengue virus. J Clin Microbiol. 2001;39:4119–24. doi: 10.1128/JCM.39.11.4119-4124.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vijayakumar TS, Chandy S, Sathish N, Abraham M, Abraham P, Sridharan G. Is dengue emerging as a major public health problem? Indian J Med Res. 2005;121:100–7. [PubMed] [Google Scholar]
- 9.Goh KT. Changing epidemiology of dengue in Singapore. Lancet. 1995;346:1098. doi: 10.1016/s0140-6736(95)91771-3. [DOI] [PubMed] [Google Scholar]
- 10.Chan KL, Ng SK, Chew LM. The 1973 dengue haemorrhagic fever outbreak in Singapore and its control. Singapore Med J. 1977;18:81–93. [PubMed] [Google Scholar]
- 11.Sharma S, Sharma SK, Mohan A, Wadhwa J, Dar L, Thulkar S, et al. Clinical Profile of Dengue Haemorrhagic Fever in Adults during 1996 – Outbreak in Delhi, India. Dengue Bulletin. 1998;22:20–30. [Google Scholar]
- 12.Young PR, Hilditch PA, Bletchly C, Halloran W. An antigen capture enzyme-linked immunosorbent assay reveals high levels of the dengue virus protein NS1 in the sera of infected patients. J Clin Microbiol. 2000;38:1053–7. doi: 10.1128/jcm.38.3.1053-1057.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vajpayee M, Mohankumar K, Wali JP, Dar L, Seth P, Broor S. Dengue virus infection during post-epidemic period in Delhi, India. Southeast Asian J Trop Med Public Health. 1999;30:507–10. [PubMed] [Google Scholar]
- 14.Gupta E, Dar L, Narang P, Srivastava VK, Broor S. Serodiagnosis of dengue during an outbreak at a tertiary care hospital in Delhi. Indian J Med Res. 2005;121:36–8. [PubMed] [Google Scholar]
- 15.Dar L, Gupta E, Narang P, Broor S. Co-circulation of dengue serotypes 1,2,3 and 4 during the 2003 outbreak in Delhi, India. Emerg Infect Dis. 2006;12:352–3. doi: 10.3201/eid1202.050767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dash PK, Saxena P, Abhyankar A, Bhargava R, Jana AM. Emergence of dengue virus type-3 in northern India. Southeast Asian J Trop Med Public Health. 2005;36:370–7. [PubMed] [Google Scholar]
- 17.Rao CV. Dengue fever in India. Indian J Pediatr. 1987;54:11–4. doi: 10.1007/BF02751227. [DOI] [PubMed] [Google Scholar]
- 18.Lall R, Dhanda V. Dengue haemorrhagic fever and the dengue shock syndrome in India. Natl Med J India. 1996;9:20–3. [PubMed] [Google Scholar]
- 19.WHO: Dengue and Severe Dengue. Fact sheet No 117. [Last accessed on 2017 Apr 20]. Available from: http://www.who.int/mediacentre/factsheets/fs117/en/
- 20.Philip Samuel P, Tyagi BK. Diagnostic methods for detection & isolation of dengue viruses from vector mosquitoes. Indian J Med Res. 2006;123:615–28. [PubMed] [Google Scholar]
- 21.Cecilia D, Kakade MB, Bhagat AB, Vallentyne J, Singh A, Patil JA, et al. Detection of dengue-4 virus in Pune, Western India after an absence of 30 years – Its association with two severe cases. Virol J. 2011;8:46. doi: 10.1186/1743-422X-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar RR, Kamal S, Patnaik SK, Sharma RC. Breeding habitats and larval indices of Aedes aegypti in residential areas of Rajahmundry town, Andhra Pradesh. Indian J Med Res. 2002;115:31–6. [PubMed] [Google Scholar]
- 23.Bessoff K, Delorey M, Sun W, Hunsperger E. Comparison of two commercially available dengue virus (DENV) NS1 capture enzyme-linked immunosorbent assays using a single clinical sample for diagnosis of acute DENV infection. Clin Vaccine Immunol. 2008;15:1513–8. doi: 10.1128/CVI.00140-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hang VT, Nguyet NM, Trung DT, Tricou V, Yoksan S, Dung NM, et al. Diagnostic accuracy of NS1 ELISA and lateral flow rapid tests for dengue sensitivity, specificity and relationship to viraemia and antibody responses. PLoS Negl Trop Dis. 2009;3:e360. doi: 10.1371/journal.pntd.0000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Narayanan M, Aravind MA, Thilothammal N, Prema R, Sargunam CS, Ramamurty N. Dengue fever epidemic in Chennai – A study of clinical profile and outcome. Indian Pediatr. 2002;39:1027–33. [PubMed] [Google Scholar]
- 26.Gomber S, Ramachandran VG, Kumar S, Agarwal KN, Gupta P, Gupta P, et al. Hematological observations as diagnostic markers in dengue hemorrhagic fever – A reappraisal. Indian Pediatr. 2001;38:477–81. [PubMed] [Google Scholar]
- 27.Bethell DB, Gamble J, Pham PL, Nguyen MD, Tran TH, Ha TH, et al. Noninvasive measurement of microvascular leakage in patients with dengue hemorrhagic fever. Clin Infect Dis. 2001;32:243–53. doi: 10.1086/318453. [DOI] [PubMed] [Google Scholar]
- 28.Halstead SB. Pathogenesis of dengue: Challenges to molecular biology. Science. 1988;239:476–81. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- 29.Halstead SB. Dengue and Dengue Hemorrhagic Fever. New York: CAB International; 1997. pp. 23–44. [Google Scholar]
- 30.World Health Organization. Dengue Hemorrhagic Fever: Diagnosis, Treatment, Prevention, and Control. 2nd ed. Geneva: World Health Organization; 1997. [Google Scholar]
- 31.Chakravarti A, Kumaria R, Sharma VK, Berry N. Serodiagnosis of dengue infection by rapid immuno chromatography test in a hospital setting in Delhi, India, 1999-2001. Dengue Bull. 2002;23:109–12. [Google Scholar]
- 32.Sharma RS, Panigrahi N, Kaul SM, Shivlal, Barua K, Bhardwaj M. Status report of DF/DHF during 1998 in the National Capital Territory of Delhi, India. Dengue Bull. 1999;23:109–12. [Google Scholar]


