Abstract
Recent work has helped reconcile puzzling results from brainstem transection studies first performed over 60 years ago, which suggested the existence of a sleep-promoting system in the medullary brainstem. It was specifically shown that GABAergic neurons located in the medullary brainstem parafacial zone (PZGABA) are not only necessary for normal slow-wave-sleep (SWS) but that their selective activation is sufficient to induce SWS in behaving animals. In this review we discuss early experimental findings that inspired the hypothesis that the caudal brainstem contained SWS-promoting circuitry. We then describe the discovery of the SWS-promoting PZGABA and discuss future experimental priorities.
Introduction
Sufficient quality and quantity of sleep is the sine qua non for optimal physiologic, neurocognitive and psychologic function. Yet, the ‘how’ and ‘why’ of sleep remain among the most enduring mysteries in the neurosciences. To the former, there exist several conceptual models for how the brain achieves sleep, spanning humoral [1], local network [2], distributed network [3] and circuit-based theories [4,5]. And while these models provide varying levels of explanatory power for “whole brain sleep”, we will emphasize in this review the circuit-based model, as this model assumes that delimited nodes of sub-cortical neurons differentially and specifically contribute to the initiation and maintenance of slow-wave-sleep and its electroencephalographic (EEG) correlate.
The circuit and synaptic bases by which sub-cortical cell populations regulate behavioral and EEG SWS is complex and remains incompletely understood. Previous lesion, transection and stimulation work has suggested not only redundancy in sleep-promoting circuitry but also, and rather remarkably, that the forebrain and brainstem can independently regulate sleep. For instance, early postnatal midbrain transections in kittens revealed the ability of the forebrain and brainstem to drive, albeit often in temporal dissociation, electrocortical and behavioral rhythms, respectively, of sleep [6].
With respect to the forebrain “sleep system”, the preoptic area (POA) plays an important, if indispensable, role in the initiation and maintenance of SWS [7,8]. Within the POA region both the ventrolateral (VLPO) and median preoptic (MnPO) nuclei contain high-density clusters of sleep-active neurons [4,9,10]. These sleep-active VLPO and MnPO neurons are largely GABAergic [11,12] and project to, and hence presumably inhibit, multiple arousal-regulatory centers. Considerable convergent evidence has, in fact, identified a particularly important role for VLPO neurons in the sleep process, including: 1) VLPO neurons show a dramatic increase in firing during SWS [13-15]; 2) cell-body specific lesions of the VLPO produce a sustained and dramatic decrease (∼40-50%) in SWS in rodents [16,17]; and 3) chemo- and opto-genetic activation of VLPO neurons produces sleep (C.B.Saper, perscomm).
As indicated, transection studies - reaching back over 60 years - as well as more recent human imaging studies [18] have inferred the existence of a brainstem “sleep system” but, until recently, a brainstem “homolog” of the POA or VLPO remained elusive. This rather conspicuous temporal gap in identifying and characterizing brainstem sleep-promoting circuitry was likely a consequence of several issues. First, there is history: the seminal clinico-anatomic studies by von Economo established that relatively discrete inflammatory lesions within the anterior hypothalamus/POA could produce a chronic state of insomnia [19], setting the stage for decades of focused experimental work on the anterior hypothalamus/POA. Second, experimental approaches that proved fruitful in the forebrain, such as excitotoxic lesions, did not prove as successful in the brainstem, often, instead, resulting in subject death. Third, there is extensive interdigidation of excitatory(glutamate) and inhibitory (GABA) neurons in the cellular brainstem, rendering the interpretation of nonselective experimental ablation, stimulation or inhibition experiments challenging. Fourth, the rodent brainstemis located deep in the skull, below the cerebellum and is therefore far less accessible than the forebrain. Taken together, it is perhaps not surprising that until the recent advent of molecular–genetic tools [20,21], which enabled selective and reversible interrogation of cell populations, a considerable void existed in the literature regarding brainstem sleep systems. In this review, we will describe the seminal historical work that first inspired the hypothesis of a brainstem sleep system, and then move to more recent findings that have identified specific cells and pathways by which the brainstem may control SWS and, finally, we will discuss current knowledge gaps and thoughts on future experimental directions.
Historical studies
The first experimental evidence to suggest that the brainstem might participate in sleep control came from work by Batini who showed that transection of the brainstem just rostral to the origin of the trigeminal roots (i.e., the “pretrigeminal” preparation) produced chronic EEG activation [22]. Batini subsequently showed that the chronic EEG desynchrony/behavioral insomnia in the pretrigeminal preparation was not dependent upon sensory inflow from the auditory or visual systems [23]. And, in fact, findings by others showed that a genuine state of alertness was present, e.g., vertical object tracking, pupillary response to visual stimulus, in the pretrigeminal cats during EEG desynchronization [24-26]. Batini's findings were therefore in stark contrast to that seen following slightly more rostral (∼2-3mm) transections of the brainstem, i.e., Bremer's cerveauisolé preparation, which produced chronic EEG deactivation [27,28]. Hence Batini's findings had important implications, namely that an EEG-synchronizing/sleep-promoting influence might arise from the caudal brainstem and that this sleep-promoting influence was not dependent upon sensory inflow. Conversely, these findings also suggested that tonic sensory inflow, which was preserved in Bremer's cerveauisolé preparation, was insufficient to support EEG activation/behavioral wake. Similar results to that of Batini were obtained by Belucchi who showed using bulbar cooling in the encephalaeisolaecat* that cooling of the medullary floor of the 4th ventricle during slow wave sleep produced EEG and behavioral arousal [29,30]. Taken together, these experimental findings were consonant with Batini's prescient surmise that “a synchronizing, or possibly sleep-inducing, influence exerted by some structure in the caudal brain stem can be tentatively envisaged”.
Building upon Batini's observation in the pretrigeminal preparation stimulation and lesion studies in the early 1960s provided evidence that a “deactivating influence” might be localized within the region of the solitary tract [31-33]. For example, Magnes and colleagues showed that low rate electrical stimulation of the cat solitary tract could induce EEG synchrony, and that this synchrony was not simply due to stimulation parameters used [31]. It was similarly shown that electrical or chemical stimulation of the nucleus of the solitary tract (NTS) could produce a cortical slow-wave-sleep like state [34,35]. Importantly, however, these stimulation studies were performed in anesthetized rats and the evoked synchronized cortical EEG showed a dominant 5 Hz band whereas the typical SWS frequency band is 0.5-4 Hz. In some contrast, a recent study in freely moving cats demonstrated that electrical stimulation of the NTS enhanced cortical EEG theta and beta, but not delta, band power and increased wakefulness [36]. Given however that the NTS is a long nucleus extending most of the dorsal part of the medulla oblongata, and relays inputs from the cranial nerve V (trigeminal), VII (facial) and IX (glossopharyngeal), at different rostro-caudal levels, one might expect the cortical effect of NTS stimulation to be highly dependent upon the actual region of NTS activated. It certainly remains possible that the NTS may contain sleep-promoting cells, and it is our hope that this intriguing possibility will be explored going forward.
Recent studies
Approximately 5 years ago, our group set out to uncover the location and identity of brainstem neurons that might contribute to the regulation of SWS. In doing so, we reasoned that any SWS-promoting brainstem neurons would likely project to and inhibit neurons of the parabrachial nucleus (PB), a cell population that we had previously identified as making an especially important contribution to the maintenance of behavioral and EEG arousal [37]. We initiated our search by retrogradely labeling inputs to the medial PB, and in doing so we uncovered a substantial input from sleep-active neurons in the rostral medulla located lateral and dorsal to the facial nerve, a region we termed the parafacial zone (PZ). Based on this foundational anatomic work, we then showed that (1) cell-specific lesions of the PZ in rats resulted in insomnia, (2) the majority of sleep-active PZ neurons were GABAergic/glycinergic and (3) that targeted genetic disruption of GABAergic/glycinergic transmission from the PZ in mice resulted in large and sustained increases in wakefulness [38]. In other words, our experimental work established that PZGABA neurons were necessary for normal amounts of SWS. We subsequently showed that genetically targeted activation of PZGABA neurons was sufficient to rapidly induce SWS, increase SWS amount and produce consolidated and enhanced cortical slow-wave-activity (SWA), the latter being a marker of SWS depth and quality, in behaving mice [39]. We then used optogenetic-based mapping to define, at least in part, the synaptic and circuit basis by which PZGABA neurons could promote and maintain sleep. We specifically found that PZGABA neurons monosynaptically innervate and release synaptic GABA onto PB neurons that, in turn, project to and release synaptic glutamate onto cortically-projecting neurons of the wake-promoting magnocellular basal forebrain [37,40], suggesting a circuit substrate through which PZGABA neurons can rapidly trigger SWS and modulate the cortical EEG. In sum, by means of genetic-based disruption, activation and inhibition we demonstrated both the sufficiency and necessity of brainstem PZGABA neurons in the regulation of SWS and cortical SWA.
While beyond the scope of this mini-review, a central role for brainstem circuitry in the regulation of rapid-eye-movement (REM) sleep was established over 50 years ago [41]. And similar to the identification and characterization of the SWS-promoting PZ, technical advances have enabled a more refined understanding of the cellular and synaptic basis by which a distributed network of brainstem circuits regulation REM sleep, including the recent identification of GABAergic REM-generator cells in the ventral medulla [42-45].
Conclusions
Acute and selective activation of PZGABA neurons rapidly induces SWS cortical EEG SWA in behaving mice. In fact, both the quantity and quality (operationally defined as greater SWA power) of SWS are enhanced, independent of the time of the day, whereas rapid eye movement sleep (REMS) is inhibited. The strong reproducibility and potent sleep-induction, all in a freely behaving framework, suggest a new and unique mouse model of SWS enhancement, which has the very real potential to permit, for the first time, a direct assessment of the role of SWS per se in a myriad of neurobiologic processes.
Our understanding of how PZGABA neurons, and possibly other, as yet unidentified, brainstem sleep-promoting cell populations, regulate SWS and cortical SWA remains rudimentary. Fortunately, the development of new genetic-based tools, some of which played a fundamental role in defining the PZGABA neurons as a key SWS-promoting brainstem cell population, should enable a rapid and more expansive experimental-based understanding of PZGABA neurobiology. For example, we are currently seeking to understand how the PZGABA neurons are regulated by afferent inputs. It is also remains unclear how the PZ can drive, and even enhance, cortical EEG SWA. The firing pattern of PZGABA neurons across the sleep-wake cycle also remains to be elucidated. Finally, determining how PZGABA neurons “fit” within the different models for sleep-wake control, e.g., flip-flop switch, and how PZGABA neuronal activity may be affected by circadian and homeostatic influences have become high experimental priorities.
Figure. The sleep-promoting parafacial Zone (PZ): neuronal circuitry and behavior.
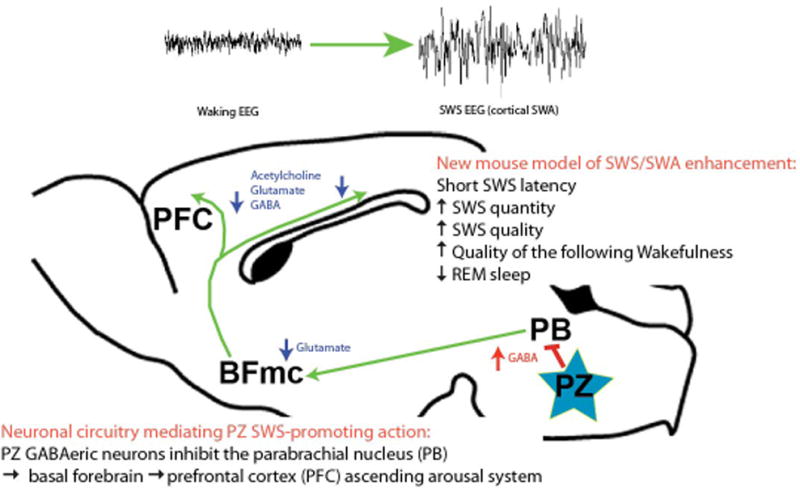
How can in vivo activation of PZGABA neurons potently and rapidly induce SWS and cortical SWA? Optogenetic-based circuit mapping suggests one possible circuit basis for these effects: activation of PZGABA neurons results in synaptic release of GABA (red arrow) onto BF-projecting PB neurons (green arrow). Inhibition of glutamatergic PB neurons reduces synaptic release of glutamate (blue arrow) onto cortically-projecting BF neurons, in turn reducing cortical levels of wake-enhancing acetylcholine, glutamate and GABA. Hence the net result is a dramatic reduction in ascending arousal influences normally provided by the PB and BF to the cortex. This reduction in arousal inputs is reflected in the cortical EEG as a decrease in the fast frequencies that are characteristic of cortical activation/desynchronization and wakefulness, the appearance of slow waves and delta waves that are characteristic of cortical synchronization and SWS and behavioral sleep. BF: basal forebrain; PB: parabrachial nucleus; PFC: prefrontal cortex; PZ: parafacial zone; SWS: slow-wave-sleep; SWA: slow-wave activity. Modified from [39].
Highlights.
- The mammalian brainstem contains sleep-promoting circuitries
- GABAergic parafacial zone (PZGABA) neurons are sleep-promoting in vivo
- The circuit basis by which PZGABA neurons induce sleep remains incompletely understood
Acknowledgments
National Institute of Health grants K99 MH103399 (C.A.) and R01 NS092652 (P.M.F.)
Footnotes
the bulbar cooling model was preferred for brainstem-based studies because it helped avoid the contaminating effects of autonomic and respiratory changes, which are typically evoked by stimulation of the medulla.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clinton JM, Davis CJ, Zielinski MR, Jewett KA, Krueger JM. Biochemical regulation of sleep and sleep biomarkers. J Clin Sleep Med. 2011;7:S38–42. doi: 10.5664/JCSM.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vyazovskiy VV, Olcese U, Hanlon EC, Nir Y, Cirelli C, Tononi G. Local sleep in awake rats. Nature. 2011;472:443–447. doi: 10.1038/nature10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gulia KK. Dynamism in Activity of the Neural Networks in Brain is the Basis of Sleep-Wakefulness Oscillations. Front Neurol. 2012;3:38. doi: 10.3389/fneur.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *4.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68:1023–1042. doi: 10.1016/j.neuron.2010.11.032. Saper et al. is an excellent review for the interested reader seeking more details on the cellular and circuit basis by which the brain regulates wake-sleep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *5.Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92:1087–1187. doi: 10.1152/physrev.00032.2011. Brown et al. is another excellent review for the interested reader seeking more details on the cellular and circuit basis by which the brain regulates wake-sleep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villablanca JR, de Andres I, Olmstead CE. Sleep-waking states develop independently in the isolated forebrain and brain stem following early postnatal midbrain transection in cats. Neuroscience. 2001;106:717–731. doi: 10.1016/s0306-4522(01)00329-3. [DOI] [PubMed] [Google Scholar]
- 7.McGinty DJ, Sterman MB. Sleep suppression after basal forebrain lesions in the cat. Science. 1968;160:1253–1255. doi: 10.1126/science.160.3833.1253. [DOI] [PubMed] [Google Scholar]
- 8.Nauta WJ. Hypothalamic regulation of sleep in rats; an experimental study. J Neurophysiol. 1946;9:285–316. doi: 10.1152/jn.1946.9.4.285. [DOI] [PubMed] [Google Scholar]
- 9.Szymusiak R, Gvilia I, McGinty D. Hypothalamic control of sleep. Sleep Med. 2007;8:291–301. doi: 10.1016/j.sleep.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271:216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- 11.Gong H, McGinty D, Guzman-Marin R, Chew KT, Stewart D, Szymusiak R. Activation of c-fos in GABAergic neurones in the preoptic area during sleep and in response to sleep deprivation. J Physiol. 2004;556:935–946. doi: 10.1113/jphysiol.2003.056622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sherin JE, Elmquist JK, Torrealba F, Saper CB. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci. 1998;18:4705–4721. doi: 10.1523/JNEUROSCI.18-12-04705.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szymusiak R, Alam N, Steininger TL, McGinty D. Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res. 1998;803:178–188. doi: 10.1016/s0006-8993(98)00631-3. [DOI] [PubMed] [Google Scholar]
- 14.Alam MA, Kumar S, McGinty D, Alam MN, Szymusiak R. Neuronal activity in the preoptic hypothalamus during sleep deprivation and recovery sleep. J Neurophysiol. 2014;111:287–299. doi: 10.1152/jn.00504.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gvilia I, Xu F, McGinty D, Szymusiak R. Homeostatic regulation of sleep: a role for preoptic area neurons. J Neurosci. 2006;26:9426–9433. doi: 10.1523/JNEUROSCI.2012-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu J, Greco MA, Shiromani P, Saper CB. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci. 2000;20:3830–3842. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vetrivelan R, Saper CB, Fuller PM. Armodafinil-induced wakefulness in animals with ventrolateral preoptic lesions. Nat Sci Sleep. 2014;6:57–63. doi: 10.2147/NSS.S53132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dang-Vu TT, Schabus M, Desseilles M, Albouy G, Boly M, Darsaud A, Gais S, Rauchs G, Sterpenich V, Vandewalle G, et al. Spontaneous neural activity during human slow wave sleep. Proc Natl Acad Sci U S A. 2008;105:15160–15165. doi: 10.1073/pnas.0801819105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Economo C. Sleep as a problem of localization. The Journal of Nervous and Mental Disease. 1930;71:1–5. [Google Scholar]
- 20.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 21.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Batini C, Moruzzi G, Palestini M, Rossi GF, Zanchetti A. Presistent patterns of wakefulness in the pretrigeminal mid pontine preparation. Science. 1958;128:30–32. doi: 10.1126/science.128.3314.30-a. [DOI] [PubMed] [Google Scholar]
- 23.Batini C, Palestini M, GF R, Zanchetti A. EEG activation patterns in the mid-pontine pretrigeminal cat following sensory deafferentation. Archives Italiennes de Biologie. 1959;97:26–32. [Google Scholar]
- 24.Affanni J, Marchiafava PL, Zernicki B. Higher nervous activity in cats with midpontine pretrigeminal transections. Science. 1962;137:126–127. doi: 10.1126/science.137.3524.126. [DOI] [PubMed] [Google Scholar]
- 25.King FA, Marchiafava PL. Ocular Movements in the Midpontine Pretrigeminal Preparation. Arch ItalBiol. 1963;101:149–160. [PubMed] [Google Scholar]
- 26.Affanni J, Marchiafava PL, Zernicki B. Orientation reactions in the midpontine pretrigeminal cat. ArchItalBiol. 1962;100:297–304. [PubMed] [Google Scholar]
- 27.Bremer F. Cerveau “isolé ” etphysiologie du sommeil. Comptesrendus de la Société de Biologie. 1935;118:1235–1241. [Google Scholar]
- 28.Bremer F. L'activitécérébrale au cours du sommeilet de la narcose. Contribution à l'étudemécanistique du sommeil Academieroyale de medecine de Belgique. 1937;2:68–86. [Google Scholar]
- 29.Berlucchi G, Maffei L, Moruzzi G, Strata P. Eeg and Behavioral Effects Elicited by Cooling of Medullaand Pons. Arch Ital Biol. 1964;102:372–392. [PubMed] [Google Scholar]
- 30.Berlucchi G. Callosal activity in unrestrained, unanesthetized cats. Arch Ital Biol. 1965;103:623–634. [PubMed] [Google Scholar]
- 31.Magnes J, Moruzzi G, Pompeiano O. Synchronization of the EEG produced by low-frequency electrical stimulation of the region of the solitary tract. Archives Italiennes de Biologie. 1961;99:33–41. [Google Scholar]
- 32.Bonvallet M, Bloch V. Bulbar Control of Cortical Arousal. Science. 1961;133:1133–1134. doi: 10.1126/science.133.3459.1133. [DOI] [PubMed] [Google Scholar]
- 33.Bonvallet M, Allen MB., Jr Prolonged Spontaneous and Evoked Reticular Activation Following Discrete Bulbar Lesions. Electroencephalogr Clin Neurophysiol. 1963;15:969–988. doi: 10.1016/0013-4694(63)90141-x. [DOI] [PubMed] [Google Scholar]
- 34.Laguzzi R, Reis DJ, Talman WT. Modulation of cardiovascular and electrocortical activity through serotonergic mechanisms in the nucleus tractus solitarius of the rat. Brain Res. 1984;304:321–328. doi: 10.1016/0006-8993(84)90336-6. [DOI] [PubMed] [Google Scholar]
- 35.Golanov EV, Reis DJ. Neurons of nucleus of the solitary tract synchronize the EEG and elevate cerebral blood flow via a novel medullary area. Brain Res. 2001;892:1–12. doi: 10.1016/s0006-8993(00)02949-8. [DOI] [PubMed] [Google Scholar]
- 36.Martinez-Vargas D, Valdes-Cruz A, Magdaleno-Madrigal VM, Fernandez-Mas R, Almazan-Alvarado S. Effect of Electrical Stimulation of the Nucleus of the Solitary Tract on Electroencephalographic Spectral Power and the Sleep-Wake Cycle in Freely Moving Cats. Brain Stimul. 2016 doi: 10.1016/j.brs.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 37.Fuller P, Sherman D, Pedersen NP, Saper CB, Lu J. Reassessment of the structural basis of the ascending arousal system. J Comp Neurol. 2011;519:933–956. doi: 10.1002/cne.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **38.Anaclet C, Lin JS, Vetrivelan R, Krenzer M, Vong L, Fuller PM, Lu J. Identification and characterization of a sleep-active cell group in the rostral medullary brainstem. J Neurosci. 2012;32:17970–17976. doi: 10.1523/JNEUROSCI.0620-12.2012. This study was the first to reveal the medullary GABAergic parafacial zone contained sleep-active neurons and that disruption of cell-body specific lesions of the PZ in rats and genetic disruption of PZ GABAergic neurotransmission in mice produced insomnia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **39.Anaclet C, Ferrari L, Arrigoni E, Bass CE, Saper CB, Lu J, Fuller PM. The GABAergic parafacial zone is a medullary slow wave sleep-promoting center. Nat Neurosci. 2014;17:1217–1224. doi: 10.1038/nn.3789. This study, which built upon the previous work by Anaclet and colleagues (2012, ref #38), used targeted chemo- and opto-genetic based approaches to show that activation of PZGABA neurons in vivo is potently sleep promoting and that a functional PZ-parabrachial-basal forebrain-cerebral cortex circuit may be the circuit substrate through which PZGABA neurons trigger sleep and cortical slow wave activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **40.Anaclet C, Pedersen NP, Ferrari LL, Venner A, Bass CE, Arrigoni E, Fuller PM. Basal forebrain control of wakefulness and cortical rhythms. Nat Commun. 2015;6:8744. doi: 10.1038/ncomms9744. This study is the first to chemogenetically parse the contributions of individual cell populations in EEG and behavioral arousal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jouvet M. Research on the neural structures and responsible mechanisms in different phases of physiological sleep. Arch Ital Biol. 1962;100:125–206. [PubMed] [Google Scholar]
- 42.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- *43.Kroeger D, Ferrari LL, Petit G, Mahoney CE, Fuller PM, Arrigoni E, Scammell TE. Cholinergic, Glutamatergic, and GABAergic Neurons of the Pedunculopontine Tegmental Nucleus Have Distinct Effects on Sleep/Wake Behavior in Mice. J Neurosci. 2017;37:1352–1366. doi: 10.1523/JNEUROSCI.1405-16.2016. This study is the first to parse the contributions of individual cell populations within the pedunculopontine tegmental nucleus in behavioral state control. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **44.Weber F, Chung S, Beier KT, Xu M, Luo L, Dan Y. Control of REM sleep by ventral medulla GABAergic neurons. Nature. 2015;526:435–438. doi: 10.1038/nature14979. This landmark study was the first to elucidate a population of REM-generator cells in the ventral medulla. The small cluster of GABAergic neurons is both sufficient and necessary for normal REM sleep amounts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *45.Chen MC, Vetrivelan R, Guo CN, Chang C, Fuller PM, Lu J. Ventral medullary control of rapid eye movement sleep and atonia. Exp Neurol. 2017;290:53–62. doi: 10.1016/j.expneurol.2017.01.002. This very recent study revealed that a population of VGAT+ neurons located in the ventromedial medulla (at a level rostral to the inferior olive) critically contributes to REM sleep and motor control. [DOI] [PMC free article] [PubMed] [Google Scholar]


