Abstract
Background:
Obstructive sleep apnea (OSA) is commonly associated with morbid obesity. Weight loss following bariatric surgery results in resolution or improvement of OSA. However, few studies have done objective assessment of the impact of bariatric surgery on OSA.
Objective:
The aim of this study was to assess the outcome of bariatric surgery on OSA.
Setting:
The study was conducted in the teaching institution of a tertiary care centre.
Methods:
Twenty-seven morbidly obese patients seeking bariatric surgery were administered Epworth Sleepiness Scale (ESS) health questionnaire and subjected to overnight polysomnography. Repeat assessment using ESS and polysomnography was done at 3–6 months after surgery.
Results:
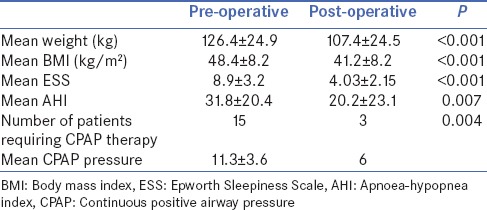
Mean age was 42.4 ± 10.5 years, and majority (77.8%) were female. The mean pre-operative weight and body mass index (BMI) were 126.4 ± 24.9 kg and 48.4 ± 8.2 kg/m2, respectively. Nearly 29.6% patients had symptoms of excessive daytime somnolence based on ESS score and overnight polysomnography detected the presence of OSA in 96.3% patients, of which 51.9% had severe OSA. At mean follow-up of 5.2 ± 2.5 months after surgery, mean weight and BMI decreased to 107.4 ± 24.5 kg and 41.2 ± 8.2 kg/m2, respectively. Mean ESS score and mean apnoea–hypopnea index declined from 8.9 ± 3.2 to 4.03 ± 2.15 (P < 0.001) and from 31.8 ± 20.4 to 20.2 ± 23.1 (P = 0.007), respectively. Number of patients requiring continuous positive airway pressure (CPAP) therapy declined from 15 to 3 and average CPAP requirement came down from 11.3 cm of H2O to 6 cm of H2O.
Conclusion:
OSA was present in a significant proportion of patients undergoing bariatric surgery. Bariatric surgery resulted in significant improvement in both subjective and objective parameters of OSA.
Keywords: Bariatric surgery, Epworth Sleepiness Scale, obstructive sleep apnoea–hypopnea syndrome, polysomnography
INTRODUCTION
Obstructive sleep apnea (OSA) is characterised by successive episodes of cessation or decrease in respiratory airflow. OSA is closely associated with obesity, its incidence is known to increase with increase in body mass index (BMI). OSA induces alveolar hypoventilation and respiratory insufficiency and thereby contributes to the development of pulmonary hypertension and cor pulmonale. Other notable health hazards of OSA include premature death, sudden death from cardiac causes and traffic accidents.[1] As OSA increases the likelihood of adverse events in post-operative period, diagnosing and treating this condition forms an integral part of pre-operative workup of morbidly obese patients undergoing bariatric surgery.[2]
OSA is suspected on the basis of characteristic history (snoring and daytime sleepiness) and physical examination (increased neck circumference); however, overnight polysomnography is needed to confirm the diagnosis. Treatment of OSA includes weight loss and administration of continuous positive airway pressure (CPAP) therapy.[1]
Weight loss by non-surgical means is non-satisfactory and non-sustainable, while bariatric surgery has been proven to be the most effective mean for durable weight loss. In addition to weight loss, bariatric surgery results in cure or improvement of OSA in a significant proportion of patients.[3] However, there is a paucity of data from Indian subcontinent regarding outcome of bariatric surgery on OSA. This study was conducted to assess the short-term outcome of bariatric surgery on OSA.
METHODS
This study was approved by our Institutional Review Board, and all the patients included gave informed written consent for their participation in the study.
Patient population
Twenty-seven morbidly obese patients who underwent bariatric surgery during October 2012 to October 2013 were included in this study. All patients underwent a detailed pre-operative evaluation by a dedicated multidisciplinary bariatric team.
Epworth Sleepiness Scale
A health questionnaire known as Epworth Sleepiness Scale (ESS) was administered to measure daytime somnolence. This validated scoring system measures the tendency to fall asleep during the day and distinguishes patients with OSA from those with primary snoring. Patients rated their tendency to fall asleep on eight items with ratings from 0 to 3, where 0 indicated no chance of dozing and 3 indicated a high chance of dozing during the activity. Thus, scores could range from 0 to 24 with higher scores indicating more sleepiness. An ESS score >10 was considered as indicative of the presence of excessive daytime sleepiness.[4,5]
Polysomnography
Irrespective of ESS scores, all patients were subjected to polysomnography. Standard overnight polysomnography was performed at the sleep laboratory of our institution using Alice 5 Acquisition System (USA). Sleep was recorded using central and occipital electroencephalographic leads, bilateral electro-oculogram and a submental electromyogram (EMG). Respiration was monitored using continuous pulse oximetry, a snoring microphone, a nasal pressure transducer, oral and nasal thermistors and chest and abdominal piezo electrodes. Heart rate was continually monitored using a modified V2 lead; bilateral anterior tibialis EMG leads were used to detect periodic limb movements.
All records were visually scored in 30 s epochs using Rechtschaffen and Kales criteria.[6] Respiratory events were scored according to ‘American Association of Sleep Medicine 2007’. Apnoea and hypopnea events and the apnoea–hypopnea index (AHI) were defined according to the recommendations of the American Academy of Sleep.[7] The severity of OSA was defined by AHI, where a frequency of 5–15 events/hour was considered mild, >15–30 as moderate and >30 events/hour as severe. OSA was considered absent when AHI was <5 events/hour. Improvement was defined as decrease in AHI accompanied by a decrease in the severity of the disease. Failure was considered when there was no decrease in AHI.
Bariatric surgery
The operative procedures offered to patients were laparoscopic sleeve gastrectomy and laparoscopic gastric bypass. Selection of operative procedure was performed in consultation with the patient after discussing in detail pros and cons of each procedure. These operations were carried out using the standard technique.
Follow-up
Patients were called for follow-up at 3–6 months after surgery. At this time, change in weight, BMI and % excess weight loss was documented and all were administered ESS questionnaire. Overnight polysomnography was carried out in all patients and CPAP titration if required.
Statistical analysis
Normally distributed variables were described as means and standard deviations. Categorical variables were given in numbers and percentages. Quantitative data were compared using Student's t-test for paired samples. Correlations were studied by Spearman's correlation coefficient. P < 0.05 was considered statistically significant. Data were analysed using IBM SPSS Statistics 20 (SPSS, Chicago, IL, USA).
RESULTS
Mean age of the study population was 42.4 ± 10.5 years and majority of patients (77.8%) were female. The mean preoperative weight and BMI of study population were 126.4 ± 24.9 kg and 48.4 ± 8.2 kg/m2, respectively.
Bariatric surgery
Laparoscopic sleeve gastrectomy was the predominant bariatric procedure performed in 22 patients (81.5%) and the remaining 5 patients (18.5%) underwent laparoscopic Roux-en-Y gastric bypass. The perioperative period was uneventful with no major complication seen in any of the patients. All patients were extubated in the operative room after conclusion of the operative procedure. No major cardiopulmonary event was encountered in the post-operative setting and none required intensive care unit care.
Epworth Sleepiness Scale
On pre-operative assessment, the mean ESS score was 8.9 ± 3.2. Excessive daytime somnolence was considered to be present if ESS score >10. Based on ESS score, only eight patients (29.6%) had excessive daytime somnolence.
Polysomnography
On further evaluation with polysomnography, out of 27 patients, 26 were found to have OSA and mean AHI was 31.8 ± 20.4. Fourteen patients (51.9%) were diagnosed to have severe OSA, six (22.2%) had moderate OSA and another six (22.2%) had mild OSA. Only one (3.7%) patient did not have polysomnographic evidence of OSA.
Fifteen patients were recommended to use CPAP therapy for the management of moderate-to-severe OSA. The mean CPAP level required before surgery was 11.3 ± 3.6 cm of H2O (range 5–16 cm of H2O).
Follow-up
At mean follow-up of 5.2 ± 2.5 months, all patients underwent repeat evaluation by ESS scoring and overnight polysomnography. The change in clinical characteristics, ESS score and polysomnography findings is summarised in Table 1. Mean weight and BMI of study population decreased to 107.4 ± 24.5 kg and 41.2 ± 8.2 kg/m2, respectively. The decrease in weight and BMI was significant (P < 0.001). The mean AHI in the follow-up period was 20.2 ± 23.1 and the mean ESS score was 4.03 ± 2.15. There was a significant improvement in mean AHI (P = 0.007) and ESS (P < 0.001).
Table 1.
Pre- and post-operative clinical parameters, Epworth Sleepiness Scale score and apnoeahypopnea index after bariatric surgery
Significant change in the trend of severity of OSA was seen after surgery as depicted in Figure 1. Amongst fourteen patients with severe OSA, one was cured, four downgraded to mild OSA, another four downgraded to moderate OSA and only five patients persisted to have severe OSA. Out of six patients with moderate OSA, two were cured, three downgraded to mild and one upgraded to severe OSA. The one patient who did not have OSA (AHI-2.4) in pre-operative period developed mild OSA (AHI-9.4), and amongst six patients with mild OSA, five were cured while only one had persistent OSA.
Figure 1.
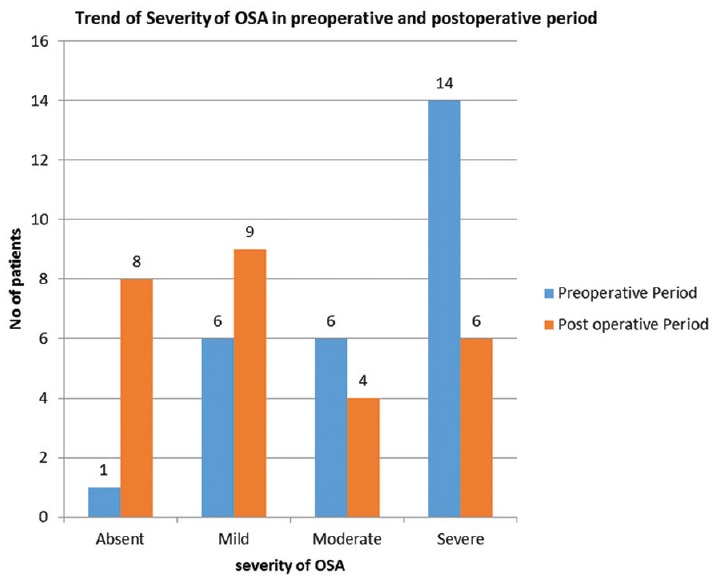
Change in the trend of severity of obstructive sleep apnoea–hypopnea syndrome after bariatric surgery
After surgery, only three patients were advised to use CPAP therapy and the mean CPAP pressure requirement decreased significantly to 6 cm.
Correlation
In this study, BMI was not found to correlate with AHI (P = 0.526) or ESS score (P = 0.332) or the requirement of CPAP levels (P = 0.481). However, requirement of CPAP levels correlated significantly with preoperative AHI and ESS scores (P = 0.001 and P < 0.001, respectively).
DISCUSSION
In this study, overnight polysomnography revealed the presence of OSA in 96.3% patients, whereas only 29.6% patients were found to have symptoms of excessive daytime somnolence. More than 70% patients had moderate-to-severe OSA. This suggests high prevalence of OSA despite the absence of patient-reported symptoms. Similar studies have also reported high prevalence of OSA amongst patients undergoing bariatric surgery ranging between 77% and 88%.[8,9] Previous studies also have shown a high prevalence of OSA in patients with obesity despite the absence of patient-reported symptoms.[10] Patients’ planning bariatric surgery may under-report symptoms for various reasons. Possible explanation for the low reports of functional impairment is the inability to recognise sleepiness. Cognitive deficits have been well documented in patients with obesity.[11]
Documentation and optimisation of OSA before bariatric surgery is warranted to minimise untoward consequences of OSA in the post-operative period.[12] Based on our study, we recommend routine polysomnographic evaluation of all patients undergoing bariatric surgery. It is our routine practice to encourage these patients to lose 5–6 kg weight before bariatric surgery by putting them on very low calorie liquid diet, and application of CPAP device is recommended at least 3–4 weeks before surgery. These two measures result in subjective improvement of OSA before surgery and minimise pulmonary complications after surgery.
Despite the high prevalence of OSA amongst morbidly obese patients undergoing bariatric surgery, correlation between pre-operative BMI and AHI was not statistically significant in this study which could be attributed to small sample size of the study population.
Our data show that weight loss achieved by bariatric surgery provided substantial benefits to morbidly obese patients with OSA reflected by improvement in both subjective parameters and objective parameters. Benefits included significant AHI reductions, reduction or discontinuation of CPAP therapy and improvement in ESS scores reflecting improvement in excessive daytime somnolence. Following bariatric surgery, some patients no longer had sleep apnoea (30%), the majority markedly improved (70%) and only minority (30%) showed no improvement or worsening of OSA. Even when sleep apnoea remains unchanged or worsened, there was an improvement in subjective parameters, measured by ESS scores. All of them (100%) did not show symptoms of excessive daytime somnolence after surgery.
Haines et al. evaluated 349 patients undergoing bariatric surgery with preoperative polysomnography and 289 (83%) patient had evidence of OSA. Post-operative polysomnography was performed in 101 patients, and at median duration of 11 months, mean respiratory disturbance index (RDI) decreased from 51 ± 4 to 15 ± 2. ESS score decreased from 10 ± 1 to 6 ± 1 after 3 months of surgery. These results are in concordance with our results. However, the follow-up duration in their study was longer which resulted in greater reduction in RDI as compared to our study.[3] Other authors have also reported similar improvement in OSA after bariatric surgery.[13,14,15]
Resolution of sleep apnoea is related to improvement in the passage of the upper airway associated with weight loss and reduction of the upper airway fatty tissue. Decrease in visceral adiposity leads to improved diaphragmatic excursion and improved ventilation and oxygenation.[16] Patients with sleep apnoea and obesity are known to have increased levels of pro-inflammatory cytokines such as interleukin 6 (IL-6) and tumour necrosis factor-alpha (TNF-α). Bariatric surgery results in decrease of IL-6 and other systemic inflammatory markers, whereas it leads to increased anti-inflammatory cytokine such as IL-8.[17] Level of soluble TNF-α receptor 2 has been found to decrease after bariatric surgery which is an independent predictor of sleep apnoea improvement.[18]
One of the significant observations in our study was that the improvement in OSA was evident very early after surgery much before the desired weight loss. Similar phenomenon has also been reported by Varela et al. and Haines et al.[3,13] Ashrafian et al. proposed that metabolic surgery by their BRAVE effect leads to improvement in type 2 diabetes mellitus, insulin resistance and metabolic syndrome.[19] As OSA and metabolic syndrome are closely interlinked, improvement in metabolic syndrome will lead to early resolution of OSA.[20]
Few patients in our study had evidence of non-improvement or worsening of AHI despite good weight loss after surgery. As OSA is a multifactorial disease, permanent structural changes in the airway and/or central nervous system structures which cannot be reversed by bariatric surgery may be responsible for non-improvement in OSA despite good weight loss after surgery. This suggests that patient with residual OSA and worsening OSA should be kept on regular follow-up and should undergo repeat polysomnography and treatment with CPAP.
This study has some limitations. We admit that the sample size is small and follow-up duration after surgery is short, post-operative polysomnography was done much before the achievement of desired weight loss after surgery. Weight loss after surgery is gradual and patients often achieve nadir weight at 12–24 months after surgery. It is possible that these patients will have further improvement in their symptoms after subsequent weight loss, and hence the improvement in OSA after surgery could have been underestimated in this study. However, after successful weight loss following bariatric surgery and subjective improvement in the overall quality of life, patients are reluctant to undergo overnight polysomnography. Furthermore, we had consistently noticed that some comorbidity such as type 2 diabetes mellitus and sleep apnoea improve dramatically within 3 months after surgery; hence, we decided to record our results at the end of 3–6 months following surgery. We need to follow these patients longer to document long-term outcomes after surgery.
CONCLUSION
Clinically significant OSA was present in >50% of morbidly obese Indian patients undergoing bariatric surgery. Relying exclusively on patient's subjective symptom score for diagnosing OSA can miss a significant proportion of patients with OSA. Hence, routine polysomnography is recommended to document the presence and severity of OSA. Bariatric surgery leads to resolution/improvement of the patient's OSA, as measured by AHI and ESS scores. These beneficial effects are evident as early as 3–6 months after surgery. Not all patients will achieve cure in OSA; hence, they should be kept under regular follow-up and post-operative sleep study and repeat CPAP titration should be performed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360:237–45. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 2.Dixon JB, Schachter LM, O’Brien PE. Predicting sleep apnea and excessive day sleepiness in the severely obese: Indicators for polysomnography. Chest. 2003;123:1134–41. doi: 10.1378/chest.123.4.1134. [DOI] [PubMed] [Google Scholar]
- 3.Haines KL, Nelson LG, Gonzalez R, Torrella T, Martin T, Kandil A, et al. Objective evidence that bariatric surgery improves obesity-related obstructive sleep apnea. Surgery. 2007;141:354–8. doi: 10.1016/j.surg.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–81. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 5.Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth Sleepiness Scale. Chest. 1993;103:30–6. doi: 10.1378/chest.103.1.30. [DOI] [PubMed] [Google Scholar]
- 6.Rechtschaffen A, Kales A, editors. A Manual of Standardized Terminology, Techniques and Scoring System of Sleep Stages in Human Subjects. Los Angeles: Brain Information Service/Brain Research Institute, University of California; 1968. [Google Scholar]
- 7.American Academy of Sleep. Medicine sleep related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. Report of the American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 8.Frey WC, Pilcher J. Obstructive sleep-related breathing disorders in patients evaluated for bariatric surgery. Obes Surg. 2003;13:676–83. doi: 10.1381/096089203322509228. [DOI] [PubMed] [Google Scholar]
- 9.O’Keeffe T, Patterson EJ. Evidence supporting routine polysomnography before bariatric surgery. Obes Surg. 2004;14:23–6. doi: 10.1381/096089204772787248. [DOI] [PubMed] [Google Scholar]
- 10.Foster GD, Sanders MH, Millman R, Zammit G, Borradaile KE, Newman AB, et al. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care. 2009;32:1017–9. doi: 10.2337/dc08-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RB. Lower cognitive function in the presence of obesity and hypertension: The Framingham heart study. Int J Obes Relat Metab Disord. 2003;27:260–8. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- 12.Ballantyne GH, Svahn J, Capella RF, et al. Predictors of prolonged hospital stay following open and laparoscopic gastric bypass for morbid obesity: Body mass index, length of surgery, sleep apnoea, asthma and the metabolic syndrome. Obes Surg. 2004;14:1042–50. doi: 10.1381/0960892041975460. [DOI] [PubMed] [Google Scholar]
- 13.Varela JE, Hinojosa MW, Nguyen NT. Resolution of obstructive sleep apnea after laparoscopic gastric bypass. Obes Surg. 2007;17:1279–82. doi: 10.1007/s11695-007-9228-6. [DOI] [PubMed] [Google Scholar]
- 14.Fritscher LG, Mottin CC, Canani S, Chatkin JM. Obesity and obstructive sleep apnea-hypopnea syndrome: The impact of bariatric surgery. Obes Surg. 2007;17:95–9. doi: 10.1007/s11695-007-9012-7. [DOI] [PubMed] [Google Scholar]
- 15.Sarkhosh K, Switzer NJ, El-Hadi M, Birch DW, Shi X, Karmali S. The impact of bariatric surgery on obstructive sleep apnea: A systematic review. Obes Surg. 2013;23:414–23. doi: 10.1007/s11695-012-0862-2. [DOI] [PubMed] [Google Scholar]
- 16.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: A systematic review and meta-analysis. JAMA. 2004;292:1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 17.Kopp HP, Kopp CW, Festa A, Krzyzanowska K, Kriwanek S, Minar E, et al. Impact of weight loss on inflammatory proteins and their association with the insulin resistance syndrome in morbidly obese patients. Arterioscler Thromb Vasc Biol. 2003;23:1042–7. doi: 10.1161/01.ATV.0000073313.16135.21. [DOI] [PubMed] [Google Scholar]
- 18.Pallayova M, Steele KE, Magnuson TH, Schweitzer MA, Smith PL, Patil SP, et al. Sleep apnea determines soluble TNF-a receptor 2 response to massive weight loss. Obes Surg. 2011;21:1413–23. doi: 10.1007/s11695-011-0359-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashrafian H, Bueter M, Ahmed K, Suliman A, Bloom SR, Darzi A, et al. Metabolic surgery: An evolution through bariatric animal models. Obes Rev. 2010;11:907–20. doi: 10.1111/j.1467-789X.2009.00701.x. [DOI] [PubMed] [Google Scholar]
- 20.Ashrafian H, le Roux CW, Rowland SP, Ali M, Cummin AR, Darzi A, et al. Metabolic surgery and obstructive sleep apnoea: The protective effects of bariatric procedures. Thorax. 2012;67:442–9. doi: 10.1136/thx.2010.151225. [DOI] [PubMed] [Google Scholar]


