Abstract
The cornea has unique features that make it a useful model for regenerative medicine studies. It is an avascular, transparent, densely innervated tissue and any pathological changes can be easily detected by slit lamp examination. Corneal sensitivity is provided by the ophthalmic branch of the trigeminal nerve that elicits protective reflexes such as blinking and tearing and exerts trophic support by releasing neuromediators and growth factors. Corneal nerves are easily evaluated for both function and morphology using standard instruments such as corneal esthesiometer and in vivo confocal microscope. All local and systemic conditions that are associated with damage of the trigeminal nerve cause the development of neurotrophic keratitis, a rare degenerative disease. Neurotrophic keratitis is characterized by impairment of corneal sensitivity associated with development of persistent epithelial defects that may progress to corneal ulcer, melting and perforation. Current neurotrophic keratitis treatments aim at supporting corneal healing and preventing progression of corneal damage. Novel compounds able to stimulate corneal nerve recovery are in advanced development stage. Among them, nerve growth factor eye drops showed to be safe and effective in stimulating corneal healing and improving corneal sensitivity in patients with neurotrophic keratitis. Neurotrophic keratitis represents an useful model to evaluate in clinical practice novel neuro-regenerative drugs.
Keywords: neurotrophic keratitis, corneal sensitivity, nerve regeneration, nerve growth factor
Introduction
The cornea is a structure of the anterior segment of the eye that protects the inner ocular tissues and focuses the external light onto the retina. The cornea is composed of five distinct layers: a stratified squamous, non-keratinized, epithelium; an acellular layer of collagen fibers called Bowman’s layer; the stroma, a regular spaced collagen fibrils forming the 90% of cornea’s thickness, that contains fibroblast like cells and nerves; a basal lamina, the Descemet’s membrane; and a single layer of endothelial cells. The cornea has unique properties being avascular, transparent and the most densely innervated tissue of the human body (Muller et al., 2003). Corneal nerves, originating from the ophthalmic branch of the trigeminal nerve, provide mechanical, chemical and thermal sensitivity and exert also a trophic supply to this avascular structure by releasing nutrients and trophic factors (Muller et al., 2003; Shaheen et al., 2014).
Several local and systemic conditions that affect the cornea such as diabetes, dry eye, herpes simplex keratitis and neurotrophic keratitis, are associated with impairment of corneal innervation leading to decrease in tear production and impairment of wound healing (Sacchetti and Lambiase, 2014; Mastropasqua et al., 2017). The cornea is characterized by peculiar properties that make it a unique tissue to be used as a model for regenerative medicine studies, in fact, the cornea can be directly visualized and corneal structures, including nerves may be easily evaluated for both function and morphology using standard instruments such as slit lamp and in vivo confocal microscopy (IVCM) (Cruzat et al., 2016). Recently, the cornea model has been successfully used in phase I/II clinical trials to obtain regulatory approval for cell therapy in limbal stem cell deficiency, a rare corneal disease, orphan of medical treatment. In fact, the possibility of a direct visualization of corneal structures, including the epithelium, associated with well standardized clinical safety and efficacy outcomes, allowed scholars to demonstrate the regenerative effects of epithelial stem cell transplantation (Rama et al., 2017). The cornea represents also a useful model to characterize neuroregeneration and to evaluate novel neuroregenerative therapeutic approaches in humans.
Instruments for the assessment of corneal sensitivity have been developed and some of them are available for clinical use. Among them, the most used is the Cochet-Bonnet aesthesiometer, that allows scholars to easily evaluate mechanical corneal sensitivity. More recently, other clinical devices able to assess more specific sensitivity patterns, including chemical, thermal and mechanical corneal sensitivity, such as the Belmonte’s no-contact esthesiometer, have been introduced for clinical and research purposes (Belmonte et al., 2004; Golebiowski et al., 2011).
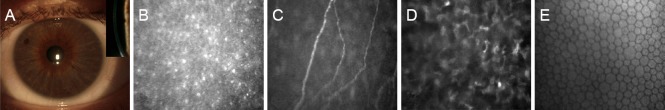
In addition, morphology of corneal nerves can be easily evaluated and visualized by IVCM, that is a rapid and non-invasive imaging method (Cruzat et al., 2017). In vivo confocal microscopy allows scholars to visualize corneal nerves in health and in several ocular and systemic pathologic conditions and to assess their morphology in terms of nerve density and length, nerve branching, reflectivity and tortuosity (Lambiase et al., 2013; Cruzat et al., 2017) (Figure 1). This technique has also been used to assess peripheral neuropathy in patients with diabetes, idiopathic small fibre neuropathy, Charcot-Marie-Tooth Neuropathy Type 1 (CMT1A), Fabry’s disease and paraneoplastic neurological syndromes (Cruzat et al., 2017). In addition, IVCM is used to evaluate in vivo the effects of treatments on corneal nerve regeneration and to evaluate recovery of corneal innervation during pathological conditions or after surgery (Tavakoli et al., 2013; Cruzat et al., 2017; Culver et al., 2017; Lewis et al., 2017).
Figure 1.
A cornea of a healthy eye observed by slit lamp exam with frontal and longitudinal (insert) view.
(A) In vivo confocal microscopy imaging shows the normal superficial corneal epithelium (B), sub-basal plexus (C), anterior stroma (D) and endothelium (E).
Recently, a neurofluorescent mouse model has been developed to study nerve regeneration in vivo. Specifically, the thy1-YFP transgenic mouse allows in vivo visualization of fluorescent sensory nerves and axonal regeneration and degeneration can be visualized and quantified in vivo allowing the possibility to investigate the neurotrophic potential of molecules and drugs (Yu and Rosenblatt, 2007; Leckelt et al., 2016).
Corneal Innervation Morphology and Function
Corneal innervation is provided by trigeminal nerve bundles that lose their perineurium and myelin sheaths and penetrate into the corneal stroma at the corneoscleral limbus, branching into the stromal plexus and then into the subepithelial and subbasal nerve plexus that provide sensitivity to the corneal epithelium (Muller et al., 2003). Corneal primary afferent neurons are represented by polymodal receptors that respond to mechanical, thermal and chemical stimuli, mechanoreceptors activated by mechanical stimuli, and cold receptors that respond to corneal cooling (Muller et al., 2003). Corneal sensory nerves react to ocular surface damage by inducing symptoms of pain and irritation and by eliciting protective reflexes such as blinking and tearing. In addition, corneal nerves provide trophic support to the ocular surface tissues, stimulate wound healing and maintain anatomic integrity by releasing neuromediators and growth factors (Muller et al., 2003; Shaheen et al., 2014; Mastropasqua et al., 2017).
The corneal nerve fibers release trophic factors that warrant trophic support and regulatory action in the corneal epithelium, maintaining the physiological renewal, anatomic integrity and stimulating corneal wound healing (Muller et al., 2003). Corneal nerves express several neuromediators including substance P (SP), calcitonin gene-related peptide (CGRP), acetylcholine, cholecystokinin, noradrenaline, serotonin, neuropeptide Y (NPY), vasointestinal peptide (VIP), met-enkephalin, brain natriuretic peptide, vasopressin and neurotensin (Shaheen et al., 2014). Among them, it has been demonstrated that SP and CGRP are able to modulate corneal epithelial cell proliferation, stratification, migration and adhesion (Mastropasqua et al., 2017). Specifically, it has been demonstrated that administration of SP associated with insulin-like growth factor-1 (IGF-1) increases corneal healing rate and stimulates corneal epithelial cell adhesion (Nishida et al., 2007). Conversely, the corneal epithelium and keratocytes release neuropeptides, neurotrophins and growth factors that influence nerve fiber survival, differentiation, and maturation including nerve growth factor (NGF), brain derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), neurotrophin 3, 4/5, epidermal growth factor (EGF), and glial cell derived neurotrophic factor (GDNF) (Mastropasqua et al., 2017). These mediators represent the main actors of the interplay between corneal epithelium and corneal nerve that mutually release neuromediators and growth factors and activate each other to produce cytokines, neuropeptides and neuromediators for thetrophism and normal healing of the cornea (Mastropasqua et al., 2017). The corneal epithelium produces and releases neurotrophic factors to support nerve trophism and healing, and corneal nerves produce trophic neuromediators for the survival, trophism and healing of the corneal epithelium (Lambiase et al., 1998; Muller et al., 2003; Mastropasqua et al., 2017). Therefore, all local and systemic conditions leading to corneal sensory nerve damage can alter this interaction, causing an impairment of the corneal epithelium physiological renewal and healing rate (Mastropasqua et al., 2017).
Production and release of neuromediators during neurodegenerative diseases of the ocular surface can be easily evaluated by non-invasive, simple techniques such as corneal and conjunctival impression cytology and tear sample collection, that allow scholars to perform biochemical and molecular evaluation (Tervo et al., 1995; Yamada et al., 2000; Lambiase et al., 2011; Sacchetti et al., 2011; Mantelli et al., 2015). Several studies using these techniques have extended results on pathogenic mechanisms and drug effects from animal models to human ocular conditions (Lee et al., 2006; Lee and Kim, 2015).
Clinical Evidence of Corneal Neuroregeneration
Total or partial loss of corneal innervation leads to the development of neurotrophic keratitis (NK). This is a rare, degenerative corneal disease characterized by corneal epithelium breakdown with scarce tendency to healing and development of epithelial defects that may progress to corneal ulcer, melting and eye perforation (Mastropasqua et al., 2017). Several ocular and systemic diseases can damage the fifth cranial nerve at various levels, from the trigeminal nucleus to the corneal nerve endings. The most common causes of NK are herpetic viral keratitis, followed by trigeminal ophthalmic branch damage due to intracranial neoplasia and/or neurosurgical procedures. Other ocular causes of corneal nerve damage include chemical burns, physical injuries, ocular surgery, corneal dystrophies, and chronic topical medications use. Systemic diseases, such as diabetes, multiple sclerosis, congenital syndromes, neurotoxic therapies and leprosy, can also impair corneal sensitivity, leading to NK (Sacchetti and Lambiase, 2014; Hsu and Modi, 2015).
Patients with NK, having a decrease of ocular surface sensitivity, rarely complain symptoms of ocular surface discomfort, while they may refer impaired vision in most severe cases (Sacchetti and Lambiase, 2014). The lack of symptoms in NK patients should be considered in the management of patients with local or systemic conditions causing trigeminal damage, and these patients should be routinely evaluated by an ophthalmologist to identify signs of corneal involvement (Sacchetti and Lambiase, 2014). NK is classified based on the severity of the corneal damage in 3 stages. Stage 1 is characterized by a cloudy appearance of the epithelium associated or not with corneal and conjunctival epithelial damage. Stage 2 is characterized by the presence of a persistent epithelial defect that may progress to corneal ulcer, perforation and/or stromal melting in stage 3.
Diagnosis of NK requires a careful clinical history collection to identify risk factors for trigeminal impairment and a multidisciplinary approach including ophthalmologist, neurologist and neuro-radiologist. The ophthalmologist will perform a complete eye examination with the slit lamp to identify corneal and conjunctival signs of NK and to classify NK severity. The diagnosis of NK is confirmed by the presence of decreased corneal sensitivity. Corneal sensitivity can be qualitatively detected by touching the cornea with the tip of a cotton swab, while severity of corneal sensitivity impairment may be quantified by a corneal aesthesiometer (Sacchetti and Lambiase, 2014). The Cochet-Bonnet aesthesiometer, the most frequently used, assesses corneal sensitivity by touching the cornea with a nylon filament: the length of the filament required to induce a blink reaction or a patient response will quantify the corneal mechanical sensitivity threshold (Sacchetti and Lambiase, 2014).
Corneal nerve imaging by IVCM may also allow the in vivo evaluation of all corneal structures including epithelium, nerves, keratocytes and endothelium (Lambiase et al., 2013). In fact, an IVCM study demonstrated that all corneal structures showed changes in patients with impairment of corneal sensitivity supporting the evidence of the key role of corneal nerves in maintaining homeostasis, survival, metabolism, and renewal of corneal cells (Lambiase et al., 2013) (Figure 2).
Figure 2.
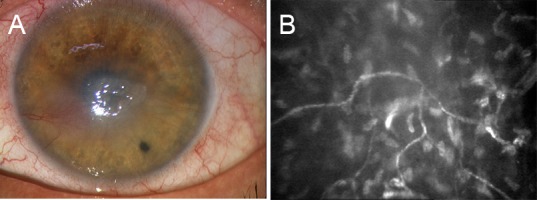
Neurotrophic corneal ulcer (A) associated with impaired corneal innervation as demonstrated by decreased nerve density and increased tortuosity (B) at in vivo confocal microscopy imaging.
Management of NK is a challenge for ophthalmologists because of the lack of specific treatments able to stimulate corneal nerve regeneration and to restore corneal nerve function. Actually, NK treatment options aim at improving corneal healing and preventing progression of corneal damage and treatments able to stimulate corneal nerve regeneration are highly sought-after (Sacchetti and Lambiase, 2014; Mastropasqua et al., 2017). Currently, the use of preservative-free ocular lubricants is a first-line therapy and can be associated with the use of therapeutic contact lens (Lambiase et al., 1999; Sacchetti and Lambiase, 2014). Recently, a new regenerating agent, Cacicol (Laboratoires Théa, France), has been proposed as therapeutic option for treatment of persistent epithelial defects (Aifa et al., 2012; Arvola et al., 2016). The results of a first open-label study evaluating the effects of Cacicol in 11 patients with refractory NK reported a complete corneal healing in 73% of cases (Aifa et al., 2012). These data were not confirmed in a second study that described a complete corneal healing in only 33% of 6 patients after 6–10 weeks of treatment (Arvola et al., 2016). Currently, unresponsive cases of NK require a surgical approach such as tarsorraphy, amniotic membrane transplantation, or conjunctival flap. These approaches are effective in achieving corneal healing, however, they result in poor cosmetic outcome and impairment of visual function (Chen et al., 2000; Hick et al., 2005; Khokhar et al., 2005; Turkoglu et al., 2014; Rajak et al., 2015; Uhlig et al., 2015; Ilic et al., 2016).
In the last decades, novel therapeutic approaches aiming at stimulating epithelial healing and/or corneal nerve regeneration and at restoring corneal nerve function and sensitivity have been proposed. Among them, several studies reported an high success rate in patients with NK treated with autologous serum eye drops, topical non-gelified platelet-rich plasma (PRP) eye drops, and umbilical cord serum eye drops. All these agents provide growth factors, neuromediators, and nutrients to the ocular surface and different studies reported corneal healing in 70% to 97% of cases (Lugo and Arentsen, 1987; Alino et al., 1998; Jeng and Dupps, 2009; Geremicca et al., 2010; Guadilla et al., 2013; Giannaccare et al., 2015; Sanchez-Avila et al., 2017). Based on these encouraging results two randomized clinical trials (RCT) are ongoing to evaluate safety and efficacy of topical plasma rich in growth factors and cord blood in patients with NK (NCT03084861, NCT02707120).
The efficacy of topical treatment with thymosin beta-4 in patients with NK was described in an open study that reported a complete corneal healing in 6 out of 9 patients. A RCT is currently ongoing to evaluate safety and efficacy of topical treatment with thymosin beta-4 in patients with NK at stage 2 and 3 (NCT02600429) (Dunn et al., 2010).
In the last decades, several growth factors and neuroptptides have been shown to exert a positive effect on corneal regeneration, including nerve growth factor (NGF), epidermal growth factor (EGF), SP and insulin-like growth factor-1 (IGF-1), vascular endothelial growth factor (VEGF), semaphorins, neurotrophins 3 and 4 (NT-3; NT-4), growth associated protein-43 (Daniele et al., 1992; Muller et al., 2003; Nishida et al., 2007; Yanai et al., 2015). Despite encouraging results showed by the different trophic factors in stimulating corneal healing in patients with NK, very few demonstrated the efficacy in restoring corneal sensitivity and nerve morphology as demonstrated by in vivo imaging.
The efficacy of topical administration of a combination of a substance P-derived peptide, phenylalanine-glycine-leucine-methionine (FGLM)-amide, with IGF-1 for the treatment of persistent epithelial defects in 11 patients with NK was evaluated in a prospective open-label study that showed a complete epithelial resurfacing in 89% of cases (Nishida et al., 2007). A larger prospective, open-label study confirmed the successful results showing a complete corneal healing in 73% of 25 patients with persistent epithelial defects associated with NK treated with topical FGLM-amide and IGF-1-derived peptide within 4 weeks of treatment (Yamada et al., 2008).
Topical treatment with NGF also represents a promising strategy to induce a durable recovery of trigeminal nerve function. A first open-label study evaluating the efficacy of NGF eye drops treatment in 12 patients with neurotrophic keratitis at stage 2 and 3 reported complete corneal healing in 100% of cases after 10 days to 6 weeks treatment associated with a recovery of corneal sensitivity in most of the patients (Lambiase et al., 1998). A subsequent open-label, non-comparative study confirmed the previous results showing a complete corneal healing in 45 out of 45 eyes with NK at stage 2 and 3 refractive to medical treatments within 6 weeks of treatment. In this study, patients also showed significant improvement of corneal sensitivity, tear function and visual acuity (Bonini et al., 2000).
In addition, the potential effect of NGF in inducing nerve regeneration has been clearly demonstrated in several human diseases, such as diabetic, traumatic and toxic neuropathy (Sacchetti and Lambiase, 2014). Recently, a novel recombinant human NGF has been developed and has successfully completed the clinical development phase and recently received marketing authorization in Europe (EU/1/17/1197) opening new prospective for its next clinical use for inducing corneal neuroregeneration (Ferrari et al., 2014).
An open study reported that treatment with oral nicergoline was effective in reaching a complete corneal healing in 23 out of 27 eyes with NK and in improving corneal sensitivity. These effects were associated with a significant increase in tear level of NGF (Lee and Kim, 2015).
Other neurotrophic factors are under investigation. Specifically, topical treatment with CNTF in mice with wounded corneas showed an increased nerve fiber density 8 weeks after wounding (Reichard et al., 2014). The administration of pigment epithelial-derived factor in association with docosahexaenoic acid after experimental surgery in rabbits showed a 75% of recovery of corneal sensitivity after 7 weeks of treatment (He et al., 2015). Subconjunctival injection of neuropeptide FF in diabetic mice promoted corneal nerve injury recovery and epithelial wound healing (Dai et al., 2015).
Conclusions
The cornea represents a unique model to evaluate in vivo the morphology and function of sensory nerves, their role in maintaining tissue trophism, the crosstalk between nerves and cells and the effect of neuroregenerative drugs. The “human model” of the corneal disease induced by sensory nerve impairment is called NK. In fact, all local and systemic conditions causing damage to the trigeminal nerve may decrease corneal sensitivity and lead to the development of NK that is characterized by the development of non-healing corneal defects associated with lack of ocular symptoms. A multidisciplinary approach to patients with NK will aid at early identifying patients with NK and at improving their clinical outcome. The management of NK is still a challenge, due to the lack of pathogenic treatments targeting nerve regeneration. The development of novel molecules stimulating corneal nerve regeneration will improve the clinical management and outcomes of this disease.
Footnotes
Conflicts of interest: None declared.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer review reports:
Reviewer 1: Rayaz Ahmed Malik, Weill Cornell Medical College in Qatar, Qatar.
Reviewer 2: Steven Levy, MD Stem Cells, USA.
Comments to authors: This is an excellent review of the physiology of the corneal nerves and the etiologies and disease manifestations of Neurotrophic Keratitis (NK). The paper also provides a well researched review of the current and previously published interventions using various neurotrophic factors. Having additional information detailing the completed studies within the paper would be helpful.
References
- Aifa A, Gueudry J, Portmann A, Delcampe A, Muraine M. Topical treatment with a new matrix therapy agent (RGTA) for the treatment of corneal neurotrophic ulcers. Invest Ophthalmol Vis Sci. 2012;53:8181–8185. doi: 10.1167/iovs.12-10476. [DOI] [PubMed] [Google Scholar]
- Alino AM, Perry HD, Kanellopoulos AJ, Donnenfeld ED, Rahn EK. Conjunctival flaps. Ophthalmology. 1998;105:1120–1123. doi: 10.1016/S0161-6420(98)96017-1. [DOI] [PubMed] [Google Scholar]
- Arvola RP, Robciuc A, Holopainen JM. Matrix regeneration therapy: a case series of corneal neurotrophic ulcers. Cornea. 2016;35:451–455. doi: 10.1097/ICO.0000000000000759. [DOI] [PubMed] [Google Scholar]
- Belmonte C, Aracil A, Acosta MC, Luna C, Gallar J. Nerves and sensations from the eye surface. Ocul Surf. 2004;2:248–253. doi: 10.1016/s1542-0124(12)70112-x. [DOI] [PubMed] [Google Scholar]
- Bonini S, Lambiase A, Rama P, Caprioglio G, Aloe L. Topical treatment with nerve growth factor for neurotrophic keratitis. Ophthalmology. 2000;107:1347–1351. doi: 10.1016/s0161-6420(00)00163-9. discussion 1351-1342. [DOI] [PubMed] [Google Scholar]
- Chen HJ, Pires RT, Tseng SC. Amniotic membrane transplantation for severe neurotrophic corneal ulcers. Br J Ophthalmol. 2000;84:826–833. doi: 10.1136/bjo.84.8.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruzat A, Qazi Y, Hamrah P. In vivo confocal microscopy of corneal nerves in health and disease. Ocul Surf. 2016;15:15–47. doi: 10.1016/j.jtos.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culver DA, Dahan A, Bajorunas D, Jeziorska M, van Velzen M, Aarts L, Tavee J, Tannemaat MR, Dunne AN, Kirk RI, Petropoulos IN, Cerami A, Malik RA, Brines M. Cibinetide improves corneal nerve fiber abundance in patients with sarcoidosis-associated small nerve fiber loss and neuropathic pain. Invest Ophthalmol Vis Sci. 2017;58:BIO52–BIO60. doi: 10.1167/iovs.16-21291. [DOI] [PubMed] [Google Scholar]
- Dai Y, Zhao X, Chen P, Yu Y, Wang Y, Xie L. Neuropeptide FF promotes recovery of corneal nerve injury associated with hyperglycemia. Invest Ophthalmol Vis Sci. 2015;56:7754–7765. doi: 10.1167/iovs.15-16513. [DOI] [PubMed] [Google Scholar]
- Daniele S, Gilbard JP, Schepens CL. Treatment of persistent epithelial defects in neurotrophic keratitis with epidermal growth factor: a preliminary open study. Graefes Arch Clin Exp Ophthalmol. 1992;230:314–317. doi: 10.1007/BF00165937. [DOI] [PubMed] [Google Scholar]
- Dunn SP, Heidemann DG, Chow CY, Crockford D, Turjman N, Angel J, Allan CB, Sosne G. Treatment of chronic nonhealing neurotrophic corneal epithelial defects with thymosin beta4. Ann N Y Acad Sci. 2010;1194:199–206. doi: 10.1111/j.1749-6632.2010.05471.x. [DOI] [PubMed] [Google Scholar]
- Ferrari MP, Mantelli F, Sacchetti M, Antonangeli MI, Cattani F, D’Anniballe G, Sinigaglia F, Ruffini PA, Lambiase A. Safety and pharmacokinetics of escalating doses of human recombinant nerve growth factor eye drops in a double-masked, randomized clinical trial. BioDrugs. 2014;28:275–283. doi: 10.1007/s40259-013-0079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geremicca W, Fonte C, Vecchio S. Blood components for topical use in tissue regeneration: evaluation of corneal lesions treated with platelet lysate and considerations on repair mechanisms. Blood Transfus. 2010;8:107–112. doi: 10.2450/2009.0091-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannaccare G, Fresina M, Vagge A, Versura P. Synergistic effect of regenerating agent plus cord blood serum eye drops for the treatment of resistant neurotrophic keratitis: a case report and a hypothesis for pathophysiologic mechanism. Int Med Case Rep J. 2015;8:277–281. doi: 10.2147/IMCRJ.S89968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golebiowski B, Papas E, Stapleton F. Assessing the sensory function of the ocular surface: implications of use of a non-contact air jet aesthesiometer versus the Cochet-Bonnet aesthesiometer. Exp Eye Res. 2011;92:408–413. doi: 10.1016/j.exer.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Guadilla AM, Balado P, Baeza A, Merino M. Effectiveness of topical autologous serum treatment in neurotrophic keratopathy. Arch Soc Esp Oftalmol. 2013;88:302–306. doi: 10.1016/j.oftal.2012.09.033. [DOI] [PubMed] [Google Scholar]
- He J, Cortina MS, Kakazu A, Bazan HE. The PEDF neuroprotective domain plus DHA induces corneal nerve regeneration after experimental surgery. Invest Ophthalmol Vis Sci. 2015;56:3505–3513. doi: 10.1167/iovs.15-16755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hick S, Demers PE, Brunette I, La C, Mabon M, Duchesne B. Amniotic membrane transplantation and fibrin glue in the management of corneal ulcers and perforations: a review of 33 cases. Cornea. 2005;24:369–377. doi: 10.1097/01.ico.0000151547.08113.d1. [DOI] [PubMed] [Google Scholar]
- Hsu HY, Modi D. Etiologies, quantitative hypoesthesia, and clinical outcomes of neurotrophic keratopathy. Eye Contact Lens. 2015;41:314–317. doi: 10.1097/ICL.0000000000000133. [DOI] [PubMed] [Google Scholar]
- Ilic D, Vicovac L, Nikolic M, Lazic Ilic E. Human amniotic membrane grafts in therapy of chronic non-healing wounds. Br Med Bull. 2016;117:59–67. doi: 10.1093/bmb/ldv053. [DOI] [PubMed] [Google Scholar]
- Jeng BH, Dupps WJ., Jr Autologous serum 50% eyedrops in the treatment of persistent corneal epithelial defects. Cornea. 2009;28:1104–1108. doi: 10.1097/ICO.0b013e3181a2a7f6. [DOI] [PubMed] [Google Scholar]
- Khokhar S, Natung T, Sony P, Sharma N, Agarwal N, Vajpayee RB. Amniotic membrane transplantation in refractory neurotrophic corneal ulcers: a randomized, controlled clinical trial. Cornea. 2005;24:654–660. doi: 10.1097/01.ico.0000153102.19776.80. [DOI] [PubMed] [Google Scholar]
- Lambiase A, Rama P, Aloe L, Bonini S. Management of neurotrophic keratopathy. Curr Opin Ophthalmol. 1999;10:270–276. doi: 10.1097/00055735-199908000-00009. [DOI] [PubMed] [Google Scholar]
- Lambiase A, Sacchetti M, Mastropasqua A, Bonini S. Corneal changes in neurosurgically induced neurotrophic keratitis. JAMA Ophthalmol. 2013;131:1547–1553. doi: 10.1001/jamaophthalmol.2013.5064. [DOI] [PubMed] [Google Scholar]
- Lambiase A, Rama P, Bonini S, Caprioglio G, Aloe L. Topical treatment with nerve growth factor for corneal neurotrophic ulcers. N Engl J Med. 1998;338:1174–1180. doi: 10.1056/NEJM199804233381702. [DOI] [PubMed] [Google Scholar]
- Lambiase A, Micera A, Sacchetti M, Cortes M, Mantelli F, Bonini S. Alterations of tear neuromediators in dry eye disease. Arch Ophthalmol. 2011;129:981–986. doi: 10.1001/archophthalmol.2011.200. [DOI] [PubMed] [Google Scholar]
- Leckelt J, Guimaraes P, Kott A, Ruggeri A, Stachs O, Baltrusch S. Early detection of diabetic neuropathy by investigating CNFL and IENFD in thy1-YFP mice. J Endocrinol. 2016;231:147–157. doi: 10.1530/JOE-16-0284. [DOI] [PubMed] [Google Scholar]
- Lee HK, Ryu IH, Seo KY, Hong S, Kim HC, Kim EK. Topical 0.1% prednisolone lowers nerve growth factor expression in keratoconjunctivitis sicca patients. Ophthalmology. 2006;113:198–205. doi: 10.1016/j.ophtha.2005.09.033. [DOI] [PubMed] [Google Scholar]
- Lee YC, Kim SY. Treatment of neurotrophic keratopathy with nicergoline. Cornea. 2015;34:303–307. doi: 10.1097/ICO.0000000000000348. [DOI] [PubMed] [Google Scholar]
- Lewis EJH, Perkins BA, Lovblom LE, Bazinet RP, Wolever TMS, Bril V. Effect of omega-3 supplementation on neuropathy in type 1 diabetes: A 12-month pilot trial. Neurology. 2017;88:2294–2301. doi: 10.1212/WNL.0000000000004033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo M, Arentsen JJ. Treatment of neurotrophic ulcers with conjunctival flaps. Am J Ophthalmol. 1987;103:711–712. doi: 10.1016/s0002-9394(14)74337-5. [DOI] [PubMed] [Google Scholar]
- Mantelli F, Lambiase A, Sacchetti M, Orlandi V, Rosa A, Casella P, Bonini S. Cocaine snorting may induce ocular surface damage through corneal sensitivity impairment. Graefes Arch Clin Exp Ophthalmol. 2015;253:765–772. doi: 10.1007/s00417-015-2938-x. [DOI] [PubMed] [Google Scholar]
- Mastropasqua L, Massaro-Giordano G, Nubile M, Sacchetti M. Understanding the pathogenesis of neurotrophic keratitis: the role of corneal nerves. J Cell Physiol. 2017;232:717–724. doi: 10.1002/jcp.25623. [DOI] [PubMed] [Google Scholar]
- Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76:521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- Nishida T, Chikama T, Morishige N, Yanai R, Yamada N, Saito J. Persistent epithelial defects due to neurotrophic keratopathy treated with a substance p-derived peptide and insulin-like growth factor 1. Jpn J Ophthalmol. 2007;51:442–447. doi: 10.1007/s10384-007-0480-z. [DOI] [PubMed] [Google Scholar]
- Rajak S, Rajak J, Selva D. Performing a tarsorrhaphy. Community Eye Health. 2015;28:10–11. [PMC free article] [PubMed] [Google Scholar]
- Rama P, Ferrari G, Pellegrini G. Cultivated limbal epithelial transplantation. Curr Opin Ophthalmol. 2017;28:387–389. doi: 10.1097/ICU.0000000000000382. [DOI] [PubMed] [Google Scholar]
- Reichard M, Hovakimyan M, Guthoff RF, Stachs O. In vivo visualisation of murine corneal nerve fibre regeneration in response to ciliary neurotrophic factor. Exp Eye Res. 2014;120:20–27. doi: 10.1016/j.exer.2013.12.015. [DOI] [PubMed] [Google Scholar]
- Sacchetti M, Lambiase A. Diagnosis and management of neurotrophic keratitis. Clin Ophthalmol. 2014;8:571–579. doi: 10.2147/OPTH.S45921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti M, Micera A, Lambiase A, Speranza S, Mantelli F, Petrachi G, Bonini S, Bonini S. Tear levels of neuropeptides increase after specific allergen challenge in allergic conjunctivitis. Mol Vis. 2011;17:47–52. [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Avila RM, Merayo-Lloves J, Riestra AC, Fernandez-Vega Cueto L, Anitua E, Begona L, Muruzabal F, Orive G. Treatment of patients with neurotrophic keratitis stages 2 and 3 with plasma rich in growth factors (PRGF-Endoret) eye-drops. Int Ophthalmol. 2017 doi: 10.1007/s10792-017-0582-7. doi:10.1007/s10792-017-0582-7. [DOI] [PubMed] [Google Scholar]
- Shaheen BS, Bakir M, Jain S. Corneal nerves in health and disease. Surv Ophthalmol. 2014;59:263–285. doi: 10.1016/j.survophthal.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakoli M, Mitu-Pretorian M, Petropoulos IN, Fadavi H, Asghar O, Alam U, Ponirakis G, Jeziorska M, Marshall A, Efron N, Boulton AJ, Augustine T, Malik RA. Corneal confocal microscopy detects early nerve regeneration in diabetic neuropathy after simultaneous pancreas and kidney transplantation. Diabetes. 2013;62:254–260. doi: 10.2337/db12-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervo TM, Mertaniemi P, Ylatupa S, Tervo K, Virtanen T, Partanen P. Release of calcitonin gene-related peptide in tears after excimer laser photorefractive keratectomy. J Refract Surg. 1995;11:126–128. doi: 10.3928/1081-597X-19950301-13. [DOI] [PubMed] [Google Scholar]
- Turkoglu E, Celik E, Alagoz G. A comparison of the efficacy of autologous serum eye drops with amniotic membrane transplantation in neurotrophic keratitis. Semin Ophthalmol. 2014;29:119–126. doi: 10.3109/08820538.2013.768678. [DOI] [PubMed] [Google Scholar]
- Uhlig CE, Frings C, Rohloff N, Harmsen-Aasman C, Schmitz R, Kiesel L, Eter N, Busse H, Alex AF. Long-term efficacy of glycerine-processed amniotic membrane transplantation in patients with corneal ulcer. Acta Ophthalmol. 2015;93:e481–487. doi: 10.1111/aos.12671. [DOI] [PubMed] [Google Scholar]
- Yamada M, Ogata M, Kawai M, Mashima Y. Decreased substance P concentrations in tears from patients with corneal hypesthesia. Am J Ophthalmol. 2000;129:671–672. doi: 10.1016/s0002-9394(00)00415-3. [DOI] [PubMed] [Google Scholar]
- Yamada N, Matsuda R, Morishige N, Yanai R, Chikama TI, Nishida T, Ishimitsu T, Kamiya A. Open clinical study of eye-drops containing tetrapeptides derived from substance P and insulin-like growth factor-1 for treatment of persistent corneal epithelial defects associated with neurotrophic keratopathy. Br J Ophthalmol. 2008;92:896–900. doi: 10.1136/bjo.2007.130013. [DOI] [PubMed] [Google Scholar]
- Yanai R, Nishida T, Chikama T, Morishige N, Yamada N, Sonoda KH. Potential new modes of treatment of neurotrophic keratopathy. Cornea 34 Suppl. 2015;11:S121–127. doi: 10.1097/ICO.0000000000000587. [DOI] [PubMed] [Google Scholar]
- Yu CQ, Rosenblatt MI. Transgenic corneal neurofluorescence in mice: a new model for in vivo investigation of nerve structure and regeneration. Invest Ophthalmol Vis Sci. 2007;48:1535–1542. doi: 10.1167/iovs.06-1192. [DOI] [PubMed] [Google Scholar]