Abstract
Leucine rich repeat proteins have gained considerable interest as therapeutic targets due to their expression and biological activity within the central nervous system. LINGO-1 has received particular attention since it inhibits axonal regeneration after spinal cord injury in a RhoA dependent manner while inhibiting leucine rich repeat and immunoglobulin-like domain-containing protein 1 (LINGO-1) disinhibits neuron outgrowth. Furthermore, LINGO-1 suppresses oligodendrocyte precursor cell maturation and myelin production. Inhibiting the action of LINGO-1 encourages remyelination both in vitro and in vivo. Accordingly, LINGO-1 antagonists show promise as therapies for demyelinating diseases. An analogous protein to LINGO-1, amphoterin-induced gene and open reading frame-3 (AMIGO3), exerts the same inhibitory effect on the axonal outgrowth of central nervous system neurons, as well as interacting with the same receptors as LINGO-1. However, AMIGO3 is upregulated more rapidly after spinal cord injury than LINGO-1. We speculate that AMIGO3 has a similar inhibitory effect on oligodendrocyte precursor cell maturation and myelin production as with axogenesis. Therefore, inhibiting AMIGO3 will likely encourage central nervous system axonal regeneration as well as the production of myelin from local oligodendrocyte precursor cell, thus providing a promising therapeutic target and an area for future investigation.
Keywords: multiple sclerosis, demyelination, oligodendrocyte, AMIGO3, LINGO1, oligodendrocyte precursor cell
Introduction
The leucine rich repeat (LRR) family of proteins has attracted interest due to their roles within both the healthy and diseased nervous system. Netrin-G-ligand and Slit are involved in axon guidance and synapse regulation, neurite outgrowth inhibitor-66 receptor 1 (NgR1) inhibits axon extension, and Tyrosine kinase (Trk) receptors signal for trophic support and the mediation of growth in neurons (Mi et al., 2004; Chen et al., 2006; Ahmed et al., 2013). More recently, interest has been focused on the LRR protein, LRR and immunoglobulin-like domain-containing protein 1 (LINGO-1), as well as a homolog, amphoterin-induced gene and open reading frame-3 (AMIGO3), with the aim of developing therapies for central nervous system (CNS) disorders. Both AMIGO3 and LINGO-1 possess an IgC-like domain, a transmembrane domain and a short cytosolic tail, with the only major difference between these proteins being the number of LRRs (6 for LINGO1, 12 for AMIGO3) contained on their ectodomain (Jepson et al., 2012). Both proteins are implicated in CNS pathology and are involved in similar molecular interactions (Kuja-Panula et al., 2003; Ahmed et al., 2013). Despite promising findings from animal models indicating that antagonism of LINGO-1 encourages axonal regeneration and remyelination, clinical trials focused on myelin repair in multiple sclerosis produced disappointing results (Ledford, 2015; MS-Society, 2016). We hypothesize that the failure of this LINGO-1 based therapy may be explained by considering the actions of AMIGO3, which can substitute for LINGO-1, and hence may compensate for therapeutically reduced levels of LINGO-1 function. This review explores the potential of LINGO-1 and AMIGO3 as therapeutic targets and examines the potential mechanisms of action of the two proteins in CNS disorders.
Expression Patterns of AMIGO3/LINGO-1
In the adult mouse, LINGO-1 expression is restricted to the CNS (Mi et al., 2004; Haines and Rigby, 2008). During development relatively low levels of LINGO-1, mRNA are detected at embryonic stages, after which there is a substantial increase in expression between late embryonic stages and postnatal day 1 in both mouse and rat brains (Mi et al., 2004, 2005; Shao et al., 2017). A similar pattern is observed in zebrafish with the peak LINGO-1 protein levels being reached 5 days after fertilization in the early larval stages (Yin and Hu, 2014). Conflicting data exists regarding the levels of LINGO-1 mRNA during murine postnatal development, with peaks observed at postnatal days 1, 4 and 21. However, levels appear to reduce and stabilize during adulthood (Mi et al., 2004; Llorens et al., 2008; Shao et al., 2017). LINGO1 protein levels show a similar distribution with a peak expression observed at postnatal day 21 (Llorens et al., 2008). The highest expression levels of LINGO-1 are within axons of CNS neurons (Mi et al., 2005; Satoh et al., 2007). A reduced but still significant level of expression is also found in oligodendrocytes (Mi et al., 2005). This restricted expression pattern can be considered an advantage for LINGO1-directed therapies since systemically delivered drugs would not be expected to produce non-CNS effects.
AMIGO3 expression is more widespread in the adult mouse with significant levels observed in the adult murine liver, intestines and lungs, however AMIGO3 is also brain enriched (Kuja-Panula et al., 2003; Ahmed et al., 2005). AMIGO3 has been identified within astrocytes, oligodendroglia and neurons, but to date its expression in inflammatory cells has not been investigated (Tickle, 2013). Interestingly, AMIGO3 mRNA is not detectable in embryonic mice (Homma et al., 2009), however, unpublished data from our laboratory suggests that AMIGO3 protein is expressed in white matter during the first 2 weeks post-natal implying that AMIGO3 is likely to regulate developmental myelination.
LRRs in CNS Axon Regeneration
LINGO-1 is an integral component of the tripartite NgR1-p75 neurotrophin receptor (p75NTR)/TROY receptor complex (Mi et al., 2004) (Figure 1). Signaling through the NgR1 complex inhibits axonal regeneration after activation by myelin-derived axon growth inhibitor ligands, such as oligodendrocyte myelin glycoprotein (OMgp), myelin associated glycoprotein (MAG), and neurite outgrowth inhibitor (Nogo). This axon growth inhibition is mediated through activated Ras homolog gene family member A (RhoA) (Mi et al., 2004; Ahmed et al., 2013) (Figure 1). Accordingly, OMgp treatment of COS-7 cells (a cell line lacking endogenous expression of the NgR1 complex as well as LINGO-1 and AMIGO3) leads to higher levels of activated RhoA, only when the cells are also transfected to express the entire combination of NgR1, p75NTR/TROY and notably, LINGO-1 (Mi et al., 2004; Ahmed et al., 2013). As expected, increasing LINGO-1 expression through transfection of full-length LINGO-1, is linked to a decrease in neurite outgrowth in cultured cerebellar granule cells (Mi et al., 2004). Additionally, antagonizing LINGO-1 in vivo with LINGO-1-Fc, results in a decrease in RhoA signaling after spinal cord injury (SCI) that correlates with increased axon regeneration (Mi et al., 2013). Counter-intuitively, LINGO-1 levels do not rise in the spinal cord until 14 days after SCI and therefore it is apparent that other NgR1 co-receptors are likely to be functional during the acute post SCI period.
Figure 1.
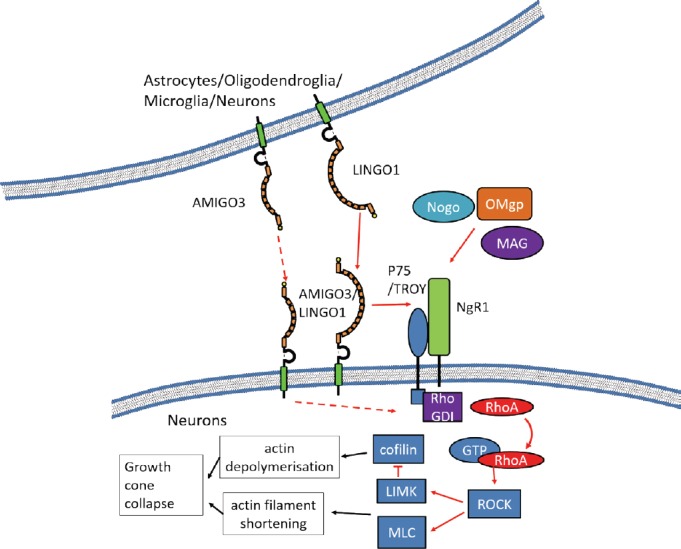
AMIGO3 and LINGO-1 signaling in neurons.
Both AMIGO3 and LINGO-1 are able to form a complex with NgR1 and P75/TROY (Mi et al., 2004; Ahmed et al., 2013). This enables the complex to activate when bound to the myelin derived proteins, Nogo, OMgp and MAG. Myelin derived protein binding permits the activation of RhoA by local RhoGDI. Activated RhoA induces ROCK signaling leading to the activation of cytosolic LIMK and myosin light chain (MLC) on actin filaments. LIMK inhibits cofilin resulting in actin depolymerization (Fujita and Yamashita, 2014). MLC activation also leads to a shortening of the actin filaments. These effects are likely to promote growth cone. A similar pathway could function in oligodendroglia as actin filaments are involved with the cell cycle and production of processes required to initiate contact with neurons (Lourenco and Graos, 2016). Both LINGO-1 and AMIGO3 can bind homophilically (Kuja-Panula et al., 2003; Chen et al., 2006). In the case of LINGO-1, this has been shown to increase RhoA signaling and it is likely that AMIGO3 could mediate a similar response (Jepson et al., 2012). AMIGO3: Amphoterin-induced gene and open reading frame-3; GTP: guanosine-5′-triphosphate; LIMK: LIM-kinase gene; LINGO-1: LRR and immunoglobulin-like domain-containing protein 1; MAG: myelin associated glycoprotein; NgR1: neurite outgrowth inhibitor-66 receptor 1; Nogo: neurite outgrowth inhibitor; OMgp: oligodendrocyte myelin glycoprotein; RhoA: Ras homolog gene family member A; RhoGDI: Rho GDP-dissociation inhibitor; ROCK: coiled-coil containing protein kinase; TROY: tumor necrosis factor receptor family member.
Ahmed et al. (2013) showed that AMIGO3 levels increased acutely after axotomy of both retinal ganglion cells and dorsal root ganglion neurons (DRGN). Additionally, a reduction in the level of AMIGO3 in these models correlates with dorsal column and optic nerve axon regeneration, while siRNA targeted against AMIGO3 enhanced neurite outgrowth of DRGN in the presence of inhibitory CNS myelin extracts. Taken together these data indicate an inhibitory influence of AMIGO3 on axon regeneration that is similar in nature to that provided by LINGO-1. Furthermore, AMIGO3, like LINGO-1, interacts with members of the NgR1 signaling complex, namely NgR1 and p75NTR, in transfected COS7 cells leading to increases in activated RhoA levels (Ahmed et al., 2013). It is likely that the analogous structures shared between AMIGO3 and LINGO-1 (Chen et al., 2006) allows for both proteins to interact with the NgR1 complex leading to the same cellular response. Interestingly, AMIGO3 is upregulated at a much faster rate than LINGO-1, with a significant increase in AMIGO3 mRNA and protein observed within 24 hours after SCI in rats, while LINGO-1 mRNA levels remained unaltered even at 10 days post-injury (Ahmed et al., 2013). Considering the earlier expression of AMIGO3 following SCI, we speculate that AMIGO3 antagonism will provide a more effective therapeutic strategy for relieving the inhibition of axonal regeneration and preventing axon growth cone collapse.
LINGO-1 and Its Role in Myelin Disorders
LINGO-1 inhibits both maturation of oligodendrocyte precursor cells (OPC) and the production of myelin. LINGO-1 inhibition or knockdown in primary rodent and human OPC increases the expression of myelin basic protein (MBP), enhances branching, and facilitates the production of myelin sheets, an in vitro analogue of the myelin sheath (Bourikas et al., 2010; Mi et al., 2013). Correspondingly, overexpression of LINGO-1 in primary OPC cultures decreases MBP levels, demonstrating the inhibitory role of LINGO-1 in myelination (Jepson et al., 2012).
In vivo, early onset myelination is observed in LINGO-1 knockout mice during development (Mi et al., 2013). Similar observations have also been reported in disease contexts. For example, both LINGO-1 blockade and LINGO-1 knockout mice demonstrate reduced clinical signs in experimental autoimmune encephalomyelitis (EAE), as well as a greater number of remyelinated axons and increased MBP expression (Bourikas et al., 2010; Mi et al., 2013; Sun et al., 2015). Furthermore, this correlates with cognitive improvements, with EAE mice showing greater spatial learning when treated with anti-LINGO-1 antibodies (Sun et al., 2015). These data provide strong evidence that inhibition of LINGO-1 enhances repair in demyelinating disease, and on this basis, LINGO-1 antagonists have advanced into clinical trials for acute optic neuritis and active relapsing multiple sclerosis (MS) (Ledford, 2015; MS-Society, 2016).
It is not clear how LINGO-1 exhibits its effects on oligodendroglia. One possibility is that LINGO-1 exerts its influence through the same mechanism as observed in neurons, through the NgR1/p75NTR/TROY receptor complex (Jepson et al., 2012) (Figure 1). Data supporting this view has been generated from non-oligodendroglial cell lines or oligodendrocytes (OL) generated from neural progenitor cells. However, it is not clear how representative these cells are of in vivo OPC populations (Jepson et al., 2012; Mi et al., 2013). There are reports stating that OL do not express NgR1 (Mei et al., 2013). Additionally, Bourikas et al. (2010) demonstrated that inhibiting NgR1 has no effect on process extension and MBP production in LINGO-1 expressing MO3.13 cells, a human hybrid oligodendroglial cell line. Interestingly, antagonism of p75NTR reverses the inhibition in these cells, suggesting that LINGO-1 may still be able to interact with p75NTR in OL despite the lack of NgR1 signaling.
A number of other potential methods of action have been suggested for the function of LINGO-1 within OL. One theory is that LINGO-1 signals intercellularly with LINGO-1 expressed on neighboring cells (Figure 1). For example, cultured rat OL show an increase in MBP expression when treated with soluble LINGO-1 as well as with astrocytes/neurons induced to overexpress LINGO-1 (Jepson et al., 2012). Conversely, a population of COS7 cells transfected solely with LINGO-1 showed no change in activated RhoA levels, suggesting that the self-interactions between neighboring COS7 cells through LINGO-1 has no downstream effects (Mi et al., 2004). Of course, it is likely that LINGO-1 homophilic interactions would merely start the signaling process and still require further transduction. COS7 cells likely lack the intracellular machinery found in oligodendroglia to transduce this signal.
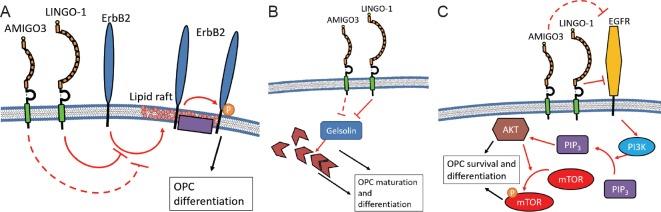
More recently, LINGO-1 has been shown to prevent phosphorylation and activation of human epidermal growth factor receptor (ErbB2). ErbB2 is a membrane bound receptor, expressed on both OPC and OL and has a positive role in OL differentiation and myelin production (Mi et al., 2013; Lee et al., 2014). LINGO-1 binds with ErbB2 through the LRR domain preventing the translocation of ErbB2 to lipid rafts in the membrane, where ErbB2 is readily phosphorylated (Lee et al., 2014) (Figure 2A). The levels of phosphorylated ErbB2 have been correlated to MBP expression and with OL maturity thereby linking the LINGO-1 induced inhibition to OL maturity (Lee et al., 2014).
Figure 2.
Potential mechanisms of action for LINGO-1 and speculative AMIGO3 signaling in oligodendroglia.
(A) LINGO-1 is able to bind to ErbB2 and prevent its translocation to the lipid rafts on oligodendroglial membranes. When ErbB2 reaches the lipid rafts, it is phosphorylated by local kinases and phosphatases. It is not clear how ErbB2 is then able to transduce its downstream effects but phosphorylated ErbB2 induces OPC differentiation (Mi et al., 2013; Lee et al., 2014). As such, LINGO-1 is observed to prevent ErbB2 from activating and thus inhibits OPC differentiation. (B) Little is known about the role of the actin severing and capping protein, gelsolin, in OPC differentiation, however activated gelsolin is correlated with increased OPC differentiation both in vivo and in vitro. This is likely due to the alteration in actin filament. However, it is possible that further protein signaling could be transducing the effects. LINGO-1 is able to bind to and directly inhibit gelsolin, thereby preventing the maturation signaling (Shao et al., 2017). (C) A further possible mechanism seen largely in dopaminergic neurons but also in oligodendroglia, is through the PI3K/AKT/m-TOR signaling pathway. LINGO-1 directly inhibits EGFR signaling, preventing the activation of intracellular PI3K (Inoue et al., 2007). Without PI3k activation, PIP2 cannot be further phosphorylated to its active form and as such, AKT and m-TOR remain inactive. Both activated AKT and m-TOR have been shown to encourage OPC survival and differentiation therefore highlighting that LINGO-1 can act to prevent OPC differentiation (Dienstmann et al., 2014). Although the ability of AMIGO3 to signal via these pathways has not been explored, the homologous structures of LINGO-1 and AMIGO3, and the fact that the proteins can replace one another in the NgR1 receptor complex, suggest it is highly likely that AMIGO3 functions through these cascades. AMIGO3: Amphoterin-induced gene and open reading frame-3; EGFR: epidermal growth factor receptor; ErbB2: epidermal growth factor receptor 2; LINGO-1: LRR and immunoglobulin-like domain-containing protein 1; m-TOR: mechanistic target of rapamycin; NgR1: neurite outgrowth inhibitor-66 receptor 1; OPC: oligodendrocyte precursor cell; PI3K: phosphoinositide 3-kinase; PIP2: phosphatidylinositol 4,5-bisphosphate.
Unsurprisingly, actin filaments have been identified as a potential downstream regulator of LINGO-1 signaling, likely due to the role of actin in the cell cycle and in the regulation of lamellipodia and filopodia (Shao et al., 2017). The actin-severing protein, gelsolin (GSN) is connected with myelin production, as identified both in primary rat OPC cultures and in Sprague-Dawley rats following lysolethicin induced demyelination, through MBP expression and counts of myelinated axons (Shao et al., 2017). Inhibiting LINGO-1 leads to higher GSN and MBP levels when primary rat OPC are stimulated to differentiate indicating gelsolin as a downstream regulator of LINGO-1 inhibition (Shao et al., 2017) (Figure 2B).
Other signaling pathways implicated for the method of action for LINGO-1 in oligodendrocytes include the phosphoinositide 3-kinase (PI3K)/AKT/mechanistic target of rapamycin (mTOR) pathway (Sun et al., 2015), the WNK lysine deficient protein kinase 1 (WNK1) pathway (Zhang et al., 2015), the brain-derived neurotrophic factor (BNDF)/tropomyosin receptor kinase B (TrkB) pathway (Meabon et al., 2016). LINGO-1 prevents the phosphorylation of both AKT and m-TOR which have both been positively implicated with myelination in mice (Sun et al., 2015) (Figure 2C). Similarly, WNK1 inhibition through siRNA treatment in primary rat OPCs leads to an increase in the area covered in MBP+ and NG2+ processes. Blocking the interaction between LINGO-1 and WNK1 reproduces this effect and leads to an increase in activated RhoA, demonstrating a RhoA mediated inhibition of OPC (Zhang et al., 2015). Finally, TrkB knockout mice show decreased myelin surrounding neurons, indicating its positive role in regulating myelination (Wong et al., 2013). LINGO-1 has since been reported to downregulate TrkB by encouraging translocation to lysosomes in vitro, thereby preventing its promotion of myelination (Meabon et al., 2016).
Despite the evidence of relevant functionality, there is some speculation as to whether LINGO-1 is expressed locally in human demyelinating diseases. Satoh et al. (2007) failed to observe LINGO-1 expression in OL from demyelinated MS tissue, directly opposing data from Mi et al. (2013). Similarly, in murine models there is a substantial lag before raised LINGO-1 expression is observed following both trauma and EAE induction (Mi et al., 2007; Ahmed et al., 2013), suggesting that alternative mechanisms are involved in the acute and chronic injury phases of OL maturation.
In spite of these differences, it appears probable that therapies aimed at inhibiting LINGO-1 have a positive effect on myelination, with phase 2 clinical trials for myelin disorders showing moderate signs of success (Ledford, 2015; MS-Society, 2016). For example, antagonizing LINGO-1 in patients suffering from optic neuritis has been found to improve the propagation rate in retinal nerves, however, this did not correlate with symptomatic relief (Ledford, 2015; MS-Society, 2016). We hypothesize that since the upregulation of LINGO-1 is delayed by at least 10 days in vivo, and that alternative LRR may be involved in the disease etiology, that antagonizing LINGO-1 alone is unlikely to be sufficient to induce rapid remyelination of the demyelinated axons. This will leave the axons open for injury and degradation.
The Potential Role of AMIGO3 in Myelin Disorders
The dramatic increase in spinal cord AMIGO3 expression shortly after SCI (Ahmed et al., 2013) suggests the possibility that this protein may also play a role in the acute stages of oligodendrocyte injury. In this context, therapies targeted against AMIGO3 may provide more rapid benefits than those focused on LINGO1 in terms of promoting OPC differentiation and remyelination in the early stages of demyelinating diseases. Earlier remyelination could provide a more effective therapy by limiting axonal injury and preventing degeneration.
The mechanism through which AMIGO3 would inhibit OPC maturation and myelin production is currently unclear. However, like LINGO-1, AMIGO3 can interact with the NgR1 receptor (Ahmed et al., 2013) (Figure 1). It is also plausible that since AMIGO3 displays homophilic binding (Kuja-Panula et al., 2003; Chen et al., 2006), it could self-interact intercellularly as similarly proposed for LINGO-1 (Jepson et al., 2012) (Figure 1). However, just as with LINGO-1, COS-7 cells transfected solely with AMIGO3 do not show increased activated RhoA (Ahmed et al., 2013) suggesting additional cellular signaling machinery is required. It is also plausible that AMIGO3 could signal through any of the other pathways highlighted for LINGO-1 inhibition in OPC (Figure 2), or alternatively due to the number of pathways involved in regulating OPC maturation, AMIGO3 may have its own separate signaling pathways.
In conclusion, we speculate that AMIGO3 could be a more effective inhibitor of OPC maturation than LINGO-1. We also believe that its antagonism may provide greater therapeutic benefits, a timely-point given that clinical trials with LINGO-1 have failed to meet the primary outcomes in MS and optic neuritis patients. Although more work is required to define its method of action, AMIGO3 seems well placed to provide an promising target for research into novel treatments for dysmyelinating diseases.
Footnotes
Funding: This work was supported by a grant from The University of Birmingham.
Conflicts of interest: None declared.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Warin Krityakiarana, Mahidol University - Ratchasuda College, Thailand; Hakan Karabagli, Selcuk University Medical Faculty, Turkey.
References
- Ahmed Z, Douglas M, John G, Berry M, Logan A. AMIGO3 Is an NgR1/p75 co-receptor signalling axon growth inhibition in the acute phase of adult central nervous system injury. PLos One. 2013;8:e61878. doi: 10.1371/journal.pone.0061878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed Z, Dent R, Suggate E, Barrett L, Seabright R, Berry M, Logan A. Disinhibition of neurotrophin-induced dorsal root ganglion cell neurite outgrowth on CNS myelin by siRNA-mediated knockdown of NgR, p75NTR and Rho-A. Mol Cell Neurosci. 2005;28:509–523. doi: 10.1016/j.mcn.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Bourikas D, Mir A, Walmsley A. LINGO-1-mediated inhibition of oligodendrocyte differentiation does not require the leucine-rich repeats and is reversed by p75(NTR) antagonists. Mol Cell Neurosci. 2010;45:363–369. doi: 10.1016/j.mcn.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Chen Y, Aulia S, Li L, Tang B. AMIGO and friends: An emerging family of brain-enriched, neuronal growth modulating, type I transmembrane proteins with leucine-rich repeats (LRR) and cell adhesion molecule motifs. Brain Res Rev. 2006;51:265–274. doi: 10.1016/j.brainresrev.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Dienstmann R, Rodon J, Serra V, Tabernero J. Picking the point of inhibition: a comparative review of PI3K/AKT/mTOR pathway inhibitors. Mol Cancer Ther. 2014;13:1021–1031. doi: 10.1158/1535-7163.MCT-13-0639. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Yamashita T. Axon growth inhibition by RhoA/ROCK in the central nervous system. Front Neurosci. 2014;8:338. doi: 10.3389/fnins.2014.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines B, Rigby P. Expression of the Lingo/LERN gene family during mouse embryogenesis. Gene Expr Patterns. 2008;8:79–86. doi: 10.1016/j.modgep.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Homma S, Shimada T, Hikake T, Yaginuma H. Expression pattern of LRR and Ig domain-containing protein (LRRIG protein) in the early mouse embryo. Gene Expr Patterns. 2009;9:1–26. doi: 10.1016/j.gep.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Inoue H, Lin L, Lee X, Shao Z, Mendes S, Snodgrass-Belt P, Sweigard H, Engber T, Pepinsky B, Yang L, Beal M, Mi S, Isacson O. Inhibition of the leucine-rich repeat protein LINGO-1 enhances survival, structure, and function of dopaminergic neurons in Parkinson’s disease models. Proc Natl Acad Sci U S A. 2007;104:14430–14435. doi: 10.1073/pnas.0700901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepson S, Vought B, Gross C, Gan L, Austen D, Frantz J, Zwahlen J, Lowe D, Markland W, Krauss R. LINGO-1, a transmembrane signaling protein, inhibits oligodendrocyte differentiation and myelination through intercellular self-interactions. J Biol Chem. 2012;287:22184–22195. doi: 10.1074/jbc.M112.366179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuja-Panula J, Kiiltomäki M, Yamashiro T, Rouhiainen A, Rauvala H. AMIGO, a transmembrane protein implicated in axon tract development, defines a novel protein family with leucine-rich repeats. JCB. 2003;160:963–973. doi: 10.1083/jcb.200209074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledford H. Drug that boosts nerve signals offers hope for multiple sclerosis. Nature. 2015;520:417. doi: 10.1038/520417a. [DOI] [PubMed] [Google Scholar]
- Lee X, Shao Z, Sheng G, Pepinsky B, Mi S. LINGO-1 regulates oligodendrocyte differentiation by inhibiting ErbB2 translocation and activation in lipid rafts. Mol Cell Neurosci. 2014;60:36–42. doi: 10.1016/j.mcn.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Llorens F, Gil V, Iraola S, Carim-Todd L, Martí E, Estivill X, Soriano E, del Rio J, Sumoy L. Developmental analysis of Lingo-1/Lern1 protein expression inthe mouse brain: Interaction of its intracellular domain with Myt1l. Dev Neurobiol. 2008;68:521–541. doi: 10.1002/dneu.20607. [DOI] [PubMed] [Google Scholar]
- Lourenco T, Graos M. Modulation of oligodendrocyte differentiation by mechanotransduction. Front Cell Neurosci. 2016;10:277. doi: 10.3389/fncel.2016.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meabon JS, de Laat R, Ieguchi K, Serbzhinsky D, Hudson MP, Huber BR, Wiley JC, Bothwell M. Intracellular LINGO-1 negatively regulates Trk neurotrophin receptor signaling. Mol Cell Neurosci. 2016;70:1–10. doi: 10.1016/j.mcn.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei F, Chong S, Chan J. Myelin-based inhibitors of oligodendrocyte myelination: clues from axonal growth and regeneration. Neurosci Bull. 2013;29:177–188. doi: 10.1007/s12264-013-1319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S, Pepinsky R, Cadavid D. Blocking LINGO-1 as a therapy to promote CNS repair: from concept to the clinic. CNS Drugs. 2013;27:493–503. doi: 10.1007/s40263-013-0068-8. [DOI] [PubMed] [Google Scholar]
- Mi S, Lee X, Shao Z, Thill G, Ji B, Relton J, Levesque M, Allaire N, Perrin S, Sands B, Crowell T, Cate R, McCoy J, Pepinsky R. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat Neurosci. 2004;7:221–228. doi: 10.1038/nn1188. [DOI] [PubMed] [Google Scholar]
- Mi S, Miller R, Lee X, Scott M, Shulag-Morskaya S, Shao Z, Chang J, Thill G, Levesque M, Zhang M, Hession C, Sah D, Trapp B, He Z, Jung V, McCoy J, Pepinsky R. LINGO-1 negatively regulates myelination by oligodendrocytes. Nat Neurosci. 2005;8:745–751. doi: 10.1038/nn1460. [DOI] [PubMed] [Google Scholar]
- Mi S, Hu B, Hahm K, Luo Y, Hui E, Yuan Q, Wong W, Wang L, Su H, Chu T, Guo J, Zhang W, So K, Pepinsky R, Shao Z, Graff C, Garber E, Jung V, Wu E, Wu W. LINGO-1 antagonist promotes spinal cord remyelination and axonal integrity in MOG-induced experimental autoimmune encephalomyelitis. Nat Med. 2007;13:1228–1233. doi: 10.1038/nm1664. [DOI] [PubMed] [Google Scholar]
- MS-Society (2016) Anti-LINGO-1. In [Google Scholar]
- Satoh J, Tabunoki H, Yamamura T, Arima K, Konno H. TROY and LINGO-1 expression in astrocytes and macrophages/microglia in multiple sclerosis lesions. Neuropathol Appl Neurobiol. 2007;33:99–107. doi: 10.1111/j.1365-2990.2006.00787.x. [DOI] [PubMed] [Google Scholar]
- Shao Z, Lee X, Huang G, Sheng G, Henderson CE, Louvard D, Sohn J, Pepinsky B, Mi S. LINGO-1 regulates oligodendrocyte differentiation through the cytoplasmic gelsolin signaling pathway. J Neurosci. 2017;37:3127–3137. doi: 10.1523/JNEUROSCI.3722-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sun J, Ren Q, Xu L, Zhang Z. LINGO-1 antibody ameliorates myelin impairment and spatial memory deficits in experimental autoimmune encephalomyelitis mice. Sci Rep. 2015;5:14235. doi: 10.1038/srep14235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tickle J. The role of amphoterin-induced gene and open reading frame (AMIGO) family in myelination and neurodegeneration. University of Birmingham. 2013 [Google Scholar]
- Wong A, Xiao J, Kemper D, Kilpatrick T, Murray S. Oligodendroglial expression of TrkB independently regulates myelination and progenitor cell proliferation. J Neurosci. 2013;33:4947–4957. doi: 10.1523/JNEUROSCI.3990-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W, Hu B. Knockdown of Lingo1b protein promotes myelination and oligodendrocyte differentiation in zebrafish. Exp Neurol. 2014;251:72–83. doi: 10.1016/j.expneurol.2013.11.012. [DOI] [PubMed] [Google Scholar]
- Zhang ZH, Li JJ, Wang QJ, Zhao WQ, Hong J, Lou SJ, Xu XH. WNK1 is involved in Nogo66 inhibition of OPC differentiation. Mol Cell Neurosci. 2015;65:135–142. doi: 10.1016/j.mcn.2015.03.003. [DOI] [PubMed] [Google Scholar]