Abstract
Background
Depression has been reported to be commonly manifested in patients with Alzheimer’s disease (AD) and is considered a risk factor for AD. The human apolipoprotein E (ApoE) gene exists in three major isoforms (coded by ε2, ε3, and ε4), and the ε4 allele has been associated with a greater incidence of both depression and AD. Although mounting evidence points to the potentially complex interaction between these two brain disorders in which ApoE might play a role, the underlying mechanisms are largely unknown.
Methods
Using human ApoE2, ApoE3, and ApoE4 gene-targeted replacement (hApoE-TR) mouse models, we investigated the role of ApoE isoforms and their potential interactions with estrogen receptor β (ERβ) signaling in modulating the brain mechanisms involved in depression.
Results
Our initial analyses in 6-month-old female hApoE-TR mice demonstrated that ApoE influenced the expression of brain-derived neurotrophic factor (BDNF) and the 5-hydroxytryptamine 2A (5-HT2A) serotonin receptor in an isoform-dependent manner, with the ApoE4 brain exhibiting the lowest level of BDNF and the highest level of 5-HT2A. In addition, both presynaptic and postsynaptic proteins were downregulated, indicating a synaptic deficit in ApoE4 brains. Our subsequent analyses revealed that a 3-month chronic treatment with an ERβ-targeted (83-fold selectivity over ERα) phytoestrogenic diet induced several changes in ApoE2 and ApoE3 brains, including a significant decrease in the expression of 5-HT2A receptors and an increase in BDNF/tropomyosin receptor kinase B and synaptic proteins. In contrast, ApoE4 brains were largely unresponsive to the treatment, with an increase only in select synaptic proteins in the treated group.
Conclusions
Taken together, these results indicate that ApoE4 negatively impacts BDNF–5-HT2A signaling in the female brain, which could in part underlie the ApoE4-mediated increased risk for depression. In a larger context, this mechanism could serve as a molecular link between depression and AD associated with ApoE4. Enhancing ERβ activity could provide a greater therapeutic benefit to non-ApoE4 carriers than to ApoE4 carriers in interventions for these brain disorders.
Keywords: Alzheimer’s disease (AD), Depression, Apolipoprotein E (ApoE), Estrogen receptor β (ERβ), Brain-derived neurotrophic factor (BDNF), 5-Hydroxytryptamine 2A (5-HT2A), Synaptic function
Background
Depression manifests in up to 50% of patients with Alzheimer’s disease (AD) [1–3] and is associated with increased neurological impairment [4] and mortality [5]. In addition, depression is an independent risk factor for the development of mild cognitive impairment (MCI) and for the progression from MCI to AD [6], contributing to at least a twofold increased AD risk compared with control subjects without depression [7–10]. Furthermore, the severity of the depressive phenotype (measured by the number of depressive symptoms in patients with depression) has been shown to be directly associated with an increased risk of developing AD, with each depressive symptom increasing the risk of AD development by approximately 20% compared with control subjects without depression [11]. Although a number of clinical studies have indicated the interaction between depression and AD, the underlying pathophysiological mechanisms are not understood.
Human apolipoprotein E (ApoE) is a 299-amino-acid lipoprotein containing an N-terminal receptor-binding region (amino acids 136–150) and a C-terminal lipid-binding region (amino acids 244–272), which are linked by a flexible hinge region [12]. The human ApoE gene, located on chromosome 19, comprises four exons, which are transcribed into an 1180-nucleotide-long ApoE messenger RNA (mRNA) transcript. Single-nucleotide polymorphisms in exon 4 of the ApoE gene result in the formation of three major ApoE protein isoforms that differ by one or two amino acids at residues 112 and 158: ApoE2 (Cys112, Cys158), ApoE3 (Cys112, Arg158), and ApoE4 (Arg112, Arg158), which have population frequencies of 8%, 75%, and 14%, respectively [13]. These amino acid differences have been shown to significantly alter ApoE protein structure and function [14, 15]. For instance, in addition to the two structural domains (i.e., receptor- and lipid-binding domains) present in ApoE2 and ApoE3, ApoE4 contains an extra domain interaction between Arg61 and Glu225 that renders ApoE4 more susceptible to proteolysis [14, 15].
The three ApoE variants have been reported to differentially impact the development and progression of late-onset Alzheimer’s disease (LOAD). Possession of one ApoE4 allele increases the risk of LOAD development by two- to threefold, and possession of two ApoE4 alleles increases the risk for LOAD by 12-fold, compared with non-ApoE4 carriers [16]. In contrast, possession of the ApoE2 allele has been reported to reduce the risk of LOAD by 50% [13, 17]. In addition, one copy of the ApoE4 allele shifts the risk curve for AD development 5 years earlier, and two copies of the ApoE4 allele shifts the curve 10 years earlier, whereas one copy of the ApoE2 allele shifts the curve 5 years later [18, 19]. Although several genome-wide association studies have identified many other loci associated with the development of LOAD, all of the studies have confirmed that ApoE is the strongest genetic risk factor associated with the development of LOAD [20–22].
In addition to contributing significantly to AD risk, ApoE has been implicated in the etiology of depressive disorders. The mean age of onset of depressive episodes in ApoE4 carriers was found to be significantly lower than the mean age of onset in non-ApoE4 carriers [23]. Moreover, ApoE4 was reported to be highly prevalent in patients with depression compared with control subjects without depression [24] and is significantly associated with the incidence of minor depression, severe depression, and depressive symptoms of any kind [25]. These data suggest that the ApoE4 allele may be a potential biomarker useful for identifying people who are at high risk of developing clinical depression [25, 26]. Similar to its protective role in LOAD, the ApoE2 allele has been associated with reduced risk for the onset of depressive disorders [27, 28].
Collectively, clinical studies have pointed toward a possible complex interaction of ApoE, AD, and depression. The goal of this study was to elucidate the mechanisms that underlie the association between the ApoE genotype and depression by examining the three ApoE isoforms’ differential modulation of brain-derived neurotrophic factor (BDNF) signaling, serotonergic signaling, and synaptic function. Moreover, to extend our previous findings on the potential role of estrogen receptor β (ERβ) in depression [29], we also analyzed ERβ-mediated effects on these signaling pathways in the presence of ApoE isoforms. The data provide a probable mechanistic rationale for the differential risk of depression associated with ApoE genetic variants, in which ERβ might play a role.
Methods
Animal models
The use of animals was approved by the Institutional Animal Care and Use Committee at the University of Kansas and followed National Institutes of Health guidelines for the care and use of laboratory animals. The study was carried out using human ApoE2, ApoE3, and ApoE4 gene-targeted replacement (hApoE2-TR, hApoE3-TR, and hApoE4-TR) mouse models. These mouse lines were created by gene targeting and carry one of the three human ApoE alleles in place of the endogenous murine ApoE gene while retaining the endogenous regulatory sequences required for modulating hApoE expression. These mice share a C57BL/6 J genetic background and express the human ApoE protein at physiological levels; thus, they provide a complete in vivo system that allows direct measurement and comparison of hApoE isoform-specific effects [30, 31]. For hApoE isoform comparison studies, cortical tissues were collected from 6-month-old hApoE2-TR, hApoE3-TR, and hApoE4-TR female mice (n = 5 for each isoform group). Dietary treatment studies were conducted with 3-month-old hApoE2-TR, hApoE3-TR, and hApoE4-TR female mice for a period of 3 months (n = 5 for each isoform/treatment group).
Animal treatment
Two rodent diets, a base/control diet and a phytoestrogenic estrogen receptor β-selective modulator (phyto-β-SERM)-supplemented diet, were custom-manufactured by Harlan Laboratories (Madison, WI, USA). Phyto-β-SERM is a rationally designed combination of three clinical phytoestrogens (genistein, daidzein, and equol) and exhibits an 83-fold binding selectivity for ERβ over ERα [32]. We have previously demonstrated that dietary supplementation with a clinically relevant dose of phyto-β-SERM resulted in significantly improved neurological outcomes in both menopausal and AD mouse models, without inducing estrogenic effects in reproductive tissues [33, 34]. The base/control diet was prepared from the Teklad Global 16% Protein Rodent Diet (Harlan Laboratories), which was ground and repelleted. This diet has a fixed formula and is nutritionally balanced, containing 16% protein and 3.6% fat. It supports the growth and maintenance of rodents and does not contain alfalfa or soybean meal, thus minimizing the levels of natural phytoestrogens. The phyto-β-SERM diet was prepared by adding equal parts of genistein, daidzein, and equol (LC Laboratories, Woburn, MA, USA) to the base diet; a total of 100 mg (genistein, daidzein, and equol) was added per 1000-g diet. This diet would deliver to each mouse a daily intake of 0.25 mg of added phyto-β-SERM formulation (genistein, daidzein, and equol) or 10 mg/kg (body weight [BW]) per mouse per day, assuming a 25-g mouse eating a 2.5-g diet per day. The diet was designed to deliver to the mice a total amount of added phytoestrogens that is biologically equivalent to a daily intake of 50 mg in humans. The conversion of a human dose to a mouse-equivalent dose was based on the conversion factor of equivalent surface area dose from human to mouse: 50 mg/60 kg (BW, human) × 12 (human-to-mouse conversion factor) = 10 mg/kg (BW, mouse). Three-month-old hApoE2-TR, hApoE3-TR, and hApoE4-TR female mice were fed one of the two custom diets for 3 months, and at the end of the treatment, mice were killed and their brain tissues were immediately collected.
Protein extraction
For tissue protein extraction, 30 mg of cortical tissue samples were homogenized using a Bullet Blender 24 homogenizer (Next Advance, Troy, NY, USA) with Pierce T-PER Tissue Protein Extraction Reagent (Thermo Fisher Scientific, Rockford, IL, USA) supplemented with protease and phosphatase inhibitors (Roche Applied Science, Indianapolis, IN, USA) and 100 μl of 0.5-mm glass beads (Next Advance) at speed 8 for 3 minutes at 4 °C, followed by centrifugation at 12,000 rpm for 8 minutes at 4 °C. Supernatants were transferred to new microcentrifuge tubes, and protein concentrations were determined using a bicinchoninic acid assay (Thermo Fisher Scientific).
Western blot analysis
Equal amounts of total protein (20 μg/lane) were loaded and separated by 10% glycine sodium dodecyl sulfate-PAGE. Resolved proteins were transferred to 0.2-μm pore-sized polyvinylidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA, USA) and blocked with 5% blotting grade blocker (Bio-Rad Laboratories) in Tris-buffered saline (TBS) with Tween 20 (TBST; 100 ml of 10× TBS [200 mM Tris, 1.5 mM NaCl, pH 7.6], 10 ml of 10% Tween 20, 890 ml of double-distilled H2O) for 1 h at room temperature (RT), followed by incubation with customized dilutions of primary antibodies at 4 °C overnight. Following overnight incubation, membranes were washed three times for 10 minutes each in TBST at RT, followed by incubation with horseradish peroxidase-conjugated secondary antibody (1:5000; Thermo Fisher Scientific) for 1 h at RT. Blots were again washed three times for 10 minutes each in TBST. Bands were visualized using chemiluminescence with an enhanced chemiluminescence detection kit (Bio-Rad Laboratories) and scanned using the C-DiGit Blot Scanner (LI-COR Biosciences, Lincoln, NE, USA). Relative intensities of the immunoreactive bands were quantified using image-digitizing software (Image Studio version 4.0; LI-COR Biosciences). Membranes were stripped in 5 ml of Restore PLUS Western Blot Stripping Buffer (Thermo Fisher Scientific) for 8 minutes at RT and reprobed with the indicated loading control. The following primary antibodies were used: rabbit polyclonal anti-BDNF (1:500; Santa Cruz Biotechnology, Dallas, TX, USA), rabbit polyclonal anti-tropomyosin receptor kinase B (anti-TrkB, 1:1000; Abcam, Cambridge, MA, USA), mouse monoclonal anti-β tubulin (1:3000; Thermo Fisher Scientific, Waltham, MA, USA), rabbit polyclonal anti-PSD95 (1:500; Alomone Labs, Jerusalem, Israel), rabbit monoclonal anti-synaptophysin (1:1000; Abcam), mouse monoclonal anti-SHANK3 (1:1000; NeuroMab/Antibodies Inc., Davis, CA, USA), rabbit polyclonal anti-synaptobrevin 2 (1:1000; Enzo Life Sciences, Farmingdale, NY, USA), rabbit polyclonal anti-5-hydroxytryptamine (serotonin) (anti-5-HT1A, 1:1000; Abcam), and rabbit polyclonal anti-5-HT2A (1:20,000; a generous gift from Dr. Nancy Muma).
Statistical analysis
Data were presented as mean ± SD. For comparisons between two groups, Student’s t test was used; for comparisons involving multiple groups, one-way analysis of variance with Tukey’s or Dunnett’s post hoc test was used. All statistical analyses were performed with Prism version 5.0 software (GraphPad Software Inc., La Jolla, CA, USA). Statistical significance was indicated by *p < 0.05, **p < 0.01, and ***p < 0.001.
Results
BDNF but not TrkB receptor expression is affected by ApoE in an isoform-dependent manner
To elucidate the role of ApoE isoforms in regulating the BDNF-TrkB pathway, we harvested cortical tissues from 6-month-old hApoE-TR female mice and probed for BDNF and TrkB immunoreactivity. The data indicate a significant decrease in BDNF expression levels in ApoE4 animals compared with ApoE2 and ApoE3 animals, whereas no significant differences were found when we compared ApoE2 with ApoE3 animals (Fig. 1a) [BDNF 14: F(2,6) = 15.07, p = 0.0046; ApoE2 vs ApoE3, p = 0.2278; ApoE2 vs ApoE4, p = 0.0040; ApoE3 vs ApoE4, p = 0.0285; BDNF 42: F(2,6) = 13.25, p = 0.0063; ApoE2 vs ApoE3, p = 0.2929; ApoE2 vs ApoE4, p = 0.0007; ApoE3 vs ApoE4; p = 0.0025]. In addition, the data revealed no differential regulation of TrkB receptor expression levels among different ApoE isoforms (Fig. 1a) [TrkB, F(2,6) = 0.0478, p = 0.9535].
Fig. 1.

Brain-derived neurotrophic factor (BDNF) and 5-hydroxytryptamine 2A (5-HT2A) expression levels are modulated by apolipoprotein E (ApoE) in an isoform-dependent manner. Expression levels of (a) BDNF and tropomyosin receptor kinase B (TrkB) and (b) 5-HT1A and 5-HT2A were examined in the cortices of 6-month-old human ApoE2, ApoE3, and ApoE4 gene-targeted replacement female mice. The integrated density value of the bands in Western blots was determined using densitometry, and data were normalized to an internal loading control (β-tubulin) and to the ApoE2 group. Data are shown as mean ± SD (n = 5). *p < 0.05, **p < 0.01 by one-way analysis of variance with Tukey’s post hoc test
5-HT2A but not 5-HT1A receptor expression is affected by ApoE in an isoform-dependent manner
In addition to BDNF/TrkB expression, we also examined the probable regulation of serotonergic signaling by different ApoE isoforms. Because 5-HT1A and 5-HT2A are the best-characterized serotonergic receptors in the field of depression, we focused our examination on the expression levels of these two receptors [35, 36]. The data indicate no significant regulation of 5-HT1A receptor expression by ApoE isoforms (Fig. 1b) [F(2,6) = 0.4805, p = 0.6404]. However, we observed differential regulation of 5-HT2A receptor expression among ApoE isoforms. Specifically, 5-HT2A expression increased by 15–20% in ApoE3 brains compared with ApoE2 brains, whereas ApoE4 brains expressed 30% more 5-HT2A than ApoE2 brains (Fig. 1b) [F(2,6) = 20.03, p = 0.0022; ApoE2 vs ApoE3, p = 0.0084; ApoE2 vs ApoE4, p = 0.0022; ApoE3 vs ApoE4, p = 0.3951].
Select presynaptic proteins are modulated by ApoE in an isoform-dependent manner
Depression has been shown to downregulate the expression levels of presynaptic proteins, a phenomenon that is reversed in patients treated with antidepressants [37]. To elucidate the possible differential regulation of presynaptic proteins among ApoE isoforms, we probed cortical tissues of hApoE-TR mice for synaptophysin (a synaptic vesicle structural protein) and synaptobrevin2 (a SNARE protein involved in the docking of synaptic vesicles to the terminal membrane). The results indicated that synaptophysin was not regulated by ApoE isoform, because no significant difference in expression levels of synaptophysin was found among the three animal groups (Fig. 2a) [F(2,6) = 0.8986, p = 0.4557]. In contrast, our data indicate that synaptobrevin2 levels were differentially modulated by ApoE isoforms. Specifically, the data demonstrated a 15% decrease in synaptobrevin2 expression in ApoE3 animals compared with ApoE2 animals, a 30% decrease in ApoE4 animals compared with ApoE2 animals, and a 15% decrease in ApoE4 animals vs ApoE3 animals (Fig. 2a) [F(2,6) = 29.16, p = 0.0008; ApoE2 vs ApoE3, p = 0.0122; ApoE2 vs ApoE4, p = 0.0007; ApoE3 vs ApoE4, p = 0.0365].
Fig. 2.
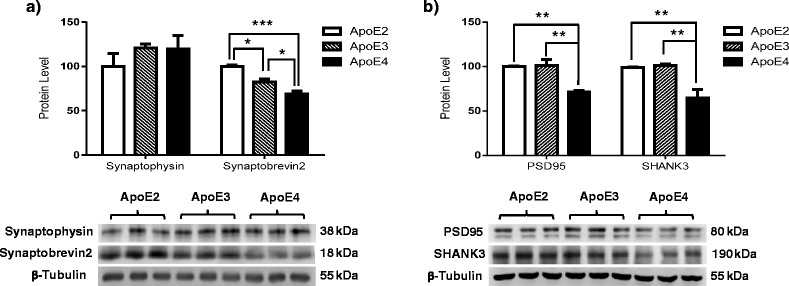
Pre- and postsynaptic proteins are differentially modulated by apolipoprotein E (ApoE) isoforms. Expression levels of (a) presynaptic proteins synaptophysin and synaptobrevin2, and (b) postsynaptic proteins PSD95 and SHANK3 were examined in the cortices of 6-month-old human ApoE2, ApoE3, and ApoE4 gene-targeted replacement female mice. The integrated density value of the bands in Western blots was determined using densitometry, and data were normalized to an internal loading control (β-tubulin) and to the ApoE2 group. Data are shown as mean ± SD (n = 5). *p < 0.05, **p < 0.01, ***p < 0.001 by one-way analysis of variance with Tukey’s post hoc test
Postsynaptic proteins are downregulated in ApoE4 brains
In addition to presynaptic proteins, the functions and expression levels of postsynaptic proteins have also been shown to be decreased in depression [38]. Therefore, to elucidate the possible differential regulation of postsynaptic proteins by ApoE isoforms, we examined the protein expression of PSD95 (a postsynaptic density protein) and SHANK3 (a multidomain scaffold protein of the postsynaptic density) in cortical tissues from hApoE-TR mice. The data indicate that PSD95 was differentially regulated by ApoE isoforms. Specifically, we observed a 25% decrease in the expression levels of PSD95 in ApoE4 animals compared with both ApoE2 and ApoE3 animals, with no significant difference occurring between ApoE2 and ApoE3 animals (Fig. 2b) [F(2,6) = 18.17, p = 0.0028; ApoE2 vs ApoE3, p = 0.9810; ApoE2 vs ApoE4, p = 0.0052; ApoE3 vs ApoE4, p = 0.0044]. Similar to these findings, SHANK3 expression levels decreased by 25% in ApoE4 animals compared with both ApoE3 and ApoE2 animals, with no difference occurring between ApoE2 and ApoE3 animals (Fig. 2b) [F(2,6) = 14.54, p = 0.0050; ApoE2 vs ApoE3, p = 0.9330; ApoE2 vs ApoE4, p = 0.0099; ApoE3 vs ApoE4, p = 0.0069].
Phyto-β-SERM treatment increases BDNF/TrkB signaling in ApoE2 and ApoE3 brains but not in ApoE4 brains
Estrogen use has been implicated in alleviating the mood-related symptoms of depression as well as in improving the cognition- and memory-related deficits associated with AD. To examine the potential effects of ERβ-mediated signaling, hApoE mice were administered a control diet or an ERβ-targeted phyto-β-SERM-supplemented diet for 3 months and killed at 6 months of age. The data indicate that phyto-β-SERM treatment significantly increased the expression levels of BDNF in ApoE3 animals but not in ApoE2 or ApoE4 animals (Fig. 3a) (BDNF 14: ApoE2, p = 0.0001; ApoE3, p = 0.0006; ApoE4, p = 0.5652; BDNF 42: ApoE2, p = 0.2586; ApoE3, p = 0.0422; ApoE4, p = 0.7721). The data also revealed that the 3-month treatment with phyto-β-SERM increased the expression levels of TrkB receptors in ApoE2 and ApoE3 animals but not in ApoE4 animals (Fig. 3a) (ApoE2, p = 0.0283; ApoE3, p = 0.0033; ApoE4, p = 0.6400).
Fig. 3.
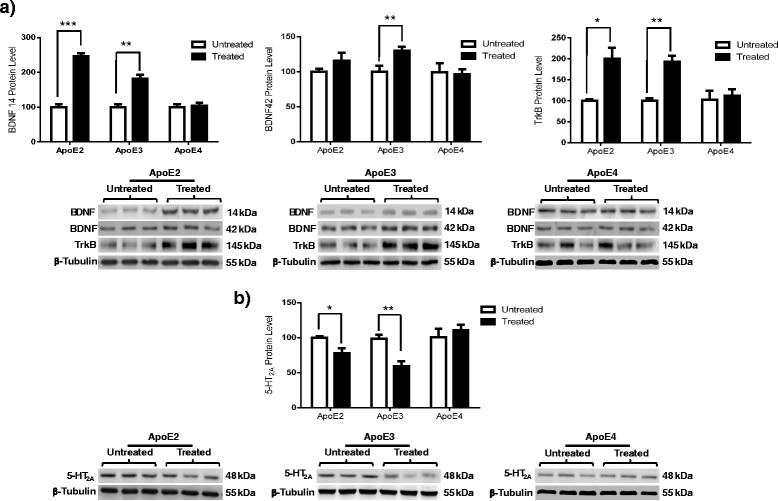
Estrogen receptor β activation modulates brain-derived neurotrophic factor (BDNF)/tropomyosin receptor kinase B (TrkB) and 5-hydroxytryptamine 2A (5-HT2A) pathways in apolipoprotein E2 isoform (ApoE2) and ApoE3 brains but not in ApoE4 brains. Three-month-old human ApoE2, ApoE3, and ApoE4 gene-targeted replacement female mice were treated with a phytoestrogenic estrogen receptor β-selective modulator (phyto-β-SERM)-supplemented diet or control diet for 3 months and killed at the age of 6 months. Cortical tissues were probed for (a) BDNF/TrkB and (b) 5-HT2A immunoreactivity. The integrated density value of the bands in Western blots was determined using densitometry, and data were normalized to an internal loading control (β-tubulin) and to the untreated group of each genotype. Data are shown as mean ± SD (n = 5). *p < 0.05, **p < 0.01 by Student’s t test
Phyto-β-SERM treatment decreases 5-HT2A expression in ApoE2 and ApoE3 brains but not in ApoE4 brains
In addition to the BDNF/TrkB pathway, we examined the effect of phyto-β-SERM treatment on the 5-HT2A receptor expression levels. The data revealed decreased expression levels of 5-HT2A receptor in ApoE2 and ApoE3 animals treated with phyto-B-SERM, but not in ApoE4 animals, compared with the control animals (Fig. 3b) (ApoE2, p = 0.0414; ApoE3, p = 0.0075; ApoE4, p = 0.3206).
Phyto-β-SERM treatment alters expression of presynaptic proteins in an ApoE isoform-dependent manner
In the context of the deleterious downregulation of functional presynaptic proteins in ApoE4 animals, we next examined whether ERβ agonism can modulate the aforementioned presynaptic proteins in 6-month-old female ApoE mice. Our data revealed that phyto-β-SERM treatment resulted in a significant increase in the expression levels of synaptophysin in ApoE2 and ApoE3 animals, but not in ApoE4 animals (Fig. 4a) (ApoE2, p = 0.0013; ApoE3, p = 0.0093; ApoE4, p = 0.6612). In contrast, the expression levels of Synaptobrevin2 increased in all animals treated with the phyto-β-SERM diet, regardless of ApoE isoform (Fig. 4b) (ApoE2, p = 0.0010; ApoE3, p = 0.0009; ApoE4, p = 0.0005).
Fig. 4.
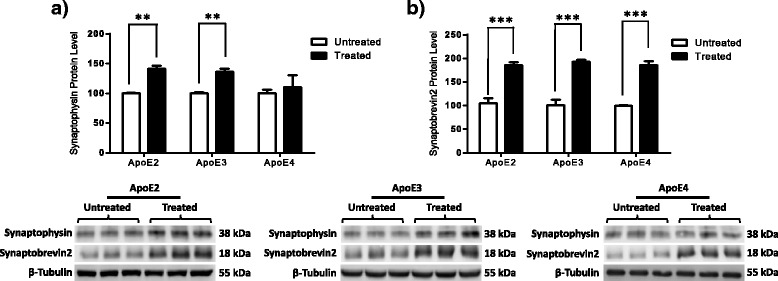
Estrogen receptor β activation leads to an upregulation of presynaptic proteins in an apolipoprotein E (ApoE) isoform-dependent manner. Three-month-old human ApoE2, ApoE3, and ApoE4 gene-targeted replacement female mice were treated with a phytoestrogenic estrogen receptor β-selective modulator (phyto-β-SERM)-supplemented diet or a control diet for 3 months and killed at the age of 6 months. Cortical tissues were probed for a) synaptophysin and b) synaptobrevin2 immunoreactivity. The integrated density value of the bands in Western blots was determined using densitometry, and data were normalized to an internal loading control (β-tubulin) and to the untreated group of each genotype. Data are shown as mean ± SD (n = 5). *p < 0.05, **p < 0.01, ***p < 0.001 by Student’s t test
Phyto-β-SERM treatment alters expression of select postsynaptic proteins independent of ApoE status
In addition to presynaptic proteins, our data revealed that ERβ agonism resulted in a significant increase in the expression levels of PSD95 in ApoE2, ApoE3, and ApoE4 animals (Fig. 5b) (ApoE2, p = 0.0076; ApoE3, p = 0.0001; ApoE4, p = 0.0013). In contrast, the expression levels of SHANK3 were not altered following chronic treatment with phyto-β-SERM, regardless of ApoE isoform (Fig. 5b) (ApoE2, p = 0.3485; ApoE3, p = 0.33; ApoE4, p = 0.5121).
Fig. 5.
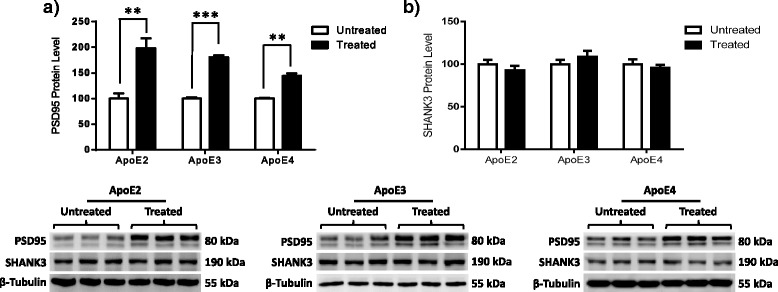
Estrogen receptor β activation leads to an upregulation of select postsynaptic proteins independent of apolipoprotein E (ApoE) status. Three-month-old human ApoE2, ApoE3, and ApoE4 gene-targeted replacement female mice were treated with a phytoestrogenic estrogen receptor β-selective modulator (phyto-β-SERM)-supplemented diet or a control diet for 3 months and killed at the age of 6 months. Cortical tissues were probed for a) PSD95 and b) SHANK3 immunoreactivity. The integrated density value of the bands in Western blots was determined using densitometry, and data were normalized to an internal loading control (β-tubulin) and to the untreated group of each genotype. Data are shown as mean ± SD (n = 5). *p < 0.05, **p < 0.01, ***p < 0.001 by Student’s t test
Discussion
Recent clinical studies have identified a significant association between depression and ApoE4, a major genetic risk factor for the development of AD [25]. Although the association has been well documented, the underlying molecular mechanisms leading to this probable association are unknown. On the basis of the literature and our own work, we hypothesize that the three ApoE isoforms differentially modulate neurotrophic and serotonergic pathways implicated in the pathophysiology of depression. The ApoE4 isoform causes significant dysregulation, thus increasing an individual’s risk of developing depression, and the ApoE2 isoform provides neuroprotection against the disease. To examine this hypothesis, we used 6-month-old human ApoE2, ApoE3, and ApoE4 gene-targeted replacement mouse models and found that BDNF and 5-HT2A receptor expression was significantly impacted in an ApoE isoform-dependent manner. Specifically, BDNF significantly decreased, whereas 5-HT2A receptor significantly increased, in ApoE4 animals compared with both ApoE2 and ApoE3 animals (Fig. 1). These data correspond with clinical findings of increased 5-HT2A mRNA and protein expression levels [39–41] and decreased BDNF expression levels in patients with depression [42, 43]. Thus, the findings imply that the ApoE4-mediated increased risk of developing depression could be partially attributed to the upregulation of 5-HT2A and downregulation of BDNF signaling in ApoE4 carriers compared with carriers of the other two isoforms.
The expression and function of synaptic proteins have also been found to be impaired in patients with depression [37]. To elucidate the probable regulation of synaptic proteins by ApoE status, we examined the modulation of the expression levels of both pre- and postsynaptic proteins in hApoE-TR animals (Fig. 2). The data indicate an isoform-based decrease in the expression levels of synaptobrevin2, a protein involved in vesicle docking and fusion, but not of synaptophysin, a protein involved in vesicular kinetics and recycling, when comparing ApoE4 animals with ApoE2 and ApoE3 animals, which suggests that ApoE isoforms regulate selective presynaptic proteins. In addition, the expression of the postsynaptic proteins PSD95 and SHANK3 was significantly reduced in ApoE4 animals compared with ApoE2 and ApoE3 animals. Collectively, these data indicate that synaptic function is compromised at both the pre- and postsynaptic levels in ApoE4 brains. The regulation of synaptic proteins in an ApoE isoform-dependent manner is well documented; ApoE4 has been associated with an overall decrease in synaptic protein content [44], including a substantial decrease in proteins such as synaptophysin [44], syntaxin [44], and PSD95 [45] in the presymptomatic stage of AD. Thus, it is highly probable that the deficit in synaptic strength and plasticity [46, 47], along with dysregulated BDNF–5-HT2A signaling, in ApoE4 carriers increases their risk of developing depression, which, in the presence of environmental stressors, can lead to the manifestation of clinical depression.
Several lines of evidence indicate a probable interaction between estrogen signaling and ApoE isoforms in the regulation of neural activities in the central nervous system [48, 49]. For instance, 17β-estradiol increased the extent of neurite outgrowth in cultured adult mouse cortical neurons that expressed the human ApoE2 or ApoE3 genes, but it had no effect on neurons from nonexpressing mice or in those supplied with exogenous ApoE4 protein [50]. Similarly, in a familial AD mouse model expressing human ApoE gene isoforms, treatment with 17β-estradiol decreased amyloid deposition in the brains of ApoE2- and ApoE3-bearing mice, whereas amyloid deposition was increased in the brains of ApoE4-expressing mice [51]. Consistent with the findings in animal models, clinical studies demonstrated that estrogen therapy (ET) was associated with lesser cognitive decline in ApoE4-negative but not ApoE4-positive individuals [49, 52]. Together, these findings indicate that estrogen may have a dual effect in the brain modulated by ApoE genotype, as well as that it tends to exert a positive outcome when ApoE4 is absent, whereas an opposite outcome could happen when ApoE4 is present. This conclusion, however, is contradicted by studies in which researchers found that estrogen use was associated with a beneficial effect in ApoE4 carriers [53–55]. In addition to its protective role against AD, ET has been reported to exert positive effects in the treatment of depression and mood-related symptomatology [56–58]. Previous work [59–61], including our own [29], strongly indicates that ERβ signaling plays a key role in the mechanism of ET in depression. To further investigate the potential interaction between ERβ signaling and ApoE genetic status, we chronically treated hApoE-TR mice with an ERβ-targeted phyto-β-SERM diet [32–34] and analyzed changes in the expression levels of serotonergic, neurotrophic, and synaptic proteins. The results of these analyses provide strong evidence that ERβ agonism potently interacts with ApoE isoforms in modulating the brain mechanisms involved in depression (Figs. 3, 4 and 5).
Conclusions
Our findings illustrate a possible mechanism involving BDNF–5-HT2A signaling pathways by which ApoE isoforms confer differential risk for depression (Fig. 6). In a larger context, this mechanism could serve as a link between depression and AD associated with ApoE4. In addition, our findings suggest that enhancing ERβ activity could provide a greater therapeutic benefit for ApoE2 and ApoE3 carriers than for ApoE4 carriers in interventions for depression. These preliminary data warrant further in-depth investigations of the pharmacological and behavioral relevance of the molecular differences identified in this study in depression models.
Fig. 6.
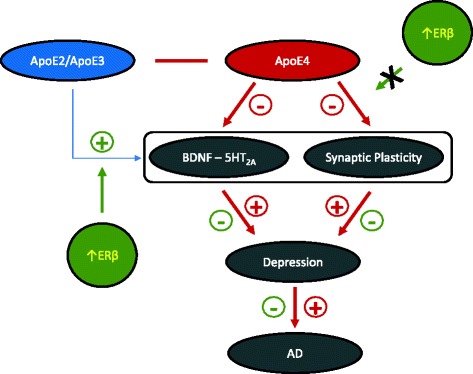
Estrogen receptor β (ERβ) interacts with apolipoprotein E (ApoE) in the regulation of brain-derived neurotrophic factor (BDNF)–5-hydroxytryptamine 2A (5-HT2A) signaling and synaptic function in the female brain. The findings presented indicate that ApoE isoforms differentially modulate the BDNF–5-HT2A signaling and synaptic function in the female brain. Specifically, compared with ApoE2 and ApoE3, the presence of the ApoE4 allele exerts a largely negative impact on these signaling pathways, which predisposes the brain to an increased risk for depression, which further increases the risk for Alzheimer’s disease (AD). ERβ signaling positively modulates these brain pathways primarily in ApoE2 and ApoE3 brains, whereas it has a minimal role in the ApoE4 brain, implicating the potential interaction of ERβ signaling and ApoE isoforms in the modulation of certain functions in the female brain
Acknowledgements
We thank Dr. Nancy Muma for providing the 5-HT2A antibody [62].
Funding
This work was supported by grants from the Alzheimer’s Association (IIRG-10-172459) and general research and start-up funds from the University of Kansas.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Abbreviations
- AD
Alzheimer’s disease
- ApoE
Apolipoprotein E
- BDNF
Brain-derived neurotrophic factor
- BW
Body weight
- ER
Estrogen receptor
- ET
Estrogen therapy
- 5-HT
5-Hydroxytryptamine (serotonin)
- LOAD
Late-onset Alzheimer’s disease
- MCI
Mild cognitive impairment
- mRNA
Messenger RNA
- Phyto-β-SERM
Phytoestrogenic estrogen receptor β-selective modulator
- RT
Room temperature
- TBS
Tris-buffered saline
- TBST
Tris-buffered saline with Tween 20
- TR
Gene-targeted replacement
- TrkB
Tropomyosin receptor kinase B
Authors’ contributions
LZ and AC conceived of and designed the experiments. AC performed the experiments. AC and LZ analyzed the data. AC and LZ wrote the manuscript. Both authors read and approved the final manuscript.
Ethics approval
The use of animals was approved by the Institutional Animal Care and Use Committee at the University of Kansas and followed National Institutes of Health guidelines for the care and use of laboratory animals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Anindit Chhibber, Email: a543c088@ku.edu.
Liqin Zhao, Phone: (785) 864-6291, Email: lzhao@ku.edu.
References
- 1.Merriam AE, et al. The psychiatric symptoms of Alzheimer’s disease. J Am Geriatr Soc. 1988;36(1):7–12. doi: 10.1111/j.1532-5415.1988.tb03427.x. [DOI] [PubMed] [Google Scholar]
- 2.Migliorelli R, et al. Prevalence and correlates of dysthymia and major depression among patients with Alzheimer’s disease. Am J Psychiatry. 1995;152(1):37–44. doi: 10.1176/ajp.152.1.37. [DOI] [PubMed] [Google Scholar]
- 3.Burns A, Jacoby R, Levy R. Psychiatric phenomena in Alzheimer’s disease. III: Disorders of mood. Br J Psychiatry. 1990;157:81–6. doi: 10.1192/bjp.157.1.81. [DOI] [PubMed] [Google Scholar]
- 4.Starkstein SE, et al. The construct of minor and major depression in Alzheimer’s disease. Am J Psychiatry. 2005;162(11):2086–93. doi: 10.1176/appi.ajp.162.11.2086. [DOI] [PubMed] [Google Scholar]
- 5.Suh GH, et al. Mortality in Alzheimer’s disease: a comparative prospective Korean study in the community and nursing homes. Int J Geriatr Psychiatry. 2005;20(1):26–34. doi: 10.1002/gps.1256. [DOI] [PubMed] [Google Scholar]
- 6.Steenland K, et al. Late-life depression as a risk factor for mild cognitive impairment or Alzheimer’s disease in 30 US Alzheimer’s disease centers. J Alzheimers Dis. 2012;31(2):265–75. doi: 10.3233/JAD-2012-111922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ownby RL, et al. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63(5):530–8. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diniz BS, et al. Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202(5):329–35. doi: 10.1192/bjp.bp.112.118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gracia-Garcia P, et al. Depression and incident Alzheimer disease: the impact of disease severity. Am J Geriatr Psychiatr. 2015;23(2):119–29. doi: 10.1016/j.jagp.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irie F, et al. Apolipoprotein E ε4 allele genotype and the effect of depressive symptoms on the risk of dementia in men. Arch Gen Psychiatry. 2008;65(8):906–12. doi: 10.1001/archpsyc.65.8.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson RS, et al. Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology. 2002;59(3):364–70. doi: 10.1212/WNL.59.3.364. [DOI] [PubMed] [Google Scholar]
- 12.Wetterau JR, et al. Human apolipoprotein E3 in aqueous solution. I. Evidence for two structural domains. J Biol Chem. 1988;263(13):6240–8. [PubMed] [Google Scholar]
- 13.Farrer LA, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA. 1997;278(16):1349–56. doi: 10.1001/jama.1997.03550160069041. [DOI] [PubMed] [Google Scholar]
- 14.Huang YD, et al. Apolipoprotein E fragments present in Alzheimer’s disease brains induce neurofibrillary tangle-like intracellular inclusions in neurons. Proc Natl Acad Sci U S A. 2001;98(15):8838–43. doi: 10.1073/pnas.151254698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye SM, et al. Apolipoprotein (apo) E4 enhances amyloid β peptide production in cultured neuronal cells: ApoE structure as a potential therapeutic target. Proc Natl Acad Sci U S A. 2005;102(51):18700–5. doi: 10.1073/pnas.0508693102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roses AD. Apolipoprotein E, alleles as risk factors in Alzheimer’s disease. Annu Rev Med. 1996;47:387–400. doi: 10.1146/annurev.med.47.1.387. [DOI] [PubMed] [Google Scholar]
- 17.Corder EH, et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet. 1994;7(2):180–4. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- 18.Corder EH, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late-onset families. Science. 1993;261(5123):921–3. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 19.Myers RH, et al. Apolipoprotein E ε4 association with dementia in a population-based study: the Framingham Study. Neurology. 1996;46(3):673–7. doi: 10.1212/WNL.46.3.673. [DOI] [PubMed] [Google Scholar]
- 20.Harold D, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009;41(10):1088–93. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jean-Charles Lambert, Simon Heath, Gael Even, Dominique Campion, Kristel Sleegers, Mikko Hiltunen, Onofre Combarros, Diana Zelenika, Maria J Bullido, Béatrice Tavernier, Luc Letenneur, Karolien Bettens, Claudine Berr, Florence Pasquier, Nathalie Fiévet, Pascale Barberger-Gateau, Sebastiaan Engelborghs, Peter De Deyn, Ignacio Mateo, Ana Franck, Seppo Helisalmi, Elisa Porcellini, Olivier Hanon, Marian M de Pancorbo, Corinne Lendon, Carole Dufouil, Céline Jaillard, Thierry Leveillard, Victoria Alvarez, Paolo Bosco, Michelangelo Mancuso, Francesco Panza, Benedetta Nacmias, Paola Bossù, Paola Piccardi, Giorgio Annoni, Davide Seripa, Daniela Galimberti, Didier Hannequin, Federico Licastro, Hilkka Soininen, Karen Ritchie, Hélène Blanché, Jean-François Dartigues, Christophe Tzourio, Ivo Gut, Christine Van Broeckhoven, Annick Alpérovitch, Mark Lathrop, Philippe Amouyel, (2009) Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nature Genetics 41 (10):1094-1099 [DOI] [PubMed]
- 22.Sudha Seshadri, (2010) Genome-wide Analysis of Genetic Loci Associated With Alzheimer Disease. JAMA 303 (18):1832 [DOI] [PMC free article] [PubMed]
- 23.Butters MA, et al. APOE is associated with age-of-onset, but not cognition, in late-life depression. Int J Geriatr Psychiatry. 2003;18(12):1075–81. doi: 10.1002/gps.1006. [DOI] [PubMed] [Google Scholar]
- 24.Rigaud AS, et al. Association of the apolipoprotein E ε4 allele with late-onset depression. Neuroepidemiology. 2001;20(4):268–72. doi: 10.1159/000054801. [DOI] [PubMed] [Google Scholar]
- 25.Skoog I, et al. A 9-year prospective population-based study on the association between the APOE*E4 allele and late-life depression in Sweden. Biol Psychiatry. 2015;78(10):730–6. doi: 10.1016/j.biopsych.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Sureshkumar R, et al. ApoE4 and late onset depression in Indian population. J Affect Disord. 2012;136(3):244–8. doi: 10.1016/j.jad.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 27.Fan PL, et al. Protective effect of the apo epsilon 2 allele in major depressive disorder in Taiwanese. Acta Psychiatr Scand. 2006;113(1):48–53. doi: 10.1111/j.1600-0447.2005.00686.x. [DOI] [PubMed] [Google Scholar]
- 28.Julian LJ, et al. ApoE alleles, depression and positive affect in multiple sclerosis. Mult Scler. 2009;15(3):311–5. doi: 10.1177/1352458508099478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chhibber A, et al. Estrogen receptor β deficiency impairs BDNF-5-HT2A signaling in the hippocampus of female brain: a possible mechanism for menopausal depression. Psychoneuroendocrinology. 2017;82:107–16. doi: 10.1016/j.psyneuen.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knouff C, et al. Apo E structure determines VLDL clearance and atherosclerosis risk in mice. J Clin Invest. 1999;103(11):1579–86. doi: 10.1172/JCI6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sullivan PM, et al. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem. 1997;272(29):17972–80. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- 32.Zhao L, Mao Z, Brinton RD. A select combination of clinically relevant phytoestrogens enhances estrogen receptor β-binding selectivity and neuroprotective activities in vitro and in vivo. Endocrinology. 2009;150(2):770–83. doi: 10.1210/en.2008-0715. [DOI] [PubMed] [Google Scholar]
- 33.Zhao L, et al. Estrogen receptor β-selective phytoestrogenic formulation prevents physical and neurological changes in a preclinical model of human menopause. Menopause. 2011;18(10):1131–42. doi: 10.1097/gme.0b013e3182175b66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao L, et al. Early intervention with an estrogen receptor β-selective phytoestrogenic formulation prolongs survival, improves spatial recognition memory, and slows progression of amyloid pathology in a female mouse model of Alzheimer’s disease. J Alzheimers Dis. 2013;37(2):403–19. doi: 10.3233/JAD-122341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nautiyal KM, Hen R. Serotonin receptors in depression: from A to B. F1000Res. 2017;6:123. doi: 10.12688/f1000research.9736.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Celada P, et al. The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. J Psychiatry Neurosci. 2004;29(4):252–65. [PMC free article] [PubMed] [Google Scholar]
- 37.Christopher Pittenger, Ronald S Duman, (2007) Stress, Depression, and Neuroplasticity: A Convergence of Mechanisms. Neuropsychopharmacology 33 (1):88-109 [DOI] [PubMed]
- 38.Linnea Vose, Patric Stanton, (2016) Synaptic Plasticity, Metaplasticity and Depression. Current Neuropharmacology 15 (1):71-86 [DOI] [PMC free article] [PubMed]
- 39.Pandey GN, et al. Higher expression of serotonin 5-HT2A receptors in the postmortem brains of teenage suicide victims. Am J Psychiatry. 2002;159(3):419–29. doi: 10.1176/appi.ajp.159.3.419. [DOI] [PubMed] [Google Scholar]
- 40.Escriba PV, Ozaita A, Garcia-Sevilla JA. Increased mRNA expression of alpha2A-adrenoceptors, serotonin receptors and mu-opioid receptors in the brains of suicide victims. Neuropsychopharmacology. 2004;29(8):1512–21. doi: 10.1038/sj.npp.1300459. [DOI] [PubMed] [Google Scholar]
- 41.Shelton RC, et al. Elevated 5-HT 2A receptors in postmortem prefrontal cortex in major depression is associated with reduced activity of protein kinase A. Neuroscience. 2009;158(4):1406–15. doi: 10.1016/j.neuroscience.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59(12):1116–27. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 43.Dwivedi Y, et al. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60(8):804–15. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- 44.Tannenberg RK, et al. Selective loss of synaptic proteins in Alzheimer’s disease: evidence for an increased severity with APOE varepsilon4. Neurochem Int. 2006;49(7):631–9. doi: 10.1016/j.neuint.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 45.Zhu Y, et al. APOE genotype alters glial activation and loss of synaptic markers in mice. Glia. 2012;60(4):559–69. doi: 10.1002/glia.22289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ji Y, et al. Apolipoprotein E isoform-specific regulation of dendritic spine morphology in apolipoprotein E transgenic mice and Alzheimer’s disease patients. Neuroscience. 2003;122(2):305–15. doi: 10.1016/j.neuroscience.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 47.Dumanis SB, et al. ApoE4 decreases spine density and dendritic complexity in cortical neurons in vivo. J Neurosci. 2009;29(48):15317–22. doi: 10.1523/JNEUROSCI.4026-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Struble RG, et al. Apolipoprotein E may be a critical factor in hormone therapy neuroprotection. Front Biosci. 2008;13:5387–405. doi: 10.2741/3088. [DOI] [PubMed] [Google Scholar]
- 49.Yaffe K, et al. Estrogen use, APOE, and cognitive decline: evidence of gene-environment interaction. Neurology. 2000;54(10):1949–54. doi: 10.1212/WNL.54.10.1949. [DOI] [PubMed] [Google Scholar]
- 50.Nathan BP, et al. Estrogen facilitates neurite extension via apolipoprotein E in cultured adult mouse cortical neurons. Endocrinology. 2004;145(7):3065–73. doi: 10.1210/en.2003-1707. [DOI] [PubMed] [Google Scholar]
- 51.Kunzler J, et al. APOE modulates the effect of estrogen therapy on Aβ accumulation EFAD-Tg mice. Neurosci Lett. 2014;560:131–6. doi: 10.1016/j.neulet.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burkhardt MS, et al. Oestrogen replacement therapy may improve memory functioning in the absence of APOE epsilon4. J Alzheimers Dis. 2004;6(3):221–8. doi: 10.3233/JAD-2004-6302. [DOI] [PubMed] [Google Scholar]
- 53.Jacobs EG, et al. Accelerated cell aging in female APOE-epsilon4 carriers: implications for hormone therapy use. PLoS One. 2013;8(2):e54713. doi: 10.1371/journal.pone.0054713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ryan J, et al. Characteristics of hormone therapy, cognitive function, and dementia: the prospective 3C Study. Neurology. 2009;73(21):1729–37. doi: 10.1212/WNL.0b013e3181c34b0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yue Y, et al. Effects of long-term, low-dose sex hormone replacement therapy on hippocampus and cognition of postmenopausal women of different apoE genotypes. Acta Pharmacol Sin. 2007;28(8):1129–35. doi: 10.1111/j.1745-7254.2007.00618.x. [DOI] [PubMed] [Google Scholar]
- 56.Carranza-Lira S, Valentino-Figueroa ML. Estrogen therapy for depression in postmenopausal women. Int J Gynaecol Obstet. 1999;65(1):35–8. doi: 10.1016/S0020-7292(99)00017-X. [DOI] [PubMed] [Google Scholar]
- 57.Soares CN, et al. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58(6):529–34. doi: 10.1001/archpsyc.58.6.529. [DOI] [PubMed] [Google Scholar]
- 58.Rasgon NL, et al. Estrogen replacement therapy in the treatment of major depressive disorder in perimenopausal women. J Clin Psychiatry. 2002;63(Suppl 7):45–8. [PubMed] [Google Scholar]
- 59.Yang F, et al. Estradiol decreases rat depressive behavior by estrogen receptor β but not alpha: no correlation with plasma corticosterone. Neuroreport. 2014;25(2):100–4. doi: 10.1097/WNR.0000000000000052. [DOI] [PubMed] [Google Scholar]
- 60.Weiser MJ, Wu TJ, Handa RJ. Estrogen receptor-β agonist diarylpropionitrile: biological activities of R- and S-enantiomers on behavior and hormonal response to stress. Endocrinology. 2009;150(4):1817–25. doi: 10.1210/en.2008-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walf AA, Koonce CJ, Frye CA. Adult female wildtype, but not oestrogen receptor β knockout, mice have decreased depression-like behaviour during pro-oestrus and following administration of oestradiol or diarylpropionitrile. J Psychopharmacol. 2009;23(4):442–50. doi: 10.1177/0269881108089598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh RK, et al. Olanzapine increases RGS7 protein expression via stimulation of the Janus tyrosine kinase-signal transducer and activator of transcription signaling cascade. J Pharmacol Exp Ther. 2007;322(1):133–40. doi: 10.1124/jpet.107.120386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.


