Abstract
Achieving axon regeneration after nervous system injury is a challenging task. As different parts of the central nervous system (CNS) differ from each other anatomically, it is important to identify an appropriate model to use for the study of axon regeneration. By using a suitable model, we can formulate a specific treatment based on the severity of injury, the neuronal cell type of interest, and the desired spinal tract for assessing regeneration. Within the sensory pathway, DRG neurons are responsible for relaying sensory information from the periphery to the CNS. We present here a protocol that uses a DRG injection with a viral vector and a concurrent dorsal root crush injury in the lower cervical spinal cord of an adult rat as a model to study sensory axon regeneration. As demonstrated using a control virus, AAV5-GFP, we show the effectiveness of a direct DRG injection in transducing DRG neurons and tracing sensory axons into the spinal cord. We also show the effectiveness of the dorsal root crush injury in denervating the forepaw as an injury model for evaluating axon regeneration. Despite the requirement for specialized training to perform this invasive surgical procedure, the protocol is flexible, and potential users can modify many parts to accommodate their experimental requirements. Importantly, it can serve as a foundation for those in search of a suitable animal model for their studies. We believe that this article will help new users to learn the procedure in a very efficient and effective manner.
Keywords: Neuroscience, Issue 123, Dorsal Root Ganglion, Injection, Dorsal Root Crush, Injury, Axon Regeneration, Adeno-associated Virus, Spinal Cord, Sensory Nervous System
Introduction
Achieving axon regeneration after nervous system injury is a challenging task1. To study the failure of axon regeneration in the central nervous system (CNS), researchers have used a plethora of nerve injury models. As regions of the CNS differ, it is important to use an anatomically appropriate model to study axon regeneration. By using the appropriate model, researchers can formulate a specific treatment based on the severity of injury, the neuronal cell type of interest, and the desired spinal tract for assessing regeneration, as opposed to a "one-for-all" treatment strategy.
In spinal cord injury, for example, the most debilitating symptoms stem from the loss of sensation and locomotion. Loss of sensation is caused by damage to the ascending sensory pathways, while the loss of locomotion is caused by damage to the descending motor pathways. Due to cellular and anatomical differences between these two pathways, many targeted axon regeneration studies only focus on one or the other pathway, with the rationale that successful recovery of either would be of tremendous benefit to patients. In this article, we present a protocol that uses a direct dorsal root ganglia (DRG) injection with a viral vector and a concurrent dorsal root crush injury in the lower cervical spinal cord of an adult rat as a model to study sensory axon regeneration.
DRG sensory neurons are responsible for relaying sensory information, such as tactile sensation and pain, from the periphery to the CNS. The long axonal projections of sensory neurons in the spinal cord serve as a good model to study long-distance axon regeneration. In addition, as rodents can survive a sensory pathway lesion such as a dorsal root crush injury with minimal welfare complications, researchers can study CNS axon regeneration without the need to completely lesion the spinal cord. A quadruple C5 - C8 (cervical level 5 - 8) dorsal root crush injury has been shown to be a useful model for forepaw deafferentation2. Additionally, a dorsal root crush injury provides a "cleaner" model to study axon regeneration than a direct spinal cord injury because it is uncomplicated by other factors such as glial scar formation.
The use of viral gene therapy to reprogram neurons into a regenerative state has been increasingly regarded as a promising treatment strategy for many neurological conditions3. Studies have shown the application of an adeno-associated virus (AAV) vector carrying the transgene of a growth-promoting protein can achieve robust axon regeneration with behavioral recovery4,5,6. The apparent low pathogenicity of AAV in eliciting an immune response and the ability to transduce non-dividing cells, such as neurons, make it the optimal vector for gene therapy. Additionally, the recombinant AAV form is used for therapy. In this form, it is incapable of integrating its viral genome into the host genome7, reducing the risk of insertional mutagenesis as compared to other viral vectors, such as lentivirus. This makes AAV a safe choice for gene therapy applications.
As a DRG contains the cell bodies of sensory neurons, it is the most appropriate anatomical target for the administration of virus for gene therapy to study and/or promote sensory axon regeneration. In a study comparing different AAV serotypes and lentivirus, AAV serotype 5 (AAV5) was shown to be the most efficient in transducing DRG neurons over a time course of at least 12 weeks when injected directly into the DRG8. Additionally, AAV can achieve more than 40% transduction efficiency, transducing all DRG neuronal subtypes, such as the large-diameter neurofilament 200 kDa (NF200)-positive neurons and the small-diameter calcitonin gene-related peptide (CGRP)- or isolectin b4 (IB4)-positive neurons4,8.
As the surgical procedure of DRG injection and dorsal root crush injury is extremely invasive and delicate, we believe that this article will help new users to learn the procedure in a very efficient manner. In this article, we show representative results from adult rats four weeks after the injection of a control virus AAV5-GFP (green fluorescent protein) into C6 - C7 DRGs with a concurrent C5 - C8 dorsal root crush injury. This model is especially suitable for researchers who are investigating the use of viral gene therapy to promote sensory axon regeneration.
Protocol
All the following animal procedures were conducted in accordance with the United Kingdom Animals (Scientific Procedures) Act 1986. If unfamiliar with these procedures, please check with local/national regulations and seek veterinary advice before starting the protocol.
1. Choosing a Suitable Strain of Animals
NOTE: A dorsal root crush injury results in the loss of sensation and paw deafferentation. Common adverse effects of forepaw deafferentation can include over-grooming, self-mutilation, and paw autotomy.
Obtain animals for the procedure. NOTE: For rats, the more active strains, such as Lister-Hooded and Wistar, have a higher incidence of paw autotomy after deafferentation as compared to the more docile strains, such as Lewis and Sprague-Dawley. If possible, the most docile strain, Lewis, should always be considered first. Should a particularly active strain be necessary for the experiment due to genetic modification or specific behavioral assessment requirements, veterinary treatment should be in place to address expected adverse effects.
2. Preparing the Virus for Injection
CAUTION: Handle all viruses in accordance with biological and laboratory safety regulations.
Dilute or concentrate the virus to a titer of 2 x 1012 genome copy (GC)/mL following individual virus packaging protocols9. NOTE: Here, the virus was diluted in Dulbecco's phosphate-buffered saline (D-PBS) with 5% sucrose. The titer may have to be optimized depending on the type of virus, promoter, construct size, and targeted cell type.
Optional: Add 1% colored dye (e.g., Fast Green) to the virus solution for easy visualization of the injection later on. Mix the solution very gently.
Keep the solution on ice for immediate use (within 4 h) or in a -80 °C freezer for long-term storage.
3. Performing the Preoperative Preparation of the Animal
NOTE: Due to the extreme invasiveness of the surgery, aseptic techniques should be used at all times.
Prepare and sterilize all surgical tools and the spinal stereotaxic frame prior to starting the surgery. For example, sterilize the tools in an autoclave at 134 °C. Use a fresh set of sterilized tools for each animal.
- Anesthetize the animal. Confirm proper anesthetization by pinching the paw to test for the withdrawal reflex or by touching the cornea for the eyelid reflex.
- For adult rats using inhalation anesthesia such as isoflurane, use 4% isoflurane in 2.0 L/min of oxygen for induction. For maintenance throughout the surgery, use 2 - 3% isoflurane in 1.5 L/min of oxygen, varying the isoflurane concentration according to the breathing pattern of the animal to avoid over- or under-dosing.
Once the animal is initially anesthetized, record the preoperative weight of the animal.
For the cervical DRG injection, shave off the fur on the neck from between the ears to just distal to the scapulae. Disinfect the shaved area with an antiseptic product.
Inject 2 mL of saline subcutaneously on the flank of the animal, along with an appropriate dose of analgesic (e.g., 5 mg/kg carprofen) and antibiotic (e.g., 5 mg/kg enrofloxacin).
Apply eye ointment to both the eyes to prevent corneal drying during the procedure.
Proceed to surgery on a spinal stereotaxic frame with a heating pad at 37 °C placed underneath the animal.
4. Injecting DRG and Performing a Dorsal Root Crush Injury
NOTE: This is an extremely delicate surgery. It is advisable to practice on a few dead animals first to familiarize with the anatomy before advancing to live animal surgery.
Locate the prominent C2 and T2 spinous processes over the skin. Make a skin incision between the C2 and T2 spinous processes with a no.10 scalpel blade (once the skin is opened, a white, fibrous-tissue midline should be visible on the first layer of muscle; the first layer of muscle has a "jelly-like" texture).
Make a similar-sized incision on the first layer of muscle along the white midline using a scalpel blade. Do not go beyond the prominent T2 spinous process, as there is a major arterial branch from the descending aorta located near there. NOTE: Heavy bleeding can occur anywhere from this step onwards. Always stop the bleeding and remove the blood by using sterilized cotton buds or surgical absorbable sponges to allow clear visualization at all times. Proceeding with the procedure "blindly" may result in unwanted damage to the spinal cord, DRG, or dorsal root, leading to unexpected adverse effects in the animal later on.
Retract the first layer of muscle using two retractors, one placed rostrally and one caudally; the second layer of muscle, with a striated appearance, should be visible.
Locate the midline of the second layer of muscle (where two longitudinal muscles can be observed connected by a thin, membranous tissue). Dissect the membranous tissue using a pair of microscissors to separate the two longitudinal muscles. Avoid using a scalpel blade if possible, as this may result in unnecessary muscle damage and bleeding.
Adjust the retractors accordingly to expose the third layer of thin muscle covering the spine. The spinous processes can be felt by lightly touching with a pair of forceps over the third layer of muscle.
Make a small incision on the third layer of muscle using microscissors and gently scrape off the muscle from the bone using a curette or scalpel in a sideway manner to clearly expose the vertebrae. If required, cut some of the muscle or tendon away to make the laminectomy easier.
To expose the left C5 - C8 DRGs, perform a left hemi-laminectomy on the C4 - T1 vertebrae by carefully removing part of the lamina and pedicle using a pair of fine rongeurs. The DRG is located near the transverse foramen of the vertebrae. NOTE: C3 - C7 vertebrae do not have a prominent spinous process. The C5 DRG is located between the C4 and C5 vertebrae, the C6 DRG is between the C5 and C6 vertebrae, and so on, while the C8 DRG is between the C7 and T1 vertebrae. There is no C8 vertebra, despite the presence of a C8 DRG and a C8 spinal cord segment.
Once enough of the DRG has been exposed for injection, prepare the syringe by placing the virus-filled microliter syringe fitted with a custom-made, 33-gauge blunt needle onto the stereotaxic syringe holder. Before injection, use a 30-gauge beveled needle to make a small superficial opening on each of the targeted DRG to assist with the insertion of the injection needle.
Insert the 33-gauge needle into the center of the DRG by turning the knobs gently to adjust the stereotaxic coordinates. Do not over-insert the needle, as this may cause fluid to leak out from the ventral side of the DRG. Should leakage occur, adjust the position of the needle immediately. NOTE: The numeric stereotaxic coordinates are not used; however, it is helpful to utilize the frame to hold the needle for injection.
Inject 1 µL of the virus into each DRG at 0.2 µL/min using an infusion syringe pump. Wait for an additional three minutes before withdrawing the needle. During the injection, the DRG will slowly change color if the virus solution contains a colored dye.
To perform a concurrent C5 - C8 dorsal root crush injury, crush each root three times for 10 s each using a pair of fine-tipped forceps (Bonn Micro), opposing the ends of the forceps completely; a white line in the tissue should appear at the crush site. Do not go deeper than required with the forceps, as this may cause damage to the ventral root.
Following the injection and/or crush, make sure that there is no bleeding or small pieces of bone fragments left at the incision site before closing up the animal. If preferred, place a small piece of surgical absorbable sponge on top of the exposed spinal cord and DRG.
Allow the third layer of the muscle to retract back naturally onto the spine without suturing. Loosely suture the two longitudinal muscles on the second layer (≈ 3 interrupted sutures) with absorbable 6-0 suture material. Suture the first layer of muscle (≈ 5 interrupted sutures) with absorbable 6-0 suture material.
Suture the skin with absorbable 5-0 suture material (≈ 10 interrupted sutures). NOTE: Make sure that the sutures are not too tight. Bulging of the skin is a sign of over-tightening and may cause discomfort to the animal.
If heavy bleeding occurred during the surgery, subcutaneously inject 1 - 2 mL of saline to replenish the loss of fluid from the animal as permitted under local regulations.
Provide edible hydrating gel and allow the animal to recover fully from anesthesia, performing regular monitoring for at least 1 h before returning the animal back to the holding area.
Keep the animal for at least 3 weeks for optimal transgene expression to assess sensory axon regeneration.
5. Performing Post-operative Care of the Animal
Provide food mash and soft cotton bedding for the animal during the first week post-surgery. If required, administer additional doses of analgesic and antibiotic to aid with the recovery in accordance with the local/national regulations and veterinary advice.
Remove the sutures on the skin after 7 - 10 days. NOTE: Common adverse effects of the surgery include the formation of seroma or hematoma at the incision site, scratch marks on the skin due to the itchiness caused by the internal absorbable sutures, subtle sensory or locomotion deficits, and signs of self-mutilation on the deafferented paw after the dorsal root crush injury. For any welfare issues that are substantially more severe than expected, seek immediate veterinary advice.
6. Performing an Anterograde CTB Injection for Axonal Tracing
NOTE: It is recommended to perform cholera toxin B subunit (CTB) axonal tracing a week prior to tissue collection.
Prepare 1% CTB solution as per the manufacturer's instruction.
Optional: Add 1% colored dye to the solution for easy visualization of the injection later on. Mix the solution very gently.
Anesthetize the animal (see step 3.2.1) and stabilize the left forepaw by taping the limb to the table.
Manually and slowly inject 1 µL of 1% CTB subcutaneously into the center of the glabrous footpad and four digits using a microliter syringe fitted with a custom-made, 33-gauge short needle. NOTE: Before injection, it helps to first use a 30-gauge beveled needle to make a small superficial opening on the skin, which assists with the insertion of the injection needle.
Allow the animal to recover fully from anesthesia before returning the animal to the holding area.
7. Collecting Tissue
To collect the virus-injected DRG and spinal cord for immunohistochemistry, administer an overdose of anesthetic and transcardially perfuse the animal with phosphate-buffered saline followed by cold 4% paraformaldehyde. Carefully perform a full laminectomy on the spine to collect the fixed tissue under a microscope. Proceed with tissue preparation, sectioning, and processing as required for analysis4,5,6. NOTE: The recovered incision site should be apparent, as observed by the presence of scar tissue.
Alternatively, to collect the virus-injected DRG for in vitro culture, sacrifice the animal humanely with an approved method, such as a rising concentration of carbon dioxide. Carefully dissect the DRG under a microscope, ensuring aseptic conditions whenever possible. Proceed with the tissue culture as desired4,5,6,10.
Representative Results
As a representation, a transverse spinal cord section with the attached DRG is presented to show the effectiveness of this protocol in transducing DRG neurons and tracing sensory axons in the spinal cord four weeks after injecting a control virus, AAV5-GFP, directly into the C7 DRG without a dorsal root crush injury (Figure 1A). Axons in both the dorsal column and the dorsal horn of the spinal cord express GFP (Figure 1B), as well as the cell bodies and axons within the injected DRG (Figure 1C). Additional anatomical analysis of the cuneate nucleus, the sensory axon terminal in the brainstem, reveals positive anterograde CTB tracing (Figure 1D).
When the DRG injection is performed concurrent with a complete C5-C8 dorsal root crush injury, no GFP-positive axons in the spinal cord or CTB-positive terminals in the cuneate nucleus are observed (Figure 2A). However, it is worth pointing out that axotomized sensory axons can still regenerate up to the dorsal root entry zone in a completely crushed dorsal root, which is a PNS environment, but not beyond into the spinal cord4,5 (Figure 2A). In the event that the injected virus contains the transgene of a potential growth-promoting protein, the presence of labelled axons in the spinal cord may represent either regeneration or an incomplete dorsal root crush injury (Figure 2B). To discriminate between these two outcomes, CTB axonal tracing in the cuneate nucleus should be analyzed. The presence of CTB-positive terminals in the cuneate nucleus highlights the likelihood of an incomplete injury (Figure 2C), while the absence of CTB-positive terminals suggests partial regeneration into the spinal cord, as regenerated axons are likely unable to grow the entire distance to reach the cuneate nucleus (Figure 2D). To date, successful sensory axon regeneration to the cuneate nucleus has mostly been reported in cases with high cervical injury11,12 or with the application of neurotrophins13,14. Any animals showing signs of incomplete injury should be excluded from axon regeneration studies.
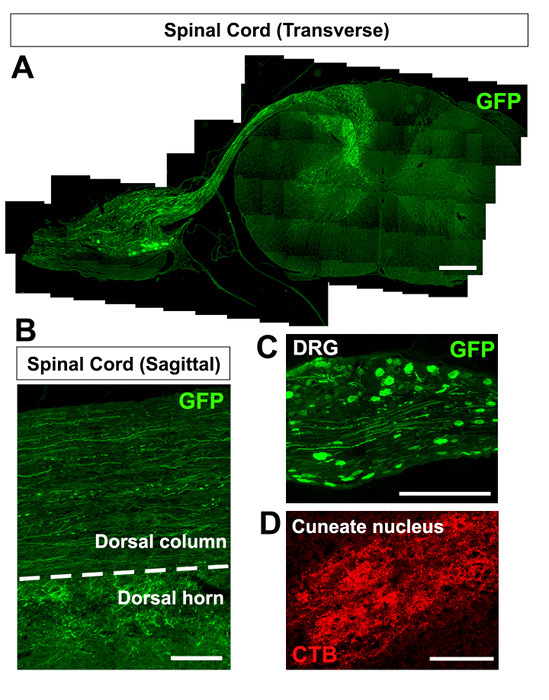 Figure 1:DRG Injection Without a Dorsal Root Crush Injury. (A-C) Spinal cord section showing GFP-positive axons in the spinal cord (A), including the dorsal column and dorsal horn (B) and cell bodies in the DRG (C) four weeks after the injection of AAV5-GFP. (D) CTB-positive sensory axon terminals in the cuneate nucleus one week following CTB injection. The scale bar is 650 µm (A-C) and 250 µm (D). Please click here to view a larger version of this figure.
Figure 1:DRG Injection Without a Dorsal Root Crush Injury. (A-C) Spinal cord section showing GFP-positive axons in the spinal cord (A), including the dorsal column and dorsal horn (B) and cell bodies in the DRG (C) four weeks after the injection of AAV5-GFP. (D) CTB-positive sensory axon terminals in the cuneate nucleus one week following CTB injection. The scale bar is 650 µm (A-C) and 250 µm (D). Please click here to view a larger version of this figure.
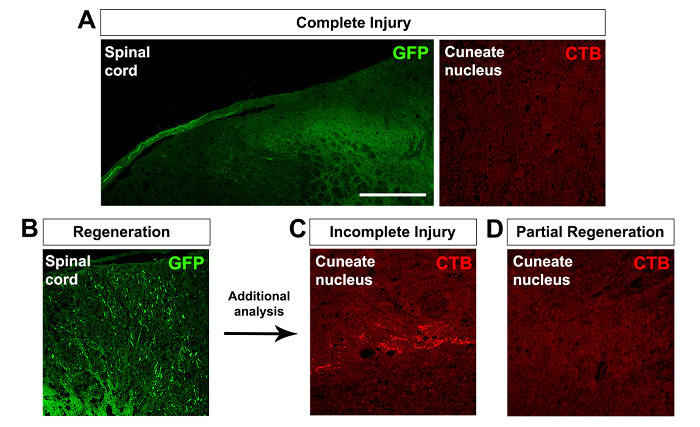 Figure 2:Assessing Dorsal Root Crush Injury and Axon Regeneration. (A) A complete dorsal root crush injury results in no labelled axons in the spinal cord or CTB-positive axon terminals in the cuneate nucleus. Axotomized sensory axons can regenerate up to the dorsal root entry zone, but not beyond into the spinal cord. (B-D) The presence of labelled axons in the spinal cord represents either incomplete injury or regeneration (B). With additional analysis, the presence of CTB-positive terminals in the cuneate nucleus suggests incomplete injury (C), while their absence suggests complete injury and potentially partial regeneration into the spinal cord (D). The scale bar is 250 µm. Please click here to view a larger version of this figure.
Figure 2:Assessing Dorsal Root Crush Injury and Axon Regeneration. (A) A complete dorsal root crush injury results in no labelled axons in the spinal cord or CTB-positive axon terminals in the cuneate nucleus. Axotomized sensory axons can regenerate up to the dorsal root entry zone, but not beyond into the spinal cord. (B-D) The presence of labelled axons in the spinal cord represents either incomplete injury or regeneration (B). With additional analysis, the presence of CTB-positive terminals in the cuneate nucleus suggests incomplete injury (C), while their absence suggests complete injury and potentially partial regeneration into the spinal cord (D). The scale bar is 250 µm. Please click here to view a larger version of this figure.
Discussion
In this article, we present a step-by-step guide to perform a DRG injection and dorsal root crush injury in the lower cervical spinal cord of an adult rat. As this is an extremely invasive and delicate surgery, we strongly recommend that all potential users obtain sufficient training and practice before advancing to live animal surgery. The users should be familiar not only with spinal cord anatomy, but also with the surrounding muscle tissues, vertebral bone structure, and vasculature. Ideally, a competent user should be able to perform the procedure with minimal damage to the surrounding tissues, carrying out a clean laminectomy by removing part of the vertebrae without inducing any damage to the spinal cord. As evident from spinal cord injury, a small lesion in the spinal cord can have a widespread detrimental effect to the entire nervous system. In addition, animals that have undergone a "clean" surgery are less likely to suffer from unexpected post-operative complications and welfare issues and are therefore less likely to have to be sacrificed before the desired experimental time point.
To administer virus into the nervous system, there are a few possible routes of administration: intravenous15, intraperitoneal16, intrathecal17, or direct injection into the target4,8. Although intravenous and intraperitoneal injections are relatively non-invasive, the crossing of the blood-brain barrier may be an issue18, and these routes result in non-specific transduction, which would not be useful in a specialized axon regeneration study. Similarly, for intrathecal injection into the subarachnoid space, a more invasive administrative route, many neuronal and non-neuronal cell types within the CNS may be transduced, potentially generating non-specific or off-target effects. Thus, the direct injection of virus into the DRG is a favorable option and likely to result in a much higher transduction efficiency than other methods. The major drawback of this option, however, is the invasiveness of the surgical procedure, which requires specialized training.
Once users have mastered the required surgical skills, this protocol offers a great amount of flexibility. In an axon regeneration study, animals can be studied in combination with other techniques, such as in vivo electrophysiology and sensory-motor behavioral tests, while the collected tissues can be used for anatomical analysis or tissue culture4. A combination of these techniques, with varied experimental time points, for example, can be used to study the progress of degeneration or regeneration of different fiber subtypes, such as NF200, CGRP, and IB4 after crush injury4. Depending on the experimental requirements, one or the other of the presented procedures can be performed alone. For example, DRG injection alone can be used for axonal tracing experiments, while dorsal root crush injury alone can be used in any studies where forepaw deafferentation is required. In addition, users can also vary the type of virus and transgene product for injection, the exact DRG to be injected, and the exact dorsal root for injury. If applicable, cell transplantation or pharmacological administration into the DRG can also be performed using this protocol. Building on acquired surgical skills, an experienced user can proceed to other techniques, such as DRG injection in the lumbar region (e.g., into L3 - L5 to assess hindlimb function)19 or dorsal column crush injury, to further study spinal cord function5.
In conclusion, we believe the DRG injection and dorsal root crush injury to be a useful model to study sensory axon regeneration. Despite the requirement for specialized training to perform the invasive surgical procedure, the protocol is flexible, and potential users can modify many parts to accommodate their experimental requirements. These procedures can serve as a foundation for those in search of a suitable animal model for sensory axon regeneration studies.
Disclosures
James Fawcett is a paid consultant for Acorda Therapeutics and Novartis.
Acknowledgments
This work was supported by grants from the Christopher and Dana Reeve Foundation, the Medical Research Council, the European Research Council ECMneuro, and the Cambridge NHMRC Biomedical Research Center. We would like to express our deepest gratitude to Heleen Merel van ’t Spijker and Justyna Barratt for their technical assistance during the filming. We would like to thank Dr. Elizabeth Moloney and Professor Joost Verhaagen (Netherlands Institute for Neuroscience) for assisting in AAV production.
References
- Chew DJ, Fawcett JW, Andrews MR. The challenges of long-distance axon regeneration in the injured CNS. Prog Brain Res. 2012;201:253–294. doi: 10.1016/B978-0-444-59544-7.00013-5. [DOI] [PubMed] [Google Scholar]
- Wu A, Lauschke JL, Morris R, Waite PM. Characterization of rat forepaw function in two models of cervical dorsal root injury. J Neurotrauma. 2009;26(1):17–29. doi: 10.1089/neu.2008.0675. [DOI] [PubMed] [Google Scholar]
- Hocquemiller M, Giersch L, Audrain M, Parker S, Cartier N. Adeno-Associated Virus-Based Gene Therapy for CNS Diseases. Hum Gene Ther. 2016;27(7):478–496. doi: 10.1089/hum.2016.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah M, et al. Expression of an Activated Integrin Promotes Long-Distance Sensory Axon Regeneration in the Spinal Cord. J Neurosci. 2016;36(27):7283–7297. doi: 10.1523/JNEUROSCI.0901-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews MR, et al. Alpha9 integrin promotes neurite outgrowth on tenascin-C and enhances sensory axon regeneration. J Neurosci. 2009;29(17):5546–5557. doi: 10.1523/JNEUROSCI.0759-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CL, et al. Kindlin-1 enhances axon growth on inhibitory chondroitin sulfate proteoglycans and promotes sensory axon regeneration. J Neurosci. 2012;32(21):7325–7335. doi: 10.1523/JNEUROSCI.5472-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty DM, Young SM, Samulski RJ. Integration of adeno-associated virus (AAV) and recombinant AAV vectors. Annu Rev Genet. 2004;38:819–845. doi: 10.1146/annurev.genet.37.110801.143717. [DOI] [PubMed] [Google Scholar]
- Mason MR, et al. Comparison of AAV serotypes for gene delivery to dorsal root ganglion neurons. Mol Ther. 2010;18(4):715–724. doi: 10.1038/mt.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermens WT, et al. Purification of recombinant adeno-associated virus by iodixanol gradient ultracentrifugation allows rapid and reproducible preparation of vector stocks for gene transfer in the nervous system. Hum Gene Ther. 1999;10(11):1885–1891. doi: 10.1089/10430349950017563. [DOI] [PubMed] [Google Scholar]
- Kappagantula S, et al. Neu3 sialidase-mediated ganglioside conversion is necessary for axon regeneration and is blocked in CNS axons. J Neurosci. 2014;34(7):2477–2492. doi: 10.1523/JNEUROSCI.4432-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto LT, et al. Chemotropic guidance facilitates axonal regeneration and synapse formation after spinal cord injury. Nat Neurosci. 2009;12(9):1106–1113. doi: 10.1038/nn.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner JF, et al. Grafted neural progenitors integrate and restore synaptic connectivity across the injured spinal cord. J Neurosci. 2011;31(12):4675–4686. doi: 10.1523/JNEUROSCI.4130-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, et al. Persistent restoration of sensory function by immediate or delayed systemic artemin after dorsal root injury. Nat Neurosci. 2008;11(4):488–496. doi: 10.1038/nn2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong LE, Gibson ME, Arnold HM, Pepinsky B, Frank E. Artemin promotes functional long-distance axonal regeneration to the brainstem after dorsal root crush. Proc Natl Acad Sci U S A. 2015;112(19):6170–6175. doi: 10.1073/pnas.1502057112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanguy Y, et al. Systemic AAVrh10 provides higher transgene expression than AAV9 in the brain and the spinal cord of neonatal mice. Front Mol Neurosci. 2015;8:36. doi: 10.3389/fnmol.2015.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust KD, Poirier A, Pacak CA, Mandel RJ, Flotte TR. Neonatal intraperitoneal or intravenous injections of recombinant adeno-associated virus type 8 transduce dorsal root ganglia and lower motor neurons. Hum Gene Ther. 2008;19(1):61–70. doi: 10.1089/hum.2007.093. [DOI] [PubMed] [Google Scholar]
- Vulchanova L, et al. Differential adeno-associated virus mediated gene transfer to sensory neurons following intrathecal delivery by direct lumbar puncture. Mol Pain. 2010;6:31. doi: 10.1186/1744-8069-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SJ, et al. Directed evolution of a novel adeno-associated virus (AAV) vector that crosses the seizure-compromised blood-brain barrier (BBB) Mol Ther. 2010;18(3):570–578. doi: 10.1038/mt.2009.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagoe ND, Attwell CL, Kouwenhoven D, Verhaagen J, Mason MR. Overexpression of ATF3 or the combination of ATF3, c-Jun, STAT3 and Smad1 promotes regeneration of the central axon branch of sensory neurons but without synergistic effects. Hum Mol Genet. 2015;24(23):6788–6800. doi: 10.1093/hmg/ddv383. [DOI] [PubMed] [Google Scholar]


