Abstract
Ixodes scapularis, the vector of Lyme disease, is one of the most important disease vectors in the eastern and Midwestern United States. This species is a three host tick that requires a blood meal from a vertebrate host for each development stage, and the adult females require a blood meal for reproduction. Larval ticks attach to their host for 3 - 5 days for feeding and drop off the host when fully engorged. This dependency on several different hosts and the lengthy attachment time for engorgement complicates tick rearing in the laboratory setting. However, to understand tick biology and tick-pathogen interactions, the production of healthy, laboratory-reared ticks is essential. Here, we demonstrate a simple, cost-effective protocol for immature tick feeding on mice. We modified the existing protocols for decreased stress on mice and increased tick feeding success and survival by using disposable cages without mesh bottoms to avoid contact of ticks with water contaminated with mice urine and feces.
Keywords: Infection, Issue 123, Ixodes scapularis, Lyme disease vector, tick rearing, tick feeding, mouse, larval tick feeding
Introduction
Ticks are obligate hematophagous ectoparasites of vertebrates and are distributed worldwide. In the United States, at least 11 species of ticks are vectors of pathogens of public health importance1. Ixodes scapularis is responsible for transmission of several pathogens such as the causative agents of Lyme disease (Borrelia burgdorferi) relapsing fever (B. miyamotoi), human granulocytic anaplasmosis (Anaplasma phagocytophilum), and babesiosis (Babesia spp.). Despite the importance of I. scapularis as a disease vector, collecting these arachnids in abundance from the wild for studies in the lab is not always feasible. Therefore, the production of healthy laboratory-reared ticks is essential to studies on tick biology, and tick-pathogen interactions.
The life cycle of all hard ticks (family Ixodidae), including I. scapularis, consists of the egg and three active stages: larva, nymph, and adult. Each active stage feeds on a vertebrate host. The complex interactions that take place between ticks and their hosts over several days of attachment and feeding are nearly impossible to replicate using artificial feeders, and are unlikely to provide enough numbers of fed ticks for mass rearing2,3,4. Therefore, live mice and rabbits are used most frequently as hosts for rearing immature (larvae and nymphs), and mature stages (adults) of ticks, respectively. The requirement of multiple hosts for blood feeding during each developmental stage complicates tick rearing, and is time and cost intensive5,6,7. Most tick rearing protocols require keeping mice in a suspended wire grid floor cage7,8 or in a cylindrical cage of such dimensions that the animal cannot move freely and groom itself6,9,10.
These cylindrical cages are later transferred to a shoebox cage with a wire grid. Engorged, detached ticks are then collected from the water underneath. However, this method results in exposing fed ticks to water contaminated with urine and feces that can increase fungal growth and tick mortality9. In addition, it increases the possibility of tick escape from the water trough, as well as causing stress to mice. To circumvent these problems, we here demonstrate larval tick feeding on mice within plastic shoebox-type disposable cages. This method allows the normal behavior of mice, increases engorged tick recovery, and decreases tick mortality due to contamination.
Protocol
The protocol (Number-00682) outlined below is approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Nevada Reno and follows the guidelines of the University of Nevada, Reno's animal research ethics committee. Briefly, mice were anesthetized with isoflurane and a nose cone was used for maintaining continuous isoflurane levels for 20 min. A vet ointment was used to prevent drying of eyes under anesthesia. Toe pinch was used to ascertain the anesthesia level and breathing rate was measured throughout the procedure. Mice were kept in individual cages and monitored until consciousness was regained. Mice were used only once for tick feeding and were euthanized post tick recovery. Euthanasia was carried out by the Laboratory of Animal Medicine staff personnel. CO2 and cervical dislocation were used for euthanizing animals.
NOTE: Working on ticks requires use of complete personal protective equipment. For counting immature ticks, wear white lab coats, long nitrile gloves to cover the sleeve opening of the lab coat capped with rubber bands, and closed-toe shoes. For infesting mice with ticks, use a hair net, disposable coveralls, long-sleeved gloves over the sleeves, and foot covers. Use white or light colored coveralls to detect wayward ticks. Periodically check gloves and sleeves for ticks.
1. Preparing Room for Animal Housing
Designate a separate room to house mice infested with ticks. Place a sticky mat or double-sided carpet tape outside and inside the door to prevent accidental tick escape.
2. Counting Ticks for Mouse Infestation
Put double-sided tape all around the top edges of a 7" x 5" x 14" plastic container and fill with water to approximately 2 cm.
Place another small box or Petri dish in the center of the container and fill with water to 1 cm creating a "moat" around this container
Store the vial containing larvae or nymphs inside the Petri dish.
Use a fine paint brush to remove larvae or nymphs from the vial and count under the microscope. Count 50 larvae or 25 nymphs into separate scintillation vials. Cover the vials immediately with nylon mesh or organdy cloth screen and close with rubber bands.
3. Infesting Mice with Immature Ticks
Use a white or light colored work bench and stick double-sided tape around the perimeter of the work area.
Anesthetize mouse with isoflurane. Check level of anesthesia by toe pinch. Once anesthetized, transfer the mouse to a heating pad covered with paper towels and attach to a nose cone for continued isoflurane supply.
Apply petroleum jelly-based eye ointment to avoid dryness. Note the breathing pattern of the mouse to adjust isoflurane levels (80 - 230 breaths per min is normal., Reduce isoflurane level if breathing rate is less than 80 breaths per min to avoid killing the animal).
Take one vial of 50 larvae or 25 nymphs and place ticks under the fur on the head between the ears with a paint brush (Figure 1). Keep mouse under anesthesia for 20 min following placement to give enough time for ticks to attach.
Move the mouse to a standard, plastic shoe box-type disposable mouse cage with static lids and white bedding. Provide toys, water, and food ad libitum as per normal mouse care.
Store the mouse cage within a larger rat or gerbil cage lined with double-sided sticky tape around top edges. Fill the outer cage with 3 cm of water (Figure 2).
4. Collecting Ticks from Mice
Immature ticks detach between days 3 and 6 of feeding. Check the cages and water moat for engorged ticks every day after day 3.
Collect detached ticks from the cage between days 4 and 6. Use a paint brush or soft forceps for picking up engorged ticks and store in clean scintillation vials capped with nylon mesh cloth secured with rubber bands.
Anesthetize and check mouse on day 7 for any remaining attached ticks.
Check the bedding, food trough, and water bottle for engorged ticks on day 7. Euthanize mice following the procedure as described above. Autoclave the disposable cages, bedding, water bottle, and food trough to avoid any escaping unfed ticks.
5. Storage of Fed Ticks
Maintain engorged ticks at 90% humidity, 20°C, and a 12:12 light:dark cycle in a humidity and temperature controlled incubator until molting occurs. This may take approximately 12 to 18 weeks to occur.
While high humidity promotes tick survival, it also makes the ticks prone to fungal overgrowth. Check the fed ticks under the microscope at least once a week for mold. If detected, wash survivors in 70% ethanol for 5 min., rinse in water, transfer to a filter paper to dry, and transfer to new, clean vials.
Representative Results
We modified existing tick rearing protocols6,10 for improved feeding efficiency and reduced stress on the mouse host. The results demonstrate that the standard shoebox style mouse cages are well suited for tick rearing. The white bedding provided a good contrast for easy collection of fed ticks. Most ticks climbed up the walls of the containers after feeding and were easy to collect. In addition, the tight fitted lid of the disposable plastic boxes (routinely used for mice housing at UNR) prevented tick escape from the box. Additionally, we did not shave mice for tick infestation (Figure 1). These changes in published protocols resulted in significant tick attachment, engorgement, and survival.
This protocol is cost effective as it does not require any special mouse cages outside of those already in use. It is also less labor intensive as we did not have to collect engorged ticks every day. We collected ticks between days 4 and 6 post infestations. Most ticks detached by day 4 and a few remaining ones detached by day 5. By day 7 all mice were free of ticks. We were able to recover an average of 67% (range 52 - 92%) engorged larval ticks (Table 1). All mice were healthy and did not show any signs of discomfort.
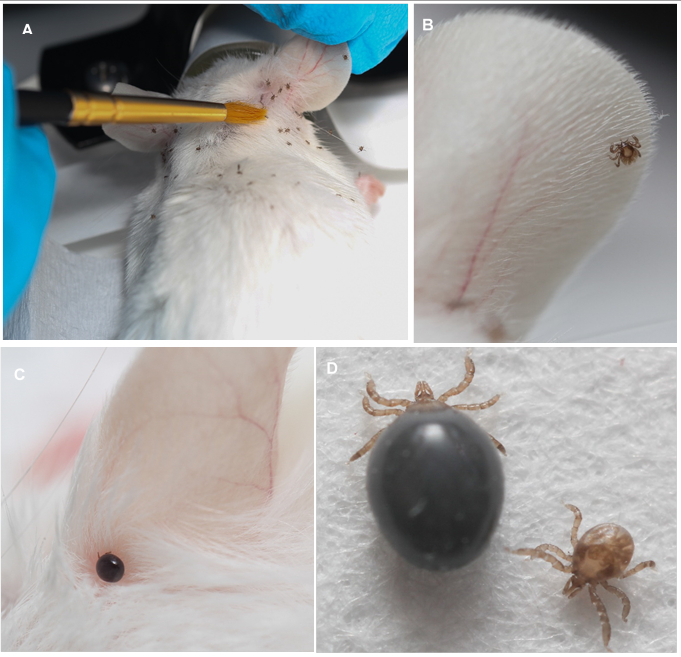 Figure 1: Feeding Ixodes scapularis Larvae on Mice. Mice were individually infested with 50 larval ticks. The engorged ticks dropped off the mouse between days 4 - 6. The cages were checked for any remaining ticks on day 7 and discarded thereafter. A: Infesting mice with unfed larval ticks. B: A larval tick attaching to the ear of a mouse. C: An engorged larval tick attached to the ear of a mouse, 3 day post infestation. D: Unfed and fully engorged tick larvae.
Figure 1: Feeding Ixodes scapularis Larvae on Mice. Mice were individually infested with 50 larval ticks. The engorged ticks dropped off the mouse between days 4 - 6. The cages were checked for any remaining ticks on day 7 and discarded thereafter. A: Infesting mice with unfed larval ticks. B: A larval tick attaching to the ear of a mouse. C: An engorged larval tick attached to the ear of a mouse, 3 day post infestation. D: Unfed and fully engorged tick larvae.
 Figure 2: Mouse Cage Set up for Tick Infestation. Two disposable mouse cages were kept inside a gerbil cage. Approximately 3 cm. water was added to the gerbil cage to make a moat. Double-sided sticky carpet tape was affixed around the top edges of the gerbil cage to avoid tick escape.
Figure 2: Mouse Cage Set up for Tick Infestation. Two disposable mouse cages were kept inside a gerbil cage. Approximately 3 cm. water was added to the gerbil cage to make a moat. Double-sided sticky carpet tape was affixed around the top edges of the gerbil cage to avoid tick escape.
| Animal (mouse) | Total number of larval ticks recovered | Percentage larval ticks recovered |
| 1 | 34 | 68% |
| 2 | 32 | 64% |
| 3 | 38 | 76% |
| 4 | 28 | 56% |
| 5 | 39 | 78% |
| 6 | 26 | 52% |
| 7 | 46 | 92% |
| 8 | 29 | 58% |
| 9 | 29 | 58% |
| 10 | 33 | 66% |
| AVERAGE | 66.8% |
Table 1: Number of Engorged Ixodes scapularis Larvae Recovered after Laboratory Infestation on Anesthetized Mouse Hosts. Each mouse was infested with 50 larvae.
Discussion
Critical Steps within the Protocol
It is important to have several levels of safety measures when rearing ticks to avoid accidental escape. Use of sticky tape and a water moat are critical to ensure safety. It is important to keep the anesthetized mouse on a heating pad to keep the body temperature constant. We also found that shaving the mouse does not provide any extra benefit for tick attachment. An individual mouse can be kept in the same cage for one week in a designated "tick room" which further limits contact of ticks with personnel.
Modifications and Troubleshooting
The production of high-quality, laboratory-reared ticks is necessary for studies on tick biology and tick-pathogen interactions. Previous feeding protocols used suspended wire floor8 or cylindrical cages to keep the mice immobile6,10 which incur extra cost as well as stress to the animal. In addition, the suspended wire cage allows the engorged ticks to fall through the wire into the water contaminated with urine and feces of the mouse thus increasing the risk of mold growth.
Limitations of the Technique
As shown in data, we successfully recovered on average 65% (up to 92%) of ticks. We occasionally found ticks in water moat outside the mouse cage but none escaped. The sticky tape on the outer walls of second container prevented the ticks from escaping.
Significance of the Technique with Respect to Existing/Alternative Methods
Our protocol of using shoe box style disposable mice cages permits free range of motion and demonstrates that mice do not need to be restrained to permit tick feeding. Most fed ticks were easily seen and collected from the transparent cage walls and any remaining ticks are readily detected among bedding at the end of the feeding cycle. This protocol does not require any custom mouse cage, thus, it is cost effective.
Literature suggests that less than 50% of ticks attach to mouse and not all attached ticks feed to completion. On an average, we collected ~ 60% engorged larval ticks. We did not find any escaped unfed or fed ticks. Occasionally we found one or two engorged larval ticks in water moat but none on the sticky tape. Our results suggest that not all ticks attach to the host and may die without feeding. Unfed, dead ticks were hard to find in litter. Occasionally, we also found blood stains on the white bedding which suggests that either mouse have killed the engorged tick after dropping off or scratched off. Our data and daily observation of cages suggest that engorged ticks either move up on the cage walls and top of the cage or hide under the bedding away from the mouse's reach. Therefore, the enclosed cage do not have any negative impact on tick survival.
Future Applications or Directions after Mastering this Technique
Our protocol provides a simplified alternative for mass rearing of ticks without adding extra cost or reducing safety of the personnel handling the animals. Future experimentation will focus on improving methods of feeding adult I. scapularis.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors acknowledge the help from the staff of Laboratory Animal Medicine, University of Nevada, Reno. MM received funding from Nevada INBRE.
References
- Gleim ER, et al. Factors associated with tick bites and pathogen prevalence in ticks parasitizing humans in Georgia, USA. Parasites & Vectors. 2016;9(125):1–13. doi: 10.1186/s13071-016-1408-6. Available from: http://doi.org/10.1186/s13071-016-1408-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krober T, Guerin PM. In vitro feeding assays for hard ticks. Trends Parasitol. 2007;23(9):445–449. doi: 10.1016/j.pt.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Kuhnert F. Feeding of Hard Ticks In Vitro: New Perspectives for Rearing and for the Identification of Systemic Acaricides. ALTEX. 1996;13(2):76–87. [PubMed] [Google Scholar]
- Voigt WP, et al. In vitro feeding of instars of the ixodid tick Amblyomma variegaturn on skin membranes and its application to the transmission of Theileria mutans and Cowdria ruminantium. Parasitol. 1993;107:257–263. doi: 10.1017/s0031182000079233. [DOI] [PubMed] [Google Scholar]
- Gregson JD. Ticks. In: Smith CN, editor. Insect Colonization and Mass Production. New York: Academic Press; 1966. pp. 49–72. [Google Scholar]
- Sonenshine DE. Biology of Ticks. Vol. 2. New York: Oxford University Press; 1993. [Google Scholar]
- Bouchard KR, Wikel SK. Care, maintenance, and experimental infestation of ticks in the laboratory setting. In: Marquaedt WC, editor. Biology of Disease Vectors. New York: Elsevier; 2005. pp. 705–711. [Google Scholar]
- Schumaker TS, Barros DM. Life cycle of Ornithodoros (Alectorobius) talaje. (Acari:Argasidae) in laboratory. J Med Entomol. 1995;32:249–254. doi: 10.1093/jmedent/32.3.249. [DOI] [PubMed] [Google Scholar]
- Banks CW, Oliver JH, Hopla CE, Dotson EM. Laboratory life cycle of Ixodes woodi (Acari:Ixodidae) J. Med. Entomol. 1998;35:177–179. doi: 10.1093/jmedent/35.2.177. [DOI] [PubMed] [Google Scholar]
- James AM, Oliver JH., Jr Feeding and host preference of immature Ixodes dammini,I.scapularis,and I.pacificus.(Acari:Ixodidae) J. Med. Entomol. 1990;27:324–330. doi: 10.1093/jmedent/27.3.324. [DOI] [PubMed] [Google Scholar]


