Abstract
Proton Transfer Reaction (PTR), combined with a Time-of-Flight (ToF) Mass Spectrometer (MS) is an analytical approach based on chemical ionization that belongs to the Direct-Injection Mass Spectrometric (DIMS) technologies. These techniques allow the rapid determination of volatile organic compounds (VOCs), assuring high sensitivity and accuracy. In general, PTR-MS requires neither sample preparation nor sample destruction, allowing real time and non-invasive analysis of samples. PTR-MS are exploited in many fields, from environmental and atmospheric chemistry to medical and biological sciences. More recently, we developed a methodology based on coupling PTR-ToF-MS with an automated sampler and tailored data analysis tools, to increase the degree of automation and, consequently, to enhance the potential of the technique. This approach allowed us to monitor bioprocesses (e.g. enzymatic oxidation, alcoholic fermentation), to screen large sample sets (e.g. different origins, entire germoplasms) and to analyze several experimental modes (e.g. different concentrations of a given ingredient, different intensities of a specific technological parameter) in terms of VOC content. Here, we report the experimental protocols exemplifying different possible applications of our methodology: i.e. the detection of VOCs released during lactic acid fermentation of yogurt (on-line bioprocess monitoring), the monitoring of VOCs associated with different apple cultivars (large-scale screening), and the in vivo study of retronasal VOC release during coffee drinking (nosespace analysis).
Keywords: Chemistry, Issue 123, Direct-Injection Mass Spectrometry (DIMS), Proton Transfer Reaction Time of Flight Mass Spectrometry (PTR-ToF-MS), autosampler, volatile organic compounds (VOCs), food, flavor, nosespace, screening, bioprocess, yogurt, coffee, apple
Introduction
Direct-Injection Mass Spectrometric (DIMS) technologies represent a class of analytical instrumental approaches that offer considerable mass and time resolution with high sensitivity and robustness, allowing the quick detection and quantification of volatile organic compounds (VOCs)1. These instrumental approaches include, among others, MS-e-noses, Atmospheric-Pressure Chemical Ionization Mass Spectrometry (APCI-MS), Proton-Transfer-Reaction Mass Spectrometry (PTR-MS), and Selected Ion-Flow-Tube Mass Spectrometry (SIFT-MS)1. The pros and the cons of each approach depend on: the kind of sample injection, the source and control of precursor ions, the control of the ionization process, and the mass analyzer1,2.
Proton-transfer-reaction mass-spectrometry (PTR-MS) was developed more than twenty years ago to monitor in real-time and with low detection limits (usually a few ppbv, part per billion by volume) most volatile organic compounds (VOCs) in air3,4. Current uses of PTR-MS range from medical applications, to food control, to environmental research5,6. The main features of this technique are: the possibility of rapid and continuous measurement, the intense and pure source of precursor ions, and the possibility to control ionization conditions (pressure, temperature and drift voltage). These features permit combining versatile uses with a high degree of standardization1,4. In fact, the method is based on reactions of hydronium ions (H3O+), which induce non-dissociative proton transfer in most volatile compounds (particularly in those characterized by a proton affinity higher than water), protonating neutral compounds (M) according to the reaction: H3O+ + M → H2O + MH+. In contrast to other techniques, e.g., APCI-MS, precursor ion generation and sample ionization are divided in two different instrumental compartments (a schematic representation of the PTR-MS instrument is given in Figure 1). An electrical discharge by water vapor in the hollow cathode ion source generates a beam of hydronium ions. After this phase, ions cross the drift tube, where the ionization of VOCs takes place7. Ions then enter a pulse extraction section and are accelerated into the TOF section. Through flight times, it is possible to determine the mass-to-charge ratios of the ions8. Each extraction pulse leads to a complete mass spectrum8 of the selected m/z range. Ion spectra are recorded by a fast data acquisition system7. A complete spectrum is typically acquired in one second although higher time resolution can be achieved according to the signal to noise level and a quantitative estimation of the VOC headspace concentration can be provided even without calibration9,10.
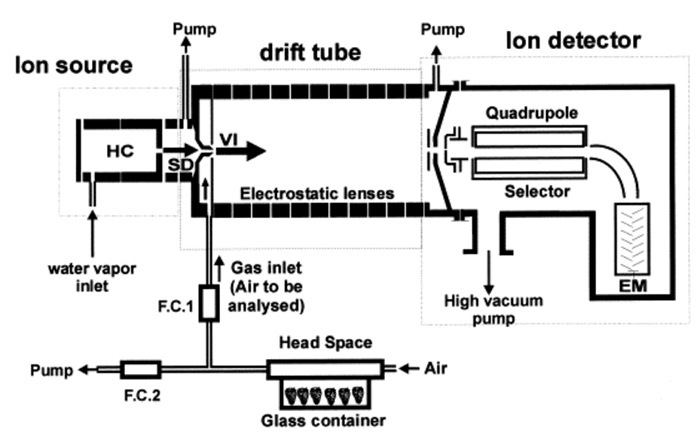 Figure 1:Schematic illustration of a PTR-MS. Schematic representation of the PTR-MS instrument. HC: external ion source with hollow cathode; SD: source drift; VI, venturi-type inlet; EM, electron multiplier; FC1-2, flow controllers. Reprinted with permission from Boschetti et al.7. Please click here to view a larger version of this figure.
Figure 1:Schematic illustration of a PTR-MS. Schematic representation of the PTR-MS instrument. HC: external ion source with hollow cathode; SD: source drift; VI, venturi-type inlet; EM, electron multiplier; FC1-2, flow controllers. Reprinted with permission from Boschetti et al.7. Please click here to view a larger version of this figure.
In general, the PTR technique assures fast analysis time, high detection sensitivity and a relatively compact instrument size, requires neither sample preparation nor sample destruction and thus allows real time investigations11. PTR is of considerable interest in the environmental, atmospheric, food, technological, medical and biological sciences12.
VOCs associated with food matrices are of outstanding interest in food science and technology because of their important role in the molecular basis of biological phenomena linked to odor and flavor perception and, thus, in food acceptance. Hence, our interest in real time and non-invasive detection of VOCs mainly deals with sensory qualities of food. In addition, if we consider the possibility to detect spoilage and pathogenic microorganisms by means of released VOCs13 and/or to monitor volatile organic compounds as markers following technological processes (e.g. Maillard by-products during thermal treatments)14, it becomes clear how VOC identification and quantification are fields of interest in food quality management6. Several recent uses of PTR-MS technologies for the rapid monitoring and quantification of VOCs in food matrices testify to the wide range of application of these analytical approaches (Table 1).
| Food matrix | Kind of application | Brief description | Reference |
| Butter | Screening/characterization | Geographical origin of European butters | 15 |
| Yogurt | Bioprocess monitoring | Evolution during lactic acid fermentation | 16 |
| Cereal bars | In vivo measurement | Nosespace during consumption of cereal bars with varying sugar composition | 17 |
| Liquid model systems | Simulated oral conditions | Evaluation of tongue pressure and oral conditions in a model mouth | 18 |
| Apple | In vivo measurement | Nosespace during consumption apple with different genetic, textural, and physicochemical parameters | 19 |
| Coffee | Screening/characterization | Differentiation of specialty coffees | 20 |
| Grape must | Screening/characterization | Effect of cooking process | 21 |
| Flavored candies | In vivo measurement | Determination on panelists using different direct mass spectrometry methods | 22 |
| Ham | Screening/characterization | Effect of the pig rearing system | 23 |
| Bread | Simulated oral conditions | Simulating bread aroma during mastication | 24 |
| Milk | Screening/characterization | Monitoring photooxidation-induced dynamic changes in milk | 25 |
| Coffee | Screening/characterization | Diversity in roasted coffees from different geographic origins | 26 |
| Bread | Bioprocess monitoring | Effect of different yeast starters during alcoholic fermentation | 27 |
| Coffee | In vivo measurement | Nosespace during consumption of different roasted coffee preparations | 28 |
| Tomatoes | Screening/characterization | Impact of Production Location, Production System, and Variety | 29 |
| Bread | Bioprocess monitoring | Effect of flour, yeast and their interaction during alcoholic fermentation | 30 |
| Mushrooms | Screening/characterization | Shelf life of dried porcini mushrooms | 31 |
| Yogurt | Bioprocess monitoring | Effect of different starter cultures during lactic fermentation | 32 |
| Apple | Screening/characterization | Diversity in an apple germplasm collection | 33 |
| Coffee | Screening/characterization | Tracing coffee origin | 34 |
| Coffee | In vivo measurement | Combination of a dynamic sensory method and in-vivo nosespace analysis to understand coffee perception | 35 |
Table 1: List of scientific studies using PTR-ToF-MS in the food sector. Non-exhaustive list of scientific studies using PTR-based approaches to monitor VOC content in food-related experiments.
In recent studies, we reported on the application of PTR-ToF-MS coupled with an automated sampling system and tailored data analysis tools to increase sampling automation and reliability and, consequently, to enhance the potential of this technique7,10,13. This allowed us to screen, in terms of VOC content, large sample sets (e.g. food of different origins with many replicates, entire germoplasms), to analyze the influence of several experimental modes on VOC release (e.g. different concentrations of a given ingredient, diverse intensities of a specific technological parameter), and to monitor VOCs associated with a given bioprocess (e.g. enzymatic oxidation, alcoholic fermentation). Here, in order to exemplify the potential of PTR-ToF-MS in the agri-food sector, we present three paradigmatic applications: the detection of VOCs released during lactic acid fermentation of yogurt induced by different microbial starter cultures (on-line bioprocess monitoring), the monitoring of VOCs associated with different apple cultivars (large-scale screening) and the in vivo study of retronasal VOC release while drinking coffee (nosespace analysis).
Protocol
The protocol follows the guidelines of our institutional committee on human research ethics.
1. Sample Preparation and Autosampler Conditions
- On-line bioprocess monitoring: detecting VOCs released during lactic acid fermentation of yogurt NOTE: This section of the protocol represents part of the procedure reported by Benozzi et al.32
- Add 5 mL of pasteurized milk to each vial (20 mL glass vials equipped with PTFE/silicone septa). Note the type of milk used and quickly heat the samples to 45 °C. Transfer them to a multifunctional GC autosampler equipped with a temperature controlled tray (45 °C).
- Use the robotic arm of the autosampler to inoculate the vials with the microbial starter cultures (according to the specifications of the starter culture manufacturer). Set the incubation time according to the typology of the desired yogurt and to the specifications reported by starter culture manufacturer. Set the autosampler to conveniently analyze one sample after the other, obtaining an on-line VOC monitoring of lactic acid fermentation during yogurt preparation.
- Large-scale screening: monitoring VOCs associated with different apple genotypes NOTE: This section of the protocol represents part of the procedure reported by Farneti et al.33,36
- Sample apples at the desired phase of ripening/conservation (e.g. at commercial harvest stage). Select at least five homogeneous fruits without any visible damage for each clone. Store the apples for the desired period at room temperature (25 °C) or refrigerate (4 °C).
- Collect five cylindrical discs (1.7 cm diameter and 1 cm thickness) from each apple with a flesh blade sampler. Include a part of cortex tissue, and avoid the core portion with seeds. Immediately homogenize the samples and freeze in liquid nitrogen. Store at -80 °C until analysis.
- Before analysis, place three replicates of the 2.5 g apple sample from each biological replicate into the vials (20 mL glass vials equipped with PTFE/silicone septa). Mix the sample with 2.5 mL of deionized water, 1 g of sodium chloride, 12.5 mg of ascorbic acid, and 12.5 mg of citric acid, and keep the samples at 4 °C until analysis (maximum 3 days).
- Incubate the samples at 40 °C and then set the autosampler to automatically analyze the VOCs.
- Nosespace analysis: studying retronasal release of VOCs during coffee drinking NOTE: This section of the protocol represents part of the procedure reported by Romano et al.28
- Prepare brewed coffee from ground coffee samples.
- Use a coffee machine: report the water/powder ratio, the type of mineral water used, the type of coffee maker, and the procedure adopted to obtain the coffee beverage (quantities are a function of the dimension of the coffee machine).
- Use a six-cup stove-top coffee maker, known in Italy as a "moka", using 450 mL water and 30 g of coffee powder. Put the brewed coffee in a vessel and transfer it in a thermostatic water bath (60 °C).
- For each coffee brew, transfer 7.5 mL aliquots to a polystyrene cup (40 mL) with a plastic cap. Have each panelist taste the beverage according to the protocol: i) 30 s of free breathing, ii) a single sip of coffee, followed by a fast swallow, and iii) 3 min of breathing into an ergonomic glass nosepiece28.
- Repeat the whole experiment for three consecutive days, randomizing the order of coffee samples and panelists each day.
- Perform sampling by applying a single-use ergonomic nosepiece in silicon rubber to the nose of the panelists. Connect the nosepiece to the PTR-ToF-MS by means of a PEEK tube that is unheated only in the first part in contact with the panelist body, then heated at 110 °C in an inlet hose, which connects the sampling interface with the PTR-MS instrument. NOTE: In Table 2 a list of products analyzed with analogous procedures to those reported by Benozzi et al.32, Farneti et al.33,36, and Romano et al.28 is reported.
| Food matrix | Number and kind of samples | Reference |
| Apple | The authors screened a collection represented by 190 accessions, composed by both old and new apple cultivars | 33 |
| Yogurt | Four starters were analyzed in terms of VOCs released during lactic fermentation of yogurt (A, FD-DVS YF-L812 Yo-Flex, Chr. Hansen; B, FD-DVS YC-380 Yo-Flex, Chr. Hansen; C, FD-DVS YC-X11 Yo-Flex, Chr. Hansen; D, YO-MIX 883, Danisco) | 32 |
| Coffee | Three different kind of ground coffee obtained from a single pure Arabica coffee blend were used: medium roast, dark roast and decaffeinated medium roast | 28 |
Table 2: List of products analyzed. List of products analyzed with analogous procedures to the ones reported by Benozzi et al.32, Farneti et al.33,36, and Romano et al.28
2. Experimental Design and Practical Precautions
Perform at least three inter-day biological replicates, each with three technical replicates, for each experimental mode.
Prior to sample incubation and analysis, flush the headspace with clean air for 1 min at 200 sccm for each vial.
Prepare a blank for every experimental mode, incubate and analyze the blank under the same conditions of the samples.
Randomize the order of samples/blanks for analysis.
Similarly to other methods used to detect VOCs, limit the use of perfumed personal care products, as well as gum and cigarettes, before using the instrument. Tightly cap all volatile chemicals in the lab, and control air drafts as much as possible during testing37.
3. PTR-MS Instrument Optimization and Analysis
NOTE: The instrumental conditions are described in the references (e.g. Makhoul et al.27).
Perform headspace measurements of the samples with a commercial PTR-ToF-MS apparatus in the standard configuration mode.
Directly inject air into the PTR-MS drift tube headspace without any treatment. There is a continuous flow of sample air through the PTR-MS so injection is achieved by simply inserting the end of the PTR-MS inlet into the sample headspace.
Set and constantly verify the following ionization conditions in the drift tube: 110 °C drift tube temperature, 2.30 mbar drift pressure, 550 V drift voltage. This leads to an E/N ratio of about 140 Td (1 Td = 10−17 cm2 V−1 s−1). The inlet line consists of a PEEK capillary tube (internal diameter 0.04 in.) heated at 110 °C. By default, set the inlet flow to 40 sccm.
Set sampling time per channel of ToF acquisition to 0.1 ns, amounting to 350,000 channels for a mass spectrum ranging up to m/z = 400. Every single spectrum is the sum of about 28,600 acquisitions lasting 35 µs each, resulting in a time resolution of 1 s. NOTE: Spectra are then continuously stored. Spectrometric signals grow from a background level to a stable value in a few seconds (the time needed to replace the gas in the inlet lines) and only the spectra acquired after this transient are considered in further analysis.
4. Tailored Data Analysis
NOTE: Tailored data analysis has been developed using a procedure in MATLAB.
Correct the count losses due to the ion detector dead time via a methodology based on Poisson statistics as described by Cappellin et al.10.
Perform internal calibration according to a procedure described by Cappellin et al.38 to achieve a good mass accuracy (up to 0.001 Th).
Carry out compound annotation comparing obtained spectral data with fragmentation data of reference standards and with data reported in the scientific literature.
Perform noise reduction, baseline removal and peak intensity extraction according to Cappellin et al.39, using modified Gaussians to fit the peaks.
Calculate peak intensity in ppbv (parts per billion by volume) via the formula described by Lindinger et al.5, using the appropriate reaction rate coefficient or a constant value for the reaction rate coefficient (k = 2.10−9 cm3 s−1), when the underlying compound is not known. The latter introduces a systematic error of up to 30% that can be accounted for if the actual coefficient is known40.
Mine the data by performing Principal Component Analysis, Analysis of Variance, Tukey's post-hoc test, and other statistical test/analysis adapting existing packages developed using R (e.g. Cappellin et al.10).
Representative Results
The volatile profile of samples resulted in a complete mass spectrum for the desired mass range acquired each second. In Figure 2, an example of the acquired average spectra during the yogurt on-line bioprocess is given32. In every spectrum, more than 300 mass peaks in the m/z range up to 250 Th can be identified32.
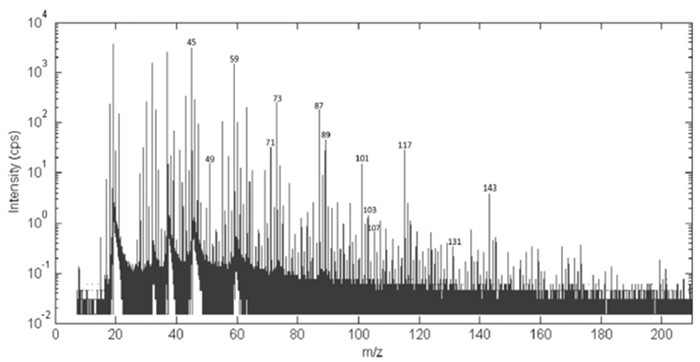 Figure 2:Average PTR-ToF-MS spectra of a sample of inoculated milk during yogurt manufacturing. Low mass region of the average PTR-ToF-MS spectra of a sample of inoculated milk during yogurt manufacturing: more than 300 mass peaks in the m/z range up to 250 Th have been identified. Reprinted with permission from Benozzi et al.32. Please click here to view a larger version of this figure.
Figure 2:Average PTR-ToF-MS spectra of a sample of inoculated milk during yogurt manufacturing. Low mass region of the average PTR-ToF-MS spectra of a sample of inoculated milk during yogurt manufacturing: more than 300 mass peaks in the m/z range up to 250 Th have been identified. Reprinted with permission from Benozzi et al.32. Please click here to view a larger version of this figure.
In the following cases, we report results obtained using the suggested tailored data analysis, for the three applications described in the protocol. We emphasize that the whole data analysis can be performed in one or a few days by tailored software developed in our laboratory10,40. In Figure 3, regarding VOC detection during lactic acid fermentation of yogurt (on-line bioprocess monitoring), we show different fermentation kinetics of nine selected mass peaks corresponding to four different commercial starter cultures32. If the molecular peak is saturated, as for acetaldehyde in this example, the corresponding 13C isotopologue can be used to estimate the concentration.
Most of these volatiles displayed classical microbial-like kinetics, with an initial lag phase, followed by a growth phase and a post-log phase32. Interestingly, the on-line analysis allowed us to highlight, for the first time, a particular depletion kinetics for four sulfur containing compounds (e.g. kinetics reported for methanethiol, Figure 3e).
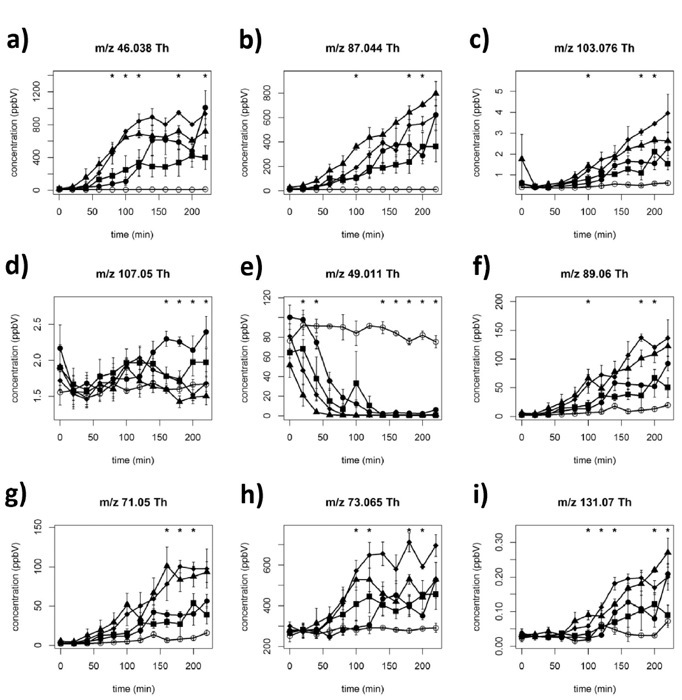 Figure 3:Fermentation kinetics of nine selected mass peaks during yogurt fermentation using four different starter cultures. Fermentation kinetics of nine selected mass peaks: (a) acetaldehyde, (b) diacetyl, (c) 2-hydroxy-3-pentanone/pentanoic acid, (d) benzaldehyde, (e) methanethiol, (f) acetoin, (g) butanoic acid, (h) 2-butanone, (i) heptanoic acid (means of three replicates ± standard deviation) (tentative identification). Open circle (○), non-inoculated milk; filled square (■), filled circle (●), filled triangle (▲), and filled rhombi (♦), correspond to four different microbial starters singularly used to pilot yogurt fermentation. Asterisks indicate statistically significant differences (ANOVA, p <0.05) among commercial starters. Reprinted with permission from Benozzi et al.32. Please click here to view a larger version of this figure.
Figure 3:Fermentation kinetics of nine selected mass peaks during yogurt fermentation using four different starter cultures. Fermentation kinetics of nine selected mass peaks: (a) acetaldehyde, (b) diacetyl, (c) 2-hydroxy-3-pentanone/pentanoic acid, (d) benzaldehyde, (e) methanethiol, (f) acetoin, (g) butanoic acid, (h) 2-butanone, (i) heptanoic acid (means of three replicates ± standard deviation) (tentative identification). Open circle (○), non-inoculated milk; filled square (■), filled circle (●), filled triangle (▲), and filled rhombi (♦), correspond to four different microbial starters singularly used to pilot yogurt fermentation. Asterisks indicate statistically significant differences (ANOVA, p <0.05) among commercial starters. Reprinted with permission from Benozzi et al.32. Please click here to view a larger version of this figure.
Recently, we detected VOCs associated with a large apple collection represented by 190 accessions (example of possible application for large-scale screening)33. The horizontal dendrogram based on the defined VOCs inventory associated to the collection highlights the presence of six main clusters, mainly determined by esters and alcohols (Figure 4). These findings led us to define an Alcohols/Esters index and to propose it as a new fruit quality descriptor suitable as an additional characterization of apples33.
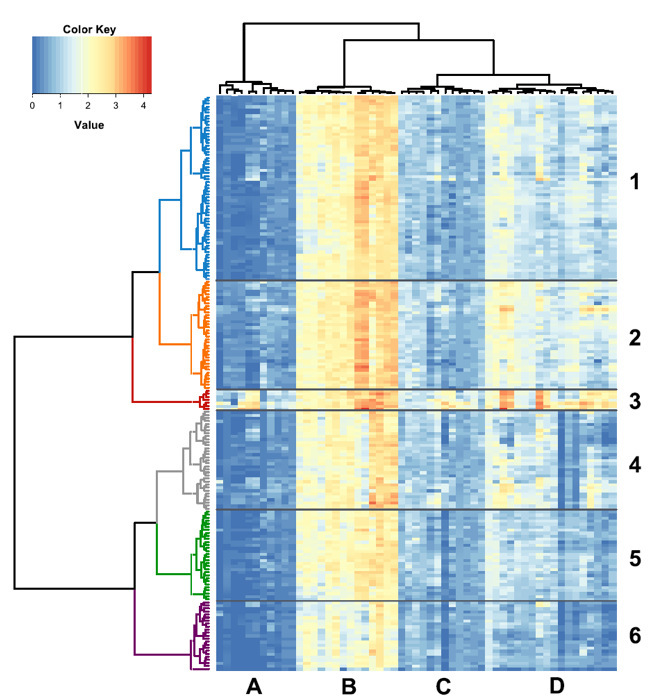 Figure 4:Heat map and two-dimensional hierarchical dendrograms of the VOC patterns assessed in 190 apple accessions by PTR-ToF-MS. Heat map and two-dimensional hierarchical dendrograms of the VOC patterns assessed in 190 apple accessions by PTR-ToF-MS (using a threshold of 25 ppbv). Apple cultivars are grouped and clustered by rows, while VOC compounds are organized by columns. Cultivar clusters are defined by numbers 1 to 6 and groups of compounds are defined by letters A to D. Reprinted with permission from Farneti et al.33. Please click here to view a larger version of this figure.
Figure 4:Heat map and two-dimensional hierarchical dendrograms of the VOC patterns assessed in 190 apple accessions by PTR-ToF-MS. Heat map and two-dimensional hierarchical dendrograms of the VOC patterns assessed in 190 apple accessions by PTR-ToF-MS (using a threshold of 25 ppbv). Apple cultivars are grouped and clustered by rows, while VOC compounds are organized by columns. Cultivar clusters are defined by numbers 1 to 6 and groups of compounds are defined by letters A to D. Reprinted with permission from Farneti et al.33. Please click here to view a larger version of this figure.
We conclude this section with results testifying to the possible application of PTR-ToF-MS in the in vivo study of retronasal VOCs release (nosespace analysis). Figure 5 (left side) describes the cumulative profiles for five coffee testers represented by means of radial plots, a graphical solution typical of sensory analysis28. In this study, medium roast, dark roast and decaffeinated medium roast ground coffee samples from a single pure Arabica blend were prepared and submitted to five panelists28. The results demonstrated the presence of reproducible and relevant differences between panelists, as evident for panelists p1 and p2 in Figure 5 (right side)28.
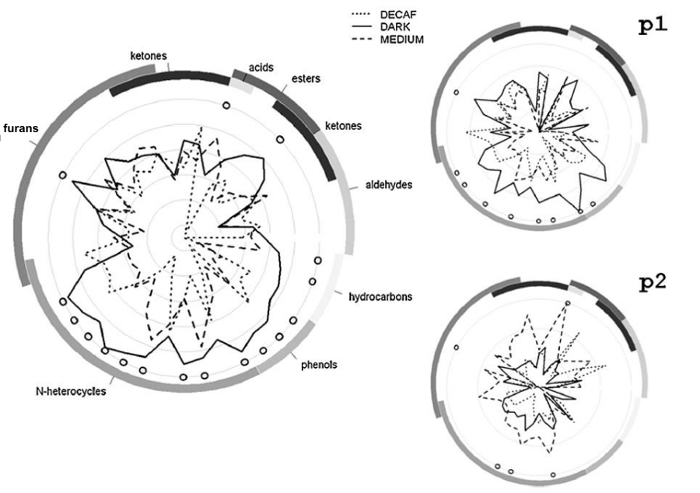 Figure 5:Radial plots representing release profiles for one selected parameter (i.e. area) and three coffee types. Radial plots representing release profiles for one selected parameter (i.e. area) and three coffee types (medium roast, dark roast and decaffeinated medium roast originating from a single pure Arabica blend). On the left: cumulative profiles for five panelists; on the right: individual profiles for two selected panelists (namely p1 and p2). Values were scaled by dividing by the respective standard deviations. The semicircular bands on the outer margins represent chemical classes, based on tentative peak identification. Circles indicate significant differences among coffee types (ANOVA and Tukey's test, p <0.05). Reprinted with permission from Romano et al.28. Please click here to view a larger version of this figure.
Figure 5:Radial plots representing release profiles for one selected parameter (i.e. area) and three coffee types. Radial plots representing release profiles for one selected parameter (i.e. area) and three coffee types (medium roast, dark roast and decaffeinated medium roast originating from a single pure Arabica blend). On the left: cumulative profiles for five panelists; on the right: individual profiles for two selected panelists (namely p1 and p2). Values were scaled by dividing by the respective standard deviations. The semicircular bands on the outer margins represent chemical classes, based on tentative peak identification. Circles indicate significant differences among coffee types (ANOVA and Tukey's test, p <0.05). Reprinted with permission from Romano et al.28. Please click here to view a larger version of this figure.
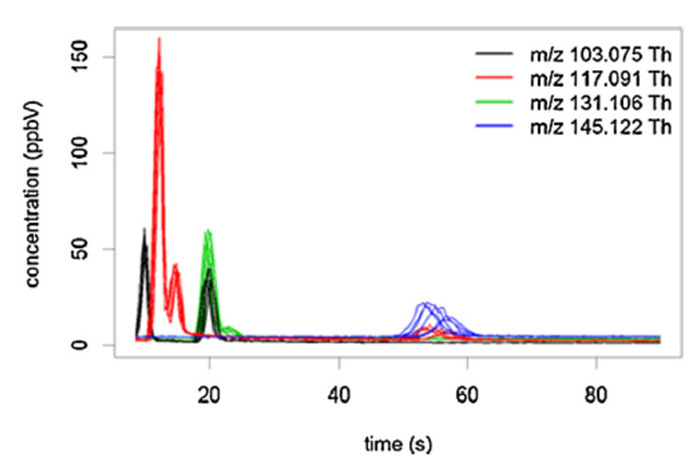 Figure 6: Example of fast-GC PTR-ToF-MS chromatograms. Chromatograms obtained from a red wine (six replicates) and four selected peaks, tentatively attributed to esters. Reprinted with permission from Romano et al.45. Please click here to view a larger version of this figure.
Figure 6: Example of fast-GC PTR-ToF-MS chromatograms. Chromatograms obtained from a red wine (six replicates) and four selected peaks, tentatively attributed to esters. Reprinted with permission from Romano et al.45. Please click here to view a larger version of this figure.
Discussion
Proton transfer reaction-mass spectrometry (PTR-MS) coupled to time of flight (ToF) mass analyzers represent a valid compromise between the need for identification and quantification of volatile organic compounds and the necessity for rapid analytical profiling. The high mass resolution that characterizes the ToF mass analyzer gives/provides relevant sensitivity and mass spectra with considerable informational content. Furthermore, the application of PTR-ToF-MS coupled with an auto-sampler and tailored data analysis tools that increase the degree of automation, enhances the potentialities of this technique.
In the many research and technological fields where volatile compound detection can be of relevance, food science and technology, in particular, require sensitivity, high-time resolution and direct analysis. On one side, the reference methods for VOC analysis are based on gas chromatography, which provides more specificity but is intrinsically slower and can achieve similar sensitivity only at the price of additional pretreatment or concentration procedures. Some rapid analysis, such as the nose-space, cannot be performed with GC based methods. Other studies screen of many samples using PTR-MS and GC as complementary approaches: PTR_MS allows the measurements of very large sample sets while GC analysis of reduced subsets provides additional information for a better interpretation of PTR-MS data40. On the other side, other rapid approaches have been proposed for VOC analysis, e.g., those based on e-noses or MS-e-noses or specific sensors. These are much less expensive as compared with PTR-MS but generally provide very low sensitivity.
PTR-ToF-MS analysis provides information on the mass of the observed spectrometric peaks, which generally is not sufficient for unambiguous compound identification. Moreover, despite the soft chemical ionization, proton transfer induced fragmentation is not always negligible. In some cases, the fragmentation pattern can be of help in tentative identification41. Nevertheless, the need arises for technological solutions which improve the analytical capability of PTR-ToF-MS. Regarding this point, an interesting development of the technique is represented by the use of primary parent ions other than H3O+. The switchable reagent ion (SRI) system4 can alternatively produce in the same hollow cathode source different parent ions, such as NO+ and O2+. This approach, changing the ionization conditions and, consequently, the fragment and cluster formation, increases the number of compounds that are detectable and allows the separation of some isomeric compounds42,43. A few applications in food science and technology are already available, such as VOC determination in dry-cured ham23, coffee34 and ethylene determination in fruits43. Another technological solution suitable to cope with difficulties in accurate compound identification is represented by the fast-GC/PTR-ToF-MS methodology44. By reason of the reduced separation times, fast-GC extends analytical capabilities, without compromising the analytical throughput of PTR-ToF-MS44. The added value of the technique is well represented in Figure 6, depicting chromatograms obtained for four peaks tentatively identified as ester fragments from the headspace of a red wine45. Besides the important separation of different isomeric fragments within the same peak, an interesting desired side effect of the application of a fast chromatographic separation step was represented by the rapid elution (and consequently, substantial elimination) of ethanol. In fact, ethanol provokes an undesired effect in PTR-based analysis of alcoholic matrices because of the reduction of hydronium ions and of the consequent formation of dimers and trimers (ethanol clusters, ethanol and water clusters, and corresponding fragments), leading to the presence of peaks that substantially compromised the correct spectra interpretation46. More recently, other developments have been proposed to improve the sensitivity of PTR-MS apparatuses which have not yet tested in food science and technology47,48.
In conclusion, rapid and non-invasive PTR-ToF-MS analysis of volatile compounds coupled with automatic sampling and tailored data handling and analysis provide a new tool which allows to efficiently address several themes in food science and technology and complements results obtainable by other techniques.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work is supported by the European Commission's 7th Framework Programme under Grant Agreement Number 287382. SY is a beneficiary of a European Commission's 7th Framework Programme Grant Agreement Number 287382. IK is a beneficiary of a FIRST doctoral school grant from the Fondazione Edmund Mach. For his work at University of Foggia, VC is supported by the Apulian Region in the framework of 'Future In Research' program (practice code 9OJ4W81).
References
- Biasioli F, Yeretzian C, Märk TD, Dewulf J, Van Langenhove H. Direct-injection mass spectrometry adds the time dimension to (B)VOC analysis. Trends Analyt Chem. 2011;30(7):1003–1017. [Google Scholar]
- Berchtold C, Bosilkovska M, Daali Y, Walder B, Zenobi R. Real-time monitoring of exhaled drugs by mass spectrometry. Mass Spectrom Rev. 2014;33(5):394–413. doi: 10.1002/mas.21393. [DOI] [PubMed] [Google Scholar]
- Hansel A, et al. Proton transfer reaction mass spectrometry: on-line trace gas analysis at the ppb level. Int J Mass Spectrom Ion Process. 1995;149:609–619. [Google Scholar]
- Jordan A, et al. An online ultra-high sensitivity Proton-transfer-reaction mass-spectrometer combined with switchable reagent ion capability PTR + SRI - MS) Int J Mass Spectrom. 2009;286(1):32–38. [Google Scholar]
- Lindinger W, Hansel A, Jordan A. On-line monitoring of volatile organic compounds at pptv levels by means of proton-transfer-reaction mass spectrometry (PTR-MS) medical applications, food control and environmental research. Int J Mass Spectrom Ion Process. 1998;173(3):191–241. [Google Scholar]
- Biasioli F, Gasperi F, Yeretzian C, Märk TD. PTR-MS monitoring of VOCs and BVOCs in food science and technology. Trends Analyt Chem. 2011;30(7):968–977. [Google Scholar]
- Campbell-Sills H, et al. Advances in wine analysis by PTR-ToF-MS: Optimization of the method and discrimination of wines from different geographical origins and fermented with different malolactic starters. Int J Mass Spectrom. 2016. pp. 42–51.
- Jordan A, et al. A high resolution and high sensitivity proton-transfer-reaction time-of-flight mass spectrometer (PTR-TOF-MS) Int J Mass Spectrom. 2009;286(2-3):122–128. [Google Scholar]
- Lindinger W, Hansel A, Jordan A. Proton-transfer-reaction mass spectrometry (PTR-MS): on-line monitoring of volatile organic compounds at pptv levels. Chem Soc Rev. 1998;27(5):347–375. [Google Scholar]
- Cappellin L, et al. On data analysis in PTR-TOF-MS: From raw spectra to data mining. Sens Actuators B Chem. 2011;155(1):183–190. [Google Scholar]
- Ellis AM, Mayhew CA. Proton Transfer Reaction Mass Spectrometry: Principles and Applications. Chichester, West Sussex: John Wiley & Sons; 2012. [Google Scholar]
- Blake RS, Monks PS, Ellis AM. Proton-Transfer Reaction Mass Spectrometry. Chem Rev. 2009;109(3):861–896. doi: 10.1021/cr800364q. [DOI] [PubMed] [Google Scholar]
- Romano A, Capozzi V, Spano G, Biasioli F. Proton transfer reaction-mass spectrometry: online and rapid determination of volatile organic compounds of microbial origin. Appl Microbiol Biotechnol. 2015;99(9):3787–3795. doi: 10.1007/s00253-015-6528-y. [DOI] [PubMed] [Google Scholar]
- Pollien P, Lindinger C, Yeretzian C, Blank I. Proton transfer reaction mass spectrometry, a tool for on-line monitoring of acrylamide formation in the headspace of maillard reaction systems and processed food. Anal Chem. 2003;75(20):5488–5494. doi: 10.1021/ac0344586. [DOI] [PubMed] [Google Scholar]
- Maçatelli M, et al. Verification of the geographical origin of European butters using PTR-MS. J Food Compost Anal. 2009;22(2):169–175. [Google Scholar]
- Soukoulis C, et al. Proton transfer reaction time-of-flight mass spectrometry monitoring of the evolution of volatile compounds during lactic acid fermentation of milk. Rapid Commun Mass Spectrom. 2010;24(14):2127–3134. doi: 10.1002/rcm.4617. [DOI] [PubMed] [Google Scholar]
- Heenan S, et al. PTR-TOF-MS monitoring of in vitro and invivo flavour release in cereal bars with varying sugar composition. Food Chem. 2012;131(2):477–484. [Google Scholar]
- Benjamin O, Silcock P, Beauchamp J, Buettner A, Everett DW. Tongue pressure and oral conditions affect volatile release from liquid systems in a model mouth. J Agric Food Chem. 2012;60(39):9918–9927. doi: 10.1021/jf3028232. [DOI] [PubMed] [Google Scholar]
- Ting VJL, et al. In vitro and in vivo flavor release from intact and fresh-cut apple in relation with genetic, textural, and physicochemical parameters. J Food Sci. 2012;77(11):1226–1233. doi: 10.1111/j.1750-3841.2012.02947.x. [DOI] [PubMed] [Google Scholar]
- Özdestan Ö, et al. Differentiation of specialty coffees by proton transfer reaction-mass spectrometry. Food Res Int. 2013;53(1):433–439. [Google Scholar]
- Dimitri G, et al. PTR-MS monitoring of volatiles fingerprint evolution during grape must cooking. LWT-Food Sci Technol. 2013;51(1):356–360. [Google Scholar]
- Déléris I, et al. Comparison of direct mass spectrometry methods for the on-line analysis of volatile compounds in foods. J Mass Spectrom. 2013;48(5):594–607. doi: 10.1002/jms.3199. [DOI] [PubMed] [Google Scholar]
- Sánchez del Pulgar J, et al. Effect of the pig rearing system on the final volatile profile of Iberian dry-cured ham as detected by PTR-ToF-MS. Meat Sci. 2013;93(3):420–428. doi: 10.1016/j.meatsci.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Onishi M, Inoue M, Araki T, Iwabuchi H, Sagara Y. A PTR-MS-based protocol for simulating bread aroma during mastication. Food Bioproc Tech. 2010;5(4):1228–1237. [Google Scholar]
- Beauchamp J, Zardin E, Silcock P, Bremer PJ. Monitoring photooxidation-induced dynamic changes in the volatile composition of extended shelf life bovine milk by PTR-MS. J Mass Spectrom. 2014;49(9):952–958. doi: 10.1002/jms.3430. [DOI] [PubMed] [Google Scholar]
- Yener S, et al. PTR-ToF-MS characterisation of roasted coffees (C. arabica) from different geographic origins. J Mass Spectrom. 2014;49(9):929–935. doi: 10.1002/jms.3455. [DOI] [PubMed] [Google Scholar]
- Makhoul S, et al. Proton-transfer-reaction mass spectrometry for the study of the production of volatile compounds by bakery yeast starters. J Mass Spectrom. 2014;49(9):850–859. doi: 10.1002/jms.3421. [DOI] [PubMed] [Google Scholar]
- Romano A, et al. Nosespace analysis by PTR-ToF-MS for the characterization of food and tasters: The case study of coffee. Int J Mass Spectrom. 2014;365:20–27. [Google Scholar]
- Muilwijk M, Heenan S, Koot A, van Ruth SM. Impact of production location, production system, and variety on the volatile organic compounds fingerprints and sensory characteristics of tomatoes. J Chem. 2015;2015:981549. [Google Scholar]
- Makhoul S, et al. Volatile compound production during the bread-making process: effect of flour, yeast and their interaction. Food Bioproc Tech. 2015;8(9):1925–1937. [Google Scholar]
- Aprea E, et al. Volatile compound changes during shelf life of dried Boletus edulis: comparison between SPME-GC-MS and PTR-ToF-MS analysis. J Mass Spectrom. 2015;50(1):56–64. doi: 10.1002/jms.3469. [DOI] [PubMed] [Google Scholar]
- Benozzi E, et al. Monitoring of lactic fermentation driven by different starter cultures via direct injection mass spectrometric analysis of flavour-related volatile compounds. Food Res Int. 2015;69:235–243. doi: 10.1016/j.foodres.2015.07.043. [DOI] [PubMed] [Google Scholar]
- Farneti B, et al. Comprehensive VOC profiling of an apple germplasm collection by PTR-ToF-MS. Metabolomics. 2014;11(4):838–850. [Google Scholar]
- Yener S, et al. Tracing coffee origin by direct injection headspace analysis with PTR/SRI-MS. Food Res Int. 2015;69:235–243. [Google Scholar]
- Charles M, et al. Understanding flavour perception of espresso coffee by the combination of a dynamic sensory method and in-vivo nosespace analysis. Food Res Int. 2015;69:9–20. [Google Scholar]
- Farneti B, et al. Untargeted metabolomics investigation of volatile compounds involved in the development of apple superficial scald by PTR-ToF-MS. Metabolomics. 2014;11(2):341–349. [Google Scholar]
- Bean HD, Zhu J, Hill JE. Characterizing Bacterial Volatiles using Secondary Electrospray Ionization Mass Spectrometry (SESI-MS) J Vis Exp. 2011. p. e2664. [DOI] [PMC free article] [PubMed]
- Cappellin L, et al. Extending the dynamic range of proton transfer reaction time-of-flight mass spectrometers by a novel dead time correction. Rapid Commun Mass Spectrom. 2011;25(1):179–183. doi: 10.1002/rcm.4819. [DOI] [PubMed] [Google Scholar]
- Cappellin L, et al. On Quantitative Determination of Volatile Organic Compound Concentrations Using Proton Transfer Reaction Time-of-Flight Mass Spectrometry. Environ Sci Technol. 2012;46(4):2283–2290. doi: 10.1021/es203985t. [DOI] [PubMed] [Google Scholar]
- Cappellin L, et al. PTR-ToF-MS and data mining methods: a new tool for fruit. Metabolomics. 2012;8(5):761–770. [Google Scholar]
- Yeretzian C, Jordan A, Lindinger W. Analysing the headspace of coffee by proton-transfer-reaction mass-spectrometry. Int J Mass Spectrom. 2003;223:115–139. [Google Scholar]
- Sulzer P, et al. From conventional proton-transfer-reaction mass spectrometry (PTR-MS) to universal trace gas analysis. Int J Mass Spectrom. 2012;321:66–70. [Google Scholar]
- Cappellin L, et al. Ethylene: Absolute real-time high-sensitivity detection with PTR/SRI-MS. The example of fruits, leaves and bacteria. Int J Mass Spectrom. 2014;365:33–41. [Google Scholar]
- Ruzsanyi V, Fischer L, Herbig J, Ager C, Amann A. Multi-capillary-column proton-transfer-reaction time-of-flight mass spectrometry. Journal of Chromatography A. 2013;1316:112–118. doi: 10.1016/j.chroma.2013.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano A, et al. Wine analysis by FastGC proton-transfer reaction-time-of-flight-mass spectrometry. Int J Mass Spectrom. 2014;369:81–86. [Google Scholar]
- Aprea E, Biasioli F, Märk TD, Gasperi F. PTR-MS study of esters in water and water/ethanol solutions: Fragmentation patterns and partition coefficients. Int J Mass Spectrom. 2007;262(1-2):114–121. [Google Scholar]
- Sulzer P, et al. A Proton Transfer Reaction-Quadrupole interface Time-Of-Flight Mass Spectrometer (PTR-QiTOF): High speed due to extreme sensitivity. Int J Mass Spectrom. 2014;368:1–5. [Google Scholar]
- Barber S, et al. Increased Sensitivity in Proton Transfer Reaction Mass Spectrometry by Incorporation of a Radio Frequency Ion Funnel. Anal Chem. 2012;84(12):5387–5391. doi: 10.1021/ac300894t. [DOI] [PubMed] [Google Scholar]


