Abstract
Background
Subjective memory complaints (SMCs) are associated with increased risk of dementia in older adults, but the role of comorbidities in modifying this risk is unknown.
Objectives
To assess whether comorbidities modify estimated dementia risk based on SMCs.
Design
The Prevention of Alzheimer’s Disease with Vitamin E and Selenium Study (PREADVISE) was designed as an ancillary study to the Selenium and Vitamin E Cancer Prevention Trial (SELECT), a randomized, multi-center prostate cancer prevention trial with sites in the Unites States, Puerto Rico, and Canada. In 2009, PREADVISE and SELECT were changed into cohort studies.
Setting
Secondary analysis of PREADVISE data.
Participants
PREADVISE recruited 7,540 non-demented male volunteers from participating SELECT sites from 2002 to 2009. SMCs, demographics, and comorbidities including hypertension, diabetes, coronary artery bypass graft (CABG), stroke, sleep apnea, and head injury were ascertained by participant interview.
Measurements
Cox models were used to investigate whether baseline comorbidities modified hazard ratios (HR) for SMC-associated dementia risk using two methods: (1) we included one interaction term between SMC and a comorbidity in the model at a time, and (2) we included all two-way interactions between SMC and covariates of interest and reduced the model by “backward” selection. SMC was operationalized as any complaint vs. no complaint.
Results
Baseline SMCs were common (23.6%). In the first analyses, with the exception of stroke, presence of self-reported comorbidities was associated with lower estimated HR for dementia based on SMC status (complaint vs. no complaint), but this difference was only significant for diabetes. In the second analysis, the two-way interactions between SMC and race as well as SMC and diabetes were significant. Here, black men without diabetes who reported SMC had the highest estimated dementia risk (HR=5.05, 95% CI 2.55–10.00), while non-black men with diabetes who reported SMC had the lowest estimated risk (HR=0.71, 95% CI 0.35–1.41).
Conclusions
SMCs were more common among men with comorbidities, but these complaints appeared to be less predictive of dementia risk than those originating from men without comorbidities, suggesting that medical conditions such as diabetes may explain SMCs that are unrelated to an underlying neurodegenerative process.
Keywords: Subjective memory complaints (SMCs), Alzheimer’s disease, dementia, comorbidities, effect measure modification
INTRODUCTION
Subjective memory complaints (SMCs) are defined as self-identified memory decline based on clinician-facilitated interviews or research studies [1,2]. The prevalence of SMC among older adults in the US approximately ranges from 25.2% to 85.7% according to previous studies [1,3–5]. SMC may reflect increased risk of premature cognitive impairment [2,4,6]. Recent studies have shown structural, functional, and neuropathological deficits among non-demented older adults with SMC [4,7–9]. Apart from growing evidence that SMC predict risk of future cognitive impairment, including dementia, studies also showed that participant characteristics may modify the association between SMC and dementia risk [2,10]. Abner et al. found an increased hazard of dementia among black participants who reported memory problem or change in comparison with those who did not report memory complaint [2]. Zwan et al. found increased risk of dementia (defined by β-amyloid burden) among APOE-ε4 carriers with SMC [10]. Joao et al. also found an interaction between education and SMC among older people [11].
However, many, and perhaps most, adults who report SMC never go on to develop dementia or any type of clinical cognitive impairment [2,4,12]. It remains a challenge to determine which SMC are meaningful and which are only a sign of a patient who is “worried well.” In addition, limited attention has been paid to the influence of comorbid health conditions on estimates of dementia risk based on SMC. Comorbidities that older adults may experience that may modify their risk of dementia include hypertension, diabetes, cardiovascular diseases, sleep apnea, and traumatic brain injury [13–18]. It is reasonable to hypothesize that the presence or absence of comorbid conditions that are themselves risk factors for dementia may modify the estimated risk of dementia associated with SMC reports.
The current study contains a large sample of initially non-demented older men, followed up to 12 years, from the longitudinal Prevention of Alzheimer’s Disease with Vitamin E and Selenium (PREADVISE) study. We hypothesized that comorbid health conditions (including hypertension, diabetes, coronary artery bypass graft (CABG), stroke, sleep apnea, and traumatic brain injury) and participant characteristics (including baseline age, educational attainment, APOE genotype, and race) may modify the association between memory complaint and the risk of dementia.
METHODS
Study Design and Participants
This secondary analysis was based on the PREADVISE study. PREADVISE was an ancillary study to the Selenium and Vitamin E Cancer Prevention Trial (SELECT), which was a large, double-blinded, randomized control trial (RCT) to prevent prostate cancer [18]. Details on the design and recruitment of the PREADVISE study can be found in Kryscio et al. and Abner et al. [2,18]. PREADVISE recruited participants from SELECT at 130 participating clinical sites in the US, Canada, and Puerto Rico between 2002 and 2009. The eligibility criteria for PREADVISE depended on active enrollment at participating SELECT study sites, and absence of dementia and other active neurologic conditions that may affect cognition. PREADVISE enrolled 7,547 non-demented men by 2009 and ceased. Due to lack of efficacy on the primary endpoint, SELECT’s Data Safety Monitoring Board recommended supplementation be stopped in 2008, and study sites began closing in 2009. PREADVISE and SELECT then transitioned into observational cohort studies [18,20]. All research activities during both the RCT and observational phases of the study were approved by the University of Kentucky Institutional Review Board (IRB) as well as the IRBs at each SELECT study site. Each participant provided written informed consent. Study supplements are not considered in this secondary analysis.
Among the original 7,547 PREADVISE participants, 4,271 of them consented to join the cohort study for annual dementia screenings. The primary screening instrument in both phases of the study was the Memory Impairment Screen (MIS) [21]. In the observational portion of the study, screening was conducted by telephone. Participants who failed the MIS screen (with scores below 5 out of 8 possible points) received a secondary screening. The secondary screening included a more in-depth cognitive assessment based on an expanded Consortium to Establish a Registry in Alzheimer’s Disease (CERADe) battery [22] during the RCT, and the modified Telephone Interview for Cognitive Status (TICS-m) [23] during observational follow-up. Additionally, some participants who did not fail the MIS were also asked to complete the CERADe battery and TICS-m. Annual screenings were completed by May 2014. All PREADVISE participants were included in the current study whether they participated in both the RCT and observational studies or just the RCT. In this report, 7 participants were excluded from our analysis because they were discovered to be ineligible for enrollment (i.e., ineligible based on MIS score at baseline). As a result, our study included 7,540 non-demented men at baseline, and followed for up to 12 years (median = 6 years).
Memory Complaint
Memory complaint information was ascertained from all PREADVISE participants during their baseline interview via self-reported memory changes [2]. Men could report no change, change, or a change they felt was a problem. Because relatively few men (<200) reported a change they felt was a problem, and our primary interest in the current study is assessing effect modifiers of memory change, we constructed a binary variable for “memory complaint” classifying participants based on no reported change vs. any reported memory change.
Participant Characteristics and Comorbidities
Baseline age, years of education, race, hypertension status, diabetes, coronary artery bypass graft (CABG), stroke, sleep apnea, and head injury with loss of consciousness less than 30 minutes were collected through participants’ self-report at baseline interviews [24]. Age and years of education were coded as continuous years. Race was coded as a binary variable (black vs. non-black). Hypertension, diabetes, CABG, stroke, sleep apnea and traumatic brain injury were coded as binary variables (“Y” vs. “N”). APOE genotypes were converted into a dummy indicator for presence or absence of any 4 alleles. Since genotyping was unavailable for 366 (4.9%) participants, we imputed missing indicators using multiple imputation [2].
Case Ascertainment
PREADVISE used two methods to identify incident cases of dementia. First, men who scored 5 or less (out of 8) on either the immediate or delayed recall portions of the annually administered primary screening instrument, the MIS, were given a secondary screen [2]. If a participant failed the secondary screen (T Score ≤ 35 on the CERADe battery, total score ≤ 35 on the TICS-m), the study investigators encouraged participants to obtain a memory workup from a local clinician and forward the medical records to PREADVISE [2]. Then participant records would be reviewed by a team of 2–3 expert neurologists and 2–3 expert neuropsychologists to form a consensus diagnosis [2]. Second, because some participants were reluctant to visit their doctors, additional longitudinal measures collected during the study were reviewed by the study investigators: the AD8 Dementia Screening Interview [25], self-reported medical history, self-reported medication use, and cognitive scores including the MIS, CERADe T-Score, NYU Paragraph Delayed Recall, and TICS-m [2,19]. Participants with an AD8 score ≥1 (at any time during follow-up) plus a self-reported diagnosis of dementia, use of memory enhancing prescription drug (i.e., donepezil, rivastigmine, galantamine, or memantine), or cognitive score below cutoffs for intact cognition (i.e., 1.5 SDs below expected performance) were diagnosed with dementia [2,19]. We recorded the date of diagnosis as the earliest occurring event [2].
Statistical Analysis
We used chi-square and t-test statistics to examine differences in categorical and continuous variables between men with and without SMCs. We conducted survival analysis to assess effect modification of memory complaint on the hazard of dementia by the covariates of interest. Survival time was calculated as the time in years between the dementia diagnosis date and the PREADVISE baseline date. Men without evidence of dementia were censored administratively at their last annual follow-up. Cox proportional hazards regression was used to estimate hazard ratios (HRs). Covariates of interest were baseline age, education, race (black vs. non-black), APOE genotype (at least one APOE-ε4 vs. no APOE-ε4), and baseline self-reported comorbidities including head injury, diabetes, stroke, coronary artery bypass graft, and sleep apnea (all coded as present/absent). To further explore the effect of comorbidities, we constructed an indicator variable for the report of any comorbidity vs. no comorbidities, which was also examined as an effect modifier of memory complaint.
We used to two methods to assess whether comorbidities modified the association between SMC and dementia risk. In the first method, we used separate models to evaluate one interaction between SMC and a given covariate at a time while adjusting for all other covariates. This provided point estimates for the adjusted HRs for memory complaint and dementia for each level of the given covariate. For example, when the interaction between SMC (yes vs. no) and hypertension (yes vs. no) was evaluated, the hazard function was specified as: λ(time to dementia) = λ0exp(β1*age + β2*education + β3*race + β4*APOE + β5*diabetes + β6*CABG + β7*stroke + β8*sleep apnea + β9*head injury + β10*SMC + β11*hypertension + β12*SMC*hypertension).
In the second method, we included all two-way interactions between memory complaint and all covariates of interest in one Cox model, which was reduced the model by “backward” selection. Here, the initial hazard function was specified as: λ(time to dementia) = λ0exp(Xj*β + β*SMC + Xi*SMC*β~), where Xj*β is the vector of covariates, excluding SMC, and their beta coefficients; and Xi*SMC*β~ is the vector of two-way interaction terms between SMC and the covariates and their beta coefficients.
The proportional hazards assumption for all models was tested by using the cumulative Martingale residual method (which has been incorporated into the “assess” statement for continuous variables in SAS 9.4® PROC PHREG). Lack of statistical significance (p>0.05) for the supreme test with the cumulative Martingale residual method was taken as support for the proportional hazards assumption. Sensitivity analyses excluding individuals with imputed APOE-ε4 information were conducted for both methods of interaction assessment. We conducted all analyses using SAS 9.4® (SAS Institute, Inc., Cary, NC).
RESULTS
The mean baseline age of all participants was 67.5±5.3 years, and participants were highly educated (Table 1). Overall, 23.6% of participants reported a memory complaint at baseline, and these participants were over twice as likely to be diagnosed with dementia than participants who reported no memory complaint (unadjusted HR = 2.32, 95% CI 1.86–2.90). Participants who reported no comorbidities were slightly but significantly younger at baseline (67.1 vs. 67.8 years, p<0.0001), had about six months more educational attainment on average (15.2 vs. 14.7 years, p<0.0001), were less likely to be black (5.9% vs. 13.4%, p<0.0001), but did not differ on proportion of APOE-ε4 carriers (27% in each group) (Table 1).
Table 1.
PREADVISE participant characteristics (N=7,540)
| Characteristic* | All Subjects (N=7,540) |
No Memory Complaint (n=5,757) |
Memory Complaint (n=1,783) |
P Value |
|---|---|---|---|---|
| Baseline age, y (mean±SD) | 67.5±5.3 | 67.2±5.2 | 68.5±5.6 | <0.001 |
| Education, y† (mean±SD) | 15.0±2.7 | 14.9±2.7 | 15.0±2.7 | 0.35 |
| Black Race | 754 (10.0) | 625 (10.9) | 129 (7.2) | <0.001 |
| APOE-ε4 | 2,031 (26.9) | 1,545 (26.8) | 486 (27.3) | 0.14 |
| Hypertension | 2,995 (39.7) | 2,287 (39.7) | 708 (39.7) | 0.99 |
| Diabetes | 858 (11.4) | 657 (11.4) | 201 (11.3) | 0.87 |
| Coronary Artery Bypass Graft | 318 (4.2) | 231 (4.0) | 87 (4.9) | 0.11 |
| Stroke | 43 (0.6) | 30 (0.5) | 13 (0.7) | 0.31 |
| Sleep Apnea | 552 (7.3) | 407 (7.1) | 145 (8.1) | 0.13 |
| Head Injury | 996 (13.2) | 649 (11.3) | 347 (19.5) | <0.001 |
| Number of comorbidities | <0.001 | |||
| 0 | 3,387 (44.9) | 2,668 (46.3) | 719 (40.3) | |
| 1 | 2,827 (37.5) | 2,118 (36.8) | 709 (39.8) | |
| 2 | 1,069 (14.2) | 786 (13.7) | 283 (15.9) | |
| 3 | 231 (3.1) | 169 (2.9) | 62 (3.5) | |
| 4 | 26 (0.3) | 16 (0.3) | 10 (0.6) | |
| Dementia Diagnosis | 325 (4.3) | 192 (3.3) | 133 (7.5) | <0.001 |
Comorbidities measured at baseline.
Note: 35 participants did not report education.
In the first set of analyses, where each interaction between SMC and a given covariate was considered separately, there was evidence that participant characteristics and comorbidities may modify the association between memory complaint and dementia risk. In particular, race, APOE, hypertension, diabetes, CABG, and stroke modified the HR for memory complaint, although only SMC*race (p=0.0395) and SMC*diabetes (p=0.0045) were significant at the 0.05 level (Figure 1). In general, presence of comorbidities was not associated with increased HR for memory complaint, with the exception of stroke (stroke: HR=9.21 (95% CI 0.95–88.8), no stroke: HR=2.01 (95% CI 1.61–2.53)). On the contrary, HRs for memory complaint among participants who reported diabetes or CABG at baseline were close to 1.00 (diabetes: HR=0.82 (95% CI 0.42–1.63), CABG: HR=0.96 (95% CI 0.42–2.20), while HRs for memory complaint among those who did not report those conditions showed a significantly elevated risk (no diabetes: HR=2.34 (95% CI 1.84–2.98), no CABG: HR=2.19 (95% CI 1.74–2.77). Although dementia risk was elevated in the presence of memory complaint for participants with and without hypertension, sleep apnea, and head injury, absence of the condition was consistently associated with higher HRs for memory complaint (hypertension: HR=1.69 (95% 1.19–2.40), no hypertension: HR=2.35 (95% CI 1.79–3.14); sleep apnea: HR=1.81 (95% CI 0.86–3.82), no sleep apnea: HR=2.08 (95% CI 1.64–2.63); head injury: HR=1.68 (95% CI 0.98–2.90), no head injury: HR=2.14 (95% CI 1.67–2.73)). Similar results were observed for participants with no comorbidities vs. any comorbidities: although both groups showed a significantly increased risk of dementia associated with memory complaint, the HR was higher for participants with no comorbidities (no comorbidities: HR=2.81 (95% CI 1.96–4.03), any comorbidities: HR=1.69 (95% CI 1.27–2.26)). Participant characteristics associated with increased HR point estimates for memory complaint were black race and presence of an APOE-ε4 allele (Figure 1). Results for these analyses remained consistent when participants with imputed APOE-ε4 data were excluded.
Figure 1. Stratum-specific adjusted hazard ratio plots for estimated effect of memory complaint on dementia risk.
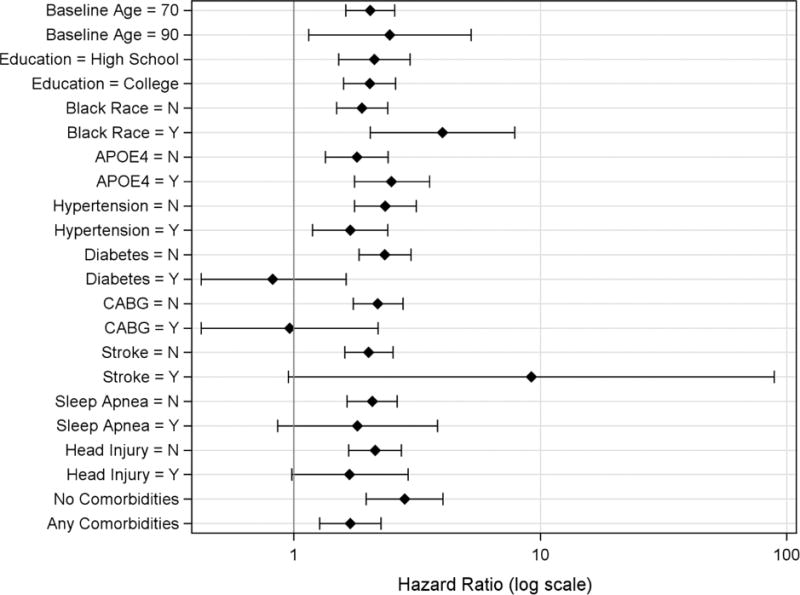
Note: Stratum-specific hazard ratios were obtained via a series of Cox regression models that assessed each interaction term separately. All models were adjusted for all variables included in the figure.
When all interactions terms with SMC were specified simultaneously and backward selection applied, the final reduced model included main effects for baseline age, APOE-ε4, CABG, and stroke, and two-way interactions between race and complaint, as well as diabetes and complaint (all significant at 0.05). Here, black men without diabetes who reported a memory complaint had the highest estimated risk of dementia, while non-Black men with diabetes who reported memory complaint had the lowest estimated risk (Table 2). Older age at baseline, presence of APOE-ε4, CABG, and stroke independently increased the hazard of dementia (Table 2) but did not significantly modify the effect of memory complaint in this analysis. Results for this analysis remained consistent when participants with imputed APOE-ε4 data were excluded.
Table 2.
Cox proportional hazards regression results (N=7,540)
| Comparison | Adjusted HR (95%CI) |
|---|---|
| Memory Complaint vs. No Complaint (Black=N, Diabetes=N) | 2.20 (1.71–2.82) |
| Memory Complaint vs. No Complaint (Black=Y, Diabetes=N) | 5.05 (2.55–10.00) |
| Memory Complaint vs. No Complaint (Black=N, Diabetes=Y) | 0.71 (0.35–1.41) |
| Memory Complaint vs. No Complaint (Black=Y, Diabetes=Y) | 1.62 (0.67–3.92) |
| Baseline Age, 10-year difference | 2.75 (2.29–3.32) |
| APOE-ε4 Carrier (Y vs. N) | 2.08 (1.66–2.60) |
| Coronary Artery Bypass Graft (Y vs. N) | 1.67 (1.13–2.47) |
| Stroke (Y vs. N) | 3.64 (1.35–9.77) |
DISCUSSION
We examined potential effect modifiers for self-reported memory complaint to further our understanding of its utility for estimating dementia risk, and we found that in most cases the effect of memory complaint was mitigated rather than amplified by the presence of comorbidities. Our results suggest complex relationships among participant characteristics, comorbidities, memory complaint, and dementia risk. For example, participants who reported no comorbidities were slightly but significantly younger at baseline, were significantly less likely to be black, but did not differ on level of education or proportion of APOE-ε4 carriers.
Only stroke plus memory complaint showed any evidence of a synergistic effect measure modification, which we interpret very cautiously given the low prevalence of history of stroke at baseline (0.6%). This general lack of synergism between memory complaint and comorbidities may indicate that comorbidity-associated memory complaints are less indicative of underlying neuropathological conditions and more indicative of memory change due to medications or condition-specific deficits (e.g., as with head injury), while memory complaints in the absence of comorbidities may be somewhat more predictive of dementia risk.
As we found in a prior analysis [2], memory complaints reported by black participants in this study were associated with greater risk of dementia. When we considered multiple interactions in the model simultaneously, we found again that the presence of comorbidity dampened the risk: black participants with memory complaints but without diabetes had a greater than five-fold increased risk of dementia, while black participants with memory complaints and with diabetes did not have a significantly increased risk of dementia. Similarly, non-black participants with memory complaints but without diabetes had over two-fold increased risk of dementia, while non-black participants with memory complaints but with diabetes had no increased risk. Although it has been shown that diabetes increases the risk of cerebrovascular pathology rather than Alzheimer’s disease pathology [29], use of hypoglycemic medications may decrease the risk of dementia [27], but the effects of different antidiabetic drugs may vary [28].
Strengths of the current study include the systematic examination of the effects of multiple common aging-related comorbidities (hypertension, diabetes, CABG, stroke, sleep apnea, and head injury) and their interactions with memory complaint on the risk of dementia. Other strengths include annual memory screening, availability of APOE genotype, large sample size, and long participant follow-up.
Importantly, we note that our measures of memory complaint and comorbidity were based on self-report, which may introduce misclassification into the analysis. Memory complaint was operationalized as a two-level variable indicating any reported memory change vs. no reported memory change, and our results may have been different had more detailed data related to memory complaint been available. Also, because PREADVISE was an ancillary study to a prostate cancer prevention trial, female participants were not included in this study. Future studies may examine consistency of the results in female populations. PREADVISE did not measure depressive symptoms at baseline, and depression may influence the risk of dementia [26]. However, we note the exclusion criteria for PREADVISE precluded enrollment for any man who had been diagnosed with or was under treatment for depression or anxiety in the four months before the baseline visit [2]. Also, dementia diagnoses may be less accurate because of the absence of a medical records review [2]. However, application of the case criteria (i.e. AD8 score ≥1 plus at least one other indicator) to participants where the diagnosis was known demonstrated good agreement [2]. Some dementia cases were likely missed due to the shift from an RCT to an observational study, particularly for those participants who did not continue in the study [2].
Limitations also include the assumptions for survival analysis. The assumption of uninformative censoring is less likely to be valid in older adult [30]. Ideally, death would be treated as a competing risk for dementia in our analyses. However, data on deaths among PREADVISE men are incomplete, and thus this analytic approach was not possible.
We believe these results shed light on the role of memory complaint, defined as a self-reported change in memory, in estimating dementia risk in the presence of common comorbidities associated with aging. Memory complaints were more common among men with comorbidities, but these complaints generally appeared to be less predictive of dementia risk than those originating from men without comorbidities. Diabetes in particular was consistently, significantly associated with reduced association between memory complaint and dementia. Additional studies of how comorbidities change the estimated association between memory complaint and dementia risk are warranted, and studies with more detailed measures of memory complaint and comorbid conditions are needed.
Acknowledgments
We gratefully acknowledge all PREADVISE volunteers for their many years of participation and Drs. Herman Buschke, John Crowley, Gregory Jicha, Gregory Cooper, Steven Estus, Donna Wilcock, and William Markesbery for their contributions to the study. We also thank the PREADVISE support staff and the Cancer Research and Biostatistics group for assistance with study procedures and data management.
FUNDING
PREADVISE (NCT00040378) was supported by NIA R01 AG019241. SELECT (NCT00076128) was supported by NCI R01 CA37429 and UM1 CA182883.
Footnotes
CONFLICT OF INTEREST
All authors declare no conflict of interest.
ETHICS
The study protocol was approved by the University of Kentucky Institutional Review Board in addition to Institutional Review Boards at each study site. All participants provided written informed consent.
References
- 1.Gifford KA, Liu D, Lu Z, et al. The source of cognitive complaints differentially predicts diagnostic conversion in non-demented older adults. Alzheimer’s & Dement. 2014;10(3):319–327. doi: 10.1016/j.jalz.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abner EL, Kryscio RJ, Caban-Holt AM, Schmitt FA. Baseline subjective memory complaints associate with increased risk of incident dementia: the PREADVISE trial. J Prevention of Alzheimer’s Dis. 2015;2(1):11–16. doi: 10.14283/jpad.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amariglio RE, Townsend MK, Grodstein F, Sperling RA, Rentz DM. Specific subjective memory complaints in older persons may indicate poor cognitive function. J Am Geriatr Soc. 2011;59(9):1612–1617. doi: 10.1111/j.1532-5415.2011.03543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kryscio RJ, Abner EL, Cooper GE, et al. Self-reported memory complaints: implications from a longitudinal cohort with autopsies. Neurology. 2014;83:1359–1365. doi: 10.1212/WNL.0000000000000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fritsch T, McClendon MJ, Wallendal MS, Hyde TF, Larsen JD. Prevalence and cognitive bases of subjective memory complaints in older adults: evidence from a community sample. J Neurodegenerative Diseases. 2014;2014 doi: 10.1155/2014/176843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckley RF, Maruff P, Ames D, et al. Subjective memory decline predicts greater rates of clinical progression in preclinical Alzheimer’s disease. Alzheimer’s & Dement. 2016;12(7):796–804. doi: 10.1016/j.jalz.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Dumas JA, Kutz AM, McDonald BC, et al. Increased working memory-related brain activity in middle-aged women with cognitive complaints. Neurobiol Aging. 2013;34:1145–1147. doi: 10.1016/j.neurobiolaging.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hafkemeijer A, Altmann-Schneider I, Oleksik AM, et al. Increased functional connectivity and brain atrophy in elderly with subjective memory complaints. Brain Connectivity. 2013;3:353–362. doi: 10.1089/brain.2013.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amariglio RE, Becker JA, Carmasin J, et al. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia. 2012;50:2880–2886. doi: 10.1016/j.neuropsychologia.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zwan MD, Villemagne VL, Dore V, et al. Subjective memory complaints in APOEε4 carriers are associated with high amyloid-b burden. J Alzheimer’s Dis. 2016;49(4):1115–1122. doi: 10.3233/JAD-150446. [DOI] [PubMed] [Google Scholar]
- 11.Joao AA, Maroco J, Gino S, Mendes T, de Mendonca A, Martins IP. Education modifies the type of subjective memory complaints in older people. Intl J Geriatr Psychiatry. 2015;31(2):153–160. doi: 10.1002/gps.4305. [DOI] [PubMed] [Google Scholar]
- 12.Kryscio RJ, Abner EL, Jicha GA, et al. Self-reported memory complaints: a comparison of demented and unparied outcomes. J Prev Alzheimers Dis. 2016;3(1):42–46. doi: 10.14283/jpad.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Degen C, Toro P, Schonknecht P, Sattler C, Schroder J. Diabetes mellitus Type II and cognitive capacity in healthy aging, mild cognitive impairment and Alzheimer’s disease. Psychiatry Res. 2016;240(30):42–46. doi: 10.1016/j.psychres.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Csiszar A, Tucsek Z, Toth P, et al. Synergistic effects of hypertension and aging on cognitive function and hippocampal expression of genes involved in b-amyloid generation and Alzheimer’s disease. Am J Physiol Heart Circ Physiol. 2013;305(8):1120–1130. doi: 10.1152/ajpheart.00288.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emamian F, Khazaie H, Tahmasian M, et al. The association between obstructive sleep apnea and Alzheimer’s disease: a meta-analysis perspective. Front Aging Neurosci. 2016;8:78. doi: 10.3389/fnagi.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sivanandam TM, Thakur MK. Traumatic brain injury: a risk factor for Alzheimer’s disease. Neuroscience & Biobehavioral Reviews. 2012;36(5):1376–1381. doi: 10.1016/j.neubiorev.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Liu G, Yao L, Liu J, et al. Cardiovascular disease contributes to Alzheimer’s disease: evidence from large-scale genome-wide association studies. Neurobiol of Aging. 2014;35(4):786–792. doi: 10.1016/j.neurobiolaging.2013.10.084. [DOI] [PubMed] [Google Scholar]
- 19.Kryscio RJ, Abner EL, Schmitt FA, et al. A randomized controlled Alzheimer’s Disease prevention trial’s evolution into an exposure trial: the PREADVISE trial. J Nutr, Health and Aging. 2013;17(1):72–75. doi: 10.1007/s12603-012-0083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodman PJ, Hartline JA, Tangen CM, et al. Moving a randomized clinical trial into an observational cohort. Clin Trials. 2013;10(1):131–142. doi: 10.1177/1740774512460345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buschke H, Kuslansky G, Katz M, et al. Screening for dementia with the Memory Impairment Screen. Neurology. 1999;52:231–238. doi: 10.1212/wnl.52.2.231. [DOI] [PubMed] [Google Scholar]
- 22.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39(9):1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 23.de Jager CA, Budge MM, Clarke R. Utility of TICS-M for the assessment of cognitive function in older adults. Int J Geriatr Psychiatry. 2003;18(4):318–324. doi: 10.1002/gps.830. [DOI] [PubMed] [Google Scholar]
- 24.Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301(1):39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65(4):559–564. doi: 10.1212/01.wnl.0000172958.95282.2a. [DOI] [PubMed] [Google Scholar]
- 26.Katon W, Lyles CR, Parker MM, Karter AJ, Huang ES, Whitmer RA. Association of depression with increased risk of dementia in patients with Type 2 Diabetes: the diabetes and aging study. Arch Gen Psychiatry. 2012;69(4):410–417. doi: 10.1001/archgenpsychiatry.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myint AA, Win KS, Aung Z. Alzheimer’s disease and type 2 diabetes mellitus: risk factors and effectiveness of antidiabetic agents in treatment of Alzheimer’s disease. Sc J Clinical Med. 2013;2(3):114–121. [Google Scholar]
- 28.Cheng C, Lin CH, Tsai YW, Tsai CJ, Chou PH, Lan TH. Type 2 diabetes and antidiabetic medications in relation to dementia diagnosis. J Gerontol: Med Sciences. 2014;2014 doi: 10.1093/gerona/glu073. [DOI] [PubMed] [Google Scholar]
- 29.Abner EL, Nelson PT, Kryscio RJ, et al. Diabetes is associated with cerebrovascular but not Alzheimer’s disease neuropathology. Alzheimers Dement. 2016;12(8):882–889. doi: 10.1016/j.jalz.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy TE, Han L, Allore HG, Peduzzi PN, Gill TM, Lin H. Treatment of death in the analysis of longitudinal studies of gerontological outcomes. J Gerontol. 2011;66A:109–114. doi: 10.1093/gerona/glq188. [DOI] [PMC free article] [PubMed] [Google Scholar]


