Abstract
Background
Women's under-representation in HIV and cardiovascular disease (CVD) research suggests a need for novel strategies to ensure robust representation of women in HIV-associated CVD research.
Objective
To elicit perspectives on CVD research participation among a community-sample of women with or at risk for HIV, and to apply acquired insights towards the development of an evidence-based campaign empowering older women with HIV to participate in a large-scale CVD prevention trial.
Methods
In a community-based setting, we surveyed 40 women with or at risk for HIV about factors which might facilitate or impede engagement in CVD research. We applied insights derived from these surveys into the development of the Follow YOUR Heart campaign, educating women about HIV-associated CVD and empowering them to learn more about a multi-site HIV-associated CVD prevention trial: REPRIEVE.
Results
Endorsed best methods for learning about a CVD research study included peer-to-peer communication (54%), provider communication (46%), and video-based communication (39%). Top endorsed non-monetary reasons for participating in research related to gaining information (63%) and helping others (47%). Top endorsed reasons for not participating related to lack of knowledge about studies (29%) and lack of request to participate (29%). Based on survey results, the REPRIEVE Follow YOUR Heart Campaign was developed. Interwoven campaign components (print materials, video, web presence) offer provider-based information/knowledge, peer-to-peer communication, and empowerment to learn more. Campaign components reflect women's self-identified motivations for research participation – education and altruism.
Conclusions
Investigation of factors influencing women's participation in HIV-associated CVD research may be usefully applied to develop evidence-based strategies for enhancing women's enrollment in disease-specific large-scale trials. If proven efficacious, such strategies may enhance conduct of large-scale research studies across disciplines.
Keywords: Women, Sex-Differences, Research Participation, HIV-Associated Cardiovascular Disease, Clinical Trials, Community-based Research, REPRIEVE
Introduction
Recent strides in the “precision” medicine movement highlight an ongoing imperative to better elucidate sex-specific differences in the pathogenesis, clinical presentation, and treatment response of acute and chronic diseases. Promoting women's participation in clinical research trials represents a critical pre-requisite to progress on this imperative. The consequences of failure are stark: Under-representation of women in clinical trials not only calls into question the generalizability of research findings1, but also fosters sex-based inequity in the development of clinical guidelines, care delivery algorithms, and health-related public policies2.
Women with HIV, as a group, are frequently underrepresented in clinical research trials, especially those evaluating new therapies 2,3,4. Worldwide, approximately 17 million women are living with HIV, representing 51% of adults with the virus5. However, according to a recent systematic review of US and international trials, women made up 19% of enrollees in antiretroviral therapeutic (ART) studies and 11% of enrollees in HIV cure studies4. Presently, in recognition of the collective aging of the population of people with HIV6, the landscape of HIV research is shifting, encompassing more work on the prevention and treatment of chronic non-communicable diseases (NCD's)7. The representation of women with HIV in NCD trials has not been rigorously studied. Moving forward, robust participation of women with HIV in NCD research will be essential to shaping sex-specific prevention and treatment algorithms for this population through the lifespan.
Older women and men with HIV, relative to age-matched peers in the general population, are at disproportionately high risk of developing NCD's, including cardiovascular disease8. In the general population, sex-specific differences in the pathogenesis, presentation and treatment response of CVD are known to exist, and yet are incompletely understood9, perhaps in part because women are underrepresented in cardiovascular clinical trials10. The REPRIEVE Trial (Randomized Trial to Prevent Vascular Events in HIV) – a 6500-person randomized controlled trial testing a strategy to prevent HIV-associated CVD11 – presents a unique opportunity to better understand how sex-specific factors (hormones, immune responsiveness) influence CVD risk and risk reduction among individuals aging with HIV. Recognizing that women have been historically under-represented in CVD research10,12,13 and in HIV research2,3,4, we identified the need for a pro-active strategy encouraging robust enrollment of women in REPRIEVE.
The primary aim of this study was to elicit perspectives on CVD research participation among a community-sample of women with or at risk for HIV and to apply acquired insights towards the development of an evidence-based campaign empowering older women with HIV to participate in a large-scale CVD prevention trial. In a community-based setting, we surveyed 40 women with or at risk for HIV about factors which might facilitate or impede engagement in CVD research. We applied insights derived from these surveys (as well as insights from conversations with women from the HIV community and from published research14) into the development of the REPRIEVE Follow YOUR Heart campaign. This multi-media campaign aims to educate women about HIV-associated CVD and empower them to learn more about participating in clinical research studies, like REPRIEVE, centered on CVD prevention.
Methods
A cross-sectional survey-based study was conducted to elicit perspectives on CVD research participation among a community-sample of women with or at risk for HIV, with a goal of channeling insights from the survey into the development of the REPRIEVE Follow YOUR Heart educational campaign.
Study Population and Strategy for Data Collection
Women with or at increased risk for HIV age 18 years and older who attended a community-based educational support group meeting offered by a non-profit organization were invited to participate in this cross-sectional survey-based study. The women's non-profit organization holds monthly meetings in an urban setting in Massachusetts and provides education on HIV and HIV prevention, as well as women's health and wellness, and domestic violence prevention. Previously collected demographics of women with or at risk for HIV attending support group meetings at this non-profit organization (approximately 68-103 attendees per month) are as follows: 56% were HIV positive, 16% were HIV negative, and 28% did not report their HIV status15. The mean age of attendees was 50.5 years (range 17-85 years)15. Sixty-one percent self-identified as Black/African American, 15% as Hispanic/Latina, 9% as Caucasian, 12% as bi-racial, and 3% as Native American. Seventy percent of the HIV-positive attendees reported living at or below the poverty level15. At the beginning of one of the monthly meetings hosted by this women's non-profit organization, information about our anonymous survey study was provided via verbal announcement, and surveys were distributed to each table of attendees. Surveys completed during the meeting were collected at meeting's end and placed in a sealed envelope. Study participation was voluntary and all information gathered was based on written self-report. We opted to conduct our survey-based study in this community setting (instead of an HIV clinic or hospital-based clinical research center) to allow for anonymous data collection from a diverse group of women with or at risk for HIV who may or may not have previously participated in research. The presently reported survey-based study was determined to be exempt by the Partners Institutional Review Board (IRB) and as per IRB guidelines for exempt protocols, consent was not obtained. Permission to conduct the study in this community-based setting was granted by the Director of the non-profit organization (HB).
Survey Content
The survey, developed by study investigators with experience in HIV-associated CVD research and HIV community education, consisted of questions about age, medical history (including CVD), and factors which might influence participation in a research study. The survey also included questions about best methods for learning about a research study about heart disease, and about methods which might enhance sustained enrollment in a longitudinal research study focused on heart disease. Two open-ended questions were included on factors which were helpful or which could have been improved during participation in an actual research study. For most questions, participants were asked to circle answers from a list of pre-populated responses (participants were free to select more than one answer for each question). Each question included an option to write in “other” free-form responses. Identifiable information was not collected from the study participants, and information on gender identification was also not collected.
Statistical Analysis of Survey Responses
Descriptive statistics were used to analyze the characteristics of the study participants. Continuous data are reported as mean + standard deviation. Percentages of participants selecting pre-populated responses to individual questions were calculated. Responses to open-ended questions are presented verbatim. Analyses were conducted with SAS JMP statistics software (version 12; SAS Institute Inc., Cary, NC).
Results
Survey-Based Study
Characteristics of the Study Sample
Forty women completed the survey at the meeting conducted in April 2015. Characteristics of the study sample are described in Table 1. The mean age of the participants was 53+13 years. Twenty-eight percent of participants reported being HIV positive. Forty-six percent of participants reported current or past treatment for depression. Common cardiovascular co-morbidities reported included hypertension (26%) and hyperlipidemia (21%).
Table 1. Clinical Characteristics of the Study Sample (N=40).
| CLINICAL CHARACTERISTICS | |
|---|---|
| Age (years) | 53±13 |
| HIV Positive | 28% (11/39) |
| Hyperlipidemia | 21% (8/39) |
| Heart Attack | 0% (0/39) |
| Chest Pain | 15% (6/39) |
| Hypertension | 26% (10/39) |
| Depression | 46% (18/39) |
| Diabetes | 13% (5/39) |
| Hepatitis B or C | 18% (7/39) |
Normally distributed data are presented as means ± SDs or percentages. N=40 for age; N=39 for other clinical characteristics due to missing data from 1 participant.
Answers to Survey Questions Regarding Participation in Research Studies
Endorsed “Best Methods” for Learning about a Research Study about Heart Disease
The five most frequently endorsed “best methods” for learning about heart disease research studies are presented in Figure 1. Greater than half of respondents (54%) endorsed “Talking to someone who did/is doing the study” as a “best method” for learning about a research study. Other self-identified best methods included: “Talking to my doctor about the study” (46%), “Watching a video about the study” (39%), “Reading a flyer about the study” (39%), and “Visiting a website with information about the study” (29%).
Figure 1. Top Endorsed “Best Methods” for Learning about a Research Study about Heart Disease.
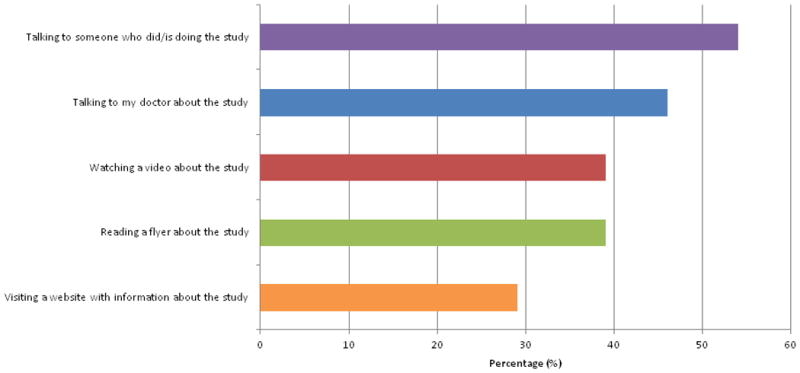
Twenty-eight women responded to this question, which included 9 pre-populated answer options about best methods for learning about and understanding a research study about heart disease. Participants were free to select more than one response. In addition to the “best methods” selections depicted in this Figure, the following methods were also endorsed: “reading about the study on Facebook” (14%), “following the study on Twitter” (4%), “reading about the study on a blog” (4%), or “seeing pictures of the tests that are being done in the study” (4%). “Other” write-in “best methods” for learning about studies were described by 7% of respondents and included “at house parties” and “the newspaper”.
Endorsed Reasons for Participating in a Research Study
The five most frequently endorsed reasons for participating in a research study are presented in Figure 2A. The majority of women endorsed “I like to get information about my health” (63%) and “The money helps” (63%) as reasons for participating in research. Nearly half of the women endorsed “To help others” as a reason for participating in research.
Figure 2A. Top Endorsed Reasons for Participating in a Research Study.
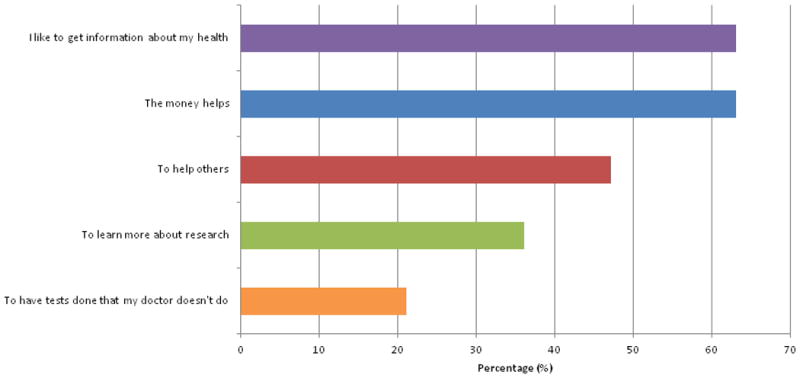
Nineteen women responded to this question, which included 7 pre-populated answer options and an “other” category. Participants were free to select more than one response. In addition to the reasons described in this Figure, 37% endorsed “to learn more about research” and 21% endorsed “to have tests done that my doctor doesn't do“. Eleven percent endorsed “I enjoyed meeting the study staff”, and 5% endorsed “to find out if there is something wrong with me”. “Other” write-in reasons were described by 11% of the respondents including “It usually improves my health”, “to help cure people”.
Endorsed Reasons for Not Participating in a Research Study
The ten most commonly endorsed reasons for not participating in research are described in Figure 2B. Leading selections were: “I didn't know where to get information about studies” (29%) and “I've never been told about studies” (29%).
Figure 2B. Top Endorsed Reasons for Not Participating in a Research Study.
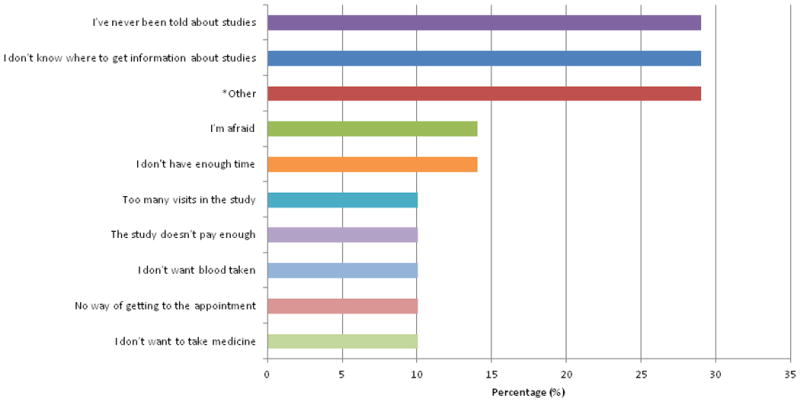
Twenty-one women responded to this question, which included 17 pre-populated answer options and an “other” category. Participants were free to select more than one response. Twenty-nine percent of the respondents described “other” write-in reasons that included “too many medical problems (on too many medications)”, “didn't qualify”, “need more information on studies,” “discriminated against due to age”, “was not eligible to do the study because on dialysis, and study had overnight visits”, and “haven't had the chance”. Less commonly endorsed reasons (not presented in this Figure) included “I don't know what research is” (5%), “I can't always understand the medical terms or tests” (5%), “I don't want x-rays” (5%), “I don't have enough money” (5%), and “I don't have childcare” (5%). None of the respondents endorsed the following reasons: “I don't trust the people who told me about studies,” “I don't want to share information about myself,” or “I'm afraid other people will find out.”
Endorsed Factors Promoting Sustained Participation in a Research Study about Heart Disease
The six most commonly endorsed factors promoting sustained participation in a longitudinal research study are presented in Figure 3. Four out of these top five responses center around good communication between study staff and participants. Additional factors endorsed included: “Access to a website that provided updates about the study” (28%), “Receipt of a payment at each study visit” (28%), “ Receive a newsletter with updates about the study” (21%), “Childcare during my visit” (7%), or “Other” related to study stipend (7%).
Figure 3. Top Factors Identified as Helpful or Warranting Improvement During Participation in a Research Study.
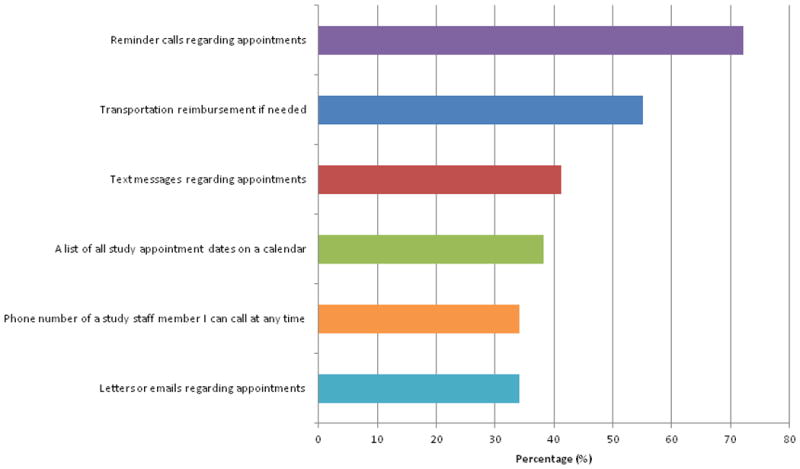
Twenty-nine women responded to this question, which included 10 pre-populated answer options and an “other” category. Participants were free to select more than one response. The question was specifically focused on factors that enhance sustained participation in a longitudinal research study about heart disease (greater than 1 year).
Factors Identified as Helpful or Warranting Improvement During Participation in a Research Study
Women were asked to list up to 3 things which were helpful and 3 things which could have been improved during participation in a previous research study. This item was an open-ended question and 10 women gave at least one response for helpful factors, and 8 women gave at least one response for factors that could have been improved.
Helpful factors identified included:
Informational: “information”; “education“; knowledge”
Communication-centered: “calls”; “reminders”; “emails”; “follow up”; “communication”; and “communication about results”
Relational: “understanding”; “experience”; “very personable staff”
Compensation-centered: “money”; “gift card”; “transportation“; “some medication”
Other: “rest”; “helping myself and others”
A summary of factors which could have been improved included:
Informational: “not enough information about the study”; “more information on different cancers”
Compensation-centered: “more services [offered in the study]; “less time of study”; “better food” “screening stipends”
Other: “better screening process”; “better HIV, bone, or diabetes studies”
Development of the Follow YOUR Heart Campaign Components
Insights from the aforementioned survey were channeled into the development of the Follow YOUR Heart campaign, aiming to educate women about HIV-associated CVD and to empower them to learn more about participating in an HIV-associated CVD prevention study, like REPRIEVE. Components of the Follow YOUR Heart campaign – including slogan, graphic/printed materials, video, web presence (including My Heart Matters heart health blog) – were developed by study investigators in consultation with community members, and with key contributions from artists (musician, graphic designer), media experts (video/sound), and public relations specialists. Feedback on the Follow YOUR Heart materials was elicited from women in the community and from HIV and CVD care providers, informing iterative changes to the materials.
Follow YOUR Heart Slogan
In developing the slogan for our educational campaign, we wished to validate women's self-identified motivations for potentially participating in a CVD trial, including the desire to gain information about one's own health and to help others. “Follow YOUR Heart” reflects how women's participation in HIV-associated CVD research can empower enhanced learning about women's heart health and fulfill an intrinsic, empathetic impulse to improve CVD preventive care for current and future generations of women aging with HIV. Feedback from women in community on the importance of empowering messaging further supported the development of the Follow YOUR Heart slogan.
Graphic/printed materials
The Follow YOUR Heart Graphics and printed materials were designed to reinforce women's self-identified motivations for potentially participating in a CVD trial (learning, giving) and to redress self-identified reasons for not participating in such a trial, namely, lack of knowledge (“I didn't know where to get information about studies”) and lack of request for information (“I've never been told about studies”). The printed materials prominently feature the Follow YOUR Heart slogan itself, as well as a graphical representation of the slogan: silhouettes of women (with red hearts) walking confidently to a destination or holding up the symbol for the REPRIEVE research trial – a red AIDS ribbon wrapped around a beating heart. The women's silhouettes reflect different body types and personal dress styles, reflecting a request for diverse representation of women in research. The printed materials also provide information about women's heart health (medical statistics on HIV-associated CVD risk in women), as well as instructions on how to learn more about the REPRIEVE research trial. (Figure 4).
Figure 4. Follow YOUR Heart Graphic.

Video
The Follow YOUR Heart video was designed to appeal to women's self-identified preferred method for learning about a research study – namely, “talking to someone who did/is doing the study.” Specifically, the video enabled virtual peer-to-peer communication about research participation. To ensure capture of candid and authentic commentary, women were filmed responding spontaneously to a series of interview questions developed by the principal investigator of the survey-based research study. These questions were grouped by the following domains: 1) Being a woman with HIV; 2) Perspective on HIV research and women's participation in HIV research; 3) Personal experience with participation in HIV research; and 4) HIV and heart disease in women. Responses to these questions were noted by study investigators to echo themes emerging from the survey-based research study.
In the Follow YOUR Heart video, an investigator-narrated voiceover is interwoven with the candid interview commentary provided by women who have previously participated in HIV research. Study investigators developed the content of the voice-over to redress self-identified reasons for not participating in research studies (lack of knowledge, lack of request for representation). Voice-over content centers on the heightened risk of CVD among women with HIV, the purpose of the REPRIEVE trial, and a “walk-through” of what participation in REPRIEVE would entail. Also included in the video is candid interview commentary by an infectious diseases clinician addressing the importance of women's participation in HIV research. This footage fulfilled another frequently endorsed “best method” for learning about research opportunities: hearing directly from a trusted medical professional. The video concludes with an invitation for women to learn more about the REPRIEVE trial, coinciding with the performance of the “Follow YOUR Heart” song specially commissioned for this project (http://followyourheart.reprievetrial.org).
Web presence, including My Heart Matters heart-health blog and social media postings
The Follow YOUR Heart web presence (including My Heart Matters heart health blog and social media postings) were designed to facilitate communication between REPRIEVE investigators and women with HIV interested in learning more about heart health, research participation, and/or the REPRIEVE trial specifically. As noted above, “communication” was described by women in our study as being helpful in research studies. The Follow YOUR Heart website features the Follow YOUR Heart video, links to web sites providing information about women's heart health and HIV-associated heart disease, and a link to the My Heart Matters heart health blog (http://followyourheart.reprievetrial.org). The blog, updated every second month, is written by study investigators and touches on issues pertaining to HIV-associated heart disease and women's participation in research. Social media postings (Facebook, Twitter) related to the REPRIEVE Follow YOUR Heart campaign are distributed via the REPRIEVE Main Trial Facebook and twitter accounts and are developed together with members of the REPRIEVE core team. Postings include links to articles and/or blogs referencing the REPRIEVE Follow YOUR Heart Campaign16.
Discussion
Our survey-based study represents the first such study, to our knowledge, querying women with or at risk for HIV about factors which may influence participation in CVD research. Moreover, ours is the first such study conducted with the express purpose of directly informing the development of an educational campaign empowering aging women with HIV to participate in a CVD prevention trial (REPRIEVE). Central themes emerging from our study suggest that women with or at risk for HIV are more likely to consider participation in heart disease research when they are made aware of research opportunities (e.g. through a peer or health care provider), when they are empowered to learn more about research opportunities, and when they are invited to participate. For those women with or at risk for HIV who have previously participated in research, key non-monetary motivations for research participation include a desire to learn more about their own health and an altruistic impulse to help facilitate advances in health care for the broader community of women.
The evidence derived from survey responses relating to factors influencing women's participation in CVD research were applied by our team in the development of the REPRIEVE Follow YOUR Heart Campaign. Specifically, we aimed to communicate with women potentially interested in participating through a screen-based strategy (video), offering both peer-to-peer communication and provider communication. Moreover, interwoven components of the campaign - including slogan, printed graphics, video, and web-based strategies (My Heart Matters heart-health blog, social media networking) – were designed to offer information/knowledge about why the REPRIEVE trial is important for women and to request women take steps to learn more about participating. Finally, all campaign components were designed to reflect women's self-identified motivations for research participation – empowerment and altruism.
Our findings that women with or at risk for HIV prefer learning about CVD research opportunities by “talking to someone who did/is doing the study” and “talking to my doctor about the study” dovetail with findings from previously published work relating to women's engagement in different types of HIV trials (not NCD-specific). Specifically, Falcoun et al. found that engagement of community advocates, including women with HIV, was an effective strategy for promoting women's participation in general HIV research trials17. Loughty et al. demonstrated that positive attributes of study staff (favoring good communication) enhanced female participation in general HIV research18 and Volkmann et al. highlighted that trust in one's provider enhanced research participation19. Meanwhile, Brown et al. demonstrated that peer referral and engagement of community agencies enhanced recruitment of women at risk for HIV to HIV vaccine trials20. Collectively, these findings highlight that peer-to-peer communication and provider/study-staff communication enhance women's participation in a variety of different HIV research trials.
In our survey, endorsed methods potentially promoting women's participation in HIV-associated CVD research included screen-based strategies (e.g. video/social media). A minority of the women we polled favored social-media based communication about research. This finding may relate to the fact that women in our study tended to be older (mean age 53) than women in previous studies examining HIV research engagement17. Moreover, access to digital media among individuals surveyed may have been limited. Indeed, the survey was distributed at a support group meeting hosted by a non-profit organization whose clients (90%) report income at or below poverty level. While social-media based communication about research was only favored by a few, the majority of women polled in our study reported desiring to learn about studies by watching a video. Previous studies have demonstrated that video-based interventions have efficacy to change health behaviors21. Ours is the first study to identify video-based presentation as a patient-endorsed means of communicating about CVD research to women with or at risk for HIV.
This study was a cross-sectional, survey-based evaluation of factors influencing HIV-associated research participation conducted among a community sample of women with or at increased risk for HIV. The study has potential limitations, as follows: Although the survey was developed by investigators with extensive experience recruiting into research studies, it is possible that the pre-populated survey responses to questions about CVD research did not encompass all possible variables. For this reason, an “other” option was provided. Although the community setting of the study allowed for recruitment of a diverse population of women, the concurrent support group-related activities that occurred at the meeting may have affected participants' ability to complete the survey in its entirety, resulting in variable response rates for each survey question. Participants' responses regarding health history (including HIV status) were not confirmed or adjudicated for accuracy. Twenty-eight percent of the participants reported having HIV, however there is a strong possibility that HIV status was underreported in surveys distributed at this community meeting centering on HIV education/ HIV prevention. Indeed, as noted, a formal report on demographics of women attending such meetings at this community center suggests that 56% report having HIV, 28% report they are not inclined to share their HIV status, and 16% report they are HIV negative15. Despite this limitation, we believe the women who completed our survey in this community setting shared with us perspectives which usefully inform the development of a campaign to enhance women's participation in HIV-CVD research. In our survey, we did not elicit information on gender identification. We acknowledge that additional work is needed to assess research participation by sex and gender and to establish evidence-based recruitment strategies which are sensitive and effective for all. Finally, the sample size of our study was relatively small, and as the survey questions were focused on methods to enhance participation in CVD research, survey findings may not be generalizable to women's participation in other types of research.
Robust representation of women in research on HIV-associated cardiovascular disease research is essential to progress in ensuring that women aging with HIV benefit from scientific advances in this field. Women's historic underrepresentation in both CVD research12,13 and HIV research 2,3,4 implies that new, more effective strategies to engage women in HIV-associated CVD research are needed. Here we applied a novel approach to developing such strategies. We surveyed women with or at risk for HIV on factors they felt would influence participation in a CVD research study, and then used their responses to inform the development of an evidence-based educational campaign to increase awareness about the largest CVD-prevention trial ever conducted among aging individuals with HIV. The reach of the Follow YOUR Heart campaign and the effectiveness of this campaign in augmenting recruitment of women to REPRIEVE will be formally evaluated at the conclusion of REPRIEVE trial enrollment. If this strategy proves efficacious, it may be applied in other large-scale research trials across disciplines. That is, investigation into patient-centered factors promoting women's engagement in clinical trials may come to be viewed as a valuable step towards successful realization of sex-specific scientific aims in research.
Acknowledgments
We would like to thank Heidi Bright, MS, APRN-BC, for her support of our research endeavor. We would also like to thank the artists (musician, graphic designer), media experts (video/sound), and public relations specialists who contributed to Follow YOUR Heart Campaign materials, as well as the research study participants and women from the community whose input helped inform the development of the campaign.
Declaration of Interest and Source of Funding: Dr. Zanni participated in a scientific advisory board meeting for Roche Diagnostics and received grant support to her institution from Gilead Sciences, both unrelated to this study. Dr. Douglas received grant support to her institution from Bristol Meyers Squibb, Edwards Lifesciences, GE HealthCare, Gilead, HeartFlow, and Roche and received royalties from UptoDate/Kluwer, all unrelated to this study. Dr. Grinspoon served as a paid consultant to Gilead Sciences, Theratechnologies, BMS, NovoNordisk, Merck, Navidea, and AstraZeneca and received grant support from Amgen, BMS, Gilead Sciences, Kowa Pharmaceuticals, and Theratechnologies, all unrelated to this study. Dr. Currier received grant support to her institution from Serono, unrelated to this study. Dr. Looby is a non-paid Board member of the community non-profit organization Healing Our Community Collaborative. For the remaining authors, no potential conflicts of interest were declared. This work is supported by 1R01AI123001-01 (NIH/NIAID: MZ and SL), The Claflin Distinguished Scholar Award (MGH Executive Committee on Research; MZ and SL), Connell Extension Grant (William F. Connell Family and the Yvonne L. Munn Center for Nursing Research; SL).
Footnotes
Clintrials registration for affiliated study: NCT02344290
References
- 1.Gandhi M, Ameli N, Bacchetti P, et al. Eligibility criteria for HIV clinical trials and generalizability of results: the gap between published reports and study protocols. AIDS. 2005 Nov 4;19(16):1885–1896. doi: 10.1097/01.aids.0000189866.67182.f7. [DOI] [PubMed] [Google Scholar]
- 2.Heidari S, Abdool Karim Q, Auerbach JD, et al. Gender-sensitive reporting in medical research. J Int AIDS Soc. 2012 Mar 08;15(1):11. doi: 10.1186/1758-2652-15-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston RE, Heitzeg MM. Sex, age, race and intervention type in clinical studies of HIV cure: a systematic review. AIDS Res Hum Retroviruses. 2015 Jan;31(1):85–97. doi: 10.1089/aid.2014.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curno MJ, Rossi S, Hodges-Mameletzis I, Johnston R, Price MA, Heidari S. A Systematic Review of the Inclusion (or Exclusion) of Women in HIV Research: From Clinical Studies of Antiretrovirals and Vaccines to Cure Strategies. J Acquir Immune Defic Syndr. 2016 Feb 1;71(2):181–188. doi: 10.1097/QAI.0000000000000842. [DOI] [PubMed] [Google Scholar]
- 5.UNAIDS. United Nations Women, Facts and Figures: HIV and AIDS: estimates from the AIDSinfo online database. [Accessed 11/11/2016, 2016];Additional disaggregations correspond to unpublished estimates for 2015 provided by UNAIDS, obtained from country-specific models of their AIDS epidemics. 2015 http://www.unwomen.org/en/what-we-do/hiv-and-aids/facts-and-figures#sthash.STWaFlZ8.dpuf.
- 6.Costagliola D. Demographics of HIV and aging. Curr Opin HIV AIDS. 2014 Jul;9(4):294–301. doi: 10.1097/COH.0000000000000076. [DOI] [PubMed] [Google Scholar]
- 7.Hunt PW. HIV and aging: emerging research issues. Curr Opin HIV AIDS. 2014 Jul;9(4):302–308. doi: 10.1097/COH.0000000000000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zanni MV, Schouten J, Grinspoon SK, Reiss P. Risk of coronary heart disease in patients with HIV infection. Nat Rev Cardiol. 2014 Dec;11(12):728–741. doi: 10.1038/nrcardio.2014.167. [DOI] [PubMed] [Google Scholar]
- 9.Garcia M, Mulvagh SL, Merz CN, Buring JE, Manson JE. Cardiovascular Disease in Women: Clinical Perspectives. Circ Res. 2016 Apr 15;118(8):1273–1293. doi: 10.1161/CIRCRESAHA.116.307547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris DJ, Douglas PS. Enrollment of women in cardiovascular clinical trials funded by the National Heart, Lung, and Blood Institute. N Engl J Med. 2000 Aug 17;343(7):475–480. doi: 10.1056/NEJM200008173430706. [DOI] [PubMed] [Google Scholar]
- 11.Mitka M. Exploring Statins to Decrease HIV-Related Heart Disease Risk. JAMA. 2015 Aug 18;314(7):657–659. doi: 10.1001/jama.2015.5498. [DOI] [PubMed] [Google Scholar]
- 12.Lee PY, Alexander KP, Hammill BG, Pasquali SK, Peterson ED. Representation of elderly persons and women in published randomized trials of acute coronary syndromes. JAMA. 2001 Aug 8;286(6):708–713. doi: 10.1001/jama.286.6.708. [DOI] [PubMed] [Google Scholar]
- 13.Kragholm K, Halim SA, Yang Q, et al. Sex-Stratified Trends in Enrollment, Patient Characteristics, Treatment, and Outcomes Among Non-ST-Segment Elevation Acute Coronary Syndrome Patients: Insights From Clinical Trials Over 17 Years. Circ Cardiovasc Qual Outcomes. 2015 Jul;8(4):357–367. doi: 10.1161/CIRCOUTCOMES.114.001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ACTG Global Community Advisory Board CSS, and Women's Enrollment Initiative. Recruitment and Retention of Women in ACTG Trials: A Guidebook of Best Practices. 2014 Summer; [Google Scholar]
- 15.Bright H. Healing Our Community Collaborative Non-profit Organization: Data from HOCC monthy meeting attendees January-June 2014. Boston: Healing Our Community Collarborative; Oct 6, 2016. [Google Scholar]
- 16.Streisand B, Fauci Anthony S. A Reprieve for Women: Embracing Inclusive Scientific Research. [Accessed 11/11/2016];Health Affairs Blog: Copyright ©2016 Health Affairs by Project HOPE– The People-to-People Health Foundation. 2016 Sep 13; http://healthaffairs.org/blog/2016/09/13/a-reprieve-for-women-embracing-inclusive-scientific-research/
- 17.Falcon R, Bridge DA, Currier J, et al. Recruitment and retention of diverse populations in antiretroviral clinical trials: practical applications from the gender, race and clinical experience study. J Womens Health (Larchmt) 2011 Jul;20(7):1043–1050. doi: 10.1089/jwh.2010.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loutfy MR, V LK, Mohammed S, et al. Recruitment of HIV-Positive Women in Research: Discussing Barriers, Facilitators, and Research Personnel's Knowledge. Open AIDS J. 2014;8:58–65. doi: 10.2174/1874613601408010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volkmann ER, Claiborne D, Currier JS. Determinants of participation in HIV clinical trials: the importance of patients' trust in their provider. HIV Clin Trials. 2009 Mar-Apr;10(2):104–109. doi: 10.1310/hct1002-104. [DOI] [PubMed] [Google Scholar]
- 20.Brown-Peterside P, Chiasson MA, Ren L, Koblin BA. Involving women in HIV vaccine efficacy trials: lessons learned from a vaccine preparedness study in New York City. J Urban Health. 2000 Sep;77(3):425–437. doi: 10.1007/BF02386751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Donnell LN, Doval AS, Duran R, O'Donnell C. Video-based sexually transmitted disease patient education: its impact on condom acquisition. Am J Public Health. 1995 Jun;85(6):817–822. doi: 10.2105/ajph.85.6.817. [DOI] [PMC free article] [PubMed] [Google Scholar]


