Abstract
Context:
The extra-intestinal manifestations of inflammatory bowel disease (IBD) are common and involve other organs or systems for example; urinary system.
Evidence Acquisitions:
For this review, we used a variety of sources by searching through Web of Science, PubMed, EMBASE, Scopus and directory of open access journals (DOAJ).
Results:
Urinary complications may occur in up to 22% of patients and nephrolithiasis or renal/kidney stones have been suggested to be a common manifestation of disease in forms of uric acid, calcium phosphate or calcium oxalate. We performed a meta-analysis on five clinical trials and reported that correlation between IBD and formation of stone in renal system is positive and significant (Fix-effect model; CI: 95%, P <0.001, and randomeffect model; CI: 95%, P = 0.03).
Conclusions:
Based on the reports of the clinical trials, calcium oxalate is more prevalent in Crohn’s disease (CD) than in ulcerative colitis (UC).
Keywords: Crohn’s disease, Nephrolithiasis, Inflammatory bowel disease, IBD, Kidney stone, Ulcerative colitis
Implication for health policy/practice/research/medical education:
In patients with IBD, ureteral obstruction, enterovesical fistulas, and urinary tract stones have the most prevalence. Ureteral obstruction, enterovesical fistulas are directly related to disease process but urinary tract stones are indirectly. Extra-intestinal urolithiasis symptoms can particularly be found in the patients with Crohn’s disease compared with UC which might be in result of steroid dependency and/or ileal involvement of CD patients. Based on the reports of the clinical trials, calcium oxalate is more prevalent in Crohn’s disease than in UC.
1. Background
Inflammatory bowel diseases (IBDs) are associated with extra-intestinal manifestations such as musculoskeletal, dermatologic, ophthalmologic, hematologic, cardiovascular, pulmonary, neurologic, pancreatic, hepatobiliary and genitourinary involvement. Crohn’s disease (CD) and ulcerative colitis (UC) are the main forms of IBD that differently affect the frequent rate of extra-intestinal complications (1).
Pathogenic factors of extra-intestinal manifestations remain unclear. Some organ disorders may be linked to immunologic origins, following intestinal bacterial overgrowth or side effects of therapy used to control bowel inflammation. In overall, pathogenesis of IBD is various including genetics, immunology responses or environmental factors (2,3).
Urinary complications in IBD patients have been reported in up to 22% of subjects that ureteral obstruction, enterovesical fistulas and kidney stones are the most common manifestations (2). Urinary tract complications including ileal masses or abscesses that may result in ureteric obstruction or fistulas to the bladder or urethra are the most widespread in CD compared with UC patients (4).
In a clinical trial 312 CD patients who were considered from urinary tract complications viewpoints, 77 (24.7%) had urologic symptoms, 51 (16%) influenced by simple cystitis and the subjects which acquired structural urinary tract abnormalities had most common manifestations (5,6).
This review article tries to discuss the relations of these parameters other renal manifestations of IBD, and also tries to find out the relation between IBD and urinary system stones. We reviewed and performed a meta-analysis on five studies of clinical trials that assessed the occurrence of renal stones in patients with IBD.
Materials and Methods
For this review, we used a variety of sources by searching through Web of Science, PubMed, EMBASE, Scopus and directory of open access journals (DOAJ). The search was performed using combinations of the following key words and or their equivalents such as; Crohn’s disease, nephrolithiasis, inflammatory bowel disease, ulcerative colitis and kidney stone.
Results
The relation between IBD and renal or urologic complications is presented in Table 1. As can be seen the complications are related to the disease process or might be iatrogenic which are discussed in more detail below.
Table 1. Renal complications of patient in inflammatory bowel disease .
| Complications directly related to disease process |
| Enterourinary fistulas |
| Ureteral obstruction |
| Genital involvement |
| Complications indirectly related to disease process |
| Nephrolithiasis |
| Glomerulonephritis |
| Amyloidosis |
| Iatrogenic complications |
| Medications |
| Aminosalicylates |
| Cyclosporine |
Entero-urinary fistulas
The prevalence of entero-urinary fistulas in patients of CD is 24-32% (1). After diverticulitis and cancer, CD is estimated to be the third cause of entero-vesical fistulas (EVF) with calculating 5%-17%, first cause of ileovesical fistulas and EVF in patients higher than 40 years with accounting for more than 75% but in patients >50 years old accounts for 1.8%. EVF can cause pneumaturia and fecaluria with frequencies of 38%–94% and 17%–63%, respectively, and epididymitis, hematuria, urinary tract infections, prostatitis, fever, abdominal pain and involuntary urine passage or urorrhea (7-13).
Management options for EVF are surgery and intervention of medications. Antibiotics and anti-inflammatory medications such as aminosalicylates and corticosteroids or cyclosporine and 6-mercaptopurine have been used successfully (14-18).
Ureteral obstruction
In 50% to 73% of patients having CD and in more than 50% of patients having UC, the obstruction ureteral is not usually occurred by stones (5). Non-calculous obstruction (NCO) in CD is due to retroperitoneal inflammation or an EVF near the ureter and surgery is the treatment choice (19).
Genital involvement
Genital involvement in IBD is uncommon and is associated with and erythema, edema, ulceration, fibrosis of vulva, scrotum penis from direct extension of underlying disease, pyoderma gangrenosum, or abscesses, phimosis, balanoposthitis, metastatic CD or granulomatous inflammation in seminal vesicles, prostate gland, and scrotum. Genital involvement may result in perineal pain, urethral strictures, urethral discharge, fever, and might parallel the activity of underlying IBD. The treatment options for this include antibiotics, systemic steroids, immunosuppression, resection of involved bowel, skin and circumcision (20-25).
Nephrolithiasis
The nephrolithiasis prevalence in IBD patients has been suggested to be more than general, in adults the prevalence is more than children and in CD patients is higher compared with UC patients and in CD patients who have extensive surgery is the highest (26-28).
Multiple kidney stones are occurred in IBD and in CD the stones are occurred often in the right ureter and terminal ileum (7%–15%) (5,29). The stones are usually composed of calcium oxalate that result in high urinary oxalate, calcium phosphate that result in increased Ca mobilization from bone, decreased tubular resorption of Ca and uric acid that result in decreased urate solubility in an acidic, concentrated urine. On the other hand, low levels of citrate, magnesium, anti-lithogenic agents, accompanying bile salt and fat malabsorption cause stone development (30).
The risk of calcium oxalate stone is increased in patients having an intact colon, due to high absorption of sodium-bound oxalate in colon, but in patients with an ileostomy, uric acid stones are increased because of frequent dehydration (31).
Despite existence of hyperoxaluria, hypocitraturia, hypomagnesuria, hypophosphaturia, low urinary pH and low urine volume in patients with IBD, but most of them have not renal stones (32), probably due to attention to individual genetic.
Glomerulonephritis
The occurrence of glomerulonephritis in CD and UC has been reported (33,34). The occurrence of glomerulonephritis is not association with duration of disease, but has correlation with level of inflammation in bowel and bile duct (35-37). For improving the renal function in patients having IBD with glomerulonephritis, the use of steroids is helpful (38,39).
Amyloidosis
Moschkowitz in 1936 reported the association between secondary amyloidosis (A) and IBD for the first time (40). It has been shown in clinical studies that amyloidosis is occurred in about 1% of IBD patients. Amyloidosis is 3-fold in male subjects compared with female ones. In CD patients the prevalence is 10-fold compared with UC patients and it correlates with extra-intestinal complications (36,41-43).
The secondary amyloidosis pathogenesis has been attributed to serum amyloid-A (SAA), which is an acute phase protein with unknown function (44) that expression level is increased in response to inflammation and may lead to amyloidosis. In patients with IBD amyloidosis may affect the kidney function and cause proteinuria followed by nephrotic syndrome and subsequently renal insufficiency (3,36,45,46). Finally, in CD with azathioprine and plasmapheresis, treatment of renal amyloidosis may be useful (47,48).
Complications of medical therapy
Drugs such as corticosteroids, azathioprine, 6-mercaptopurine, metronidazole and low dose of methotrexate have little or no nephrotoxicity (49-54). Aminosalicylates and cyclosporine are the medications with potential renal toxicity.
Aminosalicylates (5-ASA)
Sulfasalazine (5-ASA bound to sulfapyridine), mesalamine, and olsalazine are types of aminosalicylates that renal toxicities with these agents have been reported (55-58). These are associated with clinical improvement in active UC and are essential in maintaining remission. Mesalazine is arguable in CD and seems to be beneficial only in mild ileocolonic disease (59). Renal deficiency might occur in more than 1% of patients using 5-ASA, but renal clinical disturbance may occur in only 1 to 500 IBD patients (60).
Treatment with 5-ASA may cause renal toxicity that include glomerulonephritis, change in nephropathy with interstitial nephritis and nephrotic syndrome, which in these cases might be associated with nephrogenic diabetes (61,62).
Cyclosporine
Dose of cyclosporine (CsA) is related to nephrotoxicity (63,64). Cyclosporine has a role in tenacious UC, which by inhibiting calcineurin (a calmodulin and calcium dependent serine/threonine protein phosphatase) can block the production of interferon- γ and interleukin-2 interrupting the cellular immune response (65,66).
Meta-analysis
We analyzed the data of 5 clinical trials by evaluating the rate difference with their 95% confidence intervals. Then, the results were reported in form of Fix and random effect- models and the presence of heterogeneity across trials by using the I2 statistical analysis. The analysis was performed by comprehensive meta-analysis V3 software. The results of the studies are outlined in Table 2.
Table 2. Clinical trials studies considered in meta-analysis .
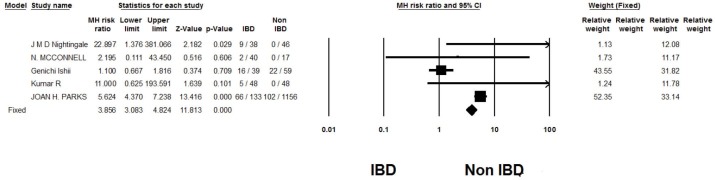
Analysis showed that correlation between IBD and renal stone is significant (Figure 1), with fix-effect model; CI: 95%, P value < 0.001, I2: 88.52 and Random-effect model; CI:95%, P value = 0.03.
Figure 1.
Forest plot of controlled trials of IBD and occur renal stone.
As it was said hyperoxaluria is a common extra intestinal complication of IBD that might be result from malabsorption and the absence of intestinal oxalate degrading bacteria. Intestinal hyperoxaluria is a cause in formation of oxalate stones in kidneys. Since probiotics in treatment of IBD are efficacious, therefore some types of probiotics should be useful in reduction of renal stone in patients with IBD (72).
Conclusion
In patients with IBD, ureteral obstruction, enterovesical fistulas, and urinary tract stones have the most prevalence. Ureteral obstruction, enterovesical fistulas are directly related to disease process but urinary tract stones are indirectly. Extra-intestinal urolithiasis symptoms can particularly be found in the patients with CD compared with UC which might be in result of steroid dependency and/or ileal involvement of CD patients. Clinical trial studies pointed that hyperoxaluria has mostly redundancy in CD that results in high urinary oxalate that would be associated with low pH and urine volume.
Authors’ contribution
All data were independently abstracted by three authors (MGA, MRK. and HN) by using a data abstraction form (author name, sex, number of patients and controls). Analysis of tests and primary draft were preformed by MGA. The manuscript was edited by MRK and HN.
Conflict of interest
The authors declared no competing interests.
Funding/Support
None declared.
Please cite this paper as: Ganji-Arjenaki M, Nasri H, Rafieian-Kopaei M. Nephrolithiasis as a common urinary system manifestation of inflammatory bowel diseases; a clinical review and meta-analysis. J Nephropathol. 2017;6(3):264-269. DOI: 10.15171/ jnp.2017.42.
References
- 1.Greenstein AJ, Janowitz HD, Sachar DB. The extra-intestinal complications of Crohn’s disease and ulcerative colitis: a study of 700 patients. Medicine. 1976;55(5):401–12. doi: 10.1097/00005792-197609000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Das KM. Relationship of extraintestinal involvements in inflammatory bowel disease (new insights into autoimmune pathogenesis) Dig Dis Sci. 1999;44(1):1–13. doi: 10.1023/a:1026629528233. [DOI] [PubMed] [Google Scholar]
- 3.Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol (NY) 2011;7(4):235–41. [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Ami H, Ginesin Y, Behar DM, Fisher D, Edoute Y, Lavy A. Diagnosis and treatment of urinary tract complication in Crohn’s disease: an experience over 15 years. Can J Gastroenterol Hepatol. 2002;16(4):225–9. doi: 10.1155/2002/204614. [DOI] [PubMed] [Google Scholar]
- 5.Pardi DS, Tremaine WJ, Sandborn WJ, McCarthy JT. Renal and urologic complications of inflammatory bowel disease. Am J Gastroenterol. 1998;93(4):504–14. doi: 10.1111/j.1572-0241.1998.156_b.x. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein CN. Treatment of the extraintestinal manifestations of inflammatory bowel disease. Curr Gastroenterol Rep. 2002;4(6):513–6. doi: 10.1007/s11894-002-0028-9. [DOI] [PubMed] [Google Scholar]
- 7.Greenstein AJ, Sachar DB, Tzakis A, Sher L, Heimann T, Aufses AH. Course of enterovesical fistulas in Crohn’s disease. Am J Surg. 1984;147(6):788–92. doi: 10.1016/0002-9610(84)90202-2. [DOI] [PubMed] [Google Scholar]
- 8.Sarr MG, Fishman EK, Goldman S, Siegelman SS, Cameron JL. Enterovesical fistula. Surg Gynecol Obstet. 1987;164(1):41–8. [PubMed] [Google Scholar]
- 9.Karamchandani MC, West CF. Vesicoenteric fistulas. Am J Surg. 1984;147(5):681–3. doi: 10.1016/0002-9610(84)90141-7. [DOI] [PubMed] [Google Scholar]
- 10.Kavanagh D, Neary P, Dodd J, Sheahan K, O’donoghue D, Hyland J. Diagnosis and treatment of enterovesical fistulae. Colorectal Dis. 2005;7(3):286–91. doi: 10.1111/j.1463-1318.2005.00786.x. [DOI] [PubMed] [Google Scholar]
- 11. Suhler A, Viville C, Leger P, Firmin F, editors. Vesico-intestinal fistulas. Annales d’urologie; 1994. [PubMed]
- 12.Solem CA, Loftus EV, Tremaine WJ, Pemberton JH, Wolff BG, Sandborn WJ. Fistulas to the urinary system in Crohn’s disease: clinical features and outcomes. Am J Gastroenterol. 2002;97(9):2300–5. doi: 10.1111/j.1572-0241.2002.05983.x. [DOI] [PubMed] [Google Scholar]
- 13.Stein RB, Lichtenstein GR. Medical therapy for Crohn’s disease: the state of the art. Surg Clin North Am. 2001;81(1):71–101. doi: 10.1016/S0039-6109(05)70274-7. [DOI] [PubMed] [Google Scholar]
- 14.Margolin ML, Korelitz BI. Management of bladder fistulas in Crohn’s disease. J Clinical Gastroenterol. 1989;11(4):399–402. doi: 10.1097/00004836-198908000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Gorcey S, Katzka I. Is operation always necessary for enterovesical fistul in Crohn’s Disease? J Clinical Gastroenterol. 1989;11(4):396–8. doi: 10.1097/00004836-198908000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson GG, Lee EW, Hunt SR, Ridley CH, Brandes SB. Management of the bladder during surgical treatment of enterovesical fistulas from benign bowel disease. J Am Coll Surg. 2008;207(4):569–72. doi: 10.1016/j.jamcollsurg.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Hanauer SB, Smith MB. Rapid closure of Crohn’s disease fistulas with continuous intravenous cyclosporin A. Am J Gastroenterol. 1993;88(5):646–9. [PubMed] [Google Scholar]
- 18.Korelitz BI, Adler DJ, Mendelsohn RA, Sacknoff AL. Long-term experience with 6-mercaptopurine in the treatment of Crohn’s disease. American Journal of Gastroenterology. 1993;88(8):1198–205. [PubMed] [Google Scholar]
- 19.Quader A. Significance and treatment of occult obstructive uropathy complicating Crohn’s disease: GE Block, WE Enker, and JB Kirsner (178: 322, 1973) Urology. 1974;3(3):395. doi: 10.1097/00000658-197309000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minocha A, Anderson M, Eaton B, Harty R. Crohn’s disease complicating male genitourinary tract without overlying cutaneous involvement. Am J Gastroenterol. 1996;91(7):1463–7. [PubMed] [Google Scholar]
- 21.Guest MGD, Fink RL. Metastatic crohn’s disease. Diseases of the colon & rectum. 2000;43(12):1764–6. doi: 10.1007/BF02236866. [DOI] [PubMed] [Google Scholar]
- 22.Andreani S, Ratnasingham K, Dang H, Gravante G, Giordano P. Crohn’s disease of the vulva. Int J Surg. 2010;8(1):2–5. doi: 10.1016/j.ijsu.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Ninan T, Aggett P, Smith F, Youngson G, Miller I. Atypical genital involvement in a child with Crohn’s disease. J Pediatr Gastroenterol Nutr. 1992;15(3):330–3. doi: 10.1097/00005176-199210000-00018. [DOI] [PubMed] [Google Scholar]
- 24.Poon KS, Gilks CB, Masterson JS. Metastatic Crohn’s disease involving the genitalia. J Urol. 2002;167(6):2541–2. [PubMed] [Google Scholar]
- 25.Wijesurendra C, Singh G, Manuel A, Morris J. Balanoposthitis—an unusual feature of Crohn’s disease? Int J STD AIDS. 1993;4(3):184. doi: 10.1177/095646249300400319. [DOI] [PubMed] [Google Scholar]
- 26.Booth I, Lander A. 6 Short bowel syndrome. Baillieres Clin Gastroenterol. 1998;12(4):739–73. doi: 10.1016/s0950-3528(98)90006-9. [DOI] [PubMed] [Google Scholar]
- 27.Clark JH, Fitzgerald JF, Bergstein JM. Nephrolithiasis in childhood inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1985;4(5):829–34. doi: 10.1097/00005176-198510000-00026. [DOI] [PubMed] [Google Scholar]
- 28.Su CG, Judge TA, Lichtenstein GR. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Clin North Am. 2002;31(1):307–27. doi: 10.1016/s0889-8553(01)00019-x. [DOI] [PubMed] [Google Scholar]
- 29.Böhles H, Beifuss O, Brandl U, Pichl J, Akcetin Z, Demling L. Urinary factors of kidney stone formation in patients with Crohn’s disease. Klin Wochenschr. 1988;66(3):87–91. doi: 10.1007/BF01774220. [DOI] [PubMed] [Google Scholar]
- 30.Coe F, Parks J, Asplin J. The pathogenesis and treatment of kidney stones. N Engl J Med. 1993;1993(328):444–5. [Google Scholar]
- 31.Present DH, Rabinowitz JG, Banks PA, Janowitz HD. Obstructive hydronephrosis: A frequent but seldom recognized complication of granulomatous disease of the bowel. N Engl J Med. 1969;280(10):523–8. doi: 10.1056/NEJM196903062801002. [DOI] [PubMed] [Google Scholar]
- 32.Hylander E, Jarnum S, Frandsen I. Urolithiasis and hyperoxaluria in chronic inflammatory bowel disease. Scand J Gastroenterol. 1978;14(4):475–9. [PubMed] [Google Scholar]
- 33.Terjung B, Worman HJ. Anti-neutrophil antibodies in primary sclerosing cholangitis. Best Pract Res Clin Gastroenterol. 2001;15(4):629–42. doi: 10.1053/bega.2001.0209. [DOI] [PubMed] [Google Scholar]
- 34.Talwalkar JA, Lindor KD. Primary sclerosing cholangitis. Inflamm Bowel Dis. 2005;11(1):62–72. doi: 10.1097/00054725-200501000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Lakatos L, Pandur T, David G, Balogh Z, Kuronya P, Tollas A. et al. Association of extraintestinal manifestations of inflammatory bowel disease in a province of western Hungary with disease phenotype: results of a 25-year follow-up study. World J Gastroenterol. 2003;9(10):2300–7. doi: 10.3748/wjg.v9.i10.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Danese S, Semeraro S, Papa A, Roberto I, Scaldaferri F, Fedeli G. et al. Extraintestinal manifestations in inflammatory bowel disease. World J Gastroenterol. 2005;11(46):7227. doi: 10.3748/wjg.v11.i46.7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Presti ME, Neuschwander-Tetri BA, Vogler CA, Janney CG, Roche JK. Case report: sclerosing cholangitis, inflammatory bowel disease, and glomerulonephritis (a case report of a rare triad) Dig Dis Sci. 1997;42(4):813–6. doi: 10.1023/a:1018824416458. [DOI] [PubMed] [Google Scholar]
- 38.Molina-Perez M, Gonzalez-Reimers E, Santolaria-Fernandez F, Maceira-Cruz B, Ravina-Cabrera M. Rapidly progressive glomerulonephritis and inflammatory bowel disease. Dis Colon Rectum. 1995;38(9):1006–7. doi: 10.1007/BF02049742. [DOI] [PubMed] [Google Scholar]
- 39.Peeters A, Van den Wall Bake A , Daha M, Breeveld F. Inflammatory bowel disease and ankylosing spondylitis associated with cutaneous vasculitis, glomerulonephritis, and circulating IgA immune complexes. Ann Rheum Dis. 1990;49(8):638–40. doi: 10.1136/ard.49.8.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moschcowitz E. The clinical aspects of amyloidosis. Ann Intern Med. 1936;10:73–88. [Google Scholar]
- 41.Wester AL, Vatn MH, Fausa O. Secondary amyloidosis in inflammatory bowel disease: a study of 18 patients admitted to Rikshospitalet University Hospital, Oslo, from 1962 to 1998. Inflamm Bowel Dis. 2001;7(4):295–300. doi: 10.1097/00054725-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Vermeire S, Van Assche G, Rutgeerts P. C-reactive protein as a marker for inflammatory bowel disease. Inflamm Bowel Dis. 2004;10(5):661–5. doi: 10.1097/00054725-200409000-00026. [DOI] [PubMed] [Google Scholar]
- 43.Fernandez-Castroagudin J, Brage VA, Lens NX, Martínez CJ, Abdulkader I. Renal amyloidosis as initial clinical manifestation of Crohn’s disease. Gastroenterologia y Hepatologia. 2001;25(6):395–7. doi: 10.1016/s0210-5705(02)70273-2. [DOI] [PubMed] [Google Scholar]
- 44.Nordling E, Abraham-Nordling M. Colonic amyloidosis, computational analysis of the major amyloidogenic species, Serum Amyloid A. Comput Biol Chem. 2012;39:29–34. doi: 10.1016/j.compbiolchem.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 45.Lovat LB, Madhoo S, Pepys MB, Hawkins PN. Long-term survival in systemic amyloid A amyloidosis complicating Crohn’s disease. Gastroenterology. 1997;112(4):1362–5. doi: 10.1016/s0016-5085(97)70150-1. [DOI] [PubMed] [Google Scholar]
- 46.Ebert EC, Nagar M. Gastrointestinal manifestations of amyloidosis. Am J Gastroenterol. 2008;103(3):776–87. doi: 10.1111/j.1572-0241.2007.01669.x. [DOI] [PubMed] [Google Scholar]
- 47.Katsanos K, Tsianos E. The kidneys in inflammatory bowel disease. Ann Gastroenterol. 2002;15:41–52. [Google Scholar]
- 48. Zorcolo L, Casula G. Urinary and Sexual Involvement in IBD. Inflammatory bowel disease and familial adenomatous polyposis. Springer; 2006:141-55.
- 49. Kjellstrant C. Side effects of steroids and their treatment. Transplant Proc. 1975 Mar;7(1):123-9. [PubMed]
- 50.Schäcke H, Döcke W-D, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96(1):23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 51.Present DH, Meltzer SJ, Krumholz MP, Wolke A, Korelitz BI. 6-Mercaptopurine in the management of inflammatory bowel disease: short-and long-term toxicity. Ann Intern Med. 1989;111(8):641–9. doi: 10.7326/0003-4819-111-8-641. [DOI] [PubMed] [Google Scholar]
- 52.Austin M, Beigi R, Meyn L, Hillier S. Microbiologic response to treatment of bacterial vaginosis with topical clindamycin or metronidazole. J Clin Microbiol. 2005;43(9):4492–7. doi: 10.1128/JCM.43.9.4492-4497.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kinder A, Hassell A, Brand J, Brownfield A, Grove M, Shadforth M. The treatment of inflammatory arthritis with methotrexate in clinical practice: treatment duration and incidence of adverse drug reactions. Rheumatology. 2005;44(1):61–6. doi: 10.1093/rheumatology/keh512. [DOI] [PubMed] [Google Scholar]
- 54.Schnabel A, Gross WL. Low-dose methotrexate in rheumatic diseases—efficacy, side effects, and risk factors for side effects. Semin Arthritis Rheum. 1994;23(5):310–27. doi: 10.1016/0049-0172(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 55.Rhodes J, Coles G. An alternative view of 5-ASA formulations. Gut. 1995;36(4):639–40. doi: 10.1136/gut.36.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loftus E, Kane S, Bjorkman D. Short-term adverse effects of 5-aminosalicylic acid agents in the treatment of ulcerative colitis. Aliment Pharmacol Ther. 2004;19(2):179–89. doi: 10.1111/j.0269-2813.2004.01827.x. [DOI] [PubMed] [Google Scholar]
- 57.Arend LJ, Springate JE. Interstitial nephritis from mesalazine: case report and literature review. Pediatr Nephrol. 2004;19(5):550–3. doi: 10.1007/s00467-004-1411-6. [DOI] [PubMed] [Google Scholar]
- 58.Ransford R, Langman M. Sulphasalazine and mesalazine: serious adverse reactions re-evaluated on the basis of suspected adverse reaction reports to the Committee on Safety of Medicines. Gut. 2002;51(4):536–9. doi: 10.1136/gut.51.4.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carter MJ, Lobo AJ, Travis SP. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53(suppl 5):v1–v16. doi: 10.1136/gut.2004.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gisbert JP, González-Lama Y, Maté J. 5-Aminosalicylates and renal function in inflammatory bowel disease: a systematic review. Inflamm Bowel Dis. 2007;13(5):629–38. doi: 10.1002/ibd.20099. [DOI] [PubMed] [Google Scholar]
- 61.Uslu N, Demir H, Saltik-Temizel IN, Topaloğlu R, Gürakan F, Yüce A. Acute tubular injury associated with mesalazine therapy in an adolescent girl with inflammatory bowel disease. Dig Dis Sci. 2007;52(11):2926–9. doi: 10.1007/s10620-006-9586-2. [DOI] [PubMed] [Google Scholar]
- 62.Oikonomou KA, Kapsoritakis AN, Stefanidis I, Potamianos SP. Drug-induced nephrotoxicity in inflammatory bowel disease. Nephron Clin Pract. 2011;119(2):c89–94. doi: 10.1159/000326682. [DOI] [PubMed] [Google Scholar]
- 63.Bennett WM. The nephrotoxicity of immunosuppressive drugs. Nephrol Dial Transplant. 1996;11(9):1899–901. [PubMed] [Google Scholar]
- 64.Kirschner BS. Safety of azathioprine and 6-mercaptopurine in pediatric patients with inflammatory bowel disease. Gastroenterology. 1998;115(4):813–21. doi: 10.1016/s0016-5085(98)70251-3. [DOI] [PubMed] [Google Scholar]
- 65.Sandborn WJ. A critical review of cyclosporine therapy in inflammatory bowel disease. Inflamm Bowel Dis. 1995;1(1):48–63. [Google Scholar]
- 66.Eckstein LA, Van Quill KR, Bui SK, Uusitalo MS, O’Brien JM. Cyclosporin a inhibits calcineurin/nuclear factor of activated T-cells signaling and induces apoptosis in retinoblastoma cells. Invest Ophthalmol Vis Sci. 2005;46(3):782–90. doi: 10.1167/iovs.04-1022. [DOI] [PubMed] [Google Scholar]
- 67.Nightingale J, Lennard-Jones J, Gertner D, Wood S, Bartram C. Colonic preservation reduces need for parenteral therapy, increases incidence of renal stones, but does not change high prevalence of gall stones in patients with a short bowel. Gut. 1992;33(11):1493–7. doi: 10.1136/gut.33.11.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McConnell N, Campbell S, Gillanders I, Rolton H, Danesh B. Risk factors for developing renal stones in inflammatory bowel disease. BJU Int. 2002;89(9):835–41. doi: 10.1046/j.1464-410x.2002.02739.x. [DOI] [PubMed] [Google Scholar]
- 69.Ishii G, Nakajima K, Tanaka N, Hara H, Kato M, Ishii N. Clinical evaluation of urolithiasis in Crohn’s disease. Int J Urol. 2009;16(5):477–80. doi: 10.1111/j.1442-2042.2009.02285.x. [DOI] [PubMed] [Google Scholar]
- 70.Kumar R, Ghoshal UC, Singh G, Mittal RD. Infrequency of colonization with Oxalobacter formigenes in inflammatory bowel disease: possible role in renal stone formation. J Gastroenterol Hepatol. 2004;19(12):1403–9. doi: 10.1111/j.1440-1746.2004.03510.x. [DOI] [PubMed] [Google Scholar]
- 71.Parks JH, Worcester EM, O’Connor RC, Coe FL. Urine stone risk factors in nephrolithiasis patients with and without bowel disease. Kidney Int. 2003;63(1):255–65. doi: 10.1046/j.1523-1755.2003.00725.x. [DOI] [PubMed] [Google Scholar]
- 72.Ganji-Arjenaki M, Rafieian-Kopaei M. Probiotics are a good choice in remission of inflammatory bowel diseases: a meta analysis and systematic review. Journal of Cellular Physiology. 2017 doi: 10.1002/jcp.25911. [DOI] [PubMed] [Google Scholar]