Abstract
In the brain, membrane trafficking systems play important roles in regulating neuronal functions, such as neuronal morphology, synaptic plasticity, survival, and glial communications. To date, numerous studies have reported that defects in these systems cause various neuronal diseases. Thus, understanding the mechanisms underlying vesicle dynamics may provide influential clues that could aid in the treatment of several neuronal disorders. Here, we describe a method for quantifying vesicle motilities, such as motility distance and rate of movement, using a software plug-in for the ImageJ platform. To obtain images for quantification, we labeled neuronal endosome-lysosome structures with EGFP-tagged vesicle marker proteins and observed the movement of vesicles using a time-lapse microscopy. This method is highly useful and simplify measuring vesicle motility in neurites, such as axons and dendrites, as well as in the soma of both neurons and glial cells. Furthermore, this method can be applied to other cell lines, such as fibroblasts and endothelial cells. This approach could provide a valuable advancement of our understanding of membrane trafficking.
Keywords: Neuroscience, Issue 123, Neuroscience, neuron, dendrite, axon, endosome, lysosome, membrane trafficking, vesicle motility
Introduction
Precise control of endosome-lysosome trafficking is indispensable for regulating neuronal function. Notably, the dynamic movements of these vesicles are a key factor underlying the regulation of neuronal morphology, development, and survival. Defects in this system cause severe neuronal disorders1,2. The molecular mechanisms that link vesicle trafficking to neuronal diseases are considered complicated, and several groups have sought to examine this relevance. For instance, it has been reported that perturbed late endosome motility is significantly associated with Niemann-Pick C disease3, an inherited neurodegenerative disorder caused by lysosome defects. Another example is a mutation in a lysosomal Ca2+ channel, trpml1, which impairs lysosomal motility, resulting in lysosomal storage diseases4,5,6. Our group has reported that dysregulation of PtdIns(3,5)P2 turnover suppresses endosome and lysosome motility in neurons, leading to an increase in vulnerability to the stress response7,8. The metabolic regulation of PtdIns(3,5)P2, which mostly localizes on late endosomes and lysosomes, plays an important role in a wide variety of cellular functions, including vesicle trafficking and fusion-fission processes9,10. Since impaired PtdIns(3,5)P2 turnover causes severe neurodegeneration11,12, the aberrant regulation of endosome-lysosome motility could be a key factor for understanding the pathogenesis of neurodegeneration. An investigation of the molecular mechanisms that underlie vesicle motility may thus provide promising clues that can deepen our understanding of several neuronal disorders.
In this paper, we introduce a valuable method to quantify vesicle motility in neurons using a free software package called Manual Tracking. The aim was to develop a fast quantification method to analyze vesicle motility. This quantification is directed through a standard approach of clicking on a reference point in each frame of a time-lapse movie. The use of the Manual Tracking software makes this approach quite simple and of broad utility, unlike other applications. Furthermore, this approach is also applicable to other cells, such as glial cells. Although this method is primitive, it can be applied to various analyses, including of cellular motility and morphological change. For instance, after defining a reference point across a sequence of images, information on the positions of the reference points and the time at each position can be extracted from sequential images using data analysis and image-processing software. Taken together, this method is simple but powerful and contributes to the development of improved efficiency in studies based on membrane trafficking, such as those examining endosome-lysosome function.
Protocol
All animal procedures were performed with the approval of the University of Tsukuba Animal Care and Use Committee (IACUC).
1. Dissection
To prepare embryonic day 13-14 ICR or C57BL/6 fetuses, euthanize pregnant mice by cervical dislocation. Remove the uterus and put it into a petri dish with Hank's Balanced Salt Solution (HBSS). Rinse it well to remove the blood.
Using forceps, transfer the uterus to a new petri dish with 70% ethanol to sterilize.
Transfer the uterus to a new petri dish with HBSS. Remove the fetuses from the uterus using sterilized dissecting forceps and surgical scissors.
Decapitate the fetuses using surgical scissors. Place them into a new petri dish with HBSS.
Remove the skin and skull and take out the brains using sterilized dissecting forceps. Put them into a petri dish with HBSS. NOTE: From this step on, it is best to conduct the procedure on a clean bench.
Remove the meninges using sterilized dissecting forceps under a stereo microscope. Cut off the basal ganglia, hippocampus, and cerebellum using forceps. Using curved-tip forceps, transfer the cortices to a new petri dish with HBSS.
Collect all cortices using a disposable dropper or a 25 mL pipette. Put them into a 15 mL tube and then remove the HBSS by pipetting.
Add 3 mL of cerebral cortical enzyme solution to the tube containing the cortices. Put this tube into a water bath and incubate at 37 °C for 5 min. NOTE: Prepare a cerebral cortical culture enzyme consisting of Phosphate-Buffered Saline (PBS) with 0.25% trypsin and 1 mM EDTA. Sterilize with a 0.22 µm filter.
Add a further 3 mL of cerebral cortical enzyme solution into the tube containing the cortices and incubate at 37 °C in a water bath for an additional 5 min.
Remove the cerebral cortical enzyme solution using a 5 mL pipette and add 5 mL of Dulbecco's Modified Eagle Medium (DMEM) containing 10% Fetal Bovine Serum (FBS).
Dissociate the cortices by gently pipetting up and down using a 5 mL pipette with a sterile P1000 tip. Pipette again using a 5 mL pipette with a P200 tip.
Put a 40 µm nylon cell strainer on a 50 mL tube and filter the suspension to remove the debris.
Centrifuge at 420 x g for 5 min at RT and then remove the DMEM medium.
Add 5 mL of cerebral cortical culture medium and gently resuspend the cell pellet. NOTE: Prepare a cerebral cortical culture medium consisting of neuron basal medium supplemented with 1x B-27 supplements, 2 mM L-glutamine, and 100 U/mL penicillin streptomycin.
Count the cells using a hemocytometer and adjust the cell concentration (as desired) using the cerebral cortical culture medium.
Plate 3.0 × 106 cortical neurons in 2.0 mL per coated 35 mm glass-bottomed dishes. NOTE: Before plating, coat the 35mmm glass-bottomed dishes with 0.1 mg/mL Poly-D-Lysine hydrobromide (PDL) or 0.01% Poly-L-Ornithine (PLO). Incubate for 3 h – O/N at 37 °C and wash with PBS at least 3x.
2. Imaging Vesicle Motility
Prepare the plasmids that will label each type of vesicle. For instance, use EGFP-Rab5 for early endosomes, EGFP-Rab7 for late endosomes, LAMP-EGFP for lysosomes, and EGFP-LC3 for autophagosomes8,13. NOTE: These plasmids are available on request from tsuruta.fuminori.fn@u.tsukuba.ac.jp. Typically,3 -5 days in vitro (DIV) neurons can be transfected with these plasmids using transfection reagents.
Mix 4.0 µg of plasmids with 200 µL of serum-free medium by pipetting. In a separate tube, dilute 8 µL of transfection reagent (see Table of Materials) in 200 µL of serum-free medium. Incubate for 5 min at RT.
Mix the plasmid solution and transfection regent solution in step 2.2 by pipetting. Incubate for 20 min at RT.
Add serum-free medium and 400 µL of the plasmid solution from step 2.3 to each coated glass-bottom dish. Incubate the neurons at 37 °C in a 5% CO2 incubator for 30 min. Replace the medium with 2 mL of cerebral cortical culture medium. Incubate the neurons at 37 °C in a 5% CO2 incubator for 1-2 days.
After 1 - 2 d of transfection, select the transfected cells for image acquisition. NOTE: Premature neurons less than 7 DIV have a tendency to exhibit dynamic vesicle motility. Select neurons that moderately express the fluorescence probe because clear images cannot be obtained when a highly expressing neuron is selected. Also, select a typical neuronal shape, such as pyramidal neurons, which have long axons and intricate dendrites, for ease of identification of the axon and dendrites (see Figure 1A).
Image acquisition using a time-lapse imaging system: For imaging, use a fluorescence microscope equipped with a Charge-Coupled Device (CCD) camera with 40X Plan Apo 0.95NA or 100X Plan Apo VC NA1.4 objective lenses. Control the temperature at 37 °C using an incubation system. Acquire neuron images at one frame/5 s over a 100 s period controlled by the image acquisition software. Alternatively, acquire images at shorter intervals and for longer time periods, such as at one frame per 2 s over a 300-s period. NOTE: A variety of applications are available for taking sequential images, many of which would be suitable for this method. Therefore, no particular application is recommended; rather, each researcher should use whatever application is available.
3. Image Analysis
Open all sequential images in ImageJ software with Manual Tracking14. NOTE: To date, Manual Tracking has been widely used in tracking experiments15,16,17,18,19. Manual Tracking was developed by Dr. Fabrice Cordelières. Detailed written instructions are available online20. For the analysis, the quality of the imaging data is improved with a sufficient number of images. It is recommended to use more than 20 images.
To combine the sequential images, choose <Image> → <Stacks> → <Images to Stack>. Click <OK>.
Choose <Plugins> → <Manual Tracking>; a tracking window will pop up.
Click on the checkbox of <Show Parameters?> and define the tracking parameters in the parameters section. NOTE: Several parameters can be set, including time interval, x/y calibration, z calibration, search square size for centering, dot size, line width, and font size. In this simplified assay, both time interval and x/y calibration were defined. Under other experimental circumstances, it may be necessary to define other parameters as well. The parameters used here are as follows: the <Time interval> is 5 s, and the <x/y calibration> is either 0.26642 µm for a 40X Plan Apo 0.95NA objective lens or 0.10657 µm for a 100X Plan Apo VC NA1.4 objective lens.
After the parameters are defined, click on <add Track> to start a new track.
After determining the vesicles of interest (e.g., the vesicles indicated by blue and red arrowheads in Figure 1A (right panel)), click on the center of the signals in the sequential images to record the xy coordinates. The results of the recorded xy coordinates, distance, and velocity show up in a new window. NOTE: In the case of lysosomes, large bright fluorescent signals (e.g., Figure 1A (right panel), orange arrowhead) are often observed in neurons. As these signals occasionally exhibit either accumulated vesicles or pathological varicosities, these signals should be avoided for quantifying motility.
Repeat step 3.6 to collect the xy coordinates of vesicles from all sequential images; each image automatically advances to the successive image after the reference point is selected.
Export these data to a new table (e.g., a spreadsheet). Use an appropriate software to create a suitable graph (see Table of Materials). NOTE: To analyze the data, select the <Distance> column, sum up all values of this column, and show a motility distance as a bar graph, such as in Figure 1B. Additionally, repeat this step, calculate the average of the total motility distance, and show as a bar graph, as in Figure 1C. The software to create a bar graph and waveform data are shown in the Table of Materials.
Representative Results
This assay was designed to measure vesicle dynamics in in vitro culture. This assay was utilized to determine the vesicle motility associated with neuronal morphology and survival. Figure 1A and B display representative data showing lysosome motility in neurons. Cortical neurons were transfected with the lysosome marker, LAMP-EGFP, and observed using a standard fluorescence microscope. It has been previously reported that specific endosome-lysosome vesicles are highly mobile, even in resting neurons8. Indeed, these results demonstrate both resting and moving vesicles in the same dendrite (Figure 1A and B). Normally, resting vesicles exhibit vibratory movement, like Brownian motion, in neuronal dendrites. In contrast, moving vesicles exhibit irregular motility. We speculate that each vesicle has a different compositional feature that underlies the determination of function, even in lysosome vesicles8. Thus, the classification of vesicles using this approach may provide useful information. Using this system, it is easy to quantify the average motility of these vesicles (Figure 1C) and to analyze the vesicle properties using this system in a time-effective manner.
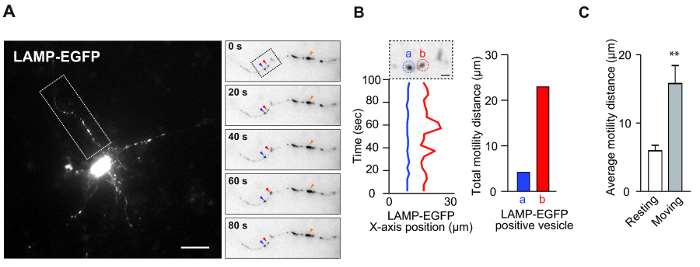 Figure 1: Lysosome Motility in Neuronal Dendrites. (A) Mouse cortical neurons (4 DIV) were transfected with LAMP-EGFP plasmid for 2 days. This image shows the motility of LAMP-EGFP-containing vesicles in the dendrites. The inset shows the time-lapse image of the LAMP-EGFP-positive vesicles. The red arrowhead indicates a moving vesicle, and the blue arrowhead indicates a resting vesicle. The orange arrowheads indicate larger fluorescent signals, as referred to above. Scale bar = 20 µm. (B) The time-dependent x-axis position of the LAMP-EGFP-positive vesicles from Figure A. The vesicle in the red circle labeled (b) is moving, and the vesicle in the blue circle labeled (a) is resting. Scale bar: 5 µm (left). The bar graph indicates the total distance of LAMP-EGFP movement (right). (C) The bar graph indicates the average motility distance of LAMP-EGFP-containing vesicles. (n = 5, mean ±standard error of the mean [SEM], **p <0.01 by Student's t-test). Please click here to view a larger version of this figure.
Figure 1: Lysosome Motility in Neuronal Dendrites. (A) Mouse cortical neurons (4 DIV) were transfected with LAMP-EGFP plasmid for 2 days. This image shows the motility of LAMP-EGFP-containing vesicles in the dendrites. The inset shows the time-lapse image of the LAMP-EGFP-positive vesicles. The red arrowhead indicates a moving vesicle, and the blue arrowhead indicates a resting vesicle. The orange arrowheads indicate larger fluorescent signals, as referred to above. Scale bar = 20 µm. (B) The time-dependent x-axis position of the LAMP-EGFP-positive vesicles from Figure A. The vesicle in the red circle labeled (b) is moving, and the vesicle in the blue circle labeled (a) is resting. Scale bar: 5 µm (left). The bar graph indicates the total distance of LAMP-EGFP movement (right). (C) The bar graph indicates the average motility distance of LAMP-EGFP-containing vesicles. (n = 5, mean ±standard error of the mean [SEM], **p <0.01 by Student's t-test). Please click here to view a larger version of this figure.
Discussion
This protocol introduces the procedure for quantifying vesicle motility. In primary neurons, endosomes and lysosomes tend to show high motility in younger neurons (4-6 DIV). Given that neurons must deliver some components to the leading edges to elongate neural processes, membrane trafficking should occur dynamically during this stage. Thus, it is important to use younger neurons to observe dynamic motility in neuronal dendrites. In addition, larger vesicles do not tend to exhibit high motility, even in younger neurons. The regulation of vesicle size is likely involved in the frequency of fusion-fission steps. It may be possible that specific vesicles that do not move dynamically easily accept other vesicles easily and terminate their movements. Thus, selecting vesicles is an important process for this analysis.
Several free plug-in software packages are available for tracking objects21. For instance, the TrackMate package can track objects22 and can be modified by numerical software, providing a range of mathematical information. On the other hand, it is slightly complicated to use for the relatively simple image analysis in this protocol. Mtrack2 is another plug-in and is based on the MultiTracker plug-in23. Mtrack2 is also useful because it can identify targets and then determine which targets in following frames are the closest. This approach is useful for quantifying enormous volumes of data; however, manual tracking may be more reliable for smaller volumes of image data. Therefore, we recommend Manual Tracking for quantifying smaller sets of data and acknowledge that it may be better to use other software to monitor larger volumes.
Several precautions should be taken in this approach. First, the sampling rate should be high in order to obtain strict data. This is because some vesicles exhibit rapid movement and abrupt changes, such as fusion-fission, and appearance-disappearance events are occasionally observed, indicating that frequent imaging is crucial to avoid miss the sign of any changes. Another point is that the use of high-end microscopes, such as scanning-disk confocal microscopes and total internal reflection fluorescence microscopes, may be key to obtaining high-resolution, interesting data. It is recommended to take images at shorter intervals using a high-end microscope, although here, images were taken every 5 s due to equipment limitations.
Substantial studies have suggested a biological link between membrane trafficking and neuronal disorders2. For example, it has been demonstrated that lysosomal defects are involved in severe neuronal disorders, such as Niemann-Pick disease and Gaucher disease24. Intriguingly, several studies have reported that lysosomal movement and localization are involved in the onset of lysosomal storage disorder6. It is possible that vesicle motility can be used as a biomarker for these disorders. One interesting approach may arise from the availability of human induced Pluripotent Stem Cells (iPSCs). Investigating vesicle motility using iPSCs-derived neurons may reveal a biomarker for lysosomal storage disorders. This approach provides some clues to the processes that underlie the risk of disease.
Since this assay is simple, it is easy to obtain motility data. Also, it is applicable to the analysis of other cellular events, including migration and morphological changes. It is important to note that this approach has several limitations due to its simplicity. As such, it is necessary to employ other approaches, including electrophysiology, biochemistry, and electron microscope analysis, to understand the precise physiological relevance of neuronal trafficking. Nevertheless, this approach is a valuable method that is suitable for researchers who want to analyze trafficking in a straightforward and time-effective manner.
Disclosures
The authors have nothing to disclose.
Acknowledgments
We thank Dr. Ricardo Dolmetsch (Stanford University School of Medicine; present affiliation: Novartis Institutes for Biomedical Research) for helping to develop this analysis and Dr. Matthew Wood, Takuma Aihara, and Dongsook Kim for their critical reading of the manuscript.
References
- Nicot AS, Laporte J. Endosomal phosphoinositides and human diseases. Traffic. 2008;9(8):1240–1249. doi: 10.1111/j.1600-0854.2008.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis MA, Luini A. Mendelian disorders of membrane trafficking. N Engl J Med. 2011;365(10):927–938. doi: 10.1056/NEJMra0910494. [DOI] [PubMed] [Google Scholar]
- Lebrand C. Late endosome motility depends on lipids via the small GTPase Rab7. EMBO J. 2002;21(6):1289–1300. doi: 10.1093/emboj/21.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CS, Bach G, Pagano RE. Abnormal transport along the lysosomal pathway in mucolipidosis, type IV disease. Proc Natl Acad Sci U S A. 1998;95(11):6373–6378. doi: 10.1073/pnas.95.11.6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal B. Neurologic, gastric, and opthalmologic pathologies in a murine model of mucolipidosis type IV. Am J Hum Genet. 2007;81(5):1070–1083. doi: 10.1086/521954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. A molecular mechanism to regulate lysosome motility for lysosome positioning and tubulation. Nat Cell Biol. 2016;18(4):404–417. doi: 10.1038/ncb3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruta F, Green EM, Rousset M, Dolmetsch RE. PIKfyve regulates CaV1.2 degradation and prevents excitotoxic cell death. J Cell Biol. 2009;187(2):279–294. doi: 10.1083/jcb.200903028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruta F, Dolmetsch RE. PIKfyve mediates the motility of late endosomes and lysosomes in neuronal dendrites. Neurosci Lett. 2015;605:18–23. doi: 10.1016/j.neulet.2015.07.021. [DOI] [PubMed] [Google Scholar]
- McCartney AJ, Zhang Y, Weisman LS. Phosphatidylinositol 3,5-bisphosphate: low abundance, high significance. Bioessays. 2014;36(1):52–64. doi: 10.1002/bies.201300012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shisheva A, Sbrissa D, Ikonomov O. Plentiful PtdIns5P from scanty PtdIns(3,5)P2 or from ample PtdIns? PIKfyve-dependent models: Evidence and speculation (response to: DOI 10.1002/bies.201300012) Bioessays. 2015;37(3):267–277. doi: 10.1002/bies.201400129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow CY. Mutation of FIG4 causes neurodegeneration in the pale tremor mouse and patients with CMT4J. Nature. 2007;448(7149):68–72. doi: 10.1038/nature05876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Loss of Vac14, a regulator of the signaling lipid phosphatidylinositol 3,5-bisphosphate, results in neurodegeneration in mice. Proc Natl Acad Sci U S A. 2007;104(44):17518–17523. doi: 10.1073/pnas.0702275104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15(3):1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordelières FP. Manual Tracking, a plug-in for ImageJ software [Internet] 2017. Available from: https://imagej.nih.gov/ij/plugins/track/track.html.
- Hu W. Exopolysaccharide-independent social motility of Myxococcus xanthus. PLoS One. 2011;6(1):e16102. doi: 10.1371/journal.pone.0016102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Z. Characterization of four type IV pilin homologues in Stigmatella aurantiaca DSM17044 by heterologous expression in Myxococcus xanthus. PLoS One. 2013;8(9):e75105. doi: 10.1371/journal.pone.0075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S. A genetic variant of cortactin linked to acute lung injury impairs lamellipodia dynamics and endothelial wound healing. Am J Physiol Lung Cell Mol Physiol. 2015;309(9):L983–L994. doi: 10.1152/ajplung.00062.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahirel M. Movement propensity and ability correlate with ecological specialization in European land snails: comparative analysis of a dispersal syndrome. J Anim Ecol. 2015;84(1):228–238. doi: 10.1111/1365-2656.12276. [DOI] [PubMed] [Google Scholar]
- Hu W. Interplay between type IV pili activity and exopolysaccharides secretion controls motility patterns in single cells of Myxococcus xanthus. Sci Rep. 2016;6:17790. doi: 10.1038/srep17790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordelières FP. Manual Tracking [Internet] 2017. Available from: https://imagej.nih.gov/ij/plugins/track/Manual Tracking plugin.pdf.
- ImageJ Tracking plug-in [Internet] 2017. Available from: https://imagej.net/Category:Tracking.
- Tinevez JY. TrackMate: An open and extensible platform for single-particle tracking. Methods. 2016. [DOI] [PubMed]
- Ekvall MT. Three-dimensional tracking of small aquatic organisms using fluorescent nanoparticles. PLoS One. 2013;8(11):e78498. doi: 10.1371/journal.pone.0078498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyakumar M, Dwek RA, Butters TD, Platt FM. Storage solutions: treating lysosomal disorders of the brain. Nat Rev Neurosci. 2005;6(9):713–725. doi: 10.1038/nrn1725. [DOI] [PubMed] [Google Scholar]


