Abstract
There is relatively limited data on outcomes of screening older adults for cancer; therefore, the decision to screen older adults requires balancing the potential harms of screening and follow-up diagnostic tests with the possibility of benefit. Harms of screening can be amplified in older and frail adults, and include discomfort from undergoing the test itself, anxiety, potential complications from diagnostic procedures resulting from a false-positive test, false reassurance from a false-negative test, and overdiagnosis of tumors that are of no threat and may result in overtreatment. In this paper, we review the evidence and guidelines on breast, colorectal, lung and prostate cancer as applied to older adults. We also provide a general framework for approaching cancer screening in older adults by incorporating evidence-based guidelines, patient preferences, and patient life expectancy estimates into shared screening decisions.
Keywords: Older adults, Aging, Geriatrics, Life expectancy, Cancer Screening
Introduction
Cancer screening is often considered a standard of preventive medical care; however, the decision to screen is less straight forward in older adults. The beneficial effects are less clear since randomized controlled trials of screening rarely include older age groups.1 Meanwhile, the harms of screening may be amplified in older and more frail adults.2 Screening in those with comorbid illness, poor functional status, or with short life expectancy may lead to overdiagnosis and treatment of cancers that otherwise would not have caused symptoms during a patient’s lifetime.3, 4 Cognitive impairment or poor education can create barriers to informed consent and distress with the subsequent “diagnostic cascade” after a positive test.5 Moreover, screening may distract from discussions on issues with more evidence of benefit such as reducing polypharmacy, healthy behavior counseling, or fall prevention.1, 2 On the other hand, there is no evidence that the benefits of screening stop at a particular age.4 To the contrary, screening detects cancer more frequently and at more advanced stages at older ages, and older adults may benefit from early treatment of localized cancer compared with advanced stages.4 Consequently, guidelines strictly based on age may underemphasize the beneficial effects of screening in a healthy 80 year old, and overemphasize these effects in an unhealthy 60 year old. Many older adults may also have strong preferences to continue screening despite risks.6–8 In summary, an ongoing challenge in deciding to screen older adults is how to balance the long-term benefits of these interventions with significant, often short-term, harms.
High quality cancer screening decisions for older adults requires individualizing the benefits and risks of these tests. Ideally, cancer screening decisions for older adults would consider their health, life expectancy, cognition, risk of disease and preferences.3, 9, 10 Simple and objective tools to estimate life expectancy that include patient age, functional status, and comorbidities are available in published literature and online (see: eprognosis.ucsf.edu).11–13 Clinicians may use such tools to determine if a patient has a lower life expectancy than the estimated lag-time to benefit from a screening intervention and therefore should not be screened.14 The lag-time to benefitting from a screening test is derived from randomized controlled trial data and represents the amount of time between when participants received a screening test and when a mortality reduction was seen for at least one in 1000 screening arm participants. Using this approach, the lag-time to benefitting from mammography and colorectal cancer screening is estimated to be approximately 10 years.15 Therefore, adults with <10 years are unlikely to live longer as a result of undergoing screening.16 Similarly, on average adults with dementia have <10 year life expectancy and would be unlikely to live longer as a result of being screened. Life expectancy estimates combined with personalized cancer risk profiles may be used to help individualize the benefits and risks of different cancer screening tests for older adults.
In this article we review national guidelines on screening older adults for colorectal, breast, prostate, and lung cancer and summarize data on the benefits and harms of screening older adults for these cancers. We also discuss approaches for discussing stopping cancer screening with older adults when appropriate, and areas of needed research.
Colorectal cancer screening
Colorectal cancer (CRC) is the third most common cancer in adults over age 70 and the second leading cause of cancer death in older adults.17 The prevalence of adenomatous polyps increases with age from 20–25% at age 50 to nearly 50% by ages 75–80,18, 19 with 1–10% of these polyps progressing to cancer in 5–10 years.20, 21 A majority of cases of CRC at older ages occur in the proximal colon or rectum.22 Several tests are available for CRC screening including fecal occult blood testing (FOBT), immunochemical-based fecal occult blood testing (FIT), fecal DNA testing, sigmoidoscopy, colonoscopy, or CT colonography with guidelines detailed in Table 1.23–26
Table 1.
Selected guidelines on cancer screening for average risk adults
| Test(s) | Guideline | Year | Recommendations | |
|---|---|---|---|---|
| Breast Cancer | Mammography | USPSTF [68] | 2016 |
|
| ACS [69] | 2015 |
|
||
| Prostate Cancer | Prostate-specific antigen test | USPSTF [44] | 2012 |
|
| ACS [45] | 2010 |
|
||
| AUA [46] | 2013 |
|
||
| Colorectal Cancer | FOBT, FIT, stool DNA, Sigmoidoscopy, CT Colonography, Colonoscopy | USPSTF [23] | 2016 |
|
| ACS, US Multisociety Task Force on Colorectal Cancer, ACR [24] | 2008 |
|
||
| ACG [25] | 2008 |
|
||
| ACP [26] | 2015 |
|
||
| Lung Cancer | Low-dose computed tomography | USPSTF [91] | 2014 |
|
| ACS [110] | 2013 |
|
||
| All cancers | AGS - Choosing Wisely [9] | 2013 |
|
|
| SGIM - Choosing Wisely [10] | 2013 |
|
Abbreviations: FOBT – fecal occult blood test, FIT – immunochemical-based fecal occult blood testing, USPSTF – United States Preventive Services Task Force, ACS – American Cancer Society, AUA – American Urological Association, ACG – American College of Gastroenterology, ACR – American College of Radiology, ACP – American College of Physicians, AGS – American Geriatrics Society, SGIM – Society of General Internal Medicine.
USPSTF Grades: A – Service recommended, high certainty that net benefit is substantial, B – Service recommended, high certainty that the net benefit is moderate, C – service recommended to selected patients based on professional judgement and patient preferences, moderate certainty that net benefit is small, D – recommends against service, moderate or high certainty that the service has no net benefit or that harms outweigh benefits, I – current evidence is insufficient to assess the balance of benefits and harms of the service.
Four trials of FOBT screening found a mortality benefit in older adults and included a combined 50,144 adults aged 70–80 years old.27 Three trials examining biennial FOBTs were conducted in Europe; an RCT in the UK had 3–6 rounds of screening with 28 years of follow-up,28, 29 an RCT in Denmark had 9 rounds of screening with 17 years of follow-up,30, 31 and a county-level trial in France had 6 rounds of screening with 11 years of follow-up.32 These studies found an 11–16% reduction in CRC-specific mortality with similar risk reductions in older adults.29, 31, 32 One RCT in the US examined annual FOBTs for 11 rounds or biennial FOBTs for 6 rounds and followed participants for 30 years.33–35 It found a reduction in CRC mortality at 30 years of 32% and 22% for those screened annually and biennially, respectively, with an overall 53% reduction among adults ≥70 years.35 The estimated number needed to screen (NNS) to prevent one CRC death through FOBT for average health men and women aged 75–79 years old is 525 and 408, respectively.16 Although the test is initially the least invasive, false-positive results are common; 86–98% of trial participants had a negative colonoscopy after a positive FOBT.4 Before screening older adults with FOBT or FIT it is therefore important to discuss false positives and whether patients are willing to consider the risks of undergoing colonoscopy.
The majority of sigmoidoscopy RCTs did not include adults >70 years old,27 but the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial which had a median follow-up of 12 years included 20,726 individuals ≥70 years old. Individuals 65–74 years old had 20% reduced CRC incidence and 35% reduced CRC mortality when screened every 3–5 years.36 The invited NNS to prevent 1 case of CRC was 282 from this trial.36 Sigmoidoscopy requires less bowel preparation than colonoscopy and the procedure can be done without sedation in the office; but technical challenges with achieving adequate depth occur more frequently at older ages. Perforation (0.1 per 1000 sigmoidoscopies) is an important harm of sigmoidoscopy.27
There are no published RCTs of colonoscopy, but one large prospective cohort showed that adults ≥75 years had a 50% reduction in rates of incident CRC diagnosis in both the proximal and distal colon if >5 years since the last endoscopy and 63% reduction if <5 years from last endoscopy.37 Age-based NNS estimations to prevent one CRC death with colonoscopy for men and women 75–79 years old of average health are 126 and 98, respectively.16 Colonoscopies are the definitive test for detection of adenomas and CRC, and have been shown to be cost-effective into older age groups because of the higher diagnostic yield.38 However, rates of procedure-related risks can increase with age and include perforation (0.4 in 1000), post-polypectomy bleeding (0.8 in 1000), cardiac events (10 in 1000) and death.15, 27, 39 Challenges with bowel prep in older adults are common and include dizziness, abdominal pain, incontinence, and nausea, and individuals can have challenges with sedation post-procedure.40
Guidelines recommend discontinuing CRC screening in adults with <10 year life expectancy due to the lag-time to benefit.15 However, in 2010 an estimated 51% of adults >75 years with life expectancies <10 years reported being screened.40 This can lead to risks from unnecessary testing as well as overdiagnosis. On autopsy studies of older adults, 10–33% are incidentally found to have colonic polyps and 2–3% have CRC suggesting the more we look the more we will find, but that we will not always improve patient quality or quantity of life.4 Therefore particular attention should be focused on exploring patient preferences and educating on risks and benefits to minimize exposure to unnecessary testing. Decision aids are effective at improving knowledge and reducing decisional conflict,41 with one tailored to CRC screening in older adults.42
Prostate Cancer Screening
Prostate cancer is the most common cancer affecting men over age 70, for whom it affects approximately 1 in 10 men and is the second leading cause of cancer related deaths.17 The rate of intermediate- and high-risk prostate cancer increases substantially with age, with one study estimating 33% of men >80 years old with prostate cancer having high-risk disease compared with 6% of men <55 years old.43 Prostate cancer screening is done through an annual or biennial Prostate Specific Antigen (PSA) test, and most organizations no longer recommend routine use of a digital rectal exam (DRE) for screening. Screening guidelines are detailed in Table 1.44–46
The evidence for benefit from PSA screening was examined in two major trials. The US PLCO trial included 76,685 men aged 55–74 years old, with approximately 10,000 men over age 70.47 Men in the intervention arm underwent annual PSA screens for 6 years and DREs for 4 years. However, there were extremely high rates of contamination in the controls where an estimated 80% of controls underwent at least one PSA test during the trial compared to 85% compliance in the intervention arm.48 This may account for the lack of a prostate cancer mortality reduction even at 15 years of follow-up.49 Post-randomization analysis by comorbidities showed a significant decrease in prostate cancer-specific mortality in men with minimal or no comorbidities with a NNS of 723, although the subgroup was significantly younger on average than trial participants.50 The European Randomized Study of Screening for Prostate Cancer (ERSPC) trial randomized men 50–74 years to PSA screening every 2–4 years and the control group received no screening.51 Results indicated an overall 20% reduction in prostate cancer-specific mortality with a NNS of 1,410 and NNT of 48, which was sustained at 13 years follow-up.52 Benefits of screening were restricted to men 55–69 years at randomization. In summary, trials on PSA screening indicate minimal evidence of mortality benefit in older adults.
A number of harms have been described from PSA screening. False positives occur at a rate of 30–40% and may lead to psychological distress and unneeded prostate biopsies.53 Prostate biopsies are associated with several short-term risks, including anxiety, moderate to severe pain (7%), moderate to severe hematuria (6%), infection requiring hospitalization (0.4–1.3%), and hospitalizations in general (7%).54–56 Overdiagnosis also represents a significant harm since a substantial percentage of prostate cancers detected are slow growing and may have remained asymptomatic during the patient’s lifetime; in the ERSPC and PLCO trials, it is estimated that 40–60% of screen-detected cancers were cases of overdiagnosis.57, 58 Overdiagnosis can lead to psychological distress and unnecessary treatments with resultant adverse effects including bowel dysfunction, urinary incontinence, erectile dysfunction, and premature death, particularly in older men with poor functional status.59
USPSTF guidelines changed in 2008 to recommending against screening for men >75 years old, then again in 2012 to recommending against screening for all men.60 USPSTF guidelines are set to change again in 2017, with draft guidelines suggesting men 55–69 years old make an individualized decision on screening after discussion with a clinician and men >70 years old not be screened.44 Prior to 2008, overall 50–60% of men >65 were screened in 2005, including 30–50% of men with <10 year life expectancy,61–63 and rates remained stable in 2010.63 After the 2012 guideline change, men >65 years old reported reduced screening rates of 37–44% in 2013, and among men >75 years with life expectancies <10 years, 32% reported being screened.64 The impact of guideline changes on prostate cancer mortality in older men remains to be seen. Nevertheless, rates of inappropriate screening highlight the continued importance of shared decision making. Decision aids are available to help with this process and have been shown to facilitate discussions and often reduce men’s interest in PSA screening.65, 66
Breast Cancer
Breast cancer is the most common life-threatening cancer to occur among women and is the second leading cause of cancer death for US women.17 While 31% of breast cancer diagnoses occur in women ≥70 years, 47% of breast cancer deaths occur among women ≥70 years.67 In addition to age, postmenopausal hormone therapy use, family history of breast cancer, history of a benign breast biopsy, age at menopause, age at first birth/parity, obesity, alcohol and cigarette use are risk factors for late-life breast cancer.68 Biennial mammograms are recommended for women 55–74 years at average risk; guidelines are detailed in Table 1.69, 70
While mammography screening is estimated to reduce breast cancer mortality by 19% among women 40–69 years,71 it is not certain whether mammography screening reduces breast cancer mortality for women ≥70 years. None of the 8 RCTs of mammography screening included women ≥75 years and only one trial included women 70–74 years.72 A subgroup analysis of these women did not find a significant reduction in breast cancer mortality associated with screening.72 Due to the lack of clinical trial data, the USPSTF states that there is insufficient evidence on whether to screen women ≥75 years old.69 The American Cancer Society (ACS) recommends continuing mammography screening as long as women are in good health and their life expectancy in >10 years.70 Despite these recommendations, in 2010, 56% of US women ≥75 years reported being screened with mammography in the past 2 years, including 36% of women with ≤5 year life expectancy.73
In the absence of clinical trial data, the benefits of mammography screening must be estimated from simulation models which estimate 1–2 fewer breast cancer deaths per 1,000 women in their 70s who are screened biennially for 10 years.74, 75 Screening may also benefit older women by finding breast cancers at an earlier stage when they may be easier to treat.76, 77 In addition, the sensitivity of mammography screening increases with age leading to fewer false positive tests.78
However there are important harms to mammography screening including anxiety resulting from false positive tests, false reassurance from an erroneously negative test, overdiagnosis, and complications from work-up and/or treatment of cancer.75 Among women ≥75 years who undergo biennial screening the cumulative probability of a false-positive mammogram over 10 years ranges from 12–27%.75, 79, 80 While follow-up tests such as diagnostic mammograms and breast ultrasounds are generally low-risk procedures, approximately 10–20% of older women that experience a false positive mammogram undergo a benign breast biopsy75, 79, 80 which can be stressful and uncomfortable for older women.81 Overdiagnosis is a particularly concerning harm since the risks of breast cancer treatment increase as women age.82 Quantifying overdiagnosis, however, remains challenging and estimates vary from 0 to 50% of screen-detected breast cancers; however most estimates tend to average around 30%.74, 83–86 Overdiagnosis likely increases with age since older women tend to have more indolent tumors and more competing mortality risks.82
Ideally, older women would consider their risk of breast cancer, life expectancy, and their preferences when deciding whether or not to continue mammography screening. A peer-reviewed decision aid is available by request from the author to help women ≥75 years old decide whether or not to continue being screened.87 As for the clinical breast examination (CBEs), no trials have compared CBE alone to no screening. The USPSTF states that there is insufficient evidence to recommend for or against CBEs and the ACS does not recommend CBEs for women at average risk for breast cancer.
Lung Cancer
Lung cancer is the second most common cancer and the leading cause of cancer death in the US,88 accounting for 1 in 4 cancer deaths.88 Risk of lung cancer increases with age and tobacco use;89 85% of lung cancers are due to smoking, and 66% are diagnosed among adults ≥65 years.88, 90, 91 Since tumor size and stage are strongly related to lung cancer survival there is strong interest in strategies that may detect lung cancer early, with guidelines detailed in Table 1.92, 93
The PLCO trial examined the effectiveness of 4 annual chest x-rays for lung cancer screening among 154,942 adults 55–74 years. After 13 years follow-up there was no significant difference in lung cancer incidence rates or mortality among those who were screened compared to those who received usual care.94, 95 Therefore, several small trials in Europe and one large trial in the US, the National Lung Cancer Screening Trial (NLST), have examined the efficacy of low-dose computed tomography (LDCT) for lung cancer screening (LCS) instead.
The NLST was a well-designed RCT involving 53,454 participants age 55–74 years with a history of at least 30 pack years of smoking who were current smokers or had quit in the past 15 years. Participants were randomized to receive either LDCT or chest x-ray annually for 3 screening rounds. The trial found LDCT was associated with a 16% relative reduction in lung cancer mortality after 6.5 years or an absolute reduction of 0.3% from 21 to 18 lung cancer deaths per 1000 participants compared to those receiving chest x-rays.96 In addition, LDCT was associated with a 6.7% reduction in all-cause mortality. The NNS to prevent one death from lung cancer was 320 (245 in adults 65–74 years compared to 364 in adults 55–64 years).97 The NNS to prevent a death overall was 219 over 6.5 years. LDCT was found to have a sensitivity of 93.8% and a specificity of 73.4%. The smaller European trials found no benefit of LDCT screening but they were not adequately powered.98 The overall average effective radiation dose used in the NLST was 1.5 millisievert (mSV) compared with 7 mSV for a standard-dose diagnostic chest CT examinations.
Despite the benefits of LCS with LDCT, LCS screening may also cause harm. Risks include false positive and false negative results,99 anxiety, unnecessary testing, radiation exposure, financial strain, and overdiagnosis (9–25% of screen-detected lung cancers are estimated to be cases of overdiagnosis).92, 97, 100–103 In the NLST, 39% of participants who had 3 annual LDCTs had at least one positive test and 96% of these results were false positives. The positive predictive value of a pulmonary nodule (≥4mm) was only 3.8%. About 2.5% of positive results in NLST require invasive diagnostic procedures (such as bronchoscopy). Complications related to diagnostic evaluation of positive results was low (1.4% in LDCT group). However, complications may be higher at less equipped medical centers.89, 104, 105 Of note, false positive rates in NLST may have been lower if the more current LDCT screening and reporting and data system standards were used.106
In NLST, no difference in mortality benefit was found for adults ≥65 years compared to those <65 years; however, only 27% of NLST participants were ≥65 years (only 10% were ≥70 years).107 An analysis comparing adults 65–74 to those 55–64 years in NLST found that adults 65–74 years had a higher prevalence of lung cancer detected but also had a higher rate of false positive results and of invasive procedures after false positive results which is concerning since older adults have higher complication rates from biopsy of pulmonary nodules and higher postoperative mortality from resection of nodules.108–110
Based on the NLST, the USPSTF and ACS recommend annual LCS with LDCT in adults 55–74 years (up to age 80 in USPSTF guidelines) who have a 30 pack-year smoking history and currently smoke or have quit within the past 15 years.92, 111 Screening is not recommended for adults with severe comorbidities or short life expectancy for whom curative surgery would not be appropriate. A prediction model for in-hospital death following thoracic surgery is available.112, 113 In addition, the USPSTF recommends smoking cessation counseling delivered with LCS,92 since quitting smoking is the most effective intervention to reduce lung cancer risk.114 Following the USPSTF’s recommendations, in 2015, Medicare began covering lung cancer screening with LDCT for adults 55–74 years who meet USPSTF criteria and have engaged in shared decision-making with their clinicians.115 Shared decision making around lung cancer screening is reimbursed annually but not required after the initial LDCT screening.
LDCT may be most beneficial to adults at high risk of lung cancer who are not at high risk for competing causes of death. A tool is available to assess patient lung cancer risk.116–118 Decision support tools are also available to help educate adults about benefits and risks of LCS.119, 120 Strategies for implementing lung cancer screening are being tested; however, a recent VA trial found implementation of LCS challenging, resource intensive, and only 58% of eligible patients chose to participate.121
A General Approach to Cancer Screening in Older Adults
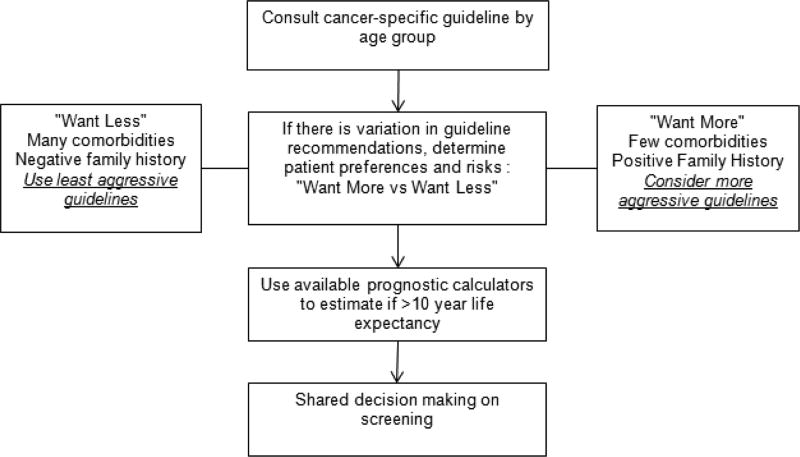
We present a general approach to integrating risks and benefits of cancer screening for older adults in shared decision making in Figure 1. We suggest clinicians start by consulting USPSTF and society guidelines to help decide whom to screen. When there is variation in guideline recommendations, patient preferences and risk factors should be included to determine which guidelines best reflect their current goals. For example, older adults who tend to be “maximizers” or prefer “more testing” despite the risks may be managed following more aggressive guidelines while those who tend to be “minimizers” can be managed following more conservative guidelines.122 Decision aids specific to particular screening modalities can be used to help elicit patient preferences.
Figure 1.
Approach to cancer screening in older adults
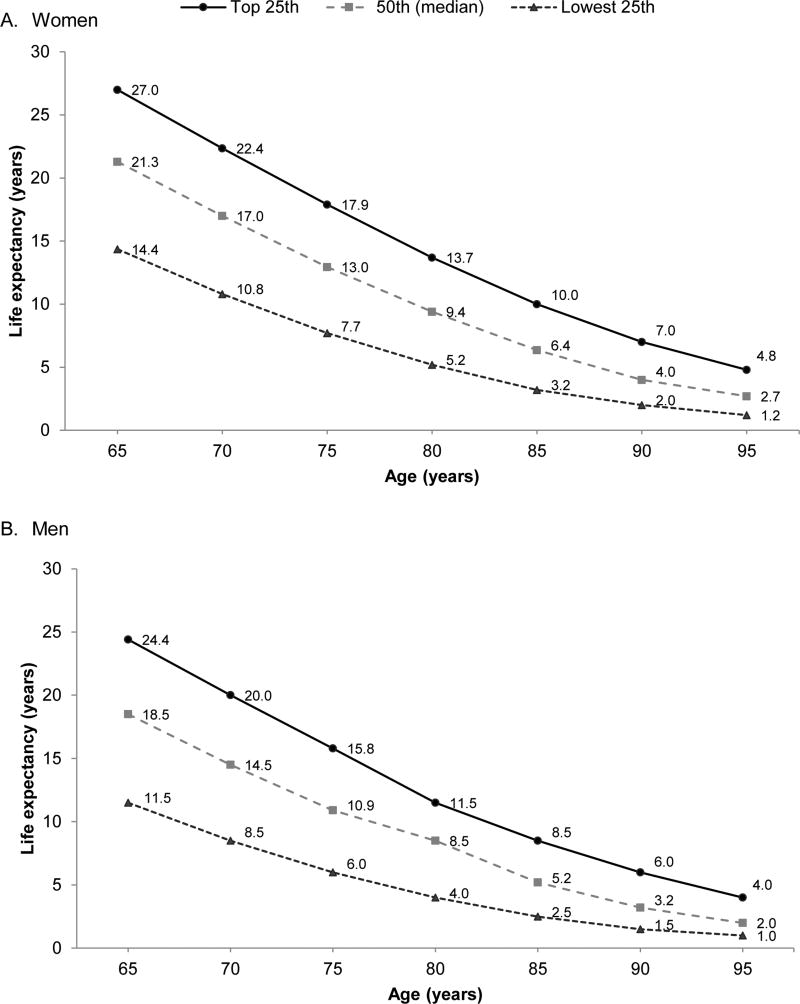
While guidelines recommend stopping cancer screening in older adults with <10 year life expectancy, prognostication can be difficult. Fortunately, several tools are available to help clinicians estimate 10-year life expectancy. The Lee-Schonberg index (available at www.ePrognosis.org) estimates whether adults have >50% risk of mortality within 10 years, considered to represent an estimated life expectancy <10 years.123 It considers factors such as age, sex, body mass index, function, comorbidities, smoking, number of hospitalizations in the past year, and perceived health.11–13 Walter and Covinsky, using data from 1997 US life tables (and updated using 2012 data in Figure 2), calculated the upper, middle and lower quartiles of life expectancy for US adults ≥65 years stratified by sex and age.3, 75, 124 Clinicians can approximate whether patients are in the top, middle, or lower quartile of health of their age group and match this to stratified estimates of average life expectancy.
Figure 2.
Upper, middle, and lower quartiles of life expectancy as estimated by 2012 US Life Tables
Figure adapted from Louise Walter and Mara Schonberg 2014 [74] using updated 2012 United States Life Table data [123].
Once a clinician estimates that his/her patient has <10 year life expectancy or that screening is not consistent with current clinical goals, it may be difficult to discuss stopping screening with older adults. As a guide, we recommend that clinicians initiate and re-initiate these discussions so that patients become aware of the need to decide when they want to stop undergoing cancer screening. It is important to discuss how the harms of cancer screening increase with rising age and worsening health while the benefits of screening become more uncertain. So that patients do not feel abandoned, it is important to encourage patients to utilize health promotion measures more likely to help them during their lifespan (e.g. exercise), and reassure older adults that it is still important to work-up concerning symptoms for cancer even if cancer screening is no longer beneficial.75
Questions/Future Work
While there are tools available to estimate older adults’ life expectancy, there is limited data on how these tools perform in clinical settings and how to incorporate patient prognosis into discussions on shared cancer screening decisions. Research is needed on how to implement existing decision aids successfully in clinical practice and on developing new decision aids for cancer screening. New technologies for cancer screening are continuously emerging, yet these technologies are often incorporated into clinical practice without knowledge of how they will impact older adults. Therefore, it is important to study the benefits and age-specific harms of new screening technologies among older adults before implementation. In addition, since overdiagnosis is a major harm of cancer screening in older adults, future studies should aim to both obtain better estimates of overdiagnosis and determine how overdiagnosis impacts quality of life in this population to better inform simulation models estimating the benefits and harms of cancer screening.
Conclusions
When discussing cancer screening with older adults, clinicians should indicate whether any data suggests screening tests improves older adults’ quality or quantity of life. Clinicians should also discuss the risks of screening, including discomfort from undergoing the test, anxiety, potential complications from diagnostic procedures resulting from a false-positive tests, and overdiagnosis of tumors that are of no threat and that may result in overtreatment. Furthermore, clinicians may want to explain that the frequency and negative impact of many risks, particularly overdiagnosis, can increase with age. Older adults should be asked how they view the potential benefits and harms of different screening tests, so that their values and preferences are considered in screening decisions.
Acknowledgments
Source of Funding:
Dr. Schonberg’s time was supported by an NCI R01 (CA181357) and an NIA R01 (AG041869).
Footnotes
Conflicts of Interest
No conflicts of interest were declared.
References
- 1.Eckstrom E, Feeny DH, Walter LC, Perdue LA, Whitlock EP. Individualizing cancer screening in older adults: a narrative review and framework for future research. Journal of general internal medicine. 2013;28(2):292–298. doi: 10.1007/s11606-012-2227-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clarfield AM. Screening in frail older people: an ounce of prevention or a pound of trouble? Journal of the American Geriatrics Society. 2010;58(10):2016–2021. doi: 10.1111/j.1532-5415.2010.03070.x. [DOI] [PubMed] [Google Scholar]
- 3.Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. Jama. 2001;285(21):2750–2756. doi: 10.1001/jama.285.21.2750. [DOI] [PubMed] [Google Scholar]
- 4.Walter LC, Lewis CL, Barton MB. Screening for colorectal, breast, and cervical cancer in the elderly: a review of the evidence. The American journal of medicine. 2005;118(10):1078–1086. doi: 10.1016/j.amjmed.2005.01.063. [DOI] [PubMed] [Google Scholar]
- 5.Sachs GA, Carter R, Holtz LR, et al. Cognitive impairment: an independent predictor of excess mortality: a cohort study. Annals of internal medicine. 2011;155(5):300–308. doi: 10.7326/0003-4819-155-5-201109060-00007. [DOI] [PubMed] [Google Scholar]
- 6.Lewis CL, Couper MP, Levin CA, Pignone MP, Zikmund-Fisher BJ. Plans to stop cancer screening tests among adults who recently considered screening. Journal of general internal medicine. 2010;25(8):859–864. doi: 10.1007/s11606-010-1346-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis CL, Kistler CE, Amick HR, et al. Older adults' attitudes about continuing cancer screening later in life: a pilot study interviewing residents of two continuing care communities. BMC geriatrics. 2006;6(1):1. doi: 10.1186/1471-2318-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torke AM, Schwartz PH, Holtz LR, Montz K, Sachs GA. Older adults and forgoing cancer screening:“I think it would be strange”. JAMA internal medicine. 2013;173(7):526–531. doi: 10.1001/jamainternmed.2013.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.AGSCW. American Geriatrics Society identifies another five things that healthcare providers and patients should question. J Am Geriatr. Soc. 2014;62(5):950–960. doi: 10.1111/jgs.12770. [DOI] [PubMed] [Google Scholar]
- 10.SGIM. Society of General Internal Medicine - Choosing Wisely. [Accessed April 5th 2017];2013 http://www.choosingwisely.org/societies/society-of-general-internal-medicine/
- 11.Schonberg MA, Davis RB, McCarthy EP, Marcantonio ER. External Validation of an Index to Predict Up to 9-Year Mortality of Community-Dwelling Adults Aged 65 and Older. Journal of the American Geriatrics Society. 2011;59(8):1444–1451. doi: 10.1111/j.1532-5415.2011.03523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schonberg MA, Davis RB, McCarthy EP, Marcantonio ER. Index to predict 5-year mortality of community-dwelling adults aged 65 and older using data from the National Health Interview Survey. Journal of general internal medicine. 2009;24(10):1115–1122. doi: 10.1007/s11606-009-1073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SJ, Lindquist K, Segal MR, Covinsky KE. Development and validation of a prognostic index for 4-year mortality in older adults. Jama. 2006;295(7):801–808. doi: 10.1001/jama.295.7.801. [DOI] [PubMed] [Google Scholar]
- 14.Lee SJ, Leipzig RM, Walter LC. Incorporating lag time to benefit into prevention decisions for older adults. Jama. 2013;310(24):2609–2610. doi: 10.1001/jama.2013.282612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SJ, Boscardin WJ, Stijacic-Cenzer I, Conell-Price J, O’Brien S, Walter LC. Time lag to benefit after screening for breast and colorectal cancer: meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. Bmj. 2013;346:e8441. doi: 10.1136/bmj.e8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ko CW, Sonnenberg A. Comparing risks and benefits of colorectal cancer screening in elderly patients. Gastroenterology. 2005;129(4):1163–1170. doi: 10.1053/j.gastro.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 17.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 18.Clipp EC, Carver EH, Pollak KI, et al. Age-related vulnerabilities of older adults with colon adenomas. Cancer. 2004;100(5):1085–1094. doi: 10.1002/cncr.20082. [DOI] [PubMed] [Google Scholar]
- 19.Winawer S, Shike M. Prevention and control of colorectal cancer. In: Greenwald P, Kramer BS, Weed D, editors. Cancer Prevention and Control. New York: Marcell-Dekker; 1995. pp. 537–560. [Google Scholar]
- 20.Brenner H, Hoffmeister M, Stegmaier C, Brenner G, Altenhofen L, Haug U. Risk of progression of advanced adenomas to colorectal cancer by age and sex: estimates based on 840 149 screening colonoscopies. Gut. 2007;56(11):1585–1589. doi: 10.1136/gut.2007.122739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.USPSTF. Screening for colorectal cancer: recommendation and rationale. Annals of internal medicine. 2002;137(2):129. doi: 10.7326/0003-4819-137-2-200207160-00014. [DOI] [PubMed] [Google Scholar]
- 22.Siegel R, DeSantis C, Jemal A. Colorectal cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64(2):104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 23.Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. Jama. 2016;315(23):2564–2575. doi: 10.1001/jama.2016.5989. [DOI] [PubMed] [Google Scholar]
- 24.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA: a cancer journal for clinicians. 2008;58(3):130–160. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 25.Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2008. The American journal of gastroenterology. 2009;104(3):739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 26.Qaseem A, Denberg TD, Hopkins RH, et al. Screening for colorectal cancer: a guidance statement from the American College of Physicians. Annals of internal medicine. 2012;156(5):378–386. doi: 10.7326/0003-4819-156-5-201203060-00010. [DOI] [PubMed] [Google Scholar]
- 27.Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. Jama. 2016;315(23):2576–2594. doi: 10.1001/jama.2016.3332. [DOI] [PubMed] [Google Scholar]
- 28.Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. The Lancet. 1996;348(9040):1472–1477. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 29.Scholefield J, Moss S, Mangham C, Whynes D, Hardcastle J. Nottingham trial of faecal occult blood testing for colorectal cancer: a 20-year follow-up. Gut. 2012;61:1036–1040. doi: 10.1136/gutjnl-2011-300774. [DOI] [PubMed] [Google Scholar]
- 30.Kronborg O, Fenger C, Olsen J, Jørgensen OD, Søndergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. The Lancet. 1996;348(9040):1467–1471. doi: 10.1016/S0140-6736(96)03430-7. [DOI] [PubMed] [Google Scholar]
- 31.Kronborg O, Jørgensen O, Fenger C, Rasmussen M. Randomized study of biennial screening with a faecal occult blood test: results after nine screening rounds. Scandinavian journal of gastroenterology. 2004;39(9):846–851. doi: 10.1080/00365520410003182. [DOI] [PubMed] [Google Scholar]
- 32.Faivre J, Dancourt V, Lejeune C, et al. Reduction in colorectal cancer mortality by fecal occult blood screening in a French controlled study. Gastroenterology. 2004;126(7):1674–1680. doi: 10.1053/j.gastro.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 33.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. New England Journal of Medicine. 1993;328(19):1365–1371. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 34.Mandel JS, Church TR, Bond JH, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. New England Journal of Medicine. 2000;343(22):1603–1607. doi: 10.1056/NEJM200011303432203. [DOI] [PubMed] [Google Scholar]
- 35.Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. New England Journal of Medicine. 2013;369(12):1106–1114. doi: 10.1056/NEJMoa1300720. [DOI] [PubMed] [Google Scholar]
- 36.Schoen RE, Pinsky PF, Weissfeld JL, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. New England Journal of Medicine. 2012;366(25):2345–2357. doi: 10.1056/NEJMoa1114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. New England Journal of Medicine. 2013;369(12):1095–1105. doi: 10.1056/NEJMoa1301969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sonnenberg A, Delcò F. Cost-effectiveness of a single colonoscopy in screening for colorectal cancer. Archives of internal medicine. 2002;162(2):163–168. doi: 10.1001/archinte.162.2.163. [DOI] [PubMed] [Google Scholar]
- 39.Warren JL, Klabunde CN, Mariotto AB, et al. Adverse events after outpatient colonoscopy in the Medicare population. Annals of internal medicine. 2009;150(12):849–857. doi: 10.7326/0003-4819-150-12-200906160-00008. [DOI] [PubMed] [Google Scholar]
- 40.Schonberg MA, Breslau ES, Hamel MB, Bellizzi KM, McCarthy EP. Colon cancer screening in US adults aged 65 and older according to life expectancy and age. Journal of the American Geriatrics Society. 2015;63(4):750–756. doi: 10.1111/jgs.13335. [DOI] [PubMed] [Google Scholar]
- 41.Volk RJ, Linder SK, Lopez-Olivo MA, et al. Patient decision aids for colorectal cancer screening: a systematic review and meta-analysis. American Journal of Preventive Medicine. 2016;51(5):779–791. doi: 10.1016/j.amepre.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewis CL, Golin CE, DeLeon C, et al. A targeted decision aid for the elderly to decide whether to undergo colorectal cancer screening: development and results of an uncontrolled trial. BMC medical informatics and decision making. 2010;10(1):1. doi: 10.1186/1472-6947-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bechis SK, Carroll PR, Cooperberg MR. Impact of Age at Diagnosis on Prostate Cancer Treatment and Survival. Journal of Clinical Oncology. 2011;29(2):235–241. doi: 10.1200/JCO.2010.30.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.USPSTF. Prostate Cancer Screening Draft Recommendations. [Accessed 4-27-2017];2017 https://screeningforprostatecancer.org/
- 45.Wolf A, Wender RC, Etzioni RB, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA: a cancer journal for clinicians. 2010;60(2):70–98. doi: 10.3322/caac.20066. [DOI] [PubMed] [Google Scholar]
- 46.Carter HB. American Urological Association (AUA) guideline on prostate cancer detection: process and rationale. BJU international. 2013;112(5):543–547. doi: 10.1111/bju.12318. [DOI] [PubMed] [Google Scholar]
- 47.Andriole GL, Crawford ED, Grubb RL, III, et al. Mortality results from a randomized prostate-cancer screening trial. New England Journal of Medicine. 2009;360(13):1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shoag JE, Mittal S, Hu JC. Reevaluating PSA testing rates in the PLCO trial. New England Journal of Medicine. 2016;374(18):1795–1796. doi: 10.1056/NEJMc1515131. [DOI] [PubMed] [Google Scholar]
- 49.Pinsky PF, Prorok PC, Yu K, et al. Extended mortality results for prostate cancer screening in the PLCO trial with median follow-up of 15 years. Cancer. 2017;123(4):592–599. doi: 10.1002/cncr.30474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crawford ED, Grubb R, III, Black A, et al. Comorbidity and mortality results from a randomized prostate cancer screening trial. Journal of Clinical Oncology. 2010;29(4):355–361. doi: 10.1200/JCO.2010.30.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl j. Med. 2009;2009(360):1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 52.Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. The Lancet. 2014;384(9959):2027–2035. doi: 10.1016/S0140-6736(14)60525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brawer MK. Prostate-specific antigen: Current status. CA: a cancer journal for clinicians. 1999;49(5):264–281. doi: 10.3322/canjclin.49.5.264. [DOI] [PubMed] [Google Scholar]
- 54.Loeb S, Carter HB, Berndt SI, Ricker W, Schaeffer EM. Complications after prostate biopsy: data from SEER-Medicare. The Journal of urology. 2011;186(5):1830–1834. doi: 10.1016/j.juro.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Loeb S, Vellekoop A, Ahmed HU, et al. Systematic review of complications of prostate biopsy. European urology. 2013;64(6):876–892. doi: 10.1016/j.eururo.2013.05.049. [DOI] [PubMed] [Google Scholar]
- 56.Rosario DJ, Lane JA, Metcalfe C, et al. Short term outcomes of prostate biopsy in men tested for cancer by prostate specific antigen: prospective evaluation within ProtecT study. Bmj. 2012;344:d7894. doi: 10.1136/bmj.d7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Welch HG, Black WC. Overdiagnosis in cancer. Journal of the National Cancer Institute. 2010;102(9):605–613. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 58.Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. Journal of the National Cancer Institute. 2009;101(6):374–383. doi: 10.1093/jnci/djp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roussel B, Ouellet GM, Mohile SG, Dale W. Prostate cancer in elderly men: Screening, active surveillance, and definitive therapy. Clinics in geriatric medicine. 2015;31(4):615–629. doi: 10.1016/j.cger.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 60.Moyer VA. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Annals of internal medicine. 2012;157(2):120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 61.Walter LC, Bertenthal D, Lindquist K, Konety BR. PSA screening among elderly men with limited life expectancies. Jama. 2006;296(19):2336–2342. doi: 10.1001/jama.296.19.2336. [DOI] [PubMed] [Google Scholar]
- 62.Kotwal AA, Mohile SG, Dale W. Remaining life expectancy measurement and PSA screening of older men. Journal of geriatric oncology. 2012;3(3):196–204. doi: 10.1016/j.jgo.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Drazer MW, Prasad SM, Huo D, et al. National trends in prostate cancer screening among older American men with limited 9-year life expectancies: Evidence of an increased need for shared decision making. Cancer. 2014;120(10):1491–1498. doi: 10.1002/cncr.28600. [DOI] [PubMed] [Google Scholar]
- 64.Drazer MW, Huo D, Eggener SE. National prostate cancer screening rates after the 2012 US Preventive Services Task Force recommendation discouraging prostate-specific antigen–based screening. Journal of Clinical Oncology. 2015;33(22):2416–2423. doi: 10.1200/JCO.2015.61.6532. [DOI] [PubMed] [Google Scholar]
- 65.Taylor KL, Williams RM, Davis K, et al. Decision making in prostate cancer screening using decision aids vs usual care: a randomized clinical trial. JAMA internal medicine. 2013;173(18):1704–1712. doi: 10.1001/jamainternmed.2013.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barry MJ, Wexler RM, Brackett CD, et al. Responses to a decision aid on prostate cancer screening in primary care practices. American Journal of Preventive Medicine. 2015;49(4):520–525. doi: 10.1016/j.amepre.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 67.ACS. American Cancer Society. Breast Cancer Facts & Figures 2015–2016. "Table 1. Estimated New Female Breast Cancer Cases and Deaths by Age, US, 2015". [Accessed January 25, 2017];2015 http://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2015–2016.pdf.
- 68.Schonberg MA, Li VW, Eliassen AH, et al. Accounting for individualized competing mortality risks in estimating postmenopausal breast cancer risk. Breast Cancer Res Treat, Dec. 2016;160(3):547–562. doi: 10.1007/s10549-016-4020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Siu AL. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Annals of internal medicine. 2016;164(4):279–296. doi: 10.7326/M15-2886. [DOI] [PubMed] [Google Scholar]
- 70.Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. Jama. 2015;314(15):1599–1614. doi: 10.1001/jama.2015.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pace LE, Keating NL. A systematic assessment of benefits and risks to guide breast cancer screening decisions. JAMA. 2014 Apr 2;311(13):1327–1335. doi: 10.1001/jama.2014.1398. [DOI] [PubMed] [Google Scholar]
- 72.Nystrom L, Andersson I, Bjurstam N, Frisell J, Nordenskjold B, Rutqvist LE. Long-term effects of mammography screening: updated overview of the Swedish randomised trials. Lancet. 2002 Mar 16;359(9310):909–919. doi: 10.1016/S0140-6736(02)08020-0. [DOI] [PubMed] [Google Scholar]
- 73.Schonberg MA, Breslau ES, McCarthy EP. Targeting of mammography screening according to life expectancy in women aged 75 and older. J Am Geriatr. Soc. 2013 Mar;61(3):388–395. doi: 10.1111/jgs.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barratt A, Howard K, Irwig L, Salkeld G, Houssami N. Model of outcomes of screening mammography: information to support informed choices. Bmj. 2005 Apr 23;330(7497):936. doi: 10.1136/bmj.38398.469479.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Walter LC, Schonberg MA. Screening mammography in older women: a review. JAMA. 2014 Apr 2;311(13):1336–1347. doi: 10.1001/jama.2014.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Henderson LM, O'Meara ES, Braithwaite D, Onega T. Performance of digital screening mammography among older women in the United States. Cancer. 2015 May 01;121(9):1379–1386. doi: 10.1002/cncr.29214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kerlikowske K, Hubbard RA, Miglioretti DL, et al. Comparative effectiveness of digital versus film-screen mammography in community practice in the United States: a cohort study. Ann Intern. Med. 2011 Oct 18;155(8):493–502. doi: 10.7326/0003-4819-155-8-201110180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McCarthy EP, Burns RB, Freund KM, et al. Mammography use, breast cancer stage at diagnosis, and survival among older women. J Am Geriatr. Soc. 2000 Oct;48(10):1226–1233. doi: 10.1111/j.1532-5415.2000.tb02595.x. [DOI] [PubMed] [Google Scholar]
- 79.Braithwaite D, Zhu W, Hubbard RA, et al. Screening outcomes in older US women undergoing multiple mammograms in community practice: does interval, age, or comorbidity score affect tumor characteristics or false positive rates? J Natl Cancer Inst. 2013 Mar 6;105(5):334–341. doi: 10.1093/jnci/djs645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schonberg MA, Silliman RA, Marcantonio ER. Weighing the benefits and burdens of mammography screening among women age 80 years or older. J Clin Oncol. 2009 Apr 10;27(11):1774–1780. doi: 10.1200/JCO.2008.19.9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schonberg MA, Silliman RA, Ngo LH, et al. Older women's experience with a benign breast biopsy-a mixed methods study. J Gen Intern. Med. 2014 Dec;29(12):1631–1640. doi: 10.1007/s11606-014-2981-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schonberg MA, Marcantonio ER, Li D, Silliman RA, Ngo L, McCarthy EP. Breast cancer among the oldest old: tumor characteristics, treatment choices, and survival. J Clin Oncol. 2010 Apr 20;28(12):2038–2045. doi: 10.1200/JCO.2009.25.9796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Van Ravesteyn NT, Stout NK, Schechter CB, et al. Benefits and harms of mammography screening after age 74 years: model estimates of overdiagnosis. Journal of the National Cancer Institute. 2015;107(7):djv103. doi: 10.1093/jnci/djv103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Etzioni R, Gulati R. Recognizing the limitations of cancer overdiagnosis studies: a first step towards overcoming them. Journal of the National Cancer Institute. 2016;108(3):djv345. doi: 10.1093/jnci/djv345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jorgensen KJ, Gotzsche PC, Kalager M, Zahl PH. Breast Cancer Screening in Denmark: A Cohort Study of Tumor Size and Overdiagnosis. Ann Intern. Med. 2017 Jan 10; doi: 10.7326/M16-0270. [DOI] [PubMed] [Google Scholar]
- 86.Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. New England Journal of Medicine. 2012;367(21):1998–2005. doi: 10.1056/NEJMoa1206809. [DOI] [PubMed] [Google Scholar]
- 87.Schonberg MA, Hamel MB, Davis RB, et al. Development and evaluation of a decision aid on mammography screening for women 75 years and older. JAMA Intern. Med. 2014 Mar 1;174(3):417–424. doi: 10.1001/jamainternmed.2013.13639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.ACS. Estimated New Cases for the Four Major Cancers by Sex and Age Group, 2016. [Accessed January 27, 2017];2016 https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2016.html.
- 89.Alberg AJ, Samet JM. Epidemiology of lung cancer. CHEST Journal. 2003;123(1_suppl):21S–49S. doi: 10.1378/chest.123.1_suppl.21s. [DOI] [PubMed] [Google Scholar]
- 90.Humphrey LL, Deffebach M, Pappas M, et al. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive services task force recommendation. Annals of internal medicine. 2013;159(6):411–420. doi: 10.7326/0003-4819-159-6-201309170-00690. [DOI] [PubMed] [Google Scholar]
- 91.CDC. Centers of Disease Control and Prevention. Current cigarette smoking among adults-United States, 2011. MMWR. Morbidity and mortality weekly report. 2012;61(44):889. [PubMed] [Google Scholar]
- 92.Moyer VA. Screening for lung cancer: US Preventive Services Task Force recommendation statement. Annals of internal medicine. 2014;160(5):330–338. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 93.Wender R, Fontham ET, Barrera E, et al. American Cancer Society lung cancer screening guidelines. CA: a cancer journal for clinicians. 2013;63(2):106–117. doi: 10.3322/caac.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Prorok PC, Andriole GL, Bresalier RS, et al. Design of the prostate, lung, colorectal and ovarian (PLCO) cancer screening trial. Controlled clinical trials. 2000;21(6):273S–309S. doi: 10.1016/s0197-2456(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 95.Oken MM, Hocking WG, Kvale PA, et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. Jama. 2011;306(17):1865–1873. doi: 10.1001/jama.2011.1591. [DOI] [PubMed] [Google Scholar]
- 96.Pinsky PF, Church TR, Izmirlian G, Kramer BS. The National Lung Screening Trial: results stratified by demographics, smoking history, and lung cancer histology. Cancer. 2013;119(22):3976–3983. doi: 10.1002/cncr.28326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aberle D, Adams A, Berg C, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. The New England journal of medicine. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: a systematic review. Jama. 2012;307(22):2418–2429. doi: 10.1001/jama.2012.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li F, Sone S, Abe H, MacMahon H, Armato SG, Doi K. Lung Cancers Missed at Low-Dose Helical CT Screening in a General Population: Comparison of Clinical, Histopathologic, and Imaging Findings 1. Radiology. 2002;225(3):673–683. doi: 10.1148/radiol.2253011375. [DOI] [PubMed] [Google Scholar]
- 100.Patz EF, Pinsky P, Gatsonis C, et al. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA internal medicine. 2014;174(2):269–274. doi: 10.1001/jamainternmed.2013.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Veronesi G, Maisonneuve P, Bellomi M, et al. Estimating Overdiagnosis in Low-Dose Computed Tomography Screening for Lung CancerA Cohort Study. Annals of internal medicine. 2012;157(11):776–784. doi: 10.7326/0003-4819-157-11-201212040-00005. [DOI] [PubMed] [Google Scholar]
- 102.Tammemagi MC, Lam S. Screening for lung cancer using low dose computed tomography. Bmj. 2014;348:g2253. doi: 10.1136/bmj.g2253. [DOI] [PubMed] [Google Scholar]
- 103.Harris RP, Sheridan SL, Lewis CL, et al. The harms of screening: a proposed taxonomy and application to lung cancer screening. JAMA internal medicine. 2014;174(2):281–286. doi: 10.1001/jamainternmed.2013.12745. [DOI] [PubMed] [Google Scholar]
- 104.Lüchtenborg M, Riaz SP, Coupland VH, et al. High procedure volume is strongly associated with improved survival after lung cancer surgery. Journal of Clinical Oncology. 2013;31(25):3141–3146. doi: 10.1200/JCO.2013.49.0219. [DOI] [PubMed] [Google Scholar]
- 105.Detterbeck FC, Mazzone PJ, Naidich DP, Bach PB. Screening for lung cancer: diagnosis and management of lung cancer: American College of Chest Physicians evidence-based clinical practice guidelines. CHEST Journal. 2013;143(5_suppl):e78S–e92S. doi: 10.1378/chest.12-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pinsky PF, Gierada DS, Black W, et al. Performance of Lung-RADS in the National Lung Screening TrialA Retrospective AssessmentPerformance of Lung-RADS in the NLST. Annals of internal medicine. 2015;162(7):485–491. doi: 10.7326/M14-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aberle DR, Adams AM, Berg CD, et al. Baseline characteristics of participants in the randomized national lung screening trial. Journal of the National Cancer Institute. 2010;102(23):1771–1779. doi: 10.1093/jnci/djq434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wiener RS, Schwartz LM, Woloshin S, Welch HG. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Annals of internal medicine. 2011;155(3):137–144. doi: 10.1059/0003-4819-155-3-201108020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kozower BD, Sheng S, O'brien SM, et al. STS database risk models: predictors of mortality and major morbidity for lung cancer resection. The Annals of thoracic surgery. 2010;90(3):875–883. doi: 10.1016/j.athoracsur.2010.03.115. [DOI] [PubMed] [Google Scholar]
- 110.Pinsky PF, Gierada DS, Hocking W, Patz EF, Kramer BS. National Lung Screening Trial findings by age: Medicare-eligible versus under-65 population. Annals of internal medicine. 2014;161(9):627–633. doi: 10.7326/M14-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wender R, Fontham ET, Barrera E, Jr, et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin. 2013 Mar-Apr;63(2):107–117. doi: 10.3322/caac.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Falcoz PE, Conti M, Brouchet L, et al. The Thoracic Surgery Scoring System (Thoracoscore): risk model for in-hospital death in 15,183 patients requiring thoracic surgery. The Journal of Thoracic and Cardiovascular Surgery. 2007;133(2):325–332. e321. doi: 10.1016/j.jtcvs.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 113.Scoring systems for ICU and surgical patients:Thoracoscore (The Thoracic Surgery Scoring System) [Accessed January 30, 2017]; http://www.sfar.org/scores2/thoracoscore2.php.
- 114.McMahon PM, Kong CY, Bouzan C, et al. Cost-effectiveness of computed tomography screening for lung cancer in the United States. Journal of Thoracic oncology. 2011;6(11):1841–1848. doi: 10.1097/JTO.0b013e31822e59b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.CMS. Centers for Medicare & Medicaid Services. Decision Memo for Screening for Lung Cancer with Low Dose Computed Tomography (LDCT) [Accessed January 26, 2017];2015 https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274.
- 116.MSKCC. Lung Cancer Screening Decision Tool. [Accessed January 27, 2017];2014 http://nomograms.mskcc.org/Lung/Screening.aspx.
- 117.Kovalchik SA, Tammemagi M, Berg CD, et al. Targeting of low-dose CT screening according to the risk of lung-cancer death. New England Journal of Medicine. 2013;369(3):245–254. doi: 10.1056/NEJMoa1301851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Katki HA, Kovalchik SA, Berg CD, Cheung LC, Chaturvedi AK. Development and validation of risk models to select ever-smokers for CT lung cancer screening. Jama. 2016;315(21):2300–2311. doi: 10.1001/jama.2016.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Volk RJ, Linder SK, Leal VB. Feasibility of a patient decision aid about lung cancer screening with low-dose computed tomography. Preventive Medicine. 2014;62:60–63. doi: 10.1016/j.ypmed.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.VA. Screening for Lung Cancer. [Accessed January 26, 2017];2014 http://www.prevention.va.gov/docs/LungCancerScreeningHandout.pdf.
- 121.Kinsinger LS, Anderson C, Kim J, et al. Implementation of Lung Cancer Screening in the Veterans Health Administration. JAMA internal medicine. 2017;177(3):399–406. doi: 10.1001/jamainternmed.2016.9022. [DOI] [PubMed] [Google Scholar]
- 122.Scherer LD, Caverly TJ, Burke J, et al. Development of the Medical Maximizer-Minimizer Scale. Health Psychol. 2016 Nov;35(11):1276–1287. doi: 10.1037/hea0000417. [DOI] [PubMed] [Google Scholar]
- 123.ePrognosis: Lee Schonberg Index. [Accessed April 5th 2017]; http://eprognosis.ucsf.edu/leeschonberg.php.
- 124.CDC. United States Life Tables, 2012. National Vital Statistics Reports. 2012;65(8) [PubMed] [Google Scholar]