Abstract
Background
Clinical outcomes of octogenarians undergoing hepatectomy for colorectal liver metastases(CRLM) are poorly characterized. The current study evaluated operative morbidity, mortality and survival outcomes among a contemporary cohort of octogenarians.
Methods
Patients undergoing their first hepatectomy for CRLM were identified from institutional databases and those ≥80 years old(y) were matched 1:1 to a group of patients <80y. Data pertaining to surgical morbidity/mortality and survival were compared using standard statistical methods.
Results
From 2002–2012, 1391 hepatectomies were performed for CRLM, 55(4%) in patients ≥80y. Major complications occurred twice as frequently among patients ≥80y [10(19%)≥80y vs. 5(9%) <80y, (p=0.270)]. No matched patient <80y. died within 90d of operation, whereas, 4(7%) patients ≥80y did, p=0.125. Median follow-up was significantly longer for the <80 y. group [44 (1–146) vs. 23(0–102) mths, p=0.006]. Probability of disease recurrence was not different between groups(p=0.123) nor was the cumulative incidence of death from disease(p=0.371). However, patients ≥80y had significantly higher incidence of non-cancer related death(p=0.012).
Conclusions
Hepatectomy for CRLM among well-selected octogenarians is reasonable with cancer related survival outcomes similar to those observed in younger patients. However, it is associated with clinically significant morbidity/mortality and continued efforts directed at optimizing perioperative care are necessary to improve early outcomes among octogenarians.
Introduction
The population is aging. Over the last half-century persistent changes in health related behaviors, as well as, economic, social and medical advances have led to increases in overall life expectancy. As of 2012, persons age 80 and over accounted for 3.7% of the U.S population. Future projections suggest that by the year 2050, 20–30 million (~8%) people living in the United States will be over the age of 80. Life expectancy for this group will range from 6–7 years and the annual probability of death for octogenarians is projected to be 7–10% per annum [1]. As the population ages there will be a simultaneous rise in age related diseases such as cancer. The estimated number of new cancer diagnoses will increase up to 45%, from 1.6 million patients in 2010 to 2.3 million by 2030. The vast majority of these diagnoses will occur in older patients and the number of patients’ ≥80 y. requiring cancer treatment will rise substantially [2]. Although the overall age structure of cancer patients will change, the most common cancer types are likely to endure, such that colorectal cancer (CRC) will remain the 3nd most common cancer diagnosis [3]. Furthermore, approximately two thirds of patients with CRC will develop metastases to the liver for which surgery remains the only potentially curative treatment [4]. It follows then, that the number of elderly patients with colorectal liver metastases (CRLM) seeking surgical opinion/treatment will increase and may necessitate special consideration.
Normal physiologic ageing is associated with a gradual decline in organ system reserve and responsiveness to stressful stimuli. In terms of the liver, it has been suggested that the aging process results in a reduction in hepatic regenerative capacity, leading some to question whether advanced chronological age should be a contraindication to liver surgery [5–8]. To date, evidence regarding outcomes of elderly patients following hepatectomy are conflicting. Some suggest that there is increased morbidity and operative mortality [9–12], while others indicate no differences in clinical outcomes [13–18]. This is likely related to differences in the age cut-off studied and non-specific patient selection including both benign and malignant pathologies, +/− underlying liver dysfunction and major and minor resections [13, 15, 19–21]. However, even amongst studies specifically evaluating hepatectomy for CRLM, results differ [10, 12, 14, 22–25].
To date only two studies have exclusively evaluated outcomes among octogenarians undergoing hepatectomy [26, 27]. Unfortunately, these studies were small, unmatched, included patients with a variety of diagnoses and focused only on short-term morbidity and mortality. Consequently, knowledge of the safety and oncologic effectiveness of hepatectomy for CRLM in patients’ ≥80 y. is limited. The current study aimed to evaluate the safety and effectiveness of hepatectomy, in the modern era, for the treatment of colorectal liver metastases (CRLM) in octogenarians; and to compare morbidity, mortality and longer-term survival outcomes to a matched group of patients < 80 y.
Materials & Methods
Study Design
This study was deemed exempt from full institutional review and ethics approval was obtained via waiver of the Health Insurance Portability and Accountability Act. From January 2002 to December 2012, all patients undergoing first time liver resection for CRLM were retrospectively identified from prospectively maintained institutional and service specific databases. Patients’ ≥ 80 years of age were identified and subsequently matched 1:1 to a group of patients < 80 years of age and clinical outcomes compared. Matching criteria included, BMI, presence of comorbidities, extent of hepatic resection and clinical risk score.
Patient Selection
This study included patients operated on over a 10-year period by multiple surgeons and granular details as to how older patients were screened and selected for surgery were not available. In general, however, patient ‘frailty’ was assessed with a combination of history, physical examination, functional status evaluation and collateral history obtained from family and caregivers.
Data Collection
Standard demographic and clinicopathological variables were obtained from the database and supplemented with information from the electronic medical record. For the purposes of matching, BMI was categorized as normal (<25 kg/m2), overweight (25–30 kg/m2) and obese (>30 kg/m2). Extent of resection was defined as major (≥4 Couinaud segments) versus minor (<4 Couinaud segments). Chronic comorbid conditions at the time of operation were documented and patients were categorized as having either ≤1 or >1 comorbidity. Finally, Clinical risk score (CRS 0–5) including tumor size and number, disease free interval (DFI), lymph node status of the primary colorectal cancer and carcinoembryonic antigen (CEA) level was calculated as previously reported by Fong et al. [28] and subsequently categorized into three groups (CRS=0, CRS 1–3 (low risk) and CRS 4–5 (high risk) and matched.
Neoadjuvant and adjuvant therapy were defined as receipt of chemotherapy, systemic and/or hepatic arterial infusion, specifically for the treatment of CRLM before and after liver resection, respectively. CRLM present at the time of primary CRC diagnosis were considered synchronous. Extrahepatic disease (EHD) was documented if there was evidence on preoperative staging imaging and/or if disease was discovered outside the liver intra-operatively. Margin positivity was defined as the presence of tumor cells at the inked resection margin. Surgical complications were prospectively recorded and graded according to the Memorial Sloan Kettering Secondary Events Program database [29]. This scoring system ranges from 0-no complication to 5-complicaiton resulting in death and is consistent with the “Common Terminology Criteria for Adverse Events Version 4.0”, endorsed by the National Institute of Health. Major complications were those with severity scores ≥ 3. Post-operative blood transfusion was defined as administration of packed red blood cells (PRBC) within 48 hours of operation.
Date of CRLM diagnosis was determined radiographically. Follow-up time was calculated as the interval from surgery to last contact with the study center or death. Timing and location of disease recurrence was ascertained from surveillance imaging studies. Disease status at the time of analysis was categorized as follows; no evidence of disease (NED), alive with disease (AWD), dead of disease (DOD) or dead of unknown/unrelated causes (DUC). Recurrence and/or death were considered events in determining recurrence free survival (RFS). All-cause mortality was the event of interest for overall survival (OS) and DOD the event for disease specific survival (DSS), respectively.
Statistical Analysis
Statistical analyses were performed using SPSS software version 21 (Chicago, IL, USA). Summary statistics for baseline demographic and clinicopathological variables are presented as mean +/− standard deviation or median (range) and frequency (%) for continuous and discrete variables, respectively. For the entire cohort, comparison among categorical variables was completed using Chi-square analysis or Fischer’s exact test where appropriate. Continuous variables were compared using T-tests and Mann-Whitney U tests depending on characteristics of distribution. Older patients (≥80 y.) were matched 1:1 using exact matching method [30] with patients <80 y. and appropriate matched analysis was performed. Categorical data were compared across matched pairs using McNemar’s test (binary outcome) or marginal homogeneity test (>2 outcomes). Given the non-normal distribution of the data, the non-parametric Wilcoxon test was utilized to compare continuous data between matched groups.
To maintain the integrity of the matched data, survival analysis was complete using the Kaplan Meier method with pair wise stratification. Comparison between groups was completed using the log-rank test. Competing risk analysis was used to separate the probability of death from disease from that of death of unrelated/unknown causes and the probability of recurrence from the probability of death without recurrence. This method allows an estimate of the cumulative incidence of multiple competing events that can happen during follow-up. Gray’s method was used for estimation and testing [31] and the Fine-Gray method for regression [32]. All statistical tests were two tailed and significant was set at p=0.05.
Results
From 2002–2012, 1391 patients underwent first time liver resection for CRLM. At the time of operation, 1336 (96%) patients were <80 and 55 (4%) patients were ≥80 years old. Younger patients (<80 y.) more commonly presented with synchronous disease, >1 CRLM and had significantly higher CRS compared to patients ≥80 y (all p-value <0.001). Use of neoadjuvant and adjuvant chemotherapy was also more common among patients <80 y (p<0.001).
Of the 55 patients’ ≥80 years of age, 1:1 matching using the 4 previously stated variables was feasible in 54 patients. Baseline demographic and clinicopathologic features of the matched groups (<80 y. and ≥80 y.) are outlined in Table 1. Operative and perioperative outcomes of the matched groups are outlined in Table 2. The median length of stay (LOS), total number and type of post-operative complications were not different between groups and are summarized in Table 3. Overall, 42 patients experienced at least one post-operative complication (39%, 42/108). Of the 4 octogenarian patients who died within 90-days, all had significant smoking histories (2 current smokers, 2 previous) and three had received neoadjuvant chemotherapy and subsequently underwent lobar resection. Cause of death was multifactorial in 3 of 4 patients with early postoperative liver and cardiorespiratory dysfunction, sepsis and ultimately, multi-system organ failure. The remaining patient was discharged home in stable condition on post-operative day #7, represented to an outside hospital on post-operative day #9, rapidly decompensated, experienced cardiovascular arrest in the emergency department and died. No autopsy was performed and ultimate cause of death was not determined.
Table 1.
Baseline Demographic and clinicopathologic characteristics of matched groups stratified by age ≥ 80 years versus < 80 years undergoing hepatectomy for colorectal liver metastases [(EBL= estimated blood loss, HAI= hepatic artery infusion chemotherapy, CEA= carcinoembryonic antigen, CRS= clinical risk score); n (%), mean +/−SD, median (range)].
| Variable | < 80 years (n=54) | ≥ 80 years (n=54) | P-value |
|---|---|---|---|
| Gender | |||
| Male | 28 (51.9) | 34 (63.0) | 0.441 |
| Age (years) | 57.68 +/− 13.0 | 83.62 +/− 2.6 | <0.001 |
| BMI (kg/m2) | 25.6+/−4.56 | 26.3+/−4.31 | 0.056 |
| ASA | 0.009 | ||
| 1 | 0 (0) | 0 (0) | |
| 2 | 20 (37) | 10 (18.5) | |
| 3 | 26 (48.1) | 43 (79.6) | |
| 4 | 1 (1.9) | 1 (1.9) | |
| Number of Comorbidities | 0(0–3) | 0(0–4) | 0.080 |
| Smoking status | 0.238 | ||
| Current | 25 (46.3) | 25 (46.3) | |
| Previous | 17 (31.5) | 22 (40.7) | |
| Never | 8 (14.8) | 1 (1.9) | |
| Pre-operative Bilirubin (mg/dL) | 0.64 +/− 0.29 | 0.63 +/− 0.27 | 0.772 |
| Pre-operative Platelets (K/uL) | 229.8 +/− 63.1 | 215.1 +/− 76.9 | 0.180 |
| Pre-operative Albumin (mg/dL) | 4.20 +/− 0.30 | 3.96 +/− 0.35 | 0.001 |
| Pre-operative AST(U/L) | 188.9 +/− 266.9 | 192.2 +/− 200.5 | 0.880 |
| Pre-operative Hemoglobin (g/dL) | 12.9 +/− 1.6 | 12.5 +/− 1.4 | 0.182 |
| Pre-operative INR | 1.19 +/− 0.20 | 1.18 +/− 0.15 | 0.755 |
| Neoadjuvant Chemotherapy | |||
| Systemic | 42 (77.8) | 31 (57.4) | 0.078 |
| HAI + Systemic | 5 (9.3) | 0 (0) | 1.00 |
| Adjuvant Chemotherapy | |||
| Systemic | 47 (87) | 27 (50) | <0.001 |
| HAI + Systemic | 16 (29.6) | 6 (11.1) | 1.00 |
| Synchronous Disease | 31 (57.4) | 14 (25.9) | 0.004 |
| Disease Free Interval (mo.) | 0 (0–180) | 16 (0–204) | 0.002 |
| Disease Free Interval <12 months | 37 (68.5) | 24 (44.4) | 0.002 |
| Node Positive Primary | 31 (57.4) | 23 (42.6) | 0.078 |
| > 1 Metastasis | 29 (53.7) | 13 (24.1) | 0.008 |
| Diameter of largest Metastasis >5cm | 13 (24.1) | 15 (27.8) | 1.00 |
| Pre-op CEA >200ng/mL | 2 (3.7) | 4 (7.4) | 0.625 |
| CRS | |||
| Low (0–3) | 52(96.3) | 52(96.3) | 1.00 |
| High (4–5) | 2(3.7) | 2(3.7) | |
| Extrahepatic Disease | 2 (3.7) | 4 (7.4) | 1.00 |
| Year of Surgery | |||
| 2002–2006 | 29 (53.7) | 22 (40.7) | 0.248 |
| 2007–2012 | 25 (46.3) | 32 (59.3) |
Table 2.
Operative characteristics, early post-operative morbidity and late disease related outcomes of matched groups stratified by age ≥80 years versus < 80 years undergoing hepatectomy for colorectal liver metastases [(EBL= estimated blood loss, FUP = follow-up, NED= no evidence of disease, AWD= alive with disease, DOD= dead of disease, DUC= dead of unrelated/unknown causes; n (%), mean +/−SD, median (range)].
| Variable | < 80 years (n=54) | ≥ 80 years (n=54) | p-value |
|---|---|---|---|
| Number of Segments Resected | 2.5 (0.5–5.0) | 2.0 (0.5–5.0) | 0.160 |
| Major Hepatic Resection (≥4 segments) | 14(26) | 13(24) | 1.00 |
| EBL (mL) | 414.8 +/− 425.7 | 394.5 +/− 292.4 | 0.895 |
| Pringle Time (min) | 31.7 +/− 23.2 | 24.9 +/− 18.7 | 0.218 |
| Operative Time (min) | 251.9 +/− 113.1 | 198.9 +/− 64.1 | 0.001 |
| Perioperative PRBC Transfusion | 4 (7) | 4 (7) | 1.0 |
| Post-operative Peak Bilirubin (mg/dL) | 1.28 +/− 0.61 | 1.22 +/− 1.1 | 0.348 |
| Post-operative Nadir Platelets (K/uL) | 216.8 +/− 58.4 | 208.8 +/− 78.2 | 0.491 |
| Post-operative Peak INR | 1.23 +/− 0.18 | 1.22 +/− 0.14 | 0.967 |
| Positive Margins | 6 (11) | 4 (7) | 0.727 |
| Length of Stay (days) | 7 (5–43) | 8.0 (4–58) | 0.188 |
| Follow-up Time (months) | 44 (1–146) | 22.5 (0–102) | 0.006 |
| Disease Recurrence | |||
| Any | 39(74) | 27(50) | 0.021 |
| Liver | 26 (48) | 9 (17) | 0.049 |
| Lung | 23 (52) | 13 (24) | 1.0 |
| Other | 19 (44) | 9 (17) | 1.0 |
| Disease Status at Last FUP | |||
| NED | 17 (32) | 14 (26) | 0.054 |
| AWD | 5 (9) | 7 (13) | |
| DOD | 23 (43) | 14 (26) | |
| DUC | 9 (17) | 19 (35) |
Table 3.
Comparison of post-operative complications among patients ≥ 80 years old versus < 80 years old undergoing first time hepatectomy for colorectal liver metastases [n(%)].
| Complication | < 80 years old (n=54) | ≥ 80 years old (n=54) | p-value |
|---|---|---|---|
| Any | 18(33) | 24(44) | 0.290 |
| Major | 5(10) | 10(19) | 0.270 |
| Infectious | 3(6) | 11(20) | 0.500 |
| GI | 9(17) | 10(19) | 1.0 |
| Hepatic | 0(0) | 2(4) | 1.0 |
| VTE/PE | 1(2) | 2(4) | 1.0 |
| Hemorrhage | 0(0) | 2(4) | 1.0 |
| Pulmonary | 3(6) | 5(10) | 1.0 |
| Cardiac | 1(2) | 6(11) | 1.0 |
| Other | 9(17) | 9(17) | 1.0 |
| 90d Mortality | 0(0) | 4(7) | 0.125 |
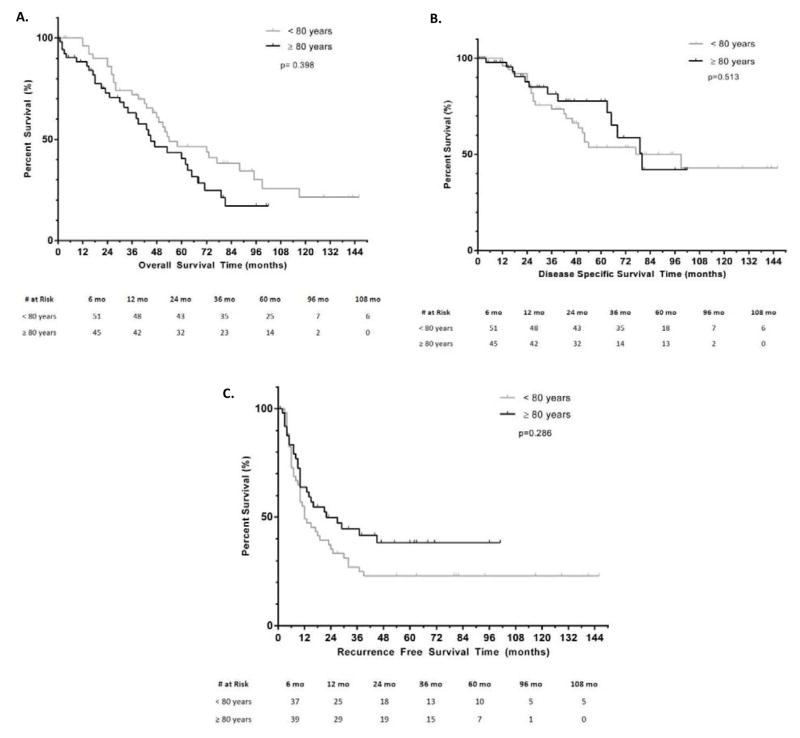
Follow-up and survival data by age group are shown in Table 2. OS, DSS and RFS are illustrated in Figure 1A–C, respectively. Median OS in the ≥80 y. group was 45(30–60) months versus 54(29–79) months for <80 y., (p=0.398). Actuarial 1-, 2- and 5-year OS was 88%, 73% and 41% in the ≥80 y. group compared to 96%, 86% and 46% in the <80 y. group. Median DSS was 80(62–98) months for the ≥80 y. group and was not different from the median DSS of <80 y. group [77(23–131) months], p=0.513. Actuarial 1-, 2- and 5-year DSS were 98%, 88% and 78% among the ≥80 y. and 96%, 88%, 54% in the <80 y. Median RFS was not different between groups [22(6–38) months, ≥80 y. versus 12(5–19) months, <80 y., p=0.286]. Actuarial 1-, 2- and 5-year RFS were 64%, 50%, and 38% among the ≥80 y. group compared to 49%, 35% and 23% in the <80 y. group.
Figure 1.
Comparison of; A-Overall; B-Disease specific; C-Recurrence free survival outcomes between patients ≥ 80y.o versus < 80y.o.
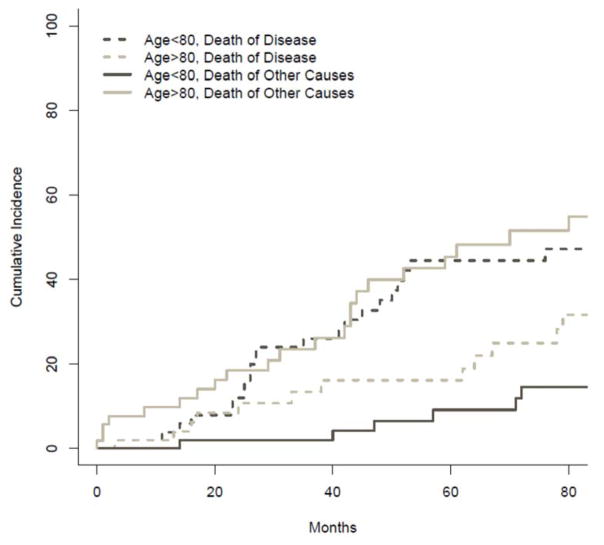
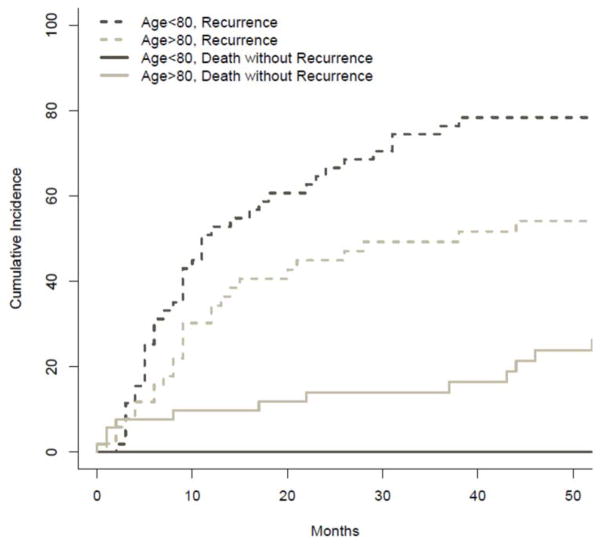
The cumulative incidence of death from disease versus death from other causes was compared between groups and is illustrated in Figure 2. The probability of death from disease at 1, 2 and 3 years was 2%, 11% and 13% among ≥80 y. patients versus, 4%, 12% and 26% in the <80 y. group (p=0.37). Conversely, the probability of death from other causes among octogenarians at 1, 2 and 3 years was 10%, 19% and 23%, respectively. This was significantly higher when compared to the 0%, 2%, and 2% probabilities observed at 1, 2 and 3 years in the <80 y. group (p=0.012). The probability of disease recurrence at 1, 2 and 3 years was 34%, 45%, 49% for ≥80 y’s. This was not significantly different when compared to the probability of recurrence observed among <80 y. at 1(53%), 2(66.5%) and 3(76.4%) years, p=0.123. Cumulative incidence of death without recurrence was significantly higher among octogenarians (p=0.025). In the ≥80 y. group, the probability of death without recurrence at 1, 2 and 3 years was 10%, 14% and 14%. Conversely, no patient <80 y. died without disease recurrence (Figure 3).
Figure 2.
Comparison of the cumulative incidence of death of disease (hashed lines) death from unrelated/other causes (solid lines) between patients ≥ 80 years old versus < 80 years old.
Figure 3.
Comparison of the cumulative incidence of recurrence (hashed lines) and death without recurrence (solid lines) between patients ≥80 years old versus < 80 years old.
Discussion
Over the past several decades advancements in all areas of surgery and perioperative care have significantly improved the safety of liver surgery [33, 34]. Furthermore, it is now well established that among patients with CRLM, those with disease amenable to complete resection are provided a survival advantage and chance for long-term cure [35–37]. However, data pertaining specifically to morbidity, morality and survival outcomes among octogenarians is lacking. The current study identified a contemporary group of patients ≥80 y. undergoing liver resection for CRLM and compared both early post-operative outcomes (morbidity/mortality) and longer term survival outcomes to a matched cohort of patients < 80 y.
Octogenarians accounted for only 4% of all liver resections for CRLM at the study institution. Though this number is small, it accurately reflects the age structure of the US population, where persons ≥80 y. account for approximately 3.7% of the total population [1]. Among the matched groups, overall morbidity rates were not different and although major complications occurred twice as frequently among patients ≥80 y., this was not statistically different, p=0.267. These findings are similar to those observed in a recent analysis of 350 consecutive patients undergoing liver resection for any indication by Shirabe et al in which no significant differences in post-operative morbidity were observed between patients ≥80y and <80 y’s[27]. Furthermore, Nojiri et al evaluated patients undergoing liver resection for CRLM specifically, and found no differences in overall morbidity rates between older and younger patients [38]. In the current study the rate of major complication among octogenarians was high (20%) and was echoed in a study by Riffat et al where a major complication rate of 27% was observed in patients 80 years and older [26]. This finding is of clinical concern and is likely a function of the overall decreased physiologic reserve and ability to tolerate a major operation.
Ninety-day outcomes following hepatectomy have been suggested as the gold-standard for surgical quality assessment [39]. In the current study 90-day mortality was not statistically different between groups; however, 4 out of 54 patients ≥80 y. died within 90-days of surgery. Conversely, no matched patient <80 y. died within 90-days. Several studies have evaluated mortality following liver resection in the ‘elderly’, albeit, using different age cut offs. Cook et al report a 90-day mortality of 7% following liver resection for CRLM among a cohort of patients’ ≥75 y. [24]. However, in a more recent evaluation of a modern (2000–2008), well-matched, cohort of patients ≥70 y. undergoing hepatectomy for CRLM, 90-day mortality was found to be 0% [16]. The lower observed mortality rates in the later study likely reflect the inclusion of patients up to a decade younger than the current study population. Of note 90-day mortality for matched patients younger than 80 years in the current study was 0% and is similar to rates of 1.3% and 0% noted in the aforementioned studies by Cook et al [24] and Cho et al [16], respectively. Though no statistical difference was observed between patients ≥80 y. and those <80 y. in the current analysis, a major complication rate nearing 20% and a 90-day mortality rate of over 7% in this highly selected group of patients over 80 is clinically relevant and warrants fastidious attention to patient selection and peri-operative care.
Median and 5-year OS were not different between octogenarians and younger matched patients (45 vs 54 months and 46% vs 41% respectively, p=0.398) and are similar to reported survival outcomes in patients over 75 years of age (44 months and 33%, respectively) [24][14, 38]. However, these later studies are somewhat historical and are a likely underestimate of survival in patients older then 75 in the modern era of cancer care. In the current study the underlying cause of death was significantly different between patient ≥80 y. vs < 80. Octogenarians had a greater probability of dying from causes unrelated to their cancer. At 1-year the estimated probability of death from other causes among the ≥80 y. group was 10% compared with 0% in <80 y. group (p=0.012). Similarly, estimates of death without disease recurrence were 14% at 3-years among octogenarians versus 0% in <80 y. patients (0.025). Although these probabilities are significantly higher when compared to their younger counterparts they are in line with the 11% probability of non-cancer death reported by Norji et al. among patients ≥75 undergoing hepatectomy for CRLM [38] as well as US census data regarding cause of death in persons over 80 years [1].
Disease recurrence is common following liver resection for CRLM, occurring in up to 60% of patients at 2 years follow-up [40]. In the current study, recurrence rates were not different between octogenarians and patients < 80y (p=0.123). Median and 5-year RFS were also not different between groups [12 vs. 22 months and 23% vs. 38%, p=0.513). These findings are similar to those observed in a study by Di Benedetto et al were no difference in disease recurrence were observed amongst a cohort of patient 70 years and older with CRLM [23]. Taken in concert these observations suggest that the biology of disease in octogenarians is similar to younger patients and that cancer related survival outcomes are not a function of chronologic age.
This study was a single center evaluation of octogenarians undergoing hepatectomy for CRLM. The matched design provides an appropriate control group that allows more specific assessment of the impact of age on outcome. However, the study is limited by the highly selected nature of the included patients and is subject to all the inherent limitations associated with retrospective investigation. More specifically, cancer treatments received before and after liver resection were not standardized and/or matched for and may impact clinical outcomes of interest. Follow-up time among the older cohort was significantly shorter (23 months vs. 44 months, p=0.006) and may have led to underestimation of recurrence/death among patients ≥ 80 y. relative to their younger counterparts. Finally, although the groups were matched for overall CRS, patients <80 y. were more likely to have synchronous disease and greater disease burden which may have negatively impacted the survival outcomes among patients <80 y.
Despite these limitations, this is the largest matched series evaluating short-term morbidity/mortality and the only series reporting on longer-term outcomes following liver resection for CRLM among octogenarians. Hepatectomy for CRLM in patients’ ≥80 y. is associated with a clinically relevant trend towards higher major morbidity and 90-day mortality and must be communicated to patients and families prior to operative intervention. Despite these increased risks of early morbidity and mortality, disease recurrence and disease specific survival outcomes observed in the current study were comparable to patients <80y and suggest hepatectomy for CRLM in well-selected patients over 80 years of age is reasonable. The coming years will see a progressive rise in the number of patients over the age of 80 seeking cancer treatment, as such, further study regarding surgical treatment in this population is necessary to optimize both short and long-term outcomes.
Acknowledgments
Funding Sources: None
Footnotes
Financial Disclosures: None
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ortman JM, VV, Hogan H. An Aging Nation: The Older Population in the United States. 2014 [cited 20154 April 6, 2015]; Available from: http://www.census.gov/prod/2014pubs/p25-1140.pdf.
- 2.Smith BD, et al. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27(17):2758–65. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 3.National Cancer Institute. SEER: Cancer Statistics Review (CSR), 1975–2012. 2015 Jun 18; ]; Available from: http://seer.cancer.gov/csr/1975_2012.
- 4.National Cancer Institute. SEER Stat Fact Sheets: Colon and Rectum Cancer. 2015 Jun 16; ]; Available from: http://seer.cancer.gov/statfacts/html/colorect.html.
- 5.Schmucker DL. Age-related changes in liver structure and function: Implications for disease ? Exp Gerontol. 2005;40(8–9):650–9. doi: 10.1016/j.exger.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Ettorre GM, et al. Postoperative liver function after elective right hepatectomy in elderly patients. Br J Surg. 2001;88(1):73–6. doi: 10.1046/j.1365-2168.2001.01629.x. [DOI] [PubMed] [Google Scholar]
- 7.Korc-Grodzicki B, et al. Surgical considerations in older adults with cancer. J Clin Oncol. 2014;32(24):2647–53. doi: 10.1200/JCO.2014.55.0962. [DOI] [PubMed] [Google Scholar]
- 8.Petrowsky H, Clavien PA. Should we deny surgery for malignant hepato-pancreatico-biliary tumors to elderly patients? World J Surg. 2005;29(9):1093–100. doi: 10.1007/s00268-005-1130-6. [DOI] [PubMed] [Google Scholar]
- 9.Koperna T, Kisser M, Schulz F. Hepatic resection in the elderly. World J Surg. 1998;22(4):406–12. doi: 10.1007/s002689900405. [DOI] [PubMed] [Google Scholar]
- 10.Figueras J, et al. Surgical treatment of liver metastases from colorectal carcinoma in elderly patients. When is it worthwhile? Clin Transl Oncol. 2007;9(6):392–400. doi: 10.1007/s12094-007-0072-x. [DOI] [PubMed] [Google Scholar]
- 11.Sulpice L, et al. Advanced age remains an achilles heel for liver resections. World J Surg. 2014;38(4):918–26. doi: 10.1007/s00268-013-2367-0. [DOI] [PubMed] [Google Scholar]
- 12.Reddy SK, et al. Major liver resection in elderly patients: a multi-institutional analysis. J Am Coll Surg. 2011;212(5):787–95. doi: 10.1016/j.jamcollsurg.2010.12.048. [DOI] [PubMed] [Google Scholar]
- 13.Aldrighetti L, et al. Liver resections in over-75-year-old patients: surgical hazard or current practice? J Surg Oncol. 2006;93(3):186–93. doi: 10.1002/jso.20342. [DOI] [PubMed] [Google Scholar]
- 14.Bockhorn M, et al. Major liver resections in the elderly-is an aggressive approach justified? Int J Colorectal Dis. 2009;24(1):83–6. doi: 10.1007/s00384-008-0571-4. [DOI] [PubMed] [Google Scholar]
- 15.Cescon M, et al. Outcome of right hepatectomies in patients older than 70 years. Arch Surg. 2003;138(5):547–52. doi: 10.1001/archsurg.138.5.547. [DOI] [PubMed] [Google Scholar]
- 16.Cho SW, et al. Safety of liver resection in the elderly: how important is age? Ann Surg Oncol. 2011;18(4):1088–95. doi: 10.1245/s10434-010-1404-6. [DOI] [PubMed] [Google Scholar]
- 17.Schiergens TS, et al. Liver resection in the elderly: significance of comorbidities and blood loss. J Gastrointest Surg. 2014;18(6):1161–70. doi: 10.1007/s11605-014-2516-2. [DOI] [PubMed] [Google Scholar]
- 18.Menon KV, et al. Outcomes after major hepatectomy in elderly patients. J Am Coll Surg. 2006;203(5):677–83. doi: 10.1016/j.jamcollsurg.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 19.Aldrighetti L, et al. Impact of age on the outcome of liver resections. Am Surg. 2004;70(5):453–60. [PubMed] [Google Scholar]
- 20.Aldrighetti L, et al. Impact of advanced age on the outcome of liver resection. World J Surg. 2003;27(10):1149–54. doi: 10.1007/s00268-003-7072-y. [DOI] [PubMed] [Google Scholar]
- 21.Mastoraki A, et al. Outcome following major hepatic resection in the elderly patients. Clin Res Hepatol Gastroenterol. 2014;38(4):462–6. doi: 10.1016/j.clinre.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Adam R, et al. Liver resection of colorectal metastases in elderly patients. Br J Surg. 2010;97(3):366–76. doi: 10.1002/bjs.6889. [DOI] [PubMed] [Google Scholar]
- 23.Di Benedetto F, et al. Liver resection for colorectal metastases in older adults: a paired matched analysis. J Am Geriatr Soc. 2011;59(12):2282–90. doi: 10.1111/j.1532-5415.2011.03734.x. [DOI] [PubMed] [Google Scholar]
- 24.Cook EJ, et al. Resection of colorectal liver metastases in the elderly: does age matter? Colorectal Dis. 2012;14(10):1210–6. doi: 10.1111/j.1463-1318.2012.02946.x. [DOI] [PubMed] [Google Scholar]
- 25.de Liguori Carino N, et al. Liver resection for colorectal liver metastases in older patients. Crit Rev Oncol Hematol. 2008;67(3):273–8. doi: 10.1016/j.critrevonc.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Riffat F, Chu F, Morris DL. Liver resection in octogenarians. HPB (Oxford) 2006;8(3):206–10. doi: 10.1080/13651820500497173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shirabe K, et al. Early outcome following hepatic resection in patients older than 80 years of age. World J Surg. 2009;33(9):1927–32. doi: 10.1007/s00268-009-0122-3. [DOI] [PubMed] [Google Scholar]
- 28.Fong Y, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309–18. doi: 10.1097/00000658-199909000-00004. discussion 318–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grobmyer SR, et al. Diagnostic laparoscopy prior to planned hepatic resection for colorectal metastases. Arch Surg. 2004;139(12):1326–30. doi: 10.1001/archsurg.139.12.1326. [DOI] [PubMed] [Google Scholar]
- 30.Jasjeet S. Multivariate and propensity score matching software with automated balance optimization. Journal of Statistical Software. 2011;42(7):1–52. [Google Scholar]
- 31.RJG A class of K-sample test for comparing the cumulative incidence of a competing risk. Annals of Statistics. 1988;16:1141–1154. [Google Scholar]
- 32.Fine JP, GR A proportional hazards model for teh subdistribution of a competing risk. J Amer Stat Assoc. 1999;94:496–509. [Google Scholar]
- 33.Jarnagin WR, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236(4):397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. discussion 406–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kingham TP, et al. Hepatic Parenchymal Preservation Surgery: Decreasing Morbidity and Mortality Rates in 4,152 Resections for Malignancy. J Am Coll Surg. 2014 doi: 10.1016/j.jamcollsurg.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomlinson J, et al. Actual 10-year Survival after resection of colorectal liver metastases. Journal of Clinical Oncology. 2007;25:4575–4582. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 36.Cummings LC, Payes JD, Cooper GS. Survival after hepatic resection in metastatic colorectal cancer: a population-based study. Cancer. 2007;109(4):718–26. doi: 10.1002/cncr.22448. [DOI] [PubMed] [Google Scholar]
- 37.Biasco G, et al. Treatment of hepatic metastases from colorectal cancer: many doubts, some certainties. Cancer Treat Rev. 2006;32(3):214–28. doi: 10.1016/j.ctrv.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 38.Nojiri K, et al. Validity of hepatic resection of colorectal liver metastases in the elderly (75 years and older) Anticancer Res. 2009;29(2):583–8. [PubMed] [Google Scholar]
- 39.Egger ME, OJ, Scoggins CR, McMasters KM, Martin RC., 2nd Assessment of the reporting of quality and outcome measures in hepatic resections: a call for 90-day reporting in all hepatectomy series. HPB (Oxford) 2015;17(9):839–45. doi: 10.1111/hpb.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Jong MC, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250(3):440–8. doi: 10.1097/SLA.0b013e3181b4539b. [DOI] [PubMed] [Google Scholar]