Abstract
Background
The objective of this study was to compare the incidence and prevalence of depressive symptoms in atypical parkinsonian (APD) syndromes versus Parkinson disease (PD).
Methods
In a large, retrospective patient cohort, the authors analyzed the incidence and prevalence of depressive symptoms using the Beck Depression Inventory and evaluated patients longitudinally on subsequent visits. For individuals who were followed at subsequent visits, incidence rates were calculated in person‐years as a measure of incidence.
Results
In total, 361 patients were identified who had APD syndromes, including progressive supranuclear palsy, corticobasal degeneration, multiple system atrophy, and dementia with Lewy bodies; and 2352 patients with PD were used as a control group. The mean Beck Depression Inventory values were significantly higher in patients with APD (F = 14.19; P < 0.001). A significantly higher proportion of those with APD screened positive for depressive symptoms both at the initial visit and on subsequent visits (P < 0.001), and depressive symptoms appeared to be more severe in the APD subgroups. Unified Parkinson's Disease Rating Scale motor scores and disease duration were correlated with depressive symptoms.
Conclusions
The current results suggest that the incidence and prevalence of depressive symptoms are higher in patients with APD syndromes and also appear to be more severe than those in patients with PD. Depressive symptoms in APD are common and affect patients regardless of disease duration or motor severity.
Keywords: atypical parkinsonism, depression, Parkinson's disease
Neuropsychiatric symptoms are common in patients with Parkinson's disease (PD) and atypical parkinsonian disorders (APDs), and they may be more severe and challenging to treat than motor symptoms. Among the neuropsychiatric comorbidities, depression is 1 of the most common nonmotor symptoms in PD; it can present in the premotor stage of the disease and affects nearly one‐half of all patients with PD.1 In addition, associated suicidal ideations are prevalent, ranging from 11% to 23%,1, 2 with a suicide‐specific mortality rate of 5.3%.2
Despite the extensive literature on depression in PD,3, 4, 5, 6, 7, 8, 9, 10 there are relative few studies in APD11, 12, 13, 14, 15 and a paucity of information about incidence or prevalence in these disorders. In progressive supranuclear palsy (PSP), depression is estimated to affect over 50% of patients16, 17 and has been identified as a possible predictor of a shorter survival.18 Depression also has been reported in 80% of patients with multiple system atrophy (MSA)19 and in over 70% of patients with corticobasal degeneration (CBD).20 Neuroimaging and postmortem studies suggest that mesolimbic dopaminergic pathways and basal ganglia dysfunction may be associated with depression, findings also encountered in patients with PD or APD who have depression.21, 22, 23, 24 However, structural and functional differences between PD and APD syndromes, including PSP, MSA, dementia with Lewy body (DLB), and CBD, have not been fully described.
In clinical practice, we recognize that patients with APD suffer from depression, anxiety, and emotional incontinence (i.e., pseudobulbar affect); and, although these comorbidities sometimes appear to be more severe than those in patients with PD, no studies have compared symptoms among populations. Therefore, the objective of the current study was to investigate the incidence and prevalence of depressive symptoms in a cohort of patients who were followed at our tertiary movement disorders referral center and to identify clinical variables that had a potential association with depression outcomes among patients who had APDs compared with those who had PD.
Patients and Methods
The study was approved by the University of Florida (UF) Institutional Review Board, and all participants provided written informed consent. This was a retrospective cohort study of patients who were followed at the UF Center for Movement Disorders and Neurorestoration, including patients collected from a clinical research database (the UF‐INFORM database). UF‐INFORM is a clinical research database that provides information on demographics as well as clinical and functional characteristics of participants who consent for enrollment during their initial visit to the center. There are currently over 8000 patients enrolled with over 700 patients who have a clinical diagnosis of APD.
Patient Selection
We selected patients who were evaluated between July 2002 and April 2015 from our cohort and fulfilled the diagnostic criteria for either PD, according to the UK Brain Bank,25 or APD. All patients were diagnosed by a movement disorders fellowship‐trained specialist. For MSA and PSP, we followed criteria established by Gilman et al.26 and Litvan et al.,27 respectively, and included patients who met possible or probable diagnostic criteria. Both MSA‐parkinsonism and MSA‐cerebellar subtypes were included and grouped together for this analysis. Although PSP subtypes have increasingly been recognized,28 given the retrospective nature and available data for this study, we included primarily classic PSP, or Richardson syndrome, in these analyses. Patients with DLB and CBD were diagnosed according to published criteria29, 30 and detailed clinical history and progression of symptoms during subsequent visits. Patients who had missing demographic or diagnostic information were excluded from the analysis. We performed a review of the electronic medical records system for each patient without a clear diagnosis and, in cases where a diagnosis was established on subsequent visits, the patient was grouped accordingly. Patients with mixed features, without a clear diagnosis, were excluded from the study.
Data Collection
At the initial clinic encounter, we documented basic demographics, including age, gender, duration/onset of symptoms, and diagnosis. We also performed a physical chart review to verify diagnosis and retrieve information regarding the use of antidepressants at the initial encounter. At each subsequent clinic visit, we collected data from clinical functional scales, including the Unified Parkinson's Disease Rating Scale (UPDRS) and the Hoehn and Yahr scale.
Outcome Measurements and Instruments
We used the Beck Depression Inventory (BDI) to identify patients with depressive symptoms. The BDI is a well‐established tool for screening depressive symptoms, and it has been previously validated in patients with PD.31 We evaluated patient BDI scores at their initial clinic visit. On the BDI, a score greater than 13 correlates with clinical depression with sensitivity of 0.67 and specificity of 0.88.31 Although there have been multiple revisions since the original version in 1961,32 the most current version of the BDI (BDI‐II from 1996) shares considerable consistency and comparability with previous versions.33, 34 Therefore, although the BDI‐II has not yet been validated in PD, we have adopted this most current version in our practice due to its comparability with the previous version and the reliability of the instrument,33 and we included patients who had been exposed to the BDI versions I, II, or both during their follow‐up period. We used the values proposed for BDI‐II to classify depressive symptoms into mild (scores from 14 to 19), moderate (scores from 20 to 28), and severe (scores >28).32
Statistical Analysis
We used descriptive statistics for continuous variables and summarized categorical variables by counts of patients and percentages using the SPSS statistical software package (version 19.0; SPSS, Inc., Chicago, IL). We compared means using independent‐sample t tests or analyses of variance for independent samples with a Welch adjustment for cases of statistically significant testing of variance homogeneity; and we used Mann‐Whitney U tests or Kruskal‐Wallis analyses of variance for variables that required nonparametric testing. Percentages were compared using χ2 tests. We conducted post‐hoc analyses when appropriate with pairwise calculations between each individual subgroups, assuming a corrected P value adjusted with the Bonferroni method to prevent type I errors, or with an analysis of adjusted standardized residuals from the contingency tables.
For prevalence and incidence analyses, we included 361 patients with APD and 2352 with PD from our database who had BDI scores collected at their initial and subsequent visits. Due to the dynamic characteristics of our cohort, we used person‐years to measure incidence.35, 36 We used the Kaplan‐Meier method to determine the cumulative probability of patients developing depressive symptoms among those without depressive symptoms at the first visit. For this analysis, we assumed the BDI values were >13 at any given point in the subsequent visits and determined the differences in incidence among those with parkinsonian syndromes using the log‐rank test. For specific correlations between continuous variables, we used univariate linear regression models and Pearson coefficients. We assumed statistical significance at a level of 0.05.
Results
Prevalence of Depressive Symptoms in Parkinsonian Syndromes
Our database search identified 361 patients who had an APD, including 69 with CBD, 60 with DLB, 130 with MSA, and 102 with PSP. We also identified 2352 patients with PD for use as a control group, yielding a combined total of 2713 patients included in the study. The mean age (± standard deviation) of patients at the first visit was youngest in the PD group (67.0 ± 9.9 years) and oldest in the DLB subgroup (71.0 ± 7.5 years). About 35% of patients in the PD group were women, whereas the proportion of women was higher in the CBD and PSP subgroups (approximately 55%). At the initial visit, the PD group had statistically significant lower mean BDI values compared with the APD subgroups, with values of 10.4 ± 7.4 points for PD, 12.6 ± 7.5 points for MSA, 13.4 ± 8.4 points for CBD, 16.2 ± 8.8 points for DLB, and 13.7 ± 7.7 points for PSP (F = 14.19; P < 0.001). The clinical and demographic data from patients at their first visit are summarized in Table 1.
Table 1.
Clinical and demographic characteristics of Patients at the initial visit
| Clinical Variables | Diagnosis | |||||
|---|---|---|---|---|---|---|
| PD, n = 2352 | MSA, n = 130 | CBD, n = 69 | DLB, n = 60 | PSP, n = 102 | P | |
| Age: Mean ± SD, y | 67.0 ± 9.9 | 70.8 ± 8.8 | 68.3 ± 8.3 | 71.0 ± 7.5 | 70.0 ± 8.0 | <0.001 |
| Gender: No. (%) | ||||||
| Male | 1532 (65.1) | 89 (68.5) | 31 (44.9) | 41 (68.3) | 46 (45.1) | <0.001 |
| Female | 820 (34.9) | 41 (31.5) | 38 (55.1) | 19 (31.7) | 56 (54.9) | |
| Duration of symptoms: Mean ± SD, ya | 7.2 ± 6.7 | 5.4 ± 4.2 | 4.1 ± 3.2 | 5.0 ± 7.0 | 3.5 ± 2.7 | <0.001 |
| H&Y score: Mean ± SDb | 2.5 ± 0.7 | 3.1 ± 0.9 | 3.3 ± 1.2 | 3.3 ± 0.5 | 3.4 ± 1.0 | <0.001 |
| UPDRS III total: Mean ± SDc | 28.5 ± 11.7 | 38.0 ± 13.6 | 42.3 ± 16.7 | 37.1 ± 11.4 | 41.6 ± 13.6 | <0.001 |
| BDI: Mean ± SD | 10.4 ± 7.4 | 12.6 ± 7.5 | 13.4 ± 8.4 | 16.2 ± 8.8 | 13.7 ± 7.6 | <0.001 |
| Apathy scale: Mean ± SDd | 12.1 ± 6.5 | 16.4 ± 8.1 | 14.4 ± 7.3 | 19.6 ± 7.2 | 17.1 ± 8.2 | <0.001 |
Available in only 1949 patients with PD, 96 with MSA, 47 with CBD, 43 with DLB, and 70 with PSP.
Available in only 905 patients with PD, 54 with MSA, 17 with CBD, 14 with DLB, and 36 with PSP.
Available in only 1041 patients with PD, 59 with MSA, 21 with CBD, 17 with DLB, 41 with PSP.
Available in only 1204 patients with PD, 55 with MSA, 32 with CBD, 30 with DLB, 50 with PSP.
PD, Parkinson's disease; MSA, multiple system atrophy; CBD, corticobasal degeneration; DLB, dementia with Lewy bodies; PSP, progressive supranuclear palsy; SD, standard deviation; H&Y, Hoehn & Yahr clinical rating scale; UPDRS III, Unified Parkinson's Disease Rating Scale, part 3 (motor part); BDI, Beck Depression Inventory.
At their initial visit to clinic, 28% of patients in the PD group screened positive for depressive symptoms compared with 40% to 55% of patients in the APD subgroups (CBD, 42%; DLB, 55%; MSA, 40%; CBD, 43%; P < 0.001) (Fig. 1A). Post‐hoc analysis demonstrated significant differences between the PD group and the DLB, MSA and PSP subgroups; however, there were no differences among the APD subgroups. When subdivided into subgroups based on severity, the PD group scored 17% and 12% in the mild and moderate‐severe ranges, respectively (Fig. 1B). In contrast, for the APD subgroups, from 20% to 23% of patients scored in the mild range, and 17% to 35% scored in the moderate‐severe range (Fig. 1B). Post‐hoc analysis revealed significantly lower percentages of PD patients in the mild, moderate, and severe ranges; significantly higher percentages of moderate depressive symptoms for the DLB and PSP subgroups; and severe depressive symptoms for the DLB and CBD subgroups (data not shown).
Figure 1.
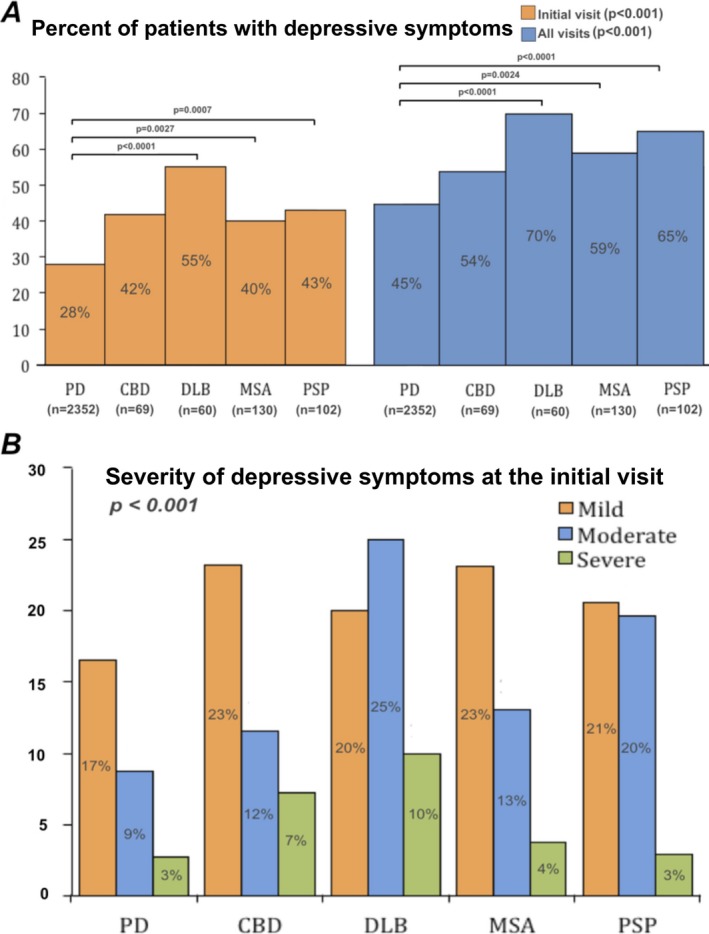
A: The percentage of patients who screened positive for depressive symptoms at the initial visit (left) and considering all clinic visits (right). Post‐hoc analysis indicates differences between subgroups (shown with respective P values). B: The severity of depressive symptoms across different parkinsonian disorders at the initial visit. PD, Parkinson's disease; CBD, corticobasal degeneration; DLB, dementia with Lewy bodies; MSA, multiple system atrophy; PSP, progressive supranuclear palsy.
Considering all visits for each patient, 45% of the patients with PD screened positive for depressive symptoms at some point in their disease. In contrast, the appearance of depressive symptoms in the APD subgroups ranged from 54% to 70% (CBD, 54%; DLB, 70%; MSA, 59%l PSP, 65%; atypical not otherwise specified, 55%; P < 0.001) (Fig. 1A). Post‐hoc analysis demonstrated a significant difference between the PD group and the DLB, MSA, and PSP APD subgroups (Fig. 1A). There were no differences among the atypical subgroups.
Cumulative Incidence of Depressive Symptoms in Parkinsonian Syndromes
To estimate the incidence of depressive symptoms in APD versus PD, we screened subsequent visits for depressive symptoms in those patients who were without depression at the initial visit. We detected an incidence rate of 14 per 100 person‐years for the PD group; whereas, for the APD subgroups, the incidence rates ranged from 22 to 53 per 100 person‐years (MSA, 22 per 100 person‐years; CBC, 27 per 100 person‐years; DLB, 27 per 100 person‐years; PSP, 53 per 100 person‐years), and the PSP subgroup had the highest incidence rate of all APD subgroups analyzed. The 1‐year survival curves were used to analyze the distribution of cumulative occurrences of depressive symptoms across different parkinsonian syndromes (Fig. 2) and demonstrated the lowest occurrence in the PD group and the highest in the PSP subgroup, corroborating the findings from the incidence rates. A log‐rank test demonstrated statistically significant differences in the cumulative occurrence curves for depressive symptoms across different diagnoses (P < 0.001).
Figure 2.
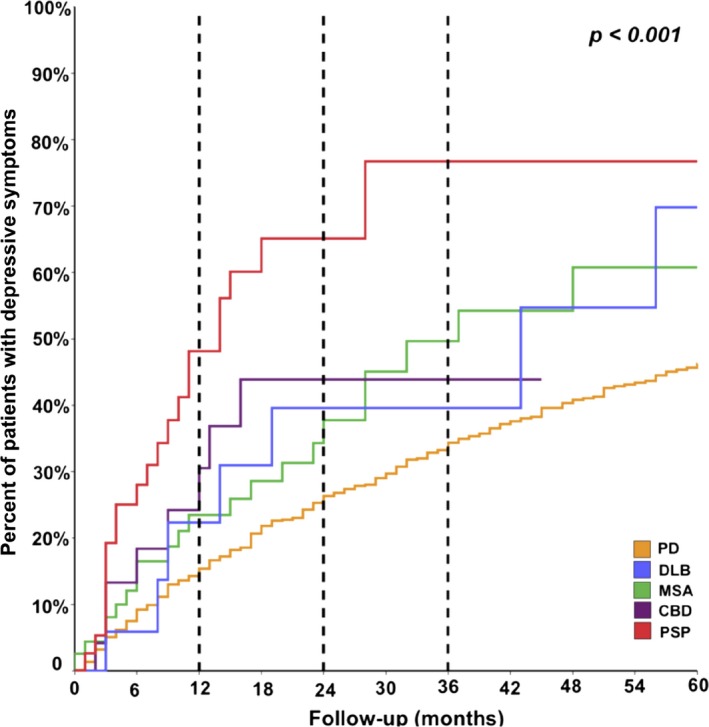
Comparison of the cumulative incidence of newly diagnosed depressive symptoms over follow‐up (in months). Dashed lines indicate differences between the cumulative incidence across different disorders at 1, 2, and 3 years of follow‐up. PD, Parkinson's disease; DLB, dementia with Lewy bodies; MSA, multiple system atrophy; CBD, corticobasal degeneration; PSP, progressive supranuclear palsy.
Influence of Motor Severity in the Occurrence of Depressive Symptoms in Parkinsonian Syndromes
To further explore the association between motor severity and the occurrence of depressive symptoms, we conducted Pearson correlations and univariate linear regressions. The results demonstrated that scores on part III (motor part) of the UPDRS (UPDRS‐III) had a weak correlation with the occurrence of depressive symptoms at the first visit (R2 [Nagelkerke] = 0.013), and calculating the Pearson coefficient between total UPDRS‐III and BDI scores confirmed a weak relationship between the 2 variables (r = 0.161; P < 0.001). Disease duration also had a weak correlation with the occurrence of depressive symptoms (R2 [Nagelkerke] = 0.024) and also was confirmed by calculating the Pearson coefficient between disease duration and BDI scores (r = 0.114; P = 0.001).
Discussion
Our results demonstrate a significantly higher prevalence of depressive symptoms in the patients who had APD syndromes compared with those who had PD in our population. In our high‐volume tertiary referral center, we analyzed and longitudinally followed a large number of patients as part of routine clinical care. The results show that a large percentage of these patients screened positive for depressive symptoms at their initial encounter and that the proportion of those who had depressive symptoms was significantly higher in the APD subgroups compared with the PD group. These findings remained significant when considering all visits. In particular, among the patients with DLB, over 50% screened positive for depressive symptoms at their initial visit, and the prevalence rate increased to nearly 70% when they were followed over a 6‐year period. These findings are highly relevant, because depression, although it is increasingly recognized as a common feature of PD, still goes undertreated and has a significant impact on quality of life.37 By comparison, although they are noted among neuropsychiatric features in patients with APD,6, 11, 12, 14, 15, 16, 19, 38, 39, 40 less is known about depressive symptoms, their prevalence, incidence, treatment differences, and impact on quality of life in this population of patients.
Mean BDI scores were significantly higher in the APD subgroups compared with the PD group, particularly in the CBD, DLB, and PSP subgroups, with mean scores in the range concerning for depression. When we compared BDI scores for depressive symptoms, scores in the PD group were primarily in the mild range; whereas, in the APD subgroups, particularly DLB and PSP, a significant portion scored in the moderate to severe range of depressive symptoms. These findings indicate not only that depressive symptoms are prevalent in APD but that they appear to be more severe than those in PD, further emphasizing the importance of screening for and treating depressive symptoms in patients with APD.
The finding that depressive symptoms in APD are more prevalent than in PD may possibly be explained by better recognition and treatment of depression in PD compared with APD, a population in which motor symptoms are often medically refractory, more severe, and can mask depressive symptoms, as surveyed by the BDI. Inferences from these data, however, are limited because of the retrospective nature of the study. It is also possible that some patients might have underestimated the occurrence of depressive symptoms due to use of antidepressants, which might not have been documented consistently in clinic notes; however, this cannot be extrapolated from our data because of its retrospective design. Further prospective studies are recommended to better clarify clinical responses to antidepressants and how screening tools can be used to monitor the treatment of depression in this particular patient population.
Our results support evidence from prior studies estimating that nearly 50% of PD patients develop depression at some point during the course of their disease.41 As for the atypical parkinsonian syndromes, previous studies have documented various results. In 2013, dell'Aquila et al. investigated predictors of a shorter survival in patients with PSP and observed that 30% of patients documented neuropsychiatric symptoms at the onset of disease (including depression, apathy, and cognitive changes).18 Previous small studies with PSP revealed a high prevalence of depression, anxiety, and various behavioral disorders, such as apathy, disinhibition, and dysphoria.16 In 2014, Bloise et al. investigated psychiatric disorders using structured questionnaires in 28 patients with PSP and identified depression in 53% of patients and 18% of healthy controls.16 Our results reflect a higher prevalence of depressive symptoms, likely related to the longitudinal aspect of the current study. A large multicentric cohort study of 154 patients attempted to establish a neuropsychiatric profile of patients with PSP.17 Depression and dysphoria were documented using the Neuropsychiatric Inventory and were identified in 58% of patients(about 41% had depression and dysphoria in the mild range). Depression also was identified in up to 80% of patients with MSA19 using the BDI; however, the cutoff used was 9 in 10, which, according to the validation studies in PD, had good sensitivity but low specificity, thus raising the possibility of false‐negative results being detected by the instrument. In the same study, Benrud‐Larson et al. also observed lower BDI scores in patients who had the MSA‐parkinsonism subtype compared with those who had the MSA‐cerebellar subtype,19 suggesting that different phenotypes of the disease may manifest different psychiatric comorbidities. In patients with CBD, Litvan et al.20 identified depression in 73% of patients using the Neuropsychiatric Inventory.
Our results also demonstrated that motor severity scores had a weak correlation with the occurrence of depressive symptoms and likely achieved statistical significance because of the large sample size. However, due to the weakness of the association between these variables and the outcome, we can assume that such an association is not clinically relevant. Similarly, Bloise et al. also failed to show a correlation between motor severity on a PSP rating scale and depression.16 These findings indicate that depressive symptoms may occur in various parkinsonian disorders independent of their motor severity and disease stage but that further studies are needed to explore this association.
It is important to recognize the close relationship between depression and apathy,42 a disorder of motivation that can present in isolation or as part of depression or dementia. Apathy has been observed in basal ganglia disorders, including PD (as high as 70% of patients42) and particularly in PSP,14, 22, 43 in which some studies have documented apathy rates as high as 91%.43 Despite the importance of distinguishing these neuropsychiatric comorbidities, most of the current literature has not differentiated between them. In 2008, the Movement Disorders Society developed a task force to establish useful tools and evidence supporting the use of the currently available apathy scales in clinical practice.41 The Apathy Scale (AS)44 has been the only instrument with the status of “recommended” by the society due to considerable evidence supporting its effectiveness in detecting apathy in patients with. Item 4 on the UPDRS (apathy), because if its frequent use, has been recommended as a tool for crude screening with a cutoff of 2 in 3; however, as a single item, it cannot be classified as a scale.42 With the purpose of identifying clinical correlates with depressive symptoms in our population, we originally attempted to correct the occurrence of depressive symptoms to apathy by using the AS in a regression model; however, due to the retrospective nature of the study, the amount of missing data affected the final analysis (unpublished data), and the subset included in the analysis likely was not representative of the total sample. Therefore, we opted not to make the appropriate adjustment and suggest that future prospective studies should be able to better differentiate apathy from depressive symptoms.
Our results also demonstrate that depressive symptoms are more commonly seen in patients who have APD compared with those who have regular PD, as demonstrated by the prevalence analysis. Nevertheless, our results show that severity of motor disability and disease duration, factors that are commonly associated with occurrence of depression, likely play a very limited role in the equation responsible for the mechanism of depression in these patients. This indicates that the global understanding of depressive symptoms and clinical depression must include variables other than disease duration and motor severity, factors that are possibly related to the underlying neurodegenerative process and are not yet fully understood. Regardless, the common occurrence of depressive symptoms in our APD subgroups raise the importance of early screening and monitoring for such comorbidity and prompt evaluation and treatment once depression is identified, because this may greatly impact quality of life in these patients.37, 45
Overall, our findings suggest that depressive symptoms likely affect patients independent of motor severity and may be related to neurodegeneration in other circuits that are disease specific. Prior studies have investigated structural and functional changes in parkinsonian patients with depression. For example, in PD, gray matter density and volume loss in the orbitofrontal cortex, the gyrus rectus, and the right superior temporal lobe have been correlated with the severity of depression.21 In patients with PSP and MSA, positron emission tomography scans have revealed decreased glucose metabolism in certain regions, including the bilateral middle frontal cortex in patients with PSP and the left superior and middle frontal areas in those with MSA.46
This study has several strengths, including the large cohort of patients with various parkinsonian syndromes and significant numbers of patients with APDs. The longitudinal aspect of this study allowed us to determine the proportion of individuals with PD versus APD who developed depressive symptoms and to compare the incidence proportions of depressive symptoms in the follow‐up period among patients with these disorders. Analyses of clinical and demographic data also allowed the identification of potential correlates with the outcome of depression. Likewise, our study included several limitations, aside from those mentioned above, related to the retrospective design. In particular, the detection of depressive symptoms was based on a structured self‐reported questionnaire (BDI), a tool that has been validated only in the PD population.31 In addition, some of the components of the BDI (i.e., sleep changes and lack of energy) overlap with common symptoms and comorbidities that are frequently encountered in parkinsonian patients, and a formal structured clinical interview would be more appropriate to better differentiate among overlapping conditions. The cutoff suggested for the BDI has good specificity (88%) but lower sensitivity (67%) and may be suboptimal as a screening tool, giving rise to an opportunity for possible underestimation of depressive symptoms in this population. We also recognize that the BDI does not differentiate apathy from depression,43 which may be a possible confound.
Conclusion
Our study indicates that depressive symptoms are more prevalent (and likely more severe) among patients who have APD syndromes compared with patients who have PD. Furthermore, depressive symptoms are prevalent in patients at their initial visit to a tertiary center and, if not present, develop within the first few years of follow‐up in a significant number of individuals with APD. The lack of a strong association between depressive symptoms and motor scores suggests that this comorbidity may affect patients independent of their disease state. These findings should raise awareness about the importance of screening and detecting depression earlier in this patient population, along with stimulating further research and the establishment of standardized practices targeting these comorbidities and improving quality of life for these patients.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
L.A.: 1A, 1B, 1C, 2B
B.A.: 1B, 1C
R.W.: 2B
S.D.J.: 1B, 1C
A.P.: 1B, 1C
D.M.R.: 1C, 3A, 3B
D.V.: 1A, 3A, 3B
D.B.: 3A, 3B
H.W.: 3A, 3B
M.S.O.: 3A, 3B
N.M.: 1A, 3A, 3B
Disclosures
Ethical Compliance Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: The authors report no sources of funding and no conflicts of interest.
Financial Disclosures for the previous 12 months: Michel S. Okun serves as a consultant for the National Parkinson Foundation and has received research grants from the National Institutes of Health, the National Parkinson Foundation, the Michael J. Fox Foundation, the Parkinson Alliance, the Smallwood Foundation, the Bachmann‐Strauss Foundation, the Tourette Syndrome Association, and the University of Florida Foundation The remaining authors report no conflicts of interest.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Marsh L. Depression and Parkinson's disease: current knowledge [serial online]. Curr Neurol Neurosci Rep 2013;13:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kostic VS, Pekmezovic T, Tomic A, et al. Suicide and suicidal ideation in Parkinson's disease. J Neurol Sci 2010;289:40–43. [DOI] [PubMed] [Google Scholar]
- 3. Aarsland D, Larsen JP, Lim NG, Janvin C, Karlsen K, Tandberg E, Cummings JL. Range of neuropsychiatric disturbances in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry 1999;67:492–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leentjens AF, Lousberg R, Verhey FR. Markers for depression in Parkinson's disease. Acta Psychiatr Scand 2002;106:196–201. [DOI] [PubMed] [Google Scholar]
- 5. McDonald WM, Richard IH, DeLong MR. Prevalence, etiology, and treatment of depression in Parkinson's disease. Biol Psychiatry 2003;54:363–375. [DOI] [PubMed] [Google Scholar]
- 6. Stefanova N, Seppi K, Scherfler C, Puschban Z, Wenning GK. Depression in alpha‐synucleinopathies: prevalence, pathophysiology and treatment. J Neural Transm Suppl 2000;60:335–343. [DOI] [PubMed] [Google Scholar]
- 7. Tandberg E, Larsen JP, Aarsland D, Cummings JL. The occurrence of depression in Parkinson's disease A community‐based study. Arch Neurol 1996;53:175–179. [DOI] [PubMed] [Google Scholar]
- 8. Richard IH. Anxiety disorders in Parkinson's disease. Adv Neurol 2005;96:42–55. [PubMed] [Google Scholar]
- 9. Dissanayaka NN, Sellbach A, Matheson S, et al. Anxiety disorders in Parkinson's disease: prevalence and risk factors. Mov Disord 2010;25:838–845. [DOI] [PubMed] [Google Scholar]
- 10. Martinez‐Martin P, Damian J. Parkinson disease: depression and anxiety in Parkinson disease. Nat Rev Neurol 2010;6:243–245. [DOI] [PubMed] [Google Scholar]
- 11. Esmonde T, Giles E, Gibson M, Hodges JR. Neuropsychological performance, disease severity, and depression in progressive supranuclear palsy. J Neurol 1996;243:638–643. [DOI] [PubMed] [Google Scholar]
- 12. Goto K, Ueki A, Shimode H, Shinjo H, Miwa C, Morita Y. Depression in multiple system atrophy: a case report. Psychiatry Clin Neurosci 2000;54:507–511. [DOI] [PubMed] [Google Scholar]
- 13. Hargrave R, Rafal R. Depression in corticobasal degeneration. Psychosomatics 1998;39:481–482. [DOI] [PubMed] [Google Scholar]
- 14. Schrag A, Sheikh S, Quinn NP, et al. A comparison of depression, anxiety, and health status in patients with progressive supranuclear palsy and multiple system atrophy. Mov Disord 2010;25:1077–1081. [DOI] [PubMed] [Google Scholar]
- 15. Tison F, Yekhlef F, Chrysostome V. Depression and self‐reported depressive symptoms in multiple system atrophy compared to Parkinson's disease. Mov Disord 2006;21:1056–1057. [DOI] [PubMed] [Google Scholar]
- 16. Bloise MC, Berardelli I, Roselli V, et al. Psychiatric disturbances in patients with progressive supranuclear palsy: a case‐control study. Parkinsonism Relat Disord 2014;20:965–968. [DOI] [PubMed] [Google Scholar]
- 17. Gerstenecker A, Duff K, Mast B, Litvan I. Behavioral abnormalities in progressive supranuclear palsy. Psychiatry Res 2013;210:1205–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. dell'Aquila C, Zoccolella S, Cardinali V, et al. Predictors of survival in a series of clinically diagnosed progressive supranuclear palsy patients. Parkinsonism Relat Disord 2013;19:980–985. [DOI] [PubMed] [Google Scholar]
- 19. Benrud‐Larson LM, Sandroni P, Schrag A, Low PA. Depressive symptoms and life satisfaction in patients with multiple system atrophy. Mov Disord 2005;20:951–957. [DOI] [PubMed] [Google Scholar]
- 20. Litvan I, Cummings JL, Mega M. Neuropsychiatric features of corticobasal degeneration. J Neurol Neurosurg Psychiatry 1998;65:717–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Benoit M, Robert PH. Imaging correlates of apathy and depression in Parkinson's disease. J Neurol Sci 2011;310:58–60. [DOI] [PubMed] [Google Scholar]
- 22. Levy R, Czernecki V. Apathy and the basal ganglia. J Neurol 2006;253(Suppl 7):VII54–VII61. [DOI] [PubMed] [Google Scholar]
- 23. Luo C, Chen Q, Song W, et al. Resting‐state fMRI study on drug‐naive patients with Parkinson's disease and with depression. J Neurol Neurosurg Psychiatry 2014;85:675–683. [DOI] [PubMed] [Google Scholar]
- 24. Wilson RS, Nag S, Boyle PA, et al. Brainstem aminergic nuclei and late‐life depressive symptoms. JAMA Psychiatry 2013;70:1320–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008;71:670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele‐Richardson‐Olszewski syndrome): report of the NINDS‐SPSP international workshop. Neurology 1996;47:1–9. [DOI] [PubMed] [Google Scholar]
- 28. Respondek G, Hoglinger GU. The phenotypic spectrum of progressive supranuclear palsy. Parkinsonism Relat Disord 2016;22(Suppl 1):S34–S36. [DOI] [PubMed] [Google Scholar]
- 29. McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863–1872. [DOI] [PubMed] [Google Scholar]
- 30. Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013;80:496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Leentjens AF, Verhey FR, Luijckx GJ, Troost J. The validity of the Beck Depression Inventory as a screening and diagnostic instrument for depression in patients with Parkinson's disease. Mov Disord 2000;15:1221–1224. [DOI] [PubMed] [Google Scholar]
- 32. Smarr KL, Keefer AL. Measures of depression and depressive symptoms: Beck Depression Inventory‐II (BDI‐II), Center for Epidemiologic Studies Depression Scale (CES‐D), Geriatric Depression Scale (GDS), Hospital Anxiety and Depression Scale (HADS), and Patient Health Questionnaire‐9 (PHQ09). Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S454–S466. [DOI] [PubMed] [Google Scholar]
- 33. Wang YP, Gorenstein C. Psychometric properties of the Beck Depression Inventory‐II: a comprehensive review. Rev Bras Psiquiatr 2013;35:416–431. [DOI] [PubMed] [Google Scholar]
- 34. Steer RA, Clark DA, Kumar G, Beck AT. Common and specific dimensions of self‐reported anxiety and depression in adolescent outpatients. J Psychopathol Behav Assess 2008;30:163–170. [Google Scholar]
- 35. Kabisch M, Ruckes C, Seibert‐Grafe M, Blettner M. Randomized controlled trials: part 17 of a series on evaluation of scientific publications. Dtsch Arzteblatt Int 2011;108:663–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vandenbroucke JP, Pearce N. Incidence rates in dynamic populations. Int J Epidemiol 2012;41:1472–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pontone GM, Bakker CC, Chen S, et al. The longitudinal impact of depression on disability in Parkinson disease. Int J Geriatr Psychiatry 2016;31:458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aarsland D, Litvan I, Larsen JP. Neuropsychiatric symptoms of patients with progressive supranuclear palsy and Parkinson's disease. J Neuropsychiatry Clin Neurosci 2001;13:42–49. [DOI] [PubMed] [Google Scholar]
- 39. Colosimo C, Morgante L, Antonini A, et al. Non‐motor symptoms in atypical and secondary parkinsonism: the PRIAMO study. J Neurol 2010;257:5–14. [DOI] [PubMed] [Google Scholar]
- 40. Geda YE, Boeve BF, Negash S, et al. Neuropsychiatric features in 36 pathologically confirmed cases of corticobasal degeneration. J Neuropsychiatry Clin Neurosci 2007;19:77–80. [DOI] [PubMed] [Google Scholar]
- 41. Reijnders JS, Ehrt U, Weber WE, Aarsland D, Leentjens AF. A systematic review of prevalence studies of depression in Parkinson's disease. Mov Disord 2008;23:183–189; quiz 313. [DOI] [PubMed] [Google Scholar]
- 42. Leentjens AF, Dujardin K, Marsh L, et al. Apathy and anhedonia rating scales in Parkinson's disease: critique and recommendations. Mov Disord 2008;23:2004–2014. [DOI] [PubMed] [Google Scholar]
- 43. Litvan I, Mega MS, Cummings JL, Fairbanks L. Neuropsychiatric aspects of progressive supranuclear palsy. Neurology 1996;47:1184–1189. [DOI] [PubMed] [Google Scholar]
- 44. Starkstein SE, Mayberg HS, Preziosi TJ, Andrezejewski P, Leiguarda R, Robinson RG. Reliability, validity, and clinical correlates of apathy in Parkinson's disease. J Neuropsychiatry Clin Neurosci 1992;4:134–139. [DOI] [PubMed] [Google Scholar]
- 45. Lawrence BJ, Gasson N, Kane R, Bucks RS, Loftus AM. Activities of daily living, depression, and quality of life in Parkinson's disease [serial online]. PLoS ONE 2014;9:e102294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Herting B, Beuthien‐Baumann B, Pottrich K, et al. Prefrontal cortex dysfunction and depression in atypical parkinsonian syndromes. Mov Disord 2007;22:490–497. [DOI] [PubMed] [Google Scholar]


