Abstract
Statement of Problem:
In order to increase the performance of glass ionomer cement, it is reinforced with metal powders, short fibers, bioceramics and other materials. Fluoroapatite (Ca10(PO4)6F2) is found in dental enamel and is usually used in dental materials due to its good chemical and physical properties.
Objectives:
In this study, the effects of the addition of synthesized fluoroapatite nanoceramic on the compressive strength and bioactivity of glass ionomer cement were investigated.
Materials and Methods:
The synthesized fluoroapatite nanoceramic particles (~ 70 nm) were incorporated into as-prepared glass ionomer powder and were characterized using X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR) and scanning electron microscopy (SEM). Moreover, the compressive strength values of the modified glass ionomer cements with 0, 1, 3 and 5 wt% of fluoroapatite were evaluated.
Results:
Results showed that glass ionomer cement containing 3 wt% fluoroapatite nanoparticles exhibited the highest compressive strength (102.6 ± 4) compared to the other groups, including control group. Furthermore, FTIR and SEM investigations indicated that after soaking the glass ionomer cement- 3 wt% fluoroapatite composite in the simulated body fluid solution, the intensity of O-H, P-O and C-O absorption bands increased as a result of the formation of apatite layer on the surface of the sample, and the rather flat and homogeneous surface of the cement became more porous and inhomogeneous.
Conclusions:
Addition of synthesized nano-fluoroapatite to as-prepared glass ionomer cement enhanced the compressive strength as well as nucleation of the calcium phosphate layer on the surface of the composite. This makes it a good candidate for dentistry and orthopedic applications.
Keywords: Fluoroapatite , Nanoparticle , Glass Ionomer Cement , Simulated Body Fluid
Introduction
This study is a continuation of our previous studies: synthesis of flouroapatite [1] and glass ionomer powders [2]. In recent years, apatites [Ca10(PO4)6(OH, F, Cl)2] have attracted considerable interest in a broad range of applications such as biomedical uses [3], drug delivery carriers [4], catalysts carriers [5] and adsorbents [6].
Among the apatite group, fluoroapatite (FA) is considered as a biomedical material due to its low solubility, excellent chemical durability, excellent biocompatibility and the moderate resistance to radiation-induced amorphization [7,8]. Although FA is structurally very similar to hydroxyapatite (HA), it has higher thermal stability, more acid solubility tolerance and greater mechanical strength than HA [9,10]. The performance of FA in biological applications is affected by the nano-scale morphology and crystallinity [7]. Hence, owing to the importance of techniques for the preparation of FA with a controlled structure, they have been widely investigated through several methods, such as precipitation [11], sol-gel [12], hydrolysis [13], hydrothermal [14], sonochemical [15] and mechanochemical [10] synthesis.
Glass ionomer cements (GICs) have recently attracted increasing interest in clinical dentistry because of their excellent properties such as biocompatibility, low cytotoxicity, ability of the material for regeneration of hard tissues, low coefficient of thermal expansion, good adhesion to moist tooth structure and anticariogenic properties due to the fluoride ion release [16-18]. Remarkable improvements have been achieved since the invention of GICs to increase their performance which include reinforcement with metal powders [19], modification with resin [20] and incorporation with short fibers [21,22], bioceramics [12,17,23] and other materials.
Moshaverinia et al. [12] synthesized nano-fluoroapatite via an ethanol based sol-gel technique using (NH4)2HPO4,Ca(NO3)2.4H2O and NH4F as the starting materials. They indicated that the addition of nano-HA and FA to Fuji II commercial GIC enhanced the mechanical properties of the resulting cements and their bond strengths to dentin.
As reported by some researchers, fluoride release is the main factor involved in the antibacterial activity of GIs [24]. In the current study, the effects of the addition of FA nanoceramic on properties of the synthesized glass ionomer cement were evaluated.
Materials and Methods
Synthesis of nanofluoroapatite
For the preparation of fluoroapatite nanoparticles via sol-gel method, 0.192 g of phosphorus pentoxide (P2O5, GR, Merck, Darmstadt, Germany) was dissolved in 20 cc of absolute ethanol (GR, Merck, Darmstadt, Germany) to a concentration of 0.5 M. Simultaneously, 7 g of calcium nitrate tetrahydrate (Ca(NO3)2.4H2O, GR, Merck, Darmstadt, Germany) was dissolved in absolute ethanol (20 cc) to form 0.5 M solution to be the Ca precursor. Subsequently, suitable amounts (1.140 cc) of hexafluorophosphoric acid (HPF6, GR, Sigma-Aldrich, St. Louis, MO, USA) were added to the P precursor and afterwards the Ca/P ratio was adjusted to 1.67 by the slow and drop- wise addition of Ca precursor in order to obtain the starting solution. The mixture was stirred on a magnetic stirrer at room temperature (r.t.) for 24 h, and the gel was aged for at least 24 h at r.t. The as-prepared FA gel was dried at 120 °C for the next 24 h. The obtained solids were grounded and finally calcined at 600 °C for 1 h (heating rate = 3 °C/min) [1].
Preparation of GI powder
The ceramic part of glass ionomer cement was synthesized by the melting method as described in the previous study [2]. In brief, having mixed a defined weight percentage of the starting materials, including aluminum oxide (Al2O3, Merck, Darmstadt, Germany), silicon oxide (SiO2, Merck, Darmstadt, Germany), fluoride strontium (SrF, Merck, Darmstadt, Germany), aluminumphosphate (AlPO4, Merck, Darmstadt, Germany), and calciumfluoride (CaF2, Merck, Darmstadt, Germany), in a ball mill, the researchers placed the powder in an electric melting furnace and heated it for three hours with the rate of 5 °C/min, to reach the temperature of 1400 °C. Melted glass, resulted from melting the mentioned crystalline materials at 1400 °C, was cooled at ambient temperature and undergone shattering process for 5 hours in a ball mill. At this stage, the obtained powder was passed through a 200 mesh sieve. The obtained powder was the ceramic part of glass ionomer cement and in the next stage, it was mixed with a polymer liquid (polyacrylicacid, Sigma-Aldrich, St. Louis, MO, USA).
Glass ionomer-fluoroapatitenanocomposite
In order to prepare glass ionomer-fluoroapatite nanocomposite, 0, 1, 3 and 5 wt% of nano-FA (~ 70 nm) were added to the synthesized glass ionomer powders and mixed in amalgamator for 30 s, separately. Then, GI-FA powders were mixed with polyacrylic acid for 30 s, where the powder/liquid (P/L) ratio was set at 2.7/1. After that, cement mixes were poured into the aluminum moulds (4 mm in diameter and 6 mm in height). The moulds were covered with glass slides, flattened and gently pressed by hand to remove air bubbles from the cements. Finally, after 1 h, the specimens were carefully removed.
The phase composition of samples was carried out by the Philips X-ray diffractometer (XRD) with Cu Kα radiation (λ=0.154 nm) at 40 kV and 30 mA.
Compressive strength measurements
Compressive strength measurement (CS; MPa) was performed on a universal testing machine (Hounsfield, Model H25KS, England) with load cell of 25 kN at 0.5 mm/min. Cylindrical specimens with 4±0.1mm diameter and 6 ± 0.1mm height were prepared according to ASTM D695, and their compressive strength was calculated by the following equation:
CS=4P/πd2 (1)
Where P is the load at fracture (N), and d is the diameter of the cylindrical specimen (mm). In each group, three samples were evaluated, and the mean values were compared.
In vitro bioactivity evaluation
In vitro bioactivity of the best performing glass ionomer-fluoroapatite nanocomposite was inves-tigated by soaking in the simulated body fluid (SBF) prepared according to the standard procedure described by Kokubo and Takadama [25] at pH 7.4 for 28 days at 37 °C.
The apatite formation on the surface of the samples as a consequence of the dissolution and precipitation process of calcium phosphate was investigated by scanning electron microscopy (SEM, Seron Technology, AIS2100, Germany) and Fourier transform infrared spectroscopy (FTIR, 6300, JASCO, Japan) over a range of 4000 - 500 cm-1at a resolution of 2.0 cm-1.
Results
Characterization of glass ionomer-fluoroapatite nanocomposite
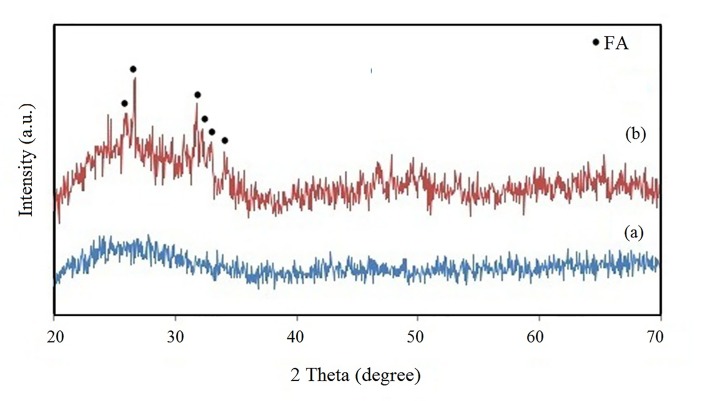
The XRD patterns of the as-prepared glass ionomer sample and FA-added GIC after being mixed with polyacrylic acid are shown in Figures 1a and b, respectively. The GIC sample (Figure 1a) does not show any sharp peaks in the XRD analysis, indicating that it is a predominantly amorphous material, whereas in the XRD pattern of the FA-added set GIC, peaks related to the crystalline apatite structure were observed between 20° and 35° (Figure 1b).
Figure1.
XRD patterns of (a) as-prepared GIC and (b) FA-added GIC after mixing with polyacrylic acid.
Additionally, the SEM photomicrographs (Figure 2a and b) showed a good mixture of the particles in the matrix and a good bonding between the glass ionomer powder and polymer liquid. Moreover, the surface morphology of the GIC-FA composite exhibited a higher degree of integrity, smoother surface and fewer surface cracks in comparison to the GIC control group.
Figure2.

SEM images of FA-added GIC after mixing with polyacrylic acid at (a) X1.0K and (b) X5.0K magnifications.
Compressive strength testing
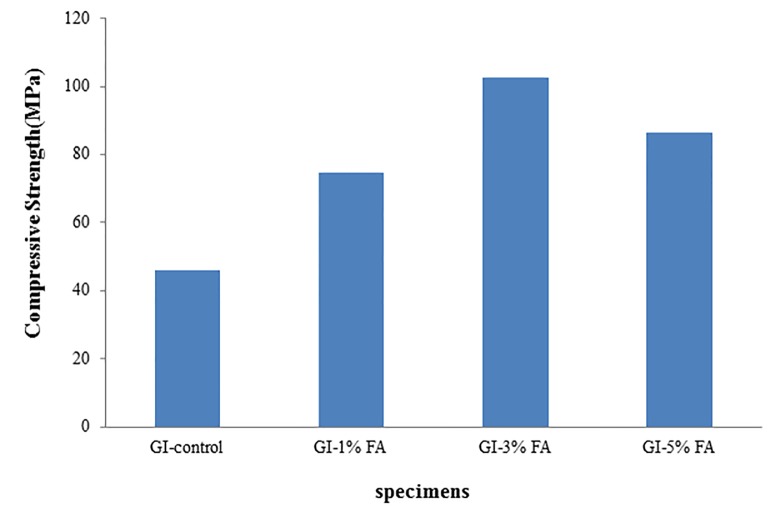
The mean values (standard deviation) of the measured compressive strength (CS) of at least three samples of each group are presented in Table 1 and graphically in Figure 3. In general, the compressive strength of GIC increased when fluoroapatite nanoparticles were added to the matrix. Mechanical results also indicated that the compressive strength increased remarkably with increasing the concentration of fluoroapatite nanoparticles up to 3 wt%.
Table 1.
Compressive strength values (mean ± standard deviation) of nano-FA-containing glass ionomer cement groups.
| Group | Compressive strength (MPa) |
|---|---|
| GI-control | 46.10 ± 3.39 |
| GI-1% (w/w) FA | 74.76 ± 5.60 |
| GI-3% (w/w) FA | 102.61 ± 4.00 |
| GI-5% (w/w) FA | 86.27 ± 4.51 |
Figure3.
Compressive strength values of nano-FA-containing glass ionomer cement groups.
In vitro bioactivity
The best performing GIC-FA nanocomposite as determined by mechanical testing, GIC-3% (w/w) FA, was chosen for the SBF trial. The qualitative investigation of precipitated apatite on the surface of GIC modified with 3 wt% fluoroapatite nanoparticles as a consequence of the dissolution and precipitation process of calciumphosphate was considered by SEM (Figure 4).
Figure4.
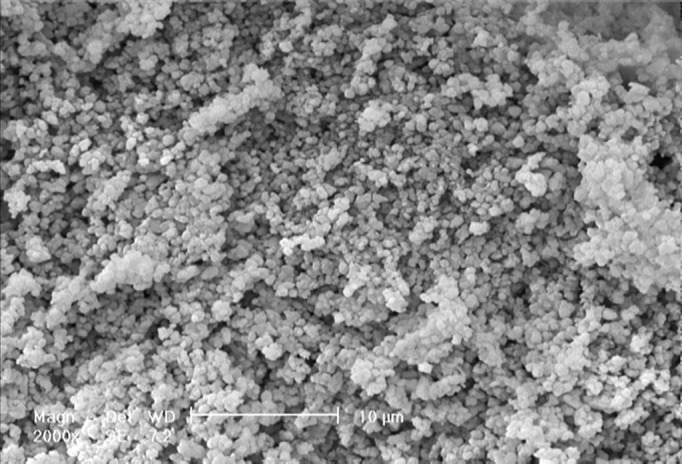
SEM micrograph of GIC-FA composite after keeping in SBF solution at 37 °C for 28 days.
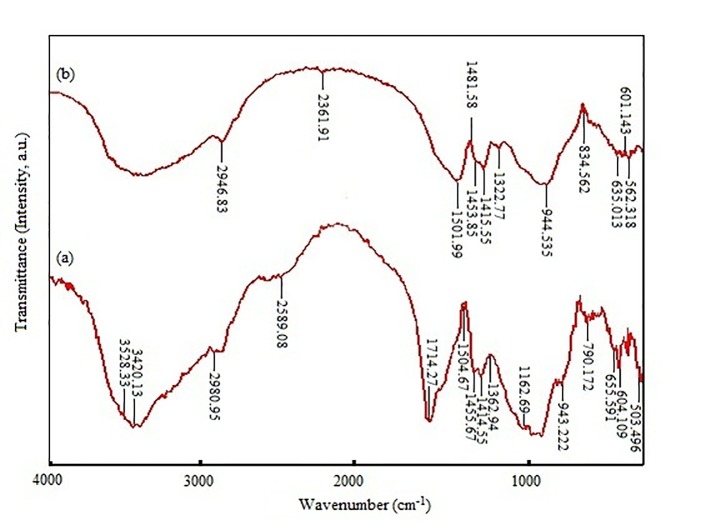
Furthermore, the results of evaluating the bioactivity of glass ionomer powder, carried out by Fourier transform infrared spectrometer, and comparing the powder sample before and after soaking in SBF for 28 days at the temperature of 37 °C, are shown in Figure 5a and b, respectively. According to the FTIR spectra of GIC and GIC-FA composite (Figure 5a and b), it is noticed that the intensity of O-H (3500 cm-1), P-O (600-500 cm-1 and 1100-950 cm-1) and C-O (1440-1335 cm-1) absorption bands [26] have increased after 28 days storage in SBF.
Figure5.
FTIR spectra of the GIC-FA composite: (a) before soaking in SBF, (b) after 28 days of soaking in SBF.
Discussion
In this study, FA particles were incorporated in the synthesized glass ionomer cement. As shown in Figure1, after incorporating the FA particles into the glass ionomer cement, the intense peaks related to the apatite structure appeared in the amorphous matrix of GIC. This was in line with the findings reported previously by Moshavernia et al. [12].
Conventional glass-ionomer cements had low mechanical properties in comparison with other restorative materials, like composite resin or dental amalgam. In order to improve the strength of these materials, the researchers added dispersing agents, such as different powders and fibrous reinforcements, to the cement mixture. Many studies suggested that the mechanical properties were affected by the porosity of the cement, bonding between dispersing agents and the cement matrix, and the properties of particles dispersed through the matrix phase [27-30]. In the present study, mechanical test results indicated that all the GIC samples became stronger when they were incorporated with fluoroapatite nanoparticles; that is to say, nano-FA/ ionomer group exhibited higher compressive strength (with the mean values 74.7 ± 5.6, 102.6 ± 4 and 86.2 ± 4.5 for GIC containing 1, 3 and 5 wt% FA, respectively) in comparison with the control group (with the mean value 46.1 ± 3.4). The increase of compressive strength in the presence of fluoroapatite nanoparticles was due to the formation of crystal phases in the amorphous structure of glass ionomer. Moreover, the combination of glass ionomer particles with larger particle size and FA particles with smaller one led to a wide distribution of particle size. This might cause a high packing density of the combined particles within the glass ionomer matrix, and therefore, a high mechanical strength [31].
Goenka et al. [32] examined the addition of synthesized nanocrystalline calcium deficient hydroxyapatite (nCDHA) by 5, 10 and 15 wt% to the commercial GIC. They concluded that ionic release percentage, weight loss and compressive strength of the nCDHA-GIC composite increased with nCDHA addition.
Li et al. [18] indicated that the mechanical strength of glass ionomer cement improved significantly when it was modified with niobium oxide (Nb2O5). In their study, the compressive strength obtained for Nb2O5- added GIC sample was about 142.25 MPa, which was about 50% higher than that of unmodified commercial GIC (94.81 MPa).
Strength reduction of the composites containing FA particles more than 3 wt% for CS is caused by the reduction of bond forces between ceramic and polymeric components of the GIC. In fact, the added fluoroapatite particles act as a barrier and prevent the complete bonding of the parts of glass ionomer cement [17,12,31]. On the other side, it should be noted that particles with a large specific surface area tend to form agglomerates. This effect can make nanoparticles more difficult to uniformly dispersed into polymer matrix, resulting in a strength reduction [33]. Similar results were also reported by Elsaka et al. [23] and Sayyedan et al. [17] for incorporating TiO2 and forsterite nanoparticles into the glass ionomer cement, respectively.
The potential of apatite formation on the surfaces of cements in the presence of SBF was considered as a measure of their bioactivity behavior. At the first stage, cement samples were immersed in SBF for 28 days, and then their surfaces were analyzed by FTIR and SEM techniques.
After investigating the FTIR spectra of GIC-FA composite, before and after soaking in the SBF for 28 days at 37 °C and pH 7.4 (Figure 5a and b). we found that by immersing the GIC-FA composite in the SBF, the intensity of O-H, P-O and C-O absorption bands increased because of the formation of carbonate hydroxyapatite on the surface of the cement [34]. Formation of apatite layer after soaking in SBF demonstrated the bioactivity behavior of this composite [2].
In addition, having qualitatively compared the scanning electron micrographs of GIC-FA cements presented in Figure 2 (before soaking in SBF) and Figure 4 (after 28 days soaking in SBF), we found that after soaking GIC-FA cements in the simulated body fluid, the rather flat and homogeneous surface of the cement transformed into a rather porous and inhomogeneous one. It is interesting to note that in Figure 4, the observable dispersed particles with the lighter colour are the apatite particles which are precipitated on the cement surface from the SBF [2]. The qualitative comparison made between Figure 4 in this study with Figure 4b and c (presented the surface of the GIC sample after 28 days of soaking in the SBF) in our previous study [2], shows that the rate of apatite’s nucleation on the surface of GIC-FA nanocomposite is more than that on the surface of GIC [34].
Based on the results obtained from scanning electron micrographs and Fourier transform infrared spectra, it can be concluded the produced composite has an appropriate bioactive behavior.
Conclusions
The findings of this study revealed that the addition of synthesized nano-FA to the as-prepared GIC enhanced the compressive strength, especially by adding 3 wt% FA. However, adding more than 3 wt% FA may decrease the compressive strength due to the high tendency of nanoparicles to form agglomerates. Moreover, FTIR and SEM investigations indicated that calcium phosphate layer nucleated on the surface of the sample, and the rather flat and homogeneous surface of the cement has transformed into a rather porous and inhomogeneous one after soaking in SBF solution for a period of time. It can be concluded from the results that the addition of synthesized nano-fluoroapatite to the as-prepared glass ionomer cement makes it a good candidate for dentistry and orthopedic applications.
Footnotes
Conflict of Interests: None declared.
References
- 1.Khaghani M, Golniya Z, Monshi A, et al. Preparation, characterization and bioactivity evaluation of fluor-hroxyapatite (FHA) nano-crystals. Adv Proc Mater. 2013;7:97–106. [Google Scholar]
- 2.Khaghani M, Doost mohammadi A, Golniya Z, et al. Preparation, physicochemical characterization, and bioactivity evaluation of strontium-containing glass ionomer cement. ISRN Ceram. 2013;2013:1–7. [Google Scholar]
- 3.Kannan S, Lemos AF, Ferreira JM. Synthesis and mechanical performance of biological-like hydroxyapatites. Chem Mater. 2006;18:2181–2186. [Google Scholar]
- 4.Komlev VS, Barinov SM, Koplik EV. A method to fabricate porous spherical hydroxyapatite granules intended for time-controlled drug release. Biomaterials. 2002;23:3449–3454. doi: 10.1016/s0142-9612(02)00049-2. [DOI] [PubMed] [Google Scholar]
- 5.Mori K, Yamaguchi K, Hara T, et al. Controlled synthesis of hydroxyapatite-supported palladium complexes as highly efficient heterogeneous catalysts. J Am Chem Soc. 2002;124:11572–11573. doi: 10.1021/ja020444q. [DOI] [PubMed] [Google Scholar]
- 6.Feng Y, Gong JL, Zeng GM, et al. Adsorption of Cd (II) and Zn (II) from aqueous solutions using magnetic hydroxyapatite nanoparticles as adsorbents. Chem Eng J. 2010;162:487–494. [Google Scholar]
- 7.Chen M, Jiang D, Li D, et al. Controllable synthesis of fluorapatite nanocrystals with various morphologies: Effects of pH value and chelating reagent. J Alloy Comp. 2009;485:396–401. [Google Scholar]
- 8.Chinthaka Silva GW, Ma L, Hemmers O, et al. Micro-structural characterization of precipitation-synthesized fluorapatite nano-material by transmission electron microscopy using different sample preparation techniques. Micron. 2008;39:269–274. doi: 10.1016/j.micron.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Sun K, Li A, et al. Size-controlled synthesis and characterization of fluorapatite nanocrystals in the presence of gelatin. Powder Tech. 2011;209:9–14. [Google Scholar]
- 10.Ebrahimi Kahrizsangi R, Nasiri Tabrizi B, Chami A. Characterization of single-crystal fluorapatite nanoparticles synthesized via mechanochemical method. Partic. 2011;9:537–544. [Google Scholar]
- 11.Chen Y, Miao X. Thermal and chemical stability of fluorohydroxyapatite ceramics with different fluorine contents. Biomaterials. 2005;26:1205–1210. doi: 10.1016/j.biomaterials.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 12.Moshaverinia A, Ansari S, Moshaverinia M, et al. Effects of incorporation of hydroxyapatite and fluoroapatite nanobioceramics into conventional glass ionomer cements (GIC) Acta Biomater. 2008;4:432–440. doi: 10.1016/j.actbio.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Kurmaev EZ, Matsuya S, Shin S, et al. Observation of fluorapatite formation under hydrolysis of tetracalcium phosphate in the presence of KF by means of soft X-ray emission and absorption spectroscopy. J Mater Sci Mater Med. 2002;13:33–36. doi: 10.1023/a:1013626316980. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez Lorenzo LM, Hart JN, Gross KA. Influence of fluorine in the synthesis of apatites. Synthesis of solid solutions of hydroxy-fluorapatite. Biomaterials 2003;24:3777–3785. doi: 10.1016/s0142-9612(03)00259-x. [DOI] [PubMed] [Google Scholar]
- 15.Barandehfard F, Keyanpour Rad M, Hosseinnia A, et al. Sonochemical synthesis of hydroxyapatite and fluoroapatite nanosized bioceramics. J Ceram Process Res. 2012;13:437–440. [Google Scholar]
- 16.Shiekh RA, Rahman IAb, Masudi SM, et al. Modification of glassionomercement by incorporating hydroxyapatite-silicanano-powderـcomposite: Sol– gel synthesis and characterization. Ceram Int. 2014;40:3165–3170. [Google Scholar]
- 17.Sayyedan FS, Fathi MH, Edris H, et al. Effect of forsterite nanoparticles on mechanical properties of glass ionomer cements. Ceram Int. 2014;40:10743–10748. [Google Scholar]
- 18.Li S, Su B, Ran J, et al. Effects of Niobium Oxide Addition on the Mechanical Properties of Glass Ionomer Cement. Mater Sci Forum. 2014;815:373–378. [Google Scholar]
- 19.Irie M, Nakai H. Mechanical properties of silver-added glass ionomers and their bond strength to human tooth. Dent Mater J. 1988;7:87–93. doi: 10.4012/dmj.7.87. [DOI] [PubMed] [Google Scholar]
- 20.Farrugia C, Camilleri J. Antimicrobial properties of conventionalrestorative filling materials and advances inantimicrobial properties of composite resins andglass ionomer cements-A literature review. Dent Mater. 2015;31:89–99. doi: 10.1016/j.dental.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Hammouda IM. Reinforcement of conventional glass-ionomer restorative material with short glass fibers. J Mech Behav Biomed Mater. 2009; 2:73–81. doi: 10.1016/j.jmbbm.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Silva RM, Santos PH, Souza LB, et al, Effects Effects of cellulose fibers on the physical and chemical properties of glass ionomer dental restorative materials. Mater Res Bull. 2013;48:118–126. [Google Scholar]
- 23.Elsaka SE, Hamouda IM, Swain MV. Titanium dioxide nanoparticles addition to a conventional glass-ionomer restorative: Influence on physical and antibacterial properties. J Dent. 2011;39:589–598. doi: 10.1016/j.jdent.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Loyola Rodriguez JP, Garcia Godoy F, Lindquist R. Growth inhibition of glass ionomer cements on mutans streptococci. Pediatr Dent. 1994;16:346–349. [PubMed] [Google Scholar]
- 25.Kokubo T, Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials. 2006;27:2907–2915. doi: 10.1016/j.biomaterials.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Wiliana T, Tam KC. Synthesis of amorphous calcium phosphate using various types of cyclodextrins. Mater Res Bull. 2007;42:820–827. [Google Scholar]
- 27.Moshaverinia A, Roohpour N, Chee WWL, et al. A review of powder modifications in conventional glass-ionomer dental cements. J Mater Chem. 2011;21:1319–1328. [Google Scholar]
- 28.Swift EJ, Dogan AU. Analysis of glass-ionomer cement with the use of electron microscopy. J Prothet Dent. 1990;64:167–174. doi: 10.1016/0022-3913(90)90173-a. [DOI] [PubMed] [Google Scholar]
- 29.Miyata N. Dispersion toughening of ceramics. Bull Ceram Soc Japan. 1986;21:605–612. [Google Scholar]
- 30.Kon M, Kawano F, Tada Y, et al. Effect of crystallization on fracture strength of castable glass-ceramics containing two crystals. Dent Mater J. 1994;13:47–54. doi: 10.4012/dmj.13.47. [DOI] [PubMed] [Google Scholar]
- 31.Gu YW, Yap AU, Cheang P, et al. Effects of incorporation of HA/ZrO2 into glass ionomer cement (GIC) Biomaterials. 2005;26:713–720. doi: 10.1016/j.biomaterials.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Goenka S, Balu R, Sampath Kumar TS. Effects of nanocrystalline calcium deficient hydroxyapatite incorporation in glass ionomer cements. J Mech Behav Biomed Mater. 2012;7:69–76. doi: 10.1016/j.jmbbm.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Zhao J, Xie D. Effect of nanoparticles on wear resistance and surface hardness of a dental glass-ionomer cement. J Compos Mater. 2009;43:2739–2752. [Google Scholar]
- 34.Sayyedan FS, Fathi MH, Edris H, et al. Fluoride release and bioactivity evaluation of glass ionomer: Forsterite nanocomposite. Dent Res J. 2013;10:452–459. [PMC free article] [PubMed] [Google Scholar]