Abstract
In this paper, an in vitro basal cytotoxicity testing strategy is described for new chemical entities that lack any pre-existing information on potential toxicity. Special attention is paid to the selection of the cellular system, cytotoxicity assay and exposure conditions. This approach is based on a newly proposed generic adverse outcome pathway from chemical insult to cell death that consists of 3 steps, including initial cell injury, mitochondrial dysfunction and cell demise. The suggested strategy to consider in vitro basal cytotoxicity as a first step in evaluating the toxicity of new chemical entities can be placed in a tiered strategy that could be continued by evaluating more specific types of toxicity.
Keywords: Adverse outcome pathway, chemical, basal cytotoxicity, in vitro experimentation
1. Introduction
Evaluation of safety is a prerequisite prior introduction of new chemical entities onto the market. Historically, animal testing has formed the basis for such risk assessment exercises. Driven by scientific and ethical constraints, and initiated more than 3 decades ago, however, there is a clear tendency worldwide to increasingly address animal-free methods for this purpose. This has been reinforced by a number of legislative changes over the past few years in the European Union, imposing a ban on animal testing for particular groups of chemicals, in casu in the cosmetics field (EU, 2003; EC, 2009). This has been followed by other parts of the world, such as in Norway, Israel, India, New Zealand and the state of São Paulo in Brazil (Laquieze et al., 2015). In response to this ubiquitous matter, the scientific community has been urged to develop animal-free methods for evaluating the safety of chemicals, including in vitro and in silico assays, being a research area that is gaining momentum. Interestingly, this has triggered a paradigm shift from classical toxicology, focusing on apical endpoints for toxicity in animal models, to predictive toxicology, relying on information on mechanisms of toxic action (NRC, 2007; Vinken, 2013).
A major tool adopted in predictive toxicology is the adverse outcome pathway (AOP) framework, which refers to a conceptual construct that portrays existing knowledge concerning the linkage between a direct molecular initiating event (MIE) and an adverse outcome (AO) via a number of key events (KEs) at a biological level of organization relevant to risk assessment. AOPs can serve several purposes pertinent to non-animal chemical risk assessment, such as read-across methods, integrated approaches to testing and assessment, quantitative structure-activity relationships or the elaboration of prioritization strategies (Vinken, 2013 and 2015). In fact, AOPs embody a number of proposed frameworks for the implementation of animal-free safety testing of chemicals. Such frameworks typically start with exposure assessment, physico-chemical profiling, read-across and biokinetic evaluation, all which dictate the subsequent selection of in vitro biomarkers and corresponding assays (Blaauboer et al., 2012). For many new chemical entities, however, such pre-existing information may be scarce, which thus impedes targeted establishment of an in vitro testing battery. In the present paper, a strategy for setting up basal in vitro cytotoxicity testing of such data-poor chemicals is outlined. This is based on a newly proposed generic AOP from chemical insult to cell death.
2. Development of a generic AOP from chemical insult to cell death
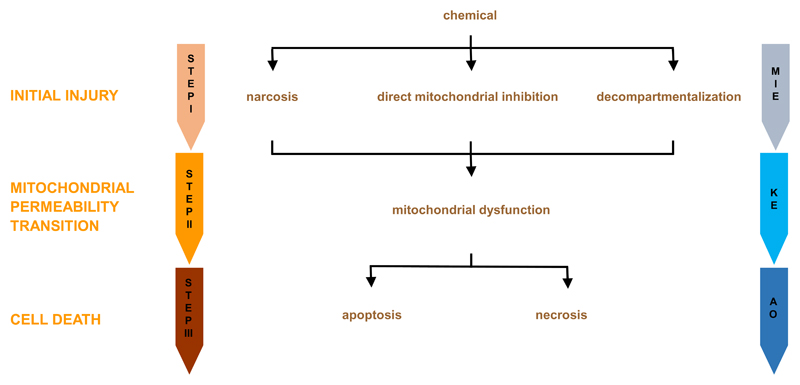
Basal cytotoxicity refers to the ability of a chemical substance to damage living cells, in particular by compromising functional and structural features related to general cellular housekeeping. Being a rather comprehensive term, it is not surprising that the pathways leading to basal cytotoxicity are quite generic (Eisenbrand et al., 2002; Ekwall, 1995; Schoonen et al., 2009). Nevertheless, a tentative AOP to basal cytotoxicity could consist of 3 consecutive steps. The first step (i.e. the MIE) involves initial cell injury caused by the parent chemical and/or its metabolites. In the second step (i.e. the KE), a mitochondrial dysfunction takes place as a consequence of the primary insult. This ultimately leads to cell death in the third step (i.e. the AO) (Figure 1). Each of these steps will be discussed in the following sections.
Figure 1. Generic adverse outcome pathway from chemical insult to cell death.
The first step or the MIE involves initial cell injury, whereby the parent chemical and/or its metabolites cause narcosis, directly impair mitochondrial function or induce decompartmentalization. In the second step, which is a KE, a MPT process takes place as a consequence of the primary insult. This ultimately leads to cell death by apoptosis or necrosis in the third step, being the actual AO.
2.1. Initial injury
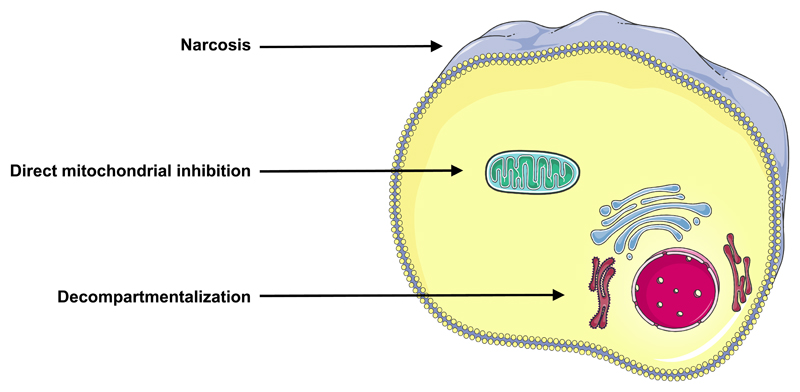
Chemicals can cause direct cell injury through a variety of mechanisms, which may involve a single specific event, such as altered activation of an ion channel (Gennari et al., 2004; Schoonen et al., 2009) or a receptor (Gennari et al., 2004; Houck and Kavlock, 2008). However, a generic AOP from chemical insult to cell death should preferably encompass more general processes that instantly disrupt cellular homeostasis (Figure 2).
Figure 2. Mechanisms of initial injury leading to basal cytotoxicity.
Chemicals can harm plasma membrane integrity through accumulation and binding to the phospholipid bilayer, called narcosis. Chemicals can negatively affect cellular energy supplies by targeting mitochondria. Chemicals can compromise subcellular architectural organization, called decompartmentalization.
A first mechanism in this respect is disturbance of plasma membrane integrity. A prerequisite for performing cellular functionality includes appropriate physical segregation between the extracellular environment and the cytosol, which contributes to selective passage of substances between both compartments. This is accomplished to a large extent by a solid double phospholipid layer. Damage to this plasma membrane induced by chemicals can occur in a number ways, of which accumulation and binding to the phospholipid bilayer, a process called narcosis, is a prominent one (Escher et al., 2002).
A second mechanism relates to interfering with subcellular architectural organization. In order to maintain homeostasis, cellular functions are restricted to specific organelles within the cell, such as the nucleus, where genetic material is stored and processed, or the rough endoplasmic reticulum, taking care of protein synthesis. This so-called compartmentalization may be compromised by chemicals, thereby jeopardizing overall cellular functionality (Eisenbrand et al., 2002; Schoonen et al., 2009).
A third mechanism involves directly negatively affecting cellular energy supplies, in particular by targeting mitochondria. Thus, chemicals may uncouple the mitochondrial respiratory chain, inhibit adenosine triphosphate (ATP) synthesis, damage mitochondrial deoxyribonucleic acid (DNA), interfere with the replication of mitochondrial DNA or decrease the synthesis and stability of mitochondrial transcripts (Jones et al., 2010; Pessayre et al., 2010).
2.2. Mytochondrial dysfunction
Mitochondria are considered as the fuel stations of the cell. In this context, pyruvate, produced from glucose through the process of glycolysis, is taken up by mitochondria and is transformed to acetylco-enzyme A. In a parallel pathway, fatty acids bound to acetylco-enzyme A enter the mitochondrion, where they are split by successive beta-oxidation cycles, also yielding acetylco-enzyme A. The latter is then converted into carbon dioxide through the tricarboxylic acid cycle. This is associated with the production of nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide. Both are oxidized, whereby electrons are generated that are used to reduce molecular oxygen to water. This reaction, catalyzed by respiratory chain complexes, is coupled with the extrusion of protons from the matrix into the inner membrane space. When energy is needed, the protons re-enter the matrix through ATP synthetase to generate ATP from adenosine diphosphate (Jones et al., 2010; Pessayre et al., 2010).
Being critical and vital, this mitochondrial process inherently is vulnerable, as there are many potential sites for toxic chemicals to act upon. An important one relates to the mitochondrial permeability transition (MPT) pore. This is a complex megachannel that spans across the inner and outer membrane of the mitochondrion and that consists of several proteins, including the voltage-dependent anion channel, adenine nucleotide translocase, cyclophilin D, creatine kinase, hexokinase, the benzodiazepine peripheral receptor and some B-cell lymphoma 2 proteins (Juhaszova et al., 2008; Tsujimoto and Shimizu, 2007). The MPT pore is usually closed, though it can be opened by some xenobiotics. This leads to the release of molecules, such as cytochrome C, into the cytosol, or the uptake of substances, including protons and water, in the mitochondrial matrix. If the MPT pore opens instantly in a large number of mitochondria, severe ATP depletion occurs. This is detrimental for many cellular functions that rely on energy and results in the disequilibrium of critical ion levels, eventually causing necrosis. Alternatively, when the MPT pore only opens in a limited set of mitochondria, unaffected mitochondria continue to produce ATP, while disrupted mitochondria release cytochrome C. This ultimately activates the apoptotic signaling cascade (Jones et al., 2010; Pessayre et al., 2012; Tsujimoto and Shimizu, 2007).
2.3. Cell death
Apoptosis is a programmed cell death mode that relies on the proteolytic activity of caspases. As such, 2 major apoptotic pathways have been described, namely the extrinsic signaling cascade and the intrinsic pathway. The latter is initiated by stimulating the release of cytochrome C from mitochondria, a process that is controlled by pro-apoptotic and anti-apoptotic B-cell lymphoma 2 proteins. Liberated cytochrome C forms a so-called apoptosome with apoptotic protease activating factor 1, deoxyadenosine triphosphate and procaspase 9. Following activation, the apoptosome triggers caspase 3. In the extrinsic pathway, specific ligands, such as Fas ligand, bind to their corresponding receptors at the cell plasma membrane surface. This induces the recruitment and cleavage of procaspase 8, which in turn activates caspase 3. Fas-expressing cells are classified as either type I or type II based upon the ability of the intrinsic pathway to contribute to the amplification of the caspase cascade activated through the extrinsic pathway. In type I cells, sufficient amounts of caspase 8 are produced through this route and caspase 3 becomes directly activated. By contrast, in type II cells, only minimal quantities of active caspase 8 can be generated and the execution of the apoptotic response requires mitochondrial amplification. In this scenario, caspase 8 activates the pro-apoptotic Bid protein, which then translocates to mitochondria, where it leads to the release of cytochrome C and apoptosome formation. The overall outcome of the apoptotic pathways is the activation of caspase 3, which is the main executor of apoptosis. In fact, caspase 3 cleaves a broad spectrum of cellular proteins, such as cytoskeletal proteins, which gives rise to the typical apoptotic phenotype, involving blebbing, cell shrinkage, cytoplasmic and nuclear condensation, DNA fragmentation and the formation of apoptotic bodies. These apoptotic bodies are rapidly engulfed by neighboring phagocytes and no inflammatory response is induced (Alkhouri et al., 2011, Au et al., 2011; Guicciardi and Gores, 2005; Mahli et al., 2006; Schulze-Bergkamen et al., 2006; St-Pierre and Dufour, 2012).
Necrosis, as opposed to apoptosis, is a rather passive and unorganized process that is caused by a plethora of stress factors. It usually starts with the loss of ion homeostasis, which activates proteases, endonucleases and phospholipases. This generally results in cell swelling, cell lysis and induction of inflammation (Au et al., 2011; Grattagliano et al., 2009; Mahli et al., 2006; Schulze-Bergkamen et al., 2006).
3. Set-up of AOP-based in vitro testing of basal cytotoxicity
In case of lack of any pre-existing information on potential toxicity of a test compound towards a specific cell type whatsoever, a general cytotoxicity testing scheme could be set up supported by the proposed generic AOP from chemical insult to cell death. A number of factors related to the selection of the cellular system, cytotoxicity assay and exposure conditions must be considered prior organizing such default in vitro cytotoxicity testing trials. These factors will be discussed in the following sections, with many of them being illustrated by knowledge gained from the use of in vitro systems based on liver, which is one of the most prominent target organs for toxicity.
3.1. Selection of the cellular system
A prerequisite for reliably predicting human real-life cytotoxicity is the use of an in vitro system in which all critical biological targets, as depicted in the generic AOP from chemical insult to cell death, are phenotypically expressed at an in vivo-like level for the entire testing regime. Human-based in vitro models are obviously strongly preferred, although this may be limited because of ethical, financial and other reasons (Fraczek et al., 2013; Vinken et al., 2012). Human-relevant information can also be obtained to a large extent when addressing systems based on other species, such as rodents. Nevertheless, this may introduce interspecies differences, which should be taken into consideration while interpreting testing results. Such inherent discrepancies between humans and rodents not only apply to tissue-specific (patho)physiological functions, but have been equally reported for seemingly more generic processes, such as inflammation (Seok et al., 2013).
Cytotoxicity sensu stricto is not a cell type-specific process, yet some tissues may be more susceptible to this process than others. As the human body consists of a large repertoire of cell types, the selection of the cellular origin of the in vitro model therefore is another critical parameter. When suspecting major mitochondrial damage induced by the chemical under investigation, one might consider a cell type that is naturally endowed with a high number of mitochondria, such as hepatocytes, myocytes or adipocytes. In most cases, however, a more common type of cell is used, such as fibroblasts.
Because of practical reasons, cell lines are more frequently used compared to primary cells for cytotoxicity testing purposes. Indeed, cell lines foresee a virtually unlimited cell supply, perform better in terms or reproducibility, are less labor-intensive and thus more users-friendly in comparison with most primary cell systems, many of which are prone to rapidly progressing dedifferentiation (Vinken et al., 2012). A number of human and rodent cell lines have been shown sensitive to chemical-induced cytotoxicity, including human embryonic kidney HEK293cells, human T-cell leukemia Jurkat cells, human neuroblastoma SH-SY5Y cells, mouse neuroblastoma N2a cells, mouse embryonic NIH3T3 fibroblasts and rat hepatoma H-4-II-E cells (Shukla et al., 2010). Nevertheless, it should be kept in mind that a majority of cell lines originate from cancers, which implies that they may perform aberrant functionality. This specifically holds true for the biotransformation machinery, which may be defective or even lacking in cell lines, but that may be indispensable for bio-activation or de-activation of chemicals (Fraczek et al., 2013; Vinken et al., 2012). Furthermore, cancer cells display altered cell death potential (Vinken et al., 2014). Regarding the latter, the background level of cell death in the selected in vitro model should be reduced as much as possible, as this may interfere with the testing outcome. This has been exemplified for conventional monolayer cultures primary of rat hepatocytes, which cope with substantial spontaneous apoptosis and necrosis (Vinken et al., 2011).
Whenever possible, it may be advisable to seed cells on small format culture plates (i.e. 96-well plates). This carries a number of advantages, including reduction of cellular and testing material, and thus of overall costs, as well as high-throughput potential. A pivotal factor in this respect is the density at which the cells are seeded, as both too low and too high densities may induce cell death (Qiao and Farrell, 1999). Similarly, the substrata used for cell seeding strongly affect viability. Thus, cultivating cells on an extracellular matrix scaffold promotes attachment and thereby cell survival (Vanhaecke et al., 2004). In the same light, the composition of the cell culture medium is of key importance while setting up cytotoxicity tests. A variety of culture media is commercially available, including Dulbecco’s modified Eagle’s medium, William’s medium E and Leibovitz’s L15 medium, all which are typically supplemented with a number of additives (Elaut et al., 2005). Among those, several ones counteract spontaneous cell death, such as serum, typically added to increase cell attachment, (Tuschl et al., 2009) and glucocorticosteroids, which positively affect the differentiated cellular phenotype (Bailly-Maitre et al., 2002). As many cytotoxicity tests are based on measuring release of components from cells into the cell culture medium as a function of time following injury, the frequency of cell culture media renewal and, linked to this, the time of sampling should be carefully selected. In this regard, while some cultivation protocols only foresee renewal of cell culture media every 3 days, others, especially those using primary cells, require daily refreshment of the cell culture medium.
3.2. Selection of cytotoxicity assays
In vitro cytotoxicity testing of chemicals for which no pre-existing toxicologically relevant information is available could rely on the proposed generic AOP from chemical insult to cell death and should include at least 2 assays to test 2 KEs. Given their indispensable role in the initiation and perpetuation of cytotoxicity, mitochondria seem ideal biomarkers of chemical-induced cellular damage. Their activity can be monitored by a number of tests, of which the [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (MTT) assay undoubtedly is the most commonly used one (Mosmann, 1983). The MTT cytotoxicity assay is a colorimetric method based on the ability of viable cells to reduce a yellow tetrazolium salt into a blue insoluble formazan that is retained inside cells. The addition of organic solvents, such as dimethylsulfoxide (DMSO), leads to solubilization of the formed formazan and its release into the culture medium allowing its colorimetric measurement. The mitochondrial enzyme succinate dehydrogenase is responsible for the reduction of the tetrazolium salt to formazan. The ability of cells to reduce MTT provides an indication of mitochondrial integrity and activity, which in turn may be interpreted as a measure of viability and/or cell number. The number of surviving cells is directly proportional to the level of the formazan product created. Typically, IC50 and IC10 values are established in MTT testing, thus concentrations of the test compound that trigger cell death in 50% and 10% of the cultured cells, respectively (Tolosa et al., 2015). A number of other tetrazolium salts, such as 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide, and water-soluble tetrazolium salts can be used for the same purpose (Gomez-Lechon et al., 2010). While setting up cell cultures for MTT testing, colored culture medium additives, such as phenol red, must be avoided, as they can interfere with formazan color development. Furthermore, for some test compounds, including chemicals that may directly reduce tetrazolium salts, the MTT assay is not applicable (Tolosa et al., 2015). A variety of alternative assays can be addressed instead, such as bioluminescent measurement of ATP content and accumulation of the supravital dye neutral red in lysosomes (Babich and Borenfreund, 1987; https://ecvam-dbalm.jrc.ec.europa.eu/methods-and-protocols/protocol/balb-c-3t3-neutral-red-uptake-cytotoxicity-assay-(3t3-nru)-protocol-no.-139/key/p_1527). In fact, the latter, like the MTT test, has been reported to be most sensitive in detecting cytotoxic events (Fotakis and Timbrell, 2006).
A second KE to be tested could be plasma membrane damage. As a consequence of the compromised cell plasma integrity, cytosolic compounds can freely move outside the cell into the cell culture medium. Among those is lactate dehydrogenase (LDH), a stable enzyme that leaks from the cell in relatively high amounts upon cell plasma membrane damage. LDH catalyzes the interconversion of pyruvate and lactate with concomitant interconversion of reduced and oxidized NADH. The consumption of NADH can be spectrophotometrically assessed and serves as a measure that is proportional to LDH activity (Bergmeyer, 1974). A parameter that is routinely used in this context is the LDH index, which is the ratio of LDH activity in the cell culture medium over the total LDH activity (i.e. in cells and in the cell culture medium). Typically, the cut-off is set at 20%, with an LDH index above this value indicating cytotoxicity (Maes et al., 2015). Other cytotoxicity techniques that are based on chemical insults to the plasma membrane surface use reporter dyes that, upon addition to the cell culture medium, move inside of damaged cells, such as propidium iodide and Trypan blue (Gomez-Lechon et al., 2010).
3.3. Selection of exposure conditions
In quantifying the cytotoxic potential of a chemical, it is important to take into account the relevance of the tested concentrations. Two issues must be considered in this respect, namely the relevant concentration in the in vitro system and its relationship to the in vivo situation. The first issue relates to the biokinetic behaviour of the compound in the in vitro system. The actual concentration causing the toxic effect is not only determined by the amount of the compound added to the cell culture medium divided by the volume thereof (i.e. the nominal concentration). Processes like evaporation, binding to culture devices or cell culture medium constituents, such as proteins, might lead to deviations in the actual cellular exposure concentrations, sometimes even higher than 2 orders of magnitude. This may result in a much lower free concentration in the cell culture medium and consequently a considerable underestimation of the compound’s cytotoxic potential (Kramer et al., 2007). On the other hand, compounds might specifically accumulate in cells or in cellular compartments, including at the outer membrane, thereby leading to a higher narcotic potential. Therefore, these in vitro biokinetic processes are essential in determining the relevance of the in vitro testing conditions (Groothuis et al, 2015; Kramer et al., 2015).
The second issue is the proper translation of the in vitro cytotoxicity results in a risk assessment context (Blaauboer et al., 2012). This is the process of so-called quantitative in vitro-in vivo extrapolation and needs to take into account any differences in the exposure of cells in the in vitro setting as compared to the in vivo situation as well as the in vivo biokinetics (Yoon et al., 2015). For the latter issue, the use of physiologically-based biokinetic modelling is an important tool (Blaauboer, 2010).
In case no information at all is available about the anticipated cytotoxic concentrations, such as to be derived from structural or physico-chemical properties, it is recommend to perform at least 2 testing rounds, each including 3 biological (i.e. different cell batches) and 3 technical (i.e. different wells on a multiwell plate) repeats. In the first run, a minimum of 10 concentrations spread over a broad concentration range (i.e. 1 nM to 10 mM) should be tested. In the second run, the concentration frame should be narrowed down, usually in the µM range, and, if necessary, further fine-tuned in additional testing rounds. Perhaps an even more important aspect of the testing regime includes the time of exposure. For some toxicological responses, critical changes in gene expression patterns are already induced and detectable within 1 hour of exposure of cultured cells to test chemicals and only vary marginally with increasing concentration (Shinde et al., 2015). In most general cytotoxicity testing procedures, however, exposure times between 1 hour and 72 hours are applied, but as holds for concentration, this parameter may also warrant optimization. Furthermore, it is strongly recommended to determine the biokinetics in vitro, such as by measuring the free concentration, for which tools like solid-phase micro-extraction are available (Kramer et al., 2007; Vaes et al., 1997).
Implementation of an appropriate set of controls is of utmost importance for sound interpretation of in vitro cytotoxicity testing results. In a majority of cases, test compounds are not fully soluble in cell culture media and thus require a co-solvent, such as DMSO, ethanol or methanol. While the latter 2 are well known to act as cytotoxicants, DMSO is sometimes added to cell culture media, such as of cultured hepatocytes, because of its beneficial effects on cell functionality. However, DMSO may also cause cell damage. In a recent study, it was found that concentrations of both DMSO and ethanol exceeding 0.5% v/v induce cyototoxicity in cultures of human breast cancer MCF-7 cells, human umbilical vein endothelial cells and mouse RAW264.7 macrophages after 24 hours of exposure as judged on MTT testing (Jamalzadeh et al., 2016). Therefore, it is strictly necessary to include a solvent control in cytotoxicity testing when using such organic liquids to improve the solubility of test compounds. Simultaneously, a positive control must be tested, being a compound known to potentially trigger the biomarker of interest. Tamoxifen has been proven an appropriate positive control in MTT testing (Tolosa et al., 2015) and ATP-based cytotoxicity assays (Shukla et al., 2010), while compounds that harm the cell plasma membrane surface, such as sodium lauryl sulphate, may be applied as positive controls for the LDH leakage assay and reporter dye uptake tests (https://ecvam-dbalm.jrc.ec.europa.eu/methods-and-protocols/protocol/balb-c-3t3-neutral-red-uptake-cytotoxicity-assay-(3t3-nru)-protocol-no.-139/key/p_1527; Maes et al., 2015). Negative controls, although sometimes ignored, are equally important as their positive counterparts. The most obvious negative control is cell culture medium, yet for a number of reasons, it may be advisable to address specific chemicals as true negative controls. Typical negative controls in in vitro cytotoxicity tests are seemingly innocuous molecules, such as mannitol. Complying with Paracelsus’ basic principle stating that the dose makes the poison, however, it should be kept in mind that such apparent harmless chemicals may become toxic in sufficiently high concentrations, being osmotic stress in case of mannitol. Furthermore, care must be taken while adding controls and test compounds to the cell culture medium as well as while handling cell culture plates, since mechanical stress can also cause cytotoxicity. In case of suspected phototoxicity or temperature instability of the test compound, specific incubation measures could be necessary (Coecke et al., 2005; Cooper-Hannan et al., 1999).
4. Conclusions and perspectives
Safety evaluation of chemicals has drastically changed in the last decades, thereby moving to testing schemes fully devoid of animal experimentation. AOPs have emerged as important drivers of such animal-free testing approaches. A wide repertoire of AOPs has been introduced in recent years for a broad variety of specific toxicological endpoints, most of which are harbored in the AOP wiki. The latter is part of the AOP Knowledge Base, which has been initiated by the Organization for Economic Cooperation and Development together with the US Environmental Protection Agency, the US Army Engineer Research and Development Center and the European Joint Research Center. It provides an open-source interface for rapid, widely accessible and collaborative sharing of established AOPs and building new AOPs (https://aopwiki.org/). However, for a number of common and highly prevalent toxicological events, such as basal cytotoxicity, AOPs are currently still lacking. For this reason, an attempt was made in the present paper to establish a generic AOP from chemical insult to cell death, consisting of 3 steps, including initial cell injury, mitochondrial dysfunction and cell demise. As holds for AOPs in general, the proposed construct should be considered as an open and flexible structure that should be continuously refined. During such iterative refinement exercises, particular attention should be paid to quantification, which is an absolute conditio sine qua non for implementation of AOPs into regulatory risk assessment. This can be achieved in several ways, such as by establishing dose/concentration-effect relationships for the MIE and/or KEs. Simultaneously, kinetic features should be included in AOPs, which may be critical for determining overall exposure and KE relationships (Vinken, 2013 and 2015).
Being pragmatic tools, the extent of AOP optimization typically depends on the intended use. In this respect, the postulated AOP from chemical insult to cell death may already be considered fit-for-purpose, namely in vitro basal cytotoxicity testing of new chemical entities with non-substantiated toxicological profiles. Such default in vitro toxicity strategy could consist of at least 2 major testing rounds. In a first run, outlined in the present paper, basal cytotoxicity can be tested using a minimum of 2 assays to assess 2 KEs. This may imply a number of repetitive runs in itself in order to optimize exposure conditions, in particular the concentration range of the test compound. This could be followed by global toxicogenomics screening, which will provide more insight into the tentative mechanism of action of the test compound. This outcome, together with the result of the first testing run, largely dictates the selection of methods in the second testing run (Blaauboer et al., 2012). In fact, the latter rather addresses suspected tissue-specific toxicity, thereby selecting biomarkers and assays that rely on the respective AOPs, such as available for specific types of hepatotoxicity, renal toxicity and neurotoxicity (https://aopwiki.org/). It should be stressed in this respect that AOPs, although presented as such, are not stand-alone linear events, but are typically part of intertwined processes of parallel cascades and crossing pathways. Consequently, AOPs can share KEs and hence a single biomarker and corresponding assay may be used for the detection of more than one AOP. This particularly holds true for mitochondrial impairment. In this regard, great promise for future in vitro toxicology lies with so-called cytomics approaches that allow simultaneous monitoring of a multitude of read-outs in real-time modus, including those related to mitochondrial dysfunction. When applied to miniaturized cell culture formats, such as 384-well or 1536-well plates, such strategies can be run in high-throughput, as already successfully initiated in the US ToxCast program (https://www.epa.gov/chemical-research/toxicity-forecasting). Further exploration of such approaches, and thus the concomitant implementation and optimization of the newly postulated AOP from chemical insult to cell death, should be strongly encouraged, as they will enable to meet the ever increasing safety measures for new chemical entities whilst reducing or even fully replacing animal experimentation.
Highlights.
-
-
A generic adverse outcome pathway from chemical insult to cell death is established, comprising 3 steps, including initial cell injury, mitochondrial dysfunction and cell demise.
-
-
An in vitro basal cytotoxicity testing strategy is proposed for new chemical entities with data-poor toxicity profiles.
Acknowledgements
This work was funded by grants of the European Research Council (Starting Grant 335476), the Fund for Scientific Research-Flanders (FWO grants G009514N and G010214N) and the University Hospital of the Vrije Universiteit Brussel-Belgium (“Willy Gepts Fonds” UZ-VUB).
Abbreviations
- AO(P)
adverse outcome (pathway)
- ATP
adenosine triphosphate
- DMSO
dimethylsulfoxide
- DNA
deoxyribonucleic acid
- KE
key event
- LDH
lactate dehydrogenase
- MIE
molecular initiating event
- MTP
mitochondrial permeability transition
- MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]
- NADH
nicotinamide adenine dinucleotide.
Footnotes
Declaration of interest
The authors report no declarations of interest.
References
- Alkhouri N, Carter-Kent C, Feldstein AE. Apoptosis in nonalcoholic fatty liver disease: diagnostic and therapeutic implications. Expert Rev Gastroenterol Hepatol. 2011;5:201–212. doi: 10.1586/egh.11.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au JS, Navarro VJ, Rossi S. Drug-induced liver injury: its pathophysiology and evolving diagnostic tools. Aliment Pharmacol Ther. 2011;34:11–20. doi: 10.1111/j.1365-2036.2011.04674.x. [DOI] [PubMed] [Google Scholar]
- Babich H, Borenfreund E. Structure-activity relationship (SAR) models established in vitro with the neutral red cytotoxicity assay. Toxicol In Vitro. 1987;1:3–9. doi: 10.1016/0887-2333(87)90031-2. [DOI] [PubMed] [Google Scholar]
- Bailly-Maitre B, de Sousa G, Zucchini N, Gugenheim J, Boulukos KE, Rahmani R. Spontaneous apoptosis in primary cultures of human and rat hepatocytes: molecular mechanisms and regulation by dexamethasone. Cell Death Differ. 2002;9:945–955. doi: 10.1038/sj.cdd.4401043. [DOI] [PubMed] [Google Scholar]
- Bergmeyer HU. Lactate dehydrogenase. In: Bergmeyer HU, editor. Methods of enzymatic analysis. New York, USA: Academic Press; 1974. pp. 574–579. [Google Scholar]
- Blaauboer BJ. Biokinetic modeling and in vitro-in vivo extrapolations. J Toxicol Environ Health B Crit Rev. 2010;13:242–252. doi: 10.1080/10937404.2010.483940. [DOI] [PubMed] [Google Scholar]
- Blaauboer BJ, Boekelheide K, Clewell HJ, Daneshian M, Dingemans MM, Goldberg AM, Heneweer M, Jaworska J, Kramer NI, Leist M, Seibert H, et al. The use of biomarkers of toxicity for integrating in vitro hazard estimates into risk assessment for humans. Altern Lab Anim. 2012;29:411–425. doi: 10.14573/altex.2012.4.411. [DOI] [PubMed] [Google Scholar]
- Coecke S, Balls M, Bowe G, Davis J, Gstraunthaler G, Hartung T, Hay R, Merten OW, Price A, Schechtman L, Stacey G, et al. Guidance on good cell culture practice. a report of the second ECVAM task force on good cell culture practice. Altern Lab Anim. 2005;33:261–287. doi: 10.1177/026119290503300313. [DOI] [PubMed] [Google Scholar]
- Cooper-Hannan R, Harbell JW, Coecke S, Balls M, Bowe G, Cervinka M, Clothier R, Hermann F, Klahm LK, de Lange J, Liebsch M, et al. The principles of good laboratory practice: application to in vitro toxicology studies. Altern Lab Anim. 1999;27:539–577. doi: 10.1177/026119299902700410. [DOI] [PubMed] [Google Scholar]
- Ekwall B. The basal cytotoxicity concept. In: Goldberg AM, van Zupthen LFM, editors. Alternative methods in toxicology and the life sciences: the World Congress on Alternatives and Animal Use in the Life Sciences: Education, Researchers, Testing. New York, USA: Mary Ann Liebert; 1995. pp. 721–725. [Google Scholar]
- Elaut G, Vanhaecke T, Heyden YV, Rogiers V. Spontaneous apoptosis, necrosis, energy status, glutathione levels and biotransformation capacities of isolated rat hepatocytes in suspension: effect of the incubation medium. Biochem Pharmacol. 2005;69:1829–1838. doi: 10.1016/j.bcp.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Eisenbrand G, Pool-Zobel B, Baker V, Balls M, Blaauboer BJ, Boobis A, Carere A, Kevekordes S, Lhuguenot JC, Pieters R, Kleiner J. Methods of in vitro toxicology. Food Chem Toxicol. 2002;40:193–236. doi: 10.1016/s0278-6915(01)00118-1. [DOI] [PubMed] [Google Scholar]
- Escher BI, Eggen RI, Schreiber U, Schreiber Z, Vye E, Wisner B, Schwarzenbach RP. Baseline toxicity (narcosis) of organic chemicals determined by in vitro membrane potential measurements in energy-transducing membranes. Environ Sci Technol. 2002;36:1971–1979. doi: 10.1021/es015844c. [DOI] [PubMed] [Google Scholar]
- EU. Directive 2003/15/EC of the European Parliament and of the Council of 27 February 2003 amending Council Directive 76/768/EEC on the approximation of the laws of the Member States relating to cosmetic products. Official Journal of the European Union. 2003;L066:26–35. [Google Scholar]
- EU. Regulation (EC) No. 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products (recast) Official Journal of the European Union. 2009;L342:59–209. [Google Scholar]
- Fotakis G, Timbrell JA. In vitro cytotoxicity assays: comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol Lett. 2006;160:171–177. doi: 10.1016/j.toxlet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Fraczek J, Bolleyn J, Vanhaecke T, Rogiers V, Vinken M. Primary hepatocyte cultures for pharmaco-toxicological studies: at the busy crossroad of various anti-dedifferentiation strategies. Arch Toxicol. 2013;87:577–610. doi: 10.1007/s00204-012-0983-3. [DOI] [PubMed] [Google Scholar]
- Gennari A, van den Berghe C, Casati S, Castell J, Clemedson C, Coecke S, Colombo A, Curren R, Dal Negro G, Goldberg A, Gosmore C, et al. Strategies to replace in vivo acute systemic toxicity testing: the report and recommendations of ECVAM Workshop 50. Altern Lab Anim. 2004;32:437–459. doi: 10.1177/026119290403200417. [DOI] [PubMed] [Google Scholar]
- Gomez-Lechon MJ, Lahoz A, Gombau L, Castell JV, Donato MT. In vitro evaluation of potential hepatotoxicity induced by drugs. Curr Pharm Des. 2010;16:1963–1977. doi: 10.2174/138161210791208910. [DOI] [PubMed] [Google Scholar]
- Grattagliano I, Bonfrate L, Diogo CV, Wang HH, Wang DQ, Portincasa P. Biochemical mechanisms in drug-induced liver injury: certainties and doubts. World J Gastroenterol. 2009;15:4865–4876. doi: 10.3748/wjg.15.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groothuis FA, Heringa MB, Nicol B, Hermens JL, Blaauboer BJ, Kramer NI. Dose metric considerations in in vitro assays to improve quantitative in vitro-in vivo dose extrapolations. Toxicology. 2015;332:30–40. doi: 10.1016/j.tox.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Guicciardi ME, Gores GJ. Apoptosis: a mechanism of acute and chronic liver injury. Gut. 2005;54:1024–1033. doi: 10.1136/gut.2004.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houck KA, Kavlock RJ. Understanding mechanisms of toxicity: insights from drug discovery research. Toxicol Appl Pharmacol. 2008;227:163–178. doi: 10.1016/j.taap.2007.10.022. [DOI] [PubMed] [Google Scholar]
- https://aopwiki.org/wiki/index.php/Main_Page (consulted November 2016)
- https://ecvam-dbalm.jrc.ec.europa.eu/methods-and-protocols/protocol/balb-c-3t3-neutral-red-uptake-cytotoxicity-assay-(3t3-nru)-protocol-no.-139/key/p_1527 (consulted November 2016)
- https://www.epa.gov/chemical-research/toxicity-forecasting (consulted November 2016)
- Jamalzadeh L, Ghafoori H, Sariri R, Rabuti H, Nasirzade J, Hasani H, Aghamaali MR. Cytotoxic effects of some common organic solvents on MCF-7, RAW-264.7 and human umbilical vein endothelial cells. Avicenna J Med Biochem. 2016:e33453. [Google Scholar]
- Jones DP, Lemasters JJ, Han D, Boelsterli UA, Kaplowitz N. Mechanisms of pathogenesis in drug hepatotoxicity putting the stress on mitochondria. Mol Interv. 2010;10:98–111. doi: 10.1124/mi.10.2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhaszova M, Wang S, Zorov DB, Nuss HB, Gleichmann M, Mattson MP, Sollott SJ. The identity and regulation of the mitochondrial permeability transition pore: where the known meets the unknown. Ann NY Acad Sci. 2008;1123:197–212. doi: 10.1196/annals.1420.023. [DOI] [PubMed] [Google Scholar]
- Kramer NI, Van Eijkeren JCH, Hermens JLM. Influence of albumin on sorption kinetics in solid-phase microextraction: consequences for chemical analyses and uptake processes. Anal Chem. 2007;79:6941–6948. doi: 10.1021/ac070574n. [DOI] [PubMed] [Google Scholar]
- Kramer NI, Di Consiglio E, Blaauboer BJ, Testai E. Biokinetics in repeated-dosing in vitro drug toxicity studies. Toxicol In Vitro. 2015;30:217–224. doi: 10.1016/j.tiv.2015.09.005. [DOI] [PubMed] [Google Scholar]
- Laquieze L, Lorencini M, Granjeiro JM. Alternative methods to animal testing and cosmetic safety: an update on regulations and ethical considerations in Brazil. Appl In Vitro Toxicol. 2015;1:243–253. [Google Scholar]
- Maes M, Vanhaecke T, Cogliati B, Yanguas SC, Willebrords J, Rogiers V, Vinken M. Measurement of apoptotic and necrotic cell death in primary hepatocyte cultures. Methods Mol Biol. 2015;1250:349–361. doi: 10.1007/978-1-4939-2074-7_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi H, Gores GJ, Lemasters JJ. Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology. 2006;43:S31–S44. doi: 10.1002/hep.21062. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- NRC. Toxicity testing in the 21st century: a vision and a strategy. The National Academies Press; Washington, DC: 2007. [Google Scholar]
- Pessayre D, Mansouri A, Berson A, Fromenty B. Mitochondrial involvement in drug-induced liver injury. Handb Exp Pharmacol. 2010;196:311–365. doi: 10.1007/978-3-642-00663-0_11. [DOI] [PubMed] [Google Scholar]
- Pessayre D, Fromenty B, Berson A, Robin MA, Letteron P, Moreau R, Mansouri A. Central role of mitochondria in druginduced liver injury. Drug Metab Rev. 2012;44:34–87. doi: 10.3109/03602532.2011.604086. [DOI] [PubMed] [Google Scholar]
- Qiao L, Farrell GC. The effects of cell density, attachment substratum and dexamethasone on spontaneous apoptosis of rat hepatocytes in primary culture. In Vitro Cell Dev Biol Anim. 1999;35:417–424. doi: 10.1007/s11626-999-0117-2. [DOI] [PubMed] [Google Scholar]
- Schoonen WG, Westerink WM, Horbach GJ. High-throughput screening for analysis of in vitro toxicity. EXS. 2009;99:401–452. doi: 10.1007/978-3-7643-8336-7_14. [DOI] [PubMed] [Google Scholar]
- Schulze-Bergkamen H, Schuchmann M, Fleischer B, Galle PR. The role of apoptosis versus oncotic necrosis in liver injury: facts or faith? J Hepatol. 2006;44:984–993. doi: 10.1016/j.jhep.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinde V, Stöber R, Nemade H, Sotiriadou I, Hescheler J, Hengstler J, Sachinidis A. Transcriptomics of hepatocytes treated with toxicants for investigating molecular Mechanisms underlying hepatotoxicity. Methods Mol Biol. 2015;1250:225–240. doi: 10.1007/978-1-4939-2074-7_16. [DOI] [PubMed] [Google Scholar]
- Shukla SJ, Huang R, Austin CP, Xia M. The future of toxicity testing: a focus on in vitro methods using a quantitative high-throughput screening platform. Drug Discov Today. 2010;15:997–1007. doi: 10.1016/j.drudis.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre MV, Dufour JF. Biomarkers for hepatocellular apoptosis in the management of liver diseases. Curr Pharm Biotechnol. 2012;13:2221–2227. doi: 10.2174/138920112802502097. [DOI] [PubMed] [Google Scholar]
- Tolosa L, Donato MT, Gomez-Lechon MJ. General cytotoxicity assessment by means of the MTT assay. Methods Mol Biol. 2015;1250:333–348. doi: 10.1007/978-1-4939-2074-7_26. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y, Shimizu S. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis. 2007;12:835–840. doi: 10.1007/s10495-006-0525-7. [DOI] [PubMed] [Google Scholar]
- Tuschl G, Hrach J, Walter Y, Hewitt PG, Mueller SO. Serum-free collagen sandwich cultures of adult rat hepatocytes maintain liver-like properties long term: a valuable model for in vitro toxicity and drug-drug interaction studies. Chem Biol Interact. 2009;181:124–137. doi: 10.1016/j.cbi.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Vaes WHJ, Ramos EU, Hamwijk C, Van Holsteijn I, Blaauboer BJ, Seinen W, Verhaar HJM, Hermens JLM. Solid phase microextraction as a tool to determine membrane/water partition coefficients and bioavailable concentrations in in vitro systems. Chem Res Toxicol. 1997;10:1067–1072. doi: 10.1021/tx970109t. [DOI] [PubMed] [Google Scholar]
- Vanhaecke T, Henkens T, Kass GE, Rogiers V. Effect of the histone deacetylase inhibitor trichostatin A on spontaneous apoptosis in various types of adult rat hepatocyte cultures. Biochem Pharmacol. 2004;68:753–760. doi: 10.1016/j.bcp.2004.05.022. [DOI] [PubMed] [Google Scholar]
- Vinken M, Decrock E, Doktorova T, Ramboer E, De Vuyst E, Vanhaecke T, Leybaert L, Rogiers V. Characterization of spontaneous cell death in monolayer cultures of primary hepatocytes. Arch Toxicol. 2011;85:1589–1596. doi: 10.1007/s00204-011-0703-4. [DOI] [PubMed] [Google Scholar]
- Vinken M, Vanhaecke T, Rogiers V. Primary hepatocyte cultures as in vitro tools for toxicity testing: quo vadis? Toxicol In Vitro. 2012;26:541–544. doi: 10.1016/j.tiv.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Vinken M. The adverse outcome pathway concept: a pragmatic tool in toxicology. Toxicology. 2013;312:158–165. doi: 10.1016/j.tox.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Vinken M, Maes M, Oliveira AG, Cogliati B, Marques PE, Menezes GB, Dagli ML, Vanhaecke T, Rogiers V. Primary hepatocytes and their cultures in liver apoptosis research. Arch Toxicol. 2014;88:199–212. doi: 10.1007/s00204-013-1123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinken M. Adverse outcome pathways and drug-induced liver injury testing. Chem Res Toxicol. 2015;28:1391–1397. doi: 10.1021/acs.chemrestox.5b00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon M, Blaauboer BJ, Clewell HJ. Quantitative in vitro to in vivo extrapolation (QIVIVE): an essential element for in vitro-based risk assessment. Toxicology. 2015;332:1–3. doi: 10.1016/j.tox.2015.02.002. [DOI] [PubMed] [Google Scholar]