Abstract
Purpose of review
Preterm birth is a significant worldwide health problem of uncertain origins. The extant body of literature examining environmental contaminant exposures in relation to preterm birth is extensive but results remain ambiguous for most organic pollutants, metals and metalloids, and air pollutants. In the present review we examine recent epidemiologic studies investigating these associations, and identify recent advances and the state of the science. Additionally, we highlight biological mechanisms of action in the pathway between chemical exposures and preterm birth, including inflammation, oxidative stress, and endocrine disruption, that deserve more attention in this context.
Recent findings
Important advances have been made in the study of the environment and preterm birth, particularly in regard to exposure assessment methods, exploration of effect modification by co-morbidities and exposures, and in identification of windows of vulnerability during gestation. There is strong evidence for an association between maternal exposure to some persistent pesticides, lead, and fine particulate matter, but data on other contaminants is sparse and only suggestive trends can be noted with the current data.
Summary
Beyond replicating current findings, further work must be done to improve understanding of mechanisms underlying the associations observed between environmental chemical exposures and preterm birth. By examining windows of vulnerability, disaggregating preterm birth by phenotypes, and measuring biomarkers of mechanistic pathways in these epidemiologic studies we can improve our ability to detect associations with exposure, provide additional evidence for causality in an observational setting, and identify opportunities for intervention.
Keywords: Preterm birth, gestational age, environment, contaminants, toxicity, epidemiology
Introduction
Preterm birth (PTB) is an extensively studied perinatal outcome due to its prevalence, high societal costs, and poorly understood origins. Defined as delivery prior to 37 weeks gestation, PTB occurs in approximately 1 in 10 pregnancies worldwide and is one of the strongest predictors of neonatal mortality and morbidity (1). Furthermore, PTB may mediate numerous later life adverse health outcomes, such as neurodevelopmental delays, asthma and allergy, and metabolic disease. There are known predictors, presentations, and hypothesized mechanisms of PTB, but knowledge of concrete causes remains minimal. The prevalence, costliness, and ambiguity surrounding this disease has led to scientific curiosity in the potential contribution of environmental chemical factors.
Exposures to organic pollutants, metals and metalloids, and air pollutants have the potential to increase risk of PTB through multiple pathways. Some of the most important mechanisms of action that have been include inflammation, oxidative stress, and endocrine disruption, and each of these in turn has been linked to PTB. A large body of literature is devoted to this research (2-4), and, importantly, there have been recent efforts to identify the relevant biological mechanisms underlying these relationships in epidemiologic studies. By measuring biomarkers of mechanism, assessing windows of vulnerability to exposure, and examining associations with PTB phenotypes, research on environmental chemicals and PTB has advanced significantly.
The intention of this review is first to describe inflammation, oxidative stress, and endocrine disruption as three potential pathways of chemical action in the pathway to PTB (Figure 1), and to highlight recent studies that have advanced understanding of these mechanisms within the context of pregnancy (Table 1). Next, we present the state of the epidemiologic evidence examining the relationship between organic pollutants, metals and metalloids, and air pollutants. We review recent studies and discuss the major findings that support or refute an association with PTB and that point toward mechanisms.
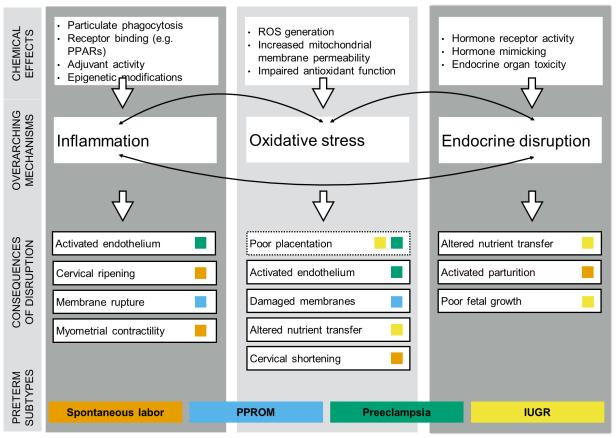
Figure 1. Potential mechanisms of environmental chemical action in the path to preterm birth.
Note: Under ‘Consequences of disruption’, dashed borders represent biological changes that may be sensitive to exposures early in pregnancy, while solid borders reflect middle to late pregnancy windows of susceptibility. Abbreviations: Peroxisome proliferator activated receptors (PPARs); Reactive oxygen species (ROS); Preterm premature rupture of the membranes (PPROM); Intrauterine growth restriction (IUGR).
Table 1.
Epidemiologic studies of environmental chemical exposures and preterm birth published since 2010.
| CLASS | CHEMICAL | REFERENCES | MAJOR FINDINGS | MAJOR FINDINGS ON MECHANISM |
|---|---|---|---|---|
|
Organic
pollutants |
Water disinfection
byproducts |
Horton 2011 (51); *Kogevinas 2016 (46); Kumar 2014 (49); Patelarou 2011 (47); Rivera- Nunez 2013 (50); Villanueva 2011 (48). |
A systematic review and meta-analysis
by Grellier in 2010 showed no association between water disinfection byproducts and PTB. Individual studies published after this review largely show null results with a few reporting small positive associations. |
In a large mother-child cohort
(n=14,005 mothers) conducted in Europe between 2002-2010, no association was seen with total trihalomethanes, chloroform, or brominated trihalomethanes; route of exposure (ingestion, inhalation, absorption); or trimester of pregnancy and PTB. |
| Persistent pollutants | Arbuckle 2012 (58); Basterrechea 2014 (67); Bergonzi 2011 (56); Chen 2012 (19); Darrow 2013 (178); Hamm 2010 (60); Kadhel 2014 (66); Pathak 2009 (57); Peltier 2015 (70); *Savitz 2012 (61); *Wesselink 2014 (68); Whitworth 2012 (63); Wojtyniak 2010 (55); Wu 2010 (64); Wu 2012 (65). |
A recent prospective cohort study of
women (n=818) exposed to chlordecone adds to past evidence that high levels of organochlorine pesticides are associated with PTB (Kadhel 2014). There are fewer studies on non-pesticide persistent compounds with mixed results. A large highly exposed population-based cohort (n=11,737 pregnancies) found no association with perflourooctonoic acid and PTB (Savitz 2012). A study of the highest know levels of residential dioxin exposure (n=617 women) also showed no association. Peltier 2015 identified an association with PBDEs and PTB. |
A case-control study examining the
relationship between PBDEs showed a dose-response relationship with increased risk of PTB with increasing levels of PBDEs in maternal plasma (Peltier 2015). |
|
| Non-persistent pollutants | Albouy-Llaty 2016 (179); *Cantonwine 2010b (180); *Ferguson 2014a (174); Ferguson 2014b (172); Forand 2012 (159); Rinsky 2012 (77); Ruckart 2014 (181); Sathyanarayana 2010 (72). |
Few studies exist for individual non-
persistent pollutants. The first study to examine longitudinal measure of phthalate metabolites during pregnancy found an association with these chemicals and PTB (Ferguson, 2014a). The association between bisphenol A and non-persistent pesticides and herbicides and PTB is less clear. |
Ferguson 2014a utilized longitudinally
collected phthalate metabolite measures during pregnancy (cases=132, controls = 352) and found an association with spontaneous PTB, particularly when restricted to exposure later in pregnancy. Behnia 2014 found an increased risk of PPROM with amniotic fluid BPA levels. |
|
|
Metals and
Metalloids |
Lead, Arsenic, Mercury,
Cadmium |
Bashore 2014 (101); Cantonwine 2010a (182); Huang 2016 (108); Myers 2010 (102); Perkins 2014 (98); Shi 2015 (183); Taylor 2015 (93); Taylor 2016 (100); Vigeh 2011 (92); Wang 2016 (106); Yang 2016 (107); Zhang 2015 (94); Zhu 2010 (99). |
Exposure to lead was consistently
associated with an increase in PTB. The most recently published study of lead exposure and PTB in a cohort of 4,285 pregnant women also shows an increased risk of PTB with prenatal lead exposure (Taylor 2015). Studies of other metals show mixed results and are inconclusive. |
Cantonwine 2010 measured lead in
each trimester during pregnancy in 235 women and found an association first trimester lead exposure and PTB. Vigeh 2011 (n=348) also found an association with PTB and 1st trimester lead exposure. |
| Air pollutants |
Criteria pollutants
(NO2, O3, CO, SO, PM10, PM2.5) |
Arroyo 2016 (146); Baker 2014 (147); Balsa 2016 (139); Candela 2013 (141); Capobussi 2016 (142); Dadvand 2015 (111); Dibben 2015 (148); Estarlich 2016 (143); Gray 2014 (134); Ha 2015 (138); Hao 2016 (120); Huang 2015 (140); Johnson 2016 (135); *Laurent 2016 (117); Lavigne 2016 (116); Le 2012 (184); Lee 2013 (185); *Li 2016 (122); Lin 2015 (128); Mendola 2016 (121); Olsson 2012 (144); Olsson 2013 (150); Olsson 2015 (149); Padula 2014 (123); Panasevich 2016 (112); Pereira 2016a (186); Poirier 2015 (151); Qian 2016 (152); Schifano 2016 (187); Stieb 2016 (113); Symanski 2015 (188); Transande 2013 (145); van den Hooven 2012 (154); *Wallace 2015 (127). |
A large number of recent studies
support an association between PTB and O3, CO, PM10, but not NO2 or SO. Work to investigate effect modification has identified an important role of co- morbidities (e.g., asthma), community level variables (e.g., urbanicity), and demographic factors. |
A study by Li 2016 in Australia
identified non-linear associations between PTB and acute exposures in the days before delivery to NO2, SO2, and CO. Wallace 2015 demonstrated associations between acute exposure to CO and SO2 with PROM, and between chronic exposure to O3 and PROM. |
| PM2.5 only | Chang 2012 (189); DeFranco 2016 (190); Fleischer 2014 (137); *Hyder 2014 (114); Kloog 2012 (115); Pereira 2014a (125); Pereira 2014b (118); Rappazzo 2014 (124); *Rappazzo 2015 (119); Symanski 2014 (136). |
Advances in exposure assessment
enabled measurement of exposure using satellite monitoring data. Hyder 2014 utilized this approach and found no association between PM2.5 and PTB in two US states (N~800,000). Recent reviews, however, conclude an association between exposure and PTB (130-133). |
Rappazzo 2015 identified differences
in association with PTB by week of gestation in which exposure was measured and by components of PM in 3 US states (N~1.75 million). Wallace 2015 identified associations between exposure hours before delivery and increased odds of PROM (N~225,000). |
|
|
Polycyclic aromatic
hydrocarbons |
Guo 2012 (158); Padula 2014b (156); Porter 2014 (160); Wilhelm 2011 (157). |
Very few studies exist and none
utilized personal air monitoring or biomarkers of PAH exposure. The largest study utilizing land use regression models (N~10,000 cases, N~100,000 controls) observed positive associations between non-traffic related PAH and PTB (Wilhelm 2011). |
Padula 2014b observed positive
associations between exposure during the last six weeks of pregnancy and very early PTB (<27 weeks) in California (N~43,000). |
|
|
Volatile organic
compounds |
Estarlich 2016 (143); Forand 2012 (159); Ghosh 2013 (161); Poirier 2015 (151); Porter 2015 (160); Wilhelm 2011(157). |
Very few studies exist, particularly by
individual compound (e.g., only one study examined trichloroethylene). The largest study by Wilhelm 2011 observed positive associations with benzene. |
Using land use regression models,
Estarlich 2016 observed that benzene exposure in the third trimester was associated with PTB in a cohort study in Spain (N=2,409). |
|
|
Environmental tobacco
smoke |
Arffin 2012 (191); Crane 2011 (165); Ghosh 2013 (161); Ion 2015 (167); Luo 2012 (192); *Qiu 2014 (166). |
Recent meta-analyses by Salmasi
2010 (163) and Cui 2016 (164) showed mixed results. |
Qiu 2014 observed increased odds of
very PTB birth (<32 weeks gestation) in association with passive smoking sustained for the duration of pregnancy in a large prospective study in China (N~10,000). No differences observed by trimester, but associations were strongest among very PTB without PPROM. |
Denotes study of importance. For criteria air pollutants and PM2.5, references are provided for papers published since the most recent comprehensive review by Stieb et al. (110). Abbreviations: PTB, preterm birth; PPROM, preterm premature rupture of the membranes; PBDEs, polybrominated diphenyl ethers.
Mechanisms of chemical action in the etiology of PTB
While rodent studies can be helpful for understanding biological processes that may be related to PTB, they offer an insufficient model, as it is very difficult to cause them to deliver preterm (5, 6). Thus, epidemiologic studies are especially valuable for studying this health outcome. This is true for studying associations with chemical exposures and for elucidating mechanisms. Beyond the utility of this model, studies of environmental chemicals and PTB that additionally investigate mechanisms can provide stronger arguments for causation in the observational setting, identify opportunities for interventions when remediating exposure is difficult or impossible, and improve the understanding of chemical toxicities in humans that may be extended to other disease. Here we examine three potential mechanistic pathways that have received some attention in the study of environmental chemicals and PTB, and deserve additional exploration in the future, including inflammation, oxidative stress, and endocrine disruption (Figure 1). Additionally, we highlight consequences of these disrupted processes in pregnancy, potentially sensitive windows of vulnerability to exposure, and phenotypic presentations of PTB that would be expected to be more strongly associated with each pathway (e.g., spontaneous preterm labor, preterm premature rupture of the membranes [PPROM], preeclampsia, and intrauterine growth restriction [IUGR]).
Inflammation
Intrauterine bacterial infection is perhaps the best established cause of PTB (7). Inflammatory pathways are thus one of the most well-studied mechanisms in the preterm pathway. Environmental contaminants have the potential to generate circulating or tissue-specific inflammation in several ways. First, particulate matter (metallic, endotoxin, or otherwise) may be engulfed by phagocytosis and lead to activation of T helper cells and release of cytokines (8). Second, xenobiotic binding to certain receptors, such as peroxisome proliferator activated receptors, can influence cytokine production as in the example of phthalate monoesters (9, 10).Third, environmental chemicals have demonstrated capacity to cause epigenetic modification by way of DNA methylation, histone modifications, and/or perturbations in miRNA expression which can lead to changes in inflammatory responses (11). Finally, these exposures can have adjuvant effects, exemplified by phthalates (12), which can increase the inflammation response to other stimuli.
While in many cases the exact cellular processes by which chemicals create inflammation are unknown, many exposures have been associated with inflammation in epidemiologic studies (13-15). Changes in the systemic maternal or the intrauterine inflammatory milieu could have downstream consequences that precipitate PTB, e.g. by initiating a cascade of events leading to cervical ripening, rupture of the amniotic sac, or increased myometrial contractility (16). These changes—sensitive to exposures later in pregnancy—may all precipitate spontaneous PTB either by initiating preterm labor or by causing PPROM.
Oxidative Stress
Oxidative stress, which can cause or be consequence of increased inflammation, is another important mechanism that could link environmental chemical exposures to PTB. Many chemicals have the capacity to induce oxidative stress, either through: overproduction of reactive oxygen species (ROS) generated enzymatically (e.g., through upregulation of cytochrome P450 pathways) or non-enzymatically (e.g., through the Fenton reaction) (17, 18); changes in mitochondrial membrane potential and permeability (19-21); and impaired antioxidant function (22, 23). Disturbance of the delicate balance between ROS and antioxidant defenses can have numerous downstream consequences in pregnancy. Early in gestation, oxidative stress can cause impaired invasion of the spiral arterioles into the maternal myometrium, resulting in poor placentation that can lead to preeclampsia or IUGR (24). Elevated levels later in pregnancy could: activate the maternal endothelium, part of a two-stage hypothesis underlying the onset of preeclampsia (25); cause damage to the membranes resulting in premature rupture (26, 27); create signaling changes in the cervix leading to shortening and spontaneous labor (28, 29); and/or impact placental protein synthesis and nutrient transport that again lead to fetal growth restriction which can result in medically indicated PTB (30, 31).
Endocrine disruption
Hormones carefully regulate nutrient transfer in pregnancy essential for growth of the fetus as well as timing of parturition. Disrupted fetal and placental thyroid hormone signaling has been observed in cases of IUGR (32). Additionally, animal studies show that glucocorticoid administration during pregnancy leads to a clear dose-dependent decrease in size of the fetus (30), and similar associations have been observed in human populations (33). Thus, thyroid and glucocorticoid hormones (e.g., cortisol) may be involved in the pathway to PTB with presentation of IUGR. Many chemicals have been linked to thyroid hormone disruption, potentially through receptor activity, particularly polychlorinated biphenyls (PCBs) and perfluorinated compounds (34, 35). Additionally, extensive in vitro and animal evidence demonstrates that environmental contaminants have the potential to interfere with glucocorticoid signaling. For example, bisphenol A (BPA) and other phenols, phthalates, perfluorinated compounds, and some pesticides can inhibit enzymes involved in the metabolism of glucocorticoids, thus raising circulating levels (37). Hormonal activity, and particularly that of the hypothalamic pituitary adrenal (HPA) axis, is additionally important in the timing of delivery. Corticotropin releasing hormone (CRH) in the placenta has been hypothesized as crucial for timing of spontaneous parturition (38, 39). Progesterone, estrogen, and cortisol pathways in the mother and fetus that interact to maintain homeostasis of CRH in pregnancy may thus be sensitive targets of chemical exposure (40).
Lastly, it is important to recognize that these pathways do not operate independently. Inflammation is tightly tied to hormonal regulation in pregnancy (16), and oxidative stress and inflammation have the potential to induce one another. These mechanisms may explain in part some of the associations observed in the following sections.
Environmental chemicals and PTB
Organic pollutants
Water disinfection byproducts (DBP)
A common method to disinfect drinking water is through the process of chlorination (41, 42). The most abundant byproducts of this process are trihalomethanes (THMs) and exposure to these along with haloacetic acids (HAAs) have been studied in relation to PTB (43). Grellier and colleagues reviewed the literature on exposure to water DBP and PTB in 2010 and concluded no association (44).
Since 2010 there have been seven additional studies (45-51). Three, conducted in Europe, used a method of exposure assessment that incorporated both measurements of THMs from public drinking water sources and individual level information on personal routes of exposure including ingestion, inhalation, and dermal absorption (45-48). The study by Costet and colleagues was novel in that exposure was quantified from a biomarker of trichloroacetic acid measured in maternal urine (45). None of these studies found an association between total THM and PTB.
The remaining three studies were US based and found small positive associations with water DBP and PTB (49-51). In New York, total THM were measured from the public water source at multiple time points during pregnancy (49). Although exposure was determined from public water source measures, this study was able to link the source with maternal residence, lending more confidence to the level of exposure being assigned to each woman. A modest association was detected between low levels of total THMs and PTB, but a null association was observed at higher levels. The remaining two studies did incorporate an individual component and used a representative water source to assign exposure, but also found similar small positive associations with some of the water DBP (51).
Persistent organic pollutants
Most of the literature examining an association between organic pollutants and PTB has focused on chemicals that persist in the environment and human body. Evidence of a relationship between persistent pesticides and PTB come from studies of populations with high levels of exposure (52, 53). A subset of studies from a recent review of environmental chemicals and PTB (54) were published since 2010 and show that overall the literature supports an association between high levels of organochlorine pesticide exposure (55-57) and PTB with weaker or null associations for the remaining persistent pollutants (54, 58-65).
Studies published since that review (54) show consistent findings of the association between persistent pesticides and PTB with high exposures associated with increased risk, whereas the relationship with lower exposures is less clear. In a study conducted in Guadeloupe where there is widespread chlordecone use and environmental contamination, authors found an association with exposure as measured by maternal blood sample at delivery and increased risk of PTB (66). However, a study conducted in Spain, where pesticide exposure was much lower, failed to show an association between 1st trimester maternal hexachlorobenzene levels and PTB (67).
Exposure to high levels of non-pesticide persistent pollutants do not show the same relationship, with generally null findings for dioxin exposure and PTB regardless of exposure burden. A chemical explosion in Seveso, Italy led to unprecedented residential dioxin exposure providing the opportunity to investigate the health effects of high levels of this chemical (68). Despite exposure to elevated levels of dioxin, an association with PTB could not be established in this cohort (68). Similarly, in a population highly exposed to perfluorooctanoic acid as a result of industrial contamination of the drinking water, no association with PTB was found.(69) In contrast, a study of flame retardants showed a dose response relationship with PTB (70). Maternal blood samples were taken at the time of delivery and an increased risk of PTB was observed with increasing levels of polybrominated diphenyl ethers.
Non-persistent organic pollutants
Investigation of organophosphate pesticides in relation to PTB has been limited and no associations with PTB have been reported (71, 72). Atrazine, another chemical used in agriculture, is applied as an herbicide, and can contaminate water supplies (73). Early studies looking at atrazine assigned exposure based on measurements from water sources and found null or weak non-significant positive associations with PTB (74, 75). Recent studies continue to use drinking water levels to assign atrazine exposure despite the ability to measure individual exposure from urine samples (76), and the evidence for atrazine being associated with increased PTB remains inconclusive (77).
Phthalates are used to increase flexibility in plastics and are also found in many personal care products. Human exposure to this group of chemicals is widespread through ingestion, dermal application, and inhalation (78). There have been conflicting findings from early studies of phthalate metabolites and PTB with one study finding a protective association (79) and another finding a harmful one (80). Since publication of these findings, there have been improvements in the assessment of phthalate exposures. In a recent nested case control study, multiple measures of nine phthalate metabolites were used to estimate exposure. In this study, the average phthalate metabolite levels from three urine samples collected from women during pregnancy (median of 10, 18, and 26 weeks gestation) was associated with increased risk of PTB (81). Further, this study examined phenotypes of PTB and observed an even stronger association between phthalate levels and spontaneous PTB. A related study characterized the variability of phthalate metabolite levels across pregnancy (82). In this study each urinary phthalate measurement was treated as a distinct exposure to assess risk of PTB in relation to different windows of susceptibility during pregnancy. Results showed the strongest association with phthalate levels measured later in pregnancy.
BPA is another chemical identified as an endocrine disrupter that is in widespread use as a component of hard plastics and epoxy resins (78). The first study to examine the relationship between BPA exposure and PTB was done using a maternal single spot urine collected during the 3rd trimester and found a modest association of BPA with PTB (84). These same investigators then used multiple measures of BPA from the same case cohort study referenced above with phthalates and found a weak and non-significant association with PTB (85).
Strengths of literature on non-persistent pollutants include several improvements in exposure assessment. Examples include the incorporation of personal information on routes of exposure of water DBP and use of multiple measurements of non-persistent chemicals like phthalates and BPA. Additionally, consideration of the timing of exposure and windows of susceptibility that may differ based on preterm phenotype aids in comparability across studies and confirmation of proposed biologic mechanisms. Limitations of the current evidence on organic pollutants and PTB include the continued use of ecological measures of exposure even when validated biomarkers exist that could quantify individual exposure. In some studies individual-level exposure was measured, but using inappropriate exposure matrices, specifically regarding non-persistent chemicals, resulting in concerns about contamination (83, 86, 87). Lastly, the consideration of combinations of related chemicals or metabolites with PTB would strengthen the literature.
Metals and metalloids
Over the past several decades, there have been many studies that have examined the association between heavy metal exposures and adverse birth outcomes. The bulk of the early literature focused on high levels of exposure (88-90) with more recent studies examining lower levels consistent with non-occupational settings.
Lead
There is convincing evidence that lead exposure in high levels is associated with PTB (88). The hypothesized mechanisms by which lead can lead to PTB fall into two categories: 1) by influencing hormone levels (91-94) and 2) inducing the production of reactive oxygen species (92, 93). Since the elimination of lead in everyday exposures like fuel and paint, examination of lower levels of lead exposure have become more relevant (95). Studies focused on these ambient levels of exposure that still persistent in the environment have shown inconsistent results possibly as the result of differences the timing of exposure assessment or type of biologic material utilized (96, 97). In a study conducted in Mexico, exposure to lead was assessed during the 1st, 2nd, and 3rd trimester in maternal whole blood and plasma and the strongest associations with PTB were seen in early pregnancy (91). Other studies with measures of lead during early pregnancy showed similar results (92, 93). Results from studies assessing lead exposure across pregnancy show less consistent results, with the possibility of lead measurements later in pregnancy attenuating the effect (98, 99). Two studies found differences in PTB by sex of the infant, but results were opposing with one finding a stronger association in girls and the other in boys (94, 98).
Mercury
The association between mercury exposure and PTB has been mixed. A study by Taylor and colleagues conducted in the UK found weak evidence of a protective effect of blood mercury levels measured in the mother early in pregnancy (median 11 weeks) and PTB, but this association attenuated in adjusted models (100). In a study population of women who were predominantly African American or of Caribbean decent, investigators found a protective effect of higher mercury levels in maternal urine during the last trimester of pregnancy, but the estimate was imprecise (101). A slightly stronger effect was seen in cord blood measures in the same population.
Arsenic
Two of the studies reviewed looked at arsenic in drinking water and PTB. Neither used individual levels of exposure, but were consistent with prior work that has found null or weak associations with PTB (102).
Cadmium
Early studies examining maternal cadmium exposure and PTB used either an ecological study design or suffered from small sample size and showed mixed results (103-105). More recently two large cohort studies conducted in China showed an association between higher levels of cadmium exposure and increased risk of PTB (106, 107). A case-cohort study of the more restrictive outcomes of preterm low birthweight also reported this association (108). Cadmium is thought to alter zinc transport, which may trigger PTB (106, 107) and may also accumulate in the placenta where it can directly or indirectly affect the fetus (108). None of the studies that reviewed mercury or arsenic proposed a hypothesized mechanism of action.
Strengths of the current literature include taking the timing of the metal exposure into account and examining lower levels of metal exposures that are more consistent with ambient exposure. Some studies measure metal exposures in more than one type of biological sample to estimate how correlated measures are across sample type, which may have implications on the interpretation of results from future studies as well as determining the best exposure matrix for each metal.
There are still many studies that use ecological data to estimate exposure to metals and metalloids, even when individual measures have been shown to be reliable, such as arsenic exposure measured in urine samples. Although some studies consider the timing of exposures, there are many studies that continue to compare measures taken from women at different times during pregnancy or only at delivery, which may not reflect exposure during pregnancy. This may be especially important for lead exposure which is thought to have a U-shaped curve during pregnancy with higher levels seen in the 1st and 3rd trimester (109).
Air pollutants
Criteria air pollutants
Papers published on air pollutants, including nitrogen dioxide (NO2), sulfur dioxide (SO2), ozone (O3), carbon monoxide (CO), and particulate matter (<2.5 μm, PM2.5; and <10um, PM10) up until January 2011 were meticulously examined by Stieb and colleagues (110). Their pooled analysis using trimester-specific as well as entire pregnancy averages concluded that the most precise effect estimates were between third trimester exposures to CO and PM10 and PTB (110). Since that time many studies have been published on criteria air pollutants with notable improvements. Exposure assessment has been optimized by more broad application of land use regression modeling (111-113), implementation of satellite-imaging technology for the assessment of PM2.5 exposure (114-116), and in studies of PM2.5 exposure, examination of the individual components within the particulate fraction in attempt to identify the most harmful constituents (117-120).The study of windows of vulnerability to exposure has expanded as well, with many studies moving beyond examining trimester or month of exposure into measurement of the preconception exposures (121) or exposures in the days or hours immediately proximate to delivery (122-124). In terms of outcome assessment, some research groups have made efforts to examine phenotypes of PTB. Potentially due to ease of assessment from medical record abstraction, the focus has been on PPROM (111, 125-127). Finally, many recent studies place an emphasis on understanding effect modifiers in these relationships. These have included co-morbidities such as asthma and diabetes (117, 121, 128), demographic factors like race, education level, and socioeconomic status (120, 123), and community-level variables, e.g. urbanicity (129).
Regarding individual criteria air pollutants, the greatest advances and the largest number of studies have focused on fine particulate matter (PM2.5). Since the review by Stieb et al., four additional reviews and meta-analyses have summarized the literature on these associations and all concluded that a positive association exists between exposure during pregnancy and PTB (130-133). The majority of papers published since that review also suggest a positive association between PM2.5 exposure and PTB, although null and in some cases protective effects have been observed in well-designed studies (113, 114, 122, 134, 135). Differences may be due to variation in study design and population, exposure assessment methods, measurement windows, and levels of exposure.
Studies examining variation in effects by characteristics of the study population have observed the greatest effect estimates in subjects with co-morbidities like asthma and diabetes (116, 121), and who have certain demographic characteristics (low education level, non-African American, residing in large metropolitan areas) (120). Width of windows of exposure vary greatly as well. Detection of positive associations between PM2.5 and PTB seem to suggest that exposure in the days or hours leading up to delivery may be most relevant (123, 124, 127, 136), although this has not been evident in all studies that had the capability of assessing this window specifically (122). Exposure assessment approaches improve dramatically year to year as well, and for this exposure two recent studies have captured exposure with satellite-based predictions, which have the ability to assign exposure to small geographic areas and more accurately capture exposures where ambient air monitors are more sparse (i.e., rural areas) (114, 115). These studies had conflicting findings; however, as these methods improve they may provide more robust data on the association between PM2.5 and PTB.
Studies of PM2.5 also vary by exposure level, source, and composition of the particulate. In a study by the World Health Organization comparing associations in African, Asian, and Latin American countries, the only clear evidence of an association between PM2.5 and PTB was observed in China, where the range of exposure was widest and where some of the highest levels in the world were observed (137). Though, this does not necessarily connote a stronger effect at higher levels. An analysis examining non-linear associations with exposure in attempt to identify a threshold effect observed no statistical association for PM2.5 in linear or non-linear single pollutant models (122). Finally, an important evolution in this research is to examine sources and components of fractionated PM2.5. One study identified power plants as important sources of PM2.5 exposure, and illustrated that proximity to these plants specifically was associated with increased risk of preterm delivery (138). Additionally, two studies examining compounds present in PM2.5 fractions suggested that elemental carbon and SO4 specifically were associated with increased risk of PTB (111, 119). Additional research studies designed to capture these elements combined will help improve the understanding of the role of exposure to PM2.5 in the etiology of PTB.
Associations with PM10 specifically are difficult to assess because not all studies examine both PM10 and PM2.5 fractions separately, thus many studies measuring PM10 alone may be capturing effects of the smaller particulate fraction. Previously Stieb et al. concluded that there was an association between PM10 and PTB, but more recent studies are ambiguous. Of the studies that do report an association with PM10, only one measured PM2.5 concurrently (123), and noted that the strongest associations were with exposure very close to the time of delivery. Notably, two studies examined these associations within natural experiments. Balsa and colleagues examined pregnancy outcomes among individuals residing near an active volcano, and observed strong associations with PM10 levels measured in the third trimester (139). On the other hand, however, a study of elevated air pollution levels occurring during the Beijing Olympic games observed no association between ambient PM10 concentrations and PTB (140).
Studies of other criteria air pollutants are less conclusive, despite having made similar advancements by way of examining more specific exposure windows, non-linearity of associations, and multi-pollutant models. In regard to NO2, positive (111, 116, 117, 120, 122, 123, 129, 141-145) and null (112, 113, 127, 128, 135, 137, 140, 146-154) associations seem to be evenly mixed. Studies that have observed positive associations point toward the second trimester or the time period immediately preceding delivery as sensitive windows of exposure (122, 129, 143). Also, evidence suggests that an association may exist only in urban populations or at a certain threshold of exposure (122, 129). Additionally, one study showed an association was between NO2 and PTB in a single-pollutant model, but when PM2.5 was added to the model the association between PTB and NO2 became null (117). Findings for O3, CO, and SO2 also remain inconclusive.
Polycyclic aromatic hydrocarbons (PAHs) and volatile organic compounds (VOCs)
Fewer studies examine PAHs or VOCs because they are not as tightly regulated by the EPA and hence are not commonly captured by air monitors. PAHs are released during combustion processes from vehicles and industrial facilities, and they are also found in tobacco smoke and grilled meats and vegetables (155). Thus, exposure occurs commonly through inhalation pathways but ambient monitors likely capture a smaller percentage of exposure compared to other air pollutants. Studies examining ambient PAH exposure in relation to PTB have identified significant associations between total PAHs as well as individual congeners, particularly with levels measured in the end of gestation (156, 157). Additionally, a study measuring PAH concentrations in cord blood identified higher levels in pregnancies with adverse birth outcomes, including PTB (158). While this research is suggestive, additional studies are necessary and would benefit from utilizing urinary biomarkers which capture total exposure through inhalation and ingestion pathways. Similarly, exposure to some VOCs in pregnancy, e.g. benzene or toluene, show some evidence of an association with PTB but the data remains very limited (143, 151, 159-161).
Environmental tobacco smoke
Lastly, environmental tobacco smoke has been examined in several studies in relation to PTB. The association between PTB and maternal smoking has been established (162), however the risk associated with indirect exposure is less clear. A meta-analysis published in 2010 showed a slight positive association with PTB that was not statistically significant in adjusted models (163). A more recent meta-analysis concluded otherwise, specifying that passive smoking in either the workplace or at home was associated with preterm delivery (164). Of the largest of these studies published in recent years (>10,000 subjects), two report positive associations with PTB, particularly delivery <32 weeks gestation (165, 166) and one had null findings (167).
In 2008, Slama et al. addressed the overarching limitations of studies examining air pollutant exposures in relation to PTB and identified major research needs (168). Many of these needs remain, including the need to better understand mechanisms underlying these observed associations, addressing co-exposure to multiple pollutants, and working from a unified methodological framework to improve comparability between studies (169). Toward this end, Pereira and colleagues are implementing a series of studies with the same design to assess the relationship between gestational exposures to PM2.5 and PTB in different regions of the world (125, 126, 170). The International Collaboration on Air Pollution and Pregnancy Outcomes is designed with the same aim, to unify methodologies across research studies to identify the relationship between ambient air pollutant exposures and adverse pregnancy outcomes (171). These collaborative research efforts will be extremely informative in the coming years.
Conclusions
Although numerous studies have been published on the relationship between environmental chemical exposures and PTB, few individual compounds have sufficient evidence to conclude an association. The data suggest a strong association between PTB and fine particulate matter (PM2.5), lead, and dichlorodiphenyltrichloroethane (DDT) exposures in pregnancy—compounds for which there have also been the largest number of publications. However, for other chemicals the overall findings are ambiguous. This is due both to small numbers of studies for some compounds as well as conflicting results. Future work on other compounds where there is strong evidence but not enough data—e.g., polycyclic aromatic hydrocarbons—is warranted.
Of the studies reviewed, many of the common limitations of environmental epidemiology studies exist. These include limited exposure assessment metrics (e.g., using a spot urine sample to assess non-persistent chemical exposures), small sample sizes and/or small numbers of cases, and failure to account for key confounders and/or co-exposures. We posit that, although there has been some concerted effort in this direction, an additional limitation to this literature is the infrequent attention to mechanistic pathways underlying the relationships of interest. It would behoove future studies to focus on disentangling these mechanisms through one or more approaches. First, by examining windows of vulnerability during gestation, investigators can examine time points of exposure that may be reflective of specific pathways to delivery. The greatest progress in this arena has been made in the study of particulate matter, where investigators have leveraged the wealth of information available from ambient air monitors to examine acute exposures (i.e., the hours or days leading up to delivery) in relation to PTB (123, 124, 127, 136). Identifying this sensitive window of exposure provides stronger evidence of a relationship between PM2.5 and PTB by highlighting a biologically plausible pathway (e.g., acute PM2.5 exposure causes oxidative stress or inflammation, which leads to membrane damage and PPROM). A second approach to identifying mechanism may be through examining associations with specific presentations of PTB, as opposed to looking at the 37-week cutoff alone, or at other cutoffs based on gestational age that may not necessarily be homogenous by mechanism. Again progress has been made here in the realm of air pollution research by groups who have focused their efforts on examining associations with PPROM (125-127), and also to some extent in the study of non-persistent organic pollutants such as BPA and phthalates (172, 173). Examining associations with phenotypes of PTB based on presentation enables both greater ability to detect effects and also identifies mechanisms that may be targeted for interventions. Finally, and most ideally, biomarkers of mechanistic intermediates can be measured concurrently with exposure to provide supportive evidence of causality if indeed an association with exposure exists.
Worth mentioning is the study published by Ferguson, McElrath, and Meeker in which mechanism is investigated under all three of these approaches (174). In that study phthalate exposure biomarkers were measured at four time points during pregnancy (windows of vulnerability); associations were examined with phenotypes of PTB (presentation phenotypes); and indices of mechanistic intermediates were measured under multiple hypothesized pathways, including inflammation, oxidative stress, and endocrine disruption (biomarkers of mechanism). In that study the authors established an association between some phthalate metabolites and specifically spontaneous PTB, with associations that were greatest in magnitude with exposure measured late in pregnancy (172). Additionally, they demonstrated that urinary phthalate metabolites were associated with oxidative stress biomarkers, particularly 8-isoprostane, and that 8-isoprostane concentrations during pregnancy were associated with spontaneous preterm delivery (175, 176). Finally, the authors developed and applied novel mediation methods and showed that, statistically, oxidative stress, as indicated by 8-isoprostane, may account for upwards of 50% of the association between some phthalate metabolites and PTB (177).
These additional steps beyond the analysis of association between exposure and outcome provide greater weight to the analysis and can provide feedback into the obstetrics community to further understanding of the complex pregnancy outcome that is PTB. Additional studies with this type of framework are necessary for establishing more concrete connections with environmental chemical exposures. By examining windows of vulnerability, presentation phenotypes, and biomarkers of mechanism, we may also reduce variability in published associations and have a greater chance at replicating study findings. This work will strengthen the evidence supporting associations between environmental chemical exposures and PTB, and help us to identify opportunities for remediating exposures or implementing interventions to prevent this serious public health problem.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Behrman RE, Butler AS. Preterm birth: causes, consequences, and prevention. National Academies Press; 2007. [PubMed] [Google Scholar]
- 2.Ferguson KK, O'Neill MS, Meeker JD. Environmental contaminant exposures and preterm birth: a comprehensive review. Journal of Toxicology and Environmental Health, Part B. 2013;16(2):69–113. doi: 10.1080/10937404.2013.775048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wigle DT, Arbuckle TE, Turner MC, Berube A, Yang Q, Liu S, et al. Epidemiologic evidence of relationships between reproductive and child health outcomes and environmental chemical contaminants. Journal of Toxicology and Environmental Health, Part B. 2008;11(5-6):373–517. doi: 10.1080/10937400801921320. [DOI] [PubMed] [Google Scholar]
- 4.Hughes CL, Waters MD. Translational Toxicology: Defining a New Therapeutic Discipline. Humana Press; 2016. [Google Scholar]
- 5.Cha J, Bartos A, Egashira M, Haraguchi H, Saito-Fujita T, Leishman E, et al. Combinatory approaches prevent preterm birth profoundly exacerbated by gene-environment interactions. The Journal of clinical investigation. 2013;123(9):4063–75. doi: 10.1172/JCI70098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaga N, Katsuki Y, Obata M, Shibutani Y. Repeated administration of low-dose lipopolysaccharide induces preterm delivery in mice: a model for human preterm parturition and for assessment of the therapeutic ability of drugs against preterm delivery. Am J Obstet Gynecol. 1996;174(2):754–9. doi: 10.1016/s0002-9378(96)70460-x. [DOI] [PubMed] [Google Scholar]
- 7.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. The lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vadillo-Ortega F, Osornio-Vargas A, Buxton MA, Sánchez BN, Rojas-Bracho L, Viveros-Alcaráz M, et al. Air pollution, inflammation and preterm birth: a potential mechanistic link. Med Hypotheses. 2014;82(2):219–24. doi: 10.1016/j.mehy.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang C, Ting AT, Seed B. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391(6662):82–6. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 10.Peraza MA, Burdick AD, Marin HE, Gonzalez FJ, Peters JM. The toxicology of ligands for peroxisome proliferator-activated receptors (PPAR) Toxicol Sci. 2006;90(2):269–95. doi: 10.1093/toxsci/kfj062. [DOI] [PubMed] [Google Scholar]
- 11.Lin VW, Baccarelli AA, Burris HH. Epigenetics—a potential mediator between air pollution and preterm birth. Environmental epigenetics. 2016;2(1):dvv008. doi: 10.1093/eep/dvv008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsen ST, Hansen JS, Hansen EW, Clausen PA, Nielsen GD. Airway inflammation and adjuvant effect after repeated airborne exposures to di-(2-ethylhexyl) phthalate and ovalbumin in BALB/c mice. Toxicology. 2007;235(1):119–29. doi: 10.1016/j.tox.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Hajat A, Allison M, Diez-Roux AV, Jenny NS, Jorgensen NW, Szpiro AA, et al. Long-term exposure to air pollution and markers of inflammation, coagulation, and endothelial activation: a repeat-measures analysis in the Multi-Ethnic Study of Atherosclerosis (MESA) Epidemiology (Cambridge, Mass) 2015;26(3):310–20. doi: 10.1097/EDE.0000000000000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kannan S, Misra DP, Dvonch JT, Krishnakumar A. Exposures to airborne particulate matter and adverse perinatal outcomes: a biologically plausible mechanistic framework for exploring potential. Ciencia & saude coletiva. 2007;12(6):1591–602. doi: 10.1590/s1413-81232007000600020. [DOI] [PubMed] [Google Scholar]
- 15.Nachman R, Mao G, Zhang X, Hong X, Chen Z, Soria C, et al. Intrauterine Inflammation and Maternal Exposure to Ambient PM2. 5 during Preconception and Specific Periods of Pregnancy: The Boston Birth Cohort. Environ Health Perspect. 2016 doi: 10.1289/EHP243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, Petraglia F. Inflammation and pregnancy. Reprod Sci. 2009;16(2):206–15. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- 17.Stohs S, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med. 1995;18(2):321–36. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- 18.Valavanidis A, Vlahogianni T, Dassenakis M, Scoullos M. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol Environ Saf. 2006;64(2):178–89. doi: 10.1016/j.ecoenv.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Wang J, Qin Q, Jiang Y, Yang G, Rao K, et al. Mono-2-ethylhexyl phthalate induced loss of mitochondrial membrane potential and activation of Caspase3 in HepG2 cells. Environ Toxicol Pharmacol. 2012;33(3):421–30. doi: 10.1016/j.etap.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Rosado-Berrios CA, Vélez C, Zayas B. Mitochondrial permeability and toxicity of diethylhexyl and monoethylhexyl phthalates on TK6 human lymphoblasts cells. Toxicol In Vitro. 2011;25(8):2010–6. doi: 10.1016/j.tiv.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29(3):222–30. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 22.López O, Hernández AF, Rodrigo L, Gil F, Pena G, Serrano JL, et al. Changes in antioxidant enzymes in humans with long-term exposure to pesticides. Toxicol Lett. 2007;171(3):146–53. doi: 10.1016/j.toxlet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Ahamed M, Siddiqui M. Low level lead exposure and oxidative stress: current opinions. Clin Chim Acta. 2007;383(1):57–64. doi: 10.1016/j.cca.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 24.Burton GJ, Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Investig. 2004;11(6):342–52. doi: 10.1016/j.jsgi.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Roberts JM, Hubel CA. Is oxidative stress the link in the two-stage model of pre-eclampsia? The Lancet. 1999;354(9181):788–9. doi: 10.1016/S0140-6736(99)80002-6. [DOI] [PubMed] [Google Scholar]
- 26.Longini M, Perrone S, Vezzosi P, Marzocchi B, Kenanidis A, Centini G, et al. Association between oxidative stress in pregnancy and preterm premature rupture of membranes. Clin Biochem. 2007;40(11):793–7. doi: 10.1016/j.clinbiochem.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Woods J. Reactive oxygen species and preterm premature rupture of membranes—a review. Placenta. 2001;22:S38–S44. doi: 10.1053/plac.2001.0638. [DOI] [PubMed] [Google Scholar]
- 28.Sanders AP, Burris HH, Just AC, Motta V, Svensson K, Mercado-Garcia A, et al. microRNA expression in the cervix during pregnancy is associated with length of gestation. Epigenetics. 2015;10(3):221–8. doi: 10.1080/15592294.2015.1006498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venkatesh K, Cantonwine D, Ferguson K, Arjona M, Meeker JD, McElrath TF. Inflammatory and oxidative stress markers associated with decreased cervical length in pregnancy. Am J Reprod Immunol. 2016;76(5):376–82. doi: 10.1111/aji.12545. [DOI] [PubMed] [Google Scholar]
- 30.Jones H, Powell T, Jansson T. Regulation of placental nutrient transport–a review. Placenta. 2007;28(8):763–74. doi: 10.1016/j.placenta.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Burton G, Yung H-W, Cindrova-Davies T, Charnock-Jones D. Placental endoplasmic reticulum stress and oxidative stress in the pathophysiology of unexplained intrauterine growth restriction and early onset preeclampsia. Placenta. 2009;30:43–8. doi: 10.1016/j.placenta.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kilby M, Verhaeg J, Gittoes N, Somerset D, Clark P, Franklyn J. Circulating thyroid hormone concentrations and placental thyroid hormone receptor expression in normal human pregnancy and pregnancy complicated by intrauterine growth restriction (IUGR) The Journal of Clinical Endocrinology & Metabolism. 1998;83(8):2964–71. doi: 10.1210/jcem.83.8.5002. [DOI] [PubMed] [Google Scholar]
- 33.Ali Khan A, Rodriguez A, Kaakinen M, Pouta A, Hartikainen AL, Jarvelin MR. Does in utero exposure to synthetic glucocorticoids influence birthweight, head circumference and birth length? A systematic review of current evidence in humans. Paediatr Perinat Epidemiol. 2011;25(1):20–36. doi: 10.1111/j.1365-3016.2010.01147.x. [DOI] [PubMed] [Google Scholar]
- 34.Boas M, Feldt-Rasmussen U, Main KM. Thyroid effects of endocrine disrupting chemicals. Mol Cell Endocrinol. 2012;355(2):240–8. doi: 10.1016/j.mce.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Steenland K, Fletcher T, Savitz DA. Epidemiologic evidence on the health effects of perfluorooctanoic acid (PFOA) Environ Health Perspect. 2010:1100–8. doi: 10.1289/ehp.0901827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sargis RM, Johnson DN, Choudhury RA, Brady MJ. Environmental endocrine disruptors promote adipogenesis in the 3t3-∣1 cell line through glucocorticoid receptor activation. Obesity. 2010;18(7):1283–8. doi: 10.1038/oby.2009.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ye L, Guo J, Ge R-S. Environmental pollutants and hydroxysteroid dehydrogenases. Vitam Horm. 2014;94:349–90. doi: 10.1016/B978-0-12-800095-3.00013-4. [DOI] [PubMed] [Google Scholar]
- 38.McLean M, Bisits A, Davies J, Woods R, Lowry P, Smith R. A placental clock controlling the length of human pregnancy. Nat Med. 1995;1(5):460–3. doi: 10.1038/nm0595-460. [DOI] [PubMed] [Google Scholar]
- 39.Wadhwa PD, Garite TJ, Porto M, Glynn L, Chicz-DeMet A, Dunkel-Schetter C, et al. Placental corticotropin-releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: a prospective investigation. Am J Obstet Gynecol. 2004;191(4):1063–9. doi: 10.1016/j.ajog.2004.06.070. [DOI] [PubMed] [Google Scholar]
- 40.Challis JR, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm 1. Endocr Rev. 2000;21(5):514–50. doi: 10.1210/edrv.21.5.0407. [DOI] [PubMed] [Google Scholar]
- 41.Cutler D, Miller G. The role of public health improvements in health advances: the twentieth-century United States. Demography. 2005;42(1):1–22. doi: 10.1353/dem.2005.0002. [DOI] [PubMed] [Google Scholar]
- 42.Wigle DT. Safe drinking water: a public health challenge. Chronic Dis Can. 1998;19(3):103–7. [PubMed] [Google Scholar]
- 43.Nieuwenhuijsen MJ, Toledano MB, Eaton NE, Fawell J, Elliott P. Chlorination disinfection byproducts in water and their association with adverse reproductive outcomes: a review. Occup Environ Med. 2000;57(2):73–85. doi: 10.1136/oem.57.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grellier J, Bennett J, Patelarou E, Smith RB, Toledano MB, Rushton L, et al. Exposure to disinfection by-products, fetal growth, and prematurity: a systematic review and meta-analysis. Epidemiology. 2010;21(3):300–13. doi: 10.1097/EDE.0b013e3181d61ffd. [DOI] [PubMed] [Google Scholar]
- 45.Costet N, Garlantezec R, Monfort C, Rouget F, Gagniere B, Chevrier C, et al. Environmental and urinary markers of prenatal exposure to drinking water disinfection by-products, fetal growth, and duration of gestation in the PELAGIE birth cohort (Brittany, France, 2002-2006) Am J Epidemiol. 2012;175(4):263–75. doi: 10.1093/aje/kwr419. [DOI] [PubMed] [Google Scholar]
- 46 •.Kogevinas M, Bustamante M, Gracia-Lavedan E, Ballester F, Cordier S, Costet N, et al. Drinking Water Disinfection By-products, Genetic Polymorphisms, and Birth Outcomes in a European Mother-Child Cohort Study. Epidemiology. 2016;27(6):903–11. doi: 10.1097/EDE.0000000000000544. Large, multi-country study investigating the association between water disinfection byproducts and preterm birth. Used individual level information on water consumption, water-related activities, and route of exposure.
- 47.Patelarou E, Kargaki S, Stephanou EG, Nieuwenhuijsen M, Sourtzi P, Gracia E, et al. Exposure to brominated trihalomethanes in drinking water and reproductive outcomes. Occup Environ Med. 2011;68(6):438–45. doi: 10.1136/oem.2010.056150. [DOI] [PubMed] [Google Scholar]
- 48.Villanueva CM, Gracia-Lavedan E, Ibarluzea J, Santa Marina L, Ballester F, Llop S, et al. Exposure to trihalomethanes through different water uses and birth weight, small for gestational age, and preterm delivery in Spain. Environ Health Perspect. 2011;119(12):1824–30. doi: 10.1289/ehp.1002425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumar S, Forand S, Babcock G, Richter W, Hart T, Hwang SA. Total trihalomethanes in public drinking water supply and birth outcomes: a cross-sectional study. Matern Child Health J. 2014;18(4):996–1006. doi: 10.1007/s10995-013-1328-4. [DOI] [PubMed] [Google Scholar]
- 50.Rivera-Nunez Z, Wright JM. Association of brominated trihalomethane and haloacetic acid exposure with fetal growth and preterm delivery in Massachusetts. J Occup Environ Med. 2013;55(10):1125–34. doi: 10.1097/JOM.0b013e3182a4ffe4. [DOI] [PubMed] [Google Scholar]
- 51.Horton BJ, Luben TJ, Herring AH, Savitz DA, Singer PC, Weinberg HS, et al. The effect of water disinfection by-products on pregnancy outcomes in two southeastern US communities. J Occup Environ Med. 2011;53(10):1172–8. doi: 10.1097/JOM.0b013e31822b8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berkowitz GS, Lapinski RH, Wolff MS. The role of DDE and polychlorinated biphenyl levels in preterm birth. Arch Environ Contam Toxicol. 1996;30(1):139–41. doi: 10.1007/BF00211340. [DOI] [PubMed] [Google Scholar]
- 53.Longnecker MP, Klebanoff MA, Zhou H, Brock JW. Association between maternal serum concentration of the DDT metabolite DDE and preterm and small-for-gestational-age babies at birth. Lancet. 2001;358(9276):110–4. doi: 10.1016/S0140-6736(01)05329-6. [DOI] [PubMed] [Google Scholar]
- 54.Ferguson KK, O'Neill MS, Meeker JD. Environmental contaminant exposures and preterm birth: a comprehensive review. J Toxicol Environ Health B Crit Rev. 2013;16(2):69–113. doi: 10.1080/10937404.2013.775048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wojtyniak BJ, Rabczenko D, Jonsson BA, Zvezday V, Pedersen HS, Rylander L, et al. Association of maternal serum concentrations of 2,2', 4,4'5,5'-hexachlorobiphenyl (CB-153) and 1,1-dichloro-2,2-bis (p-chlorophenyl)-ethylene (p,p'-DDE) levels with birth weight, gestational age and preterm births in Inuit and European populations. Environ Health. 2010;9:56. doi: 10.1186/1476-069X-9-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bergonzi R, De Palma G, Specchia C, Dinolfo M, Tomasi C, Frusca T, et al. Persistent organochlorine compounds in fetal and maternal tissues: evaluation of their potential influence on several indicators of fetal growth and health. Sci Total Environ. 2011;409(15):2888–93. doi: 10.1016/j.scitotenv.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 57.Pathak R, Ahmed RS, Tripathi AK, Guleria K, Sharma CS, Makhijani SD, et al. Maternal and cord blood levels of organochlorine pesticides: association with preterm labor. Clin Biochem. 2009;42(7-8):746–9. doi: 10.1016/j.clinbiochem.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 58.Arbuckle TE, Kubwabo C, Walker M, Davis K, Lalonde K, Kosarac I, et al. Umbilical cord blood levels of perfluoroalkyl acids and polybrominated flame retardants. Int J Hyg Environ Health. 2012 doi: 10.1016/j.ijheh.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 59.Chen MH, Ha EH, Wen TW, Su YN, Lien GW, Chen CY, et al. Perfluorinated compounds in umbilical cord blood and adverse birth outcomes. PLoS One. 2012;7(8):e42474. doi: 10.1371/journal.pone.0042474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hamm MP, Cherry NM, Chan E, Martin JW, Burstyn I. Maternal exposure to perfluorinated acids and fetal growth. J Expo Sci Environ Epidemiol. 2010;20(7):589–97. doi: 10.1038/jes.2009.57. [DOI] [PubMed] [Google Scholar]
- 61 •.Savitz DA, Stein CR, Bartell SM, Elston B, Gong J, Shin HM, et al. Perfluorooctanoic acid exposure and pregnancy outcome in a highly exposed community. Epidemiology. 2012;23(3):386–92. doi: 10.1097/EDE.0b013e31824cb93b. Used modeling techniques to improve exposure assessment of perflourinated compounds in a highly exposed cohort. Incorporated data from individual serum levels, historical exposure, environmental factors, and industrial operations to quantify exposure levels of participants in the study.
- 62.Savitz DA, Stein CR, Elston B, Wellenius GA, Bartell SM, Shin HM, et al. Relationship of perfluorooctanoic acid exposure to pregnancy outcome based on birth records in the Mid-Ohio Valley. Environ Health Persp. 2012;120:1201–7. doi: 10.1289/ehp.1104752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Whitworth KW, Haug LS, Baird DD, Becher G, Hoppin JA, Skjaerven R, et al. Perfluorinated compounds in relation to birth weight in the Norwegian mother and child cohort study. Am J Epidemiol. 2012;175:1209–16. doi: 10.1093/aje/kwr459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu K, Xu X, Liu J, Guo Y, Li Y, Huo X. Polybrominated diphenyl ethers in umbilical cord blood and relevant factors in neonates from Guiyu, China. Environ Sci Technol. 2010;44(2):813–9. doi: 10.1021/es9024518. [DOI] [PubMed] [Google Scholar]
- 65.Wu K, Xu X, Peng L, Liu J, Guo Y, Huo X. Association between maternal exposure to perfluorooctanoic acid (PFOA) from electronic waste recycling and neonatal health outcomes. Environ Int. 2012;48:1–8. doi: 10.1016/j.envint.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 66.Kadhel P, Monfort C, Costet N, Rouget F, Thome JP, Multigner L, et al. Chlordecone exposure, length of gestation, and risk of preterm birth. Am J Epidemiol. 2014;179(5):536–44. doi: 10.1093/aje/kwt313. [DOI] [PubMed] [Google Scholar]
- 67.Basterrechea M, Lertxundi A, Iniguez C, Mendez M, Murcia M, Mozo I, et al. Prenatal exposure to hexachlorobenzene (HCB) and reproductive effects in a multicentre birth cohort in Spain. Sci Total Environ. 2014;466-467:770–6. doi: 10.1016/j.scitotenv.2013.07.053. [DOI] [PubMed] [Google Scholar]
- 68 •.Wesselink A, Warner M, Samuels S, Parigi A, Brambilla P, Mocarelli P, et al. Maternal dioxin exposure and pregnancy outcomes over 30 years of follow-up in Seveso. Environ Int. 2014;63:143–8. doi: 10.1016/j.envint.2013.11.005. Utilized data from an industrial accident that allowed for the study of residential exposure to dioxin at unprecedented levels and preterm birth.
- 69.Darrow LA, Stein CR, Steenland K. Serum perfluorooctanoic acid and perfluorooctane sulfonate concentrations in relation to birth outcomes in the Mid-Ohio Valley, 2005-2010. Environmental health perspectives. 2013;121(10):1207–13. doi: 10.1289/ehp.1206372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peltier MR, Koo HC, Getahun D, Menon R. Does exposure to flame retardants increase the risk for preterm birth? J Reprod Immunol. 2015;107:20–5. doi: 10.1016/j.jri.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eskenazi B, Harley K, Bradman A, Weltzien E, Jewell NP, Barr DB, et al. Association of in utero organophosphate pesticide exposure and fetal growth and length of gestation in an agricultural population. Environ Health Perspect. 2004;112(10):1116–24. doi: 10.1289/ehp.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sathyanarayana S, Basso O, Karr CJ, Lozano P, Alavanja M, Sandler DP, et al. Maternal pesticide use and birth weight in the agricultural health study. J Agromedicine. 2010;15(2):127–36. doi: 10.1080/10599241003622699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuang Z, McConnell LL, Torrents A, Meritt D, Tobash S. Atmospheric deposition of pesticides to an agricultural watershed of the Chesapeake Bay. J Environ Qual. 2003;32(5):1611–22. doi: 10.2134/jeq2003.1611. [DOI] [PubMed] [Google Scholar]
- 74.Ochoa-Acuna H, Frankenberger J, Hahn L, Carbajo C. Drinking-water herbicide exposure in Indiana and prevalence of small-for-gestational-age and preterm delivery. Environ Health Perspect. 2009;117(10):1619–24. doi: 10.1289/ehp.0900784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Villanueva CM, Durand G, Coutte MB, Chevrier C, Cordier S. Atrazine in municipal drinking water and risk of low birth weight, preterm delivery, and small-for-gestational-age status. Occup Environ Med. 2005;62(6):400–5. doi: 10.1136/oem.2004.016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chevrier C, Limon G, Monfort C, Rouget F, Garlantezec R, Petit C, et al. Urinary biomarkers of prenatal atrazine exposure and adverse birth outcomes in the PELAGIE birth cohort. Environ Health Perspect. 2011;119(7):1034–41. doi: 10.1289/ehp.1002775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rinsky JL, Hopenhayn C, Golla V, Browning S, Bush HM. Atrazine exposure in public drinking water and preterm birth. Public Health Rep. 2012;127(1):72–80. doi: 10.1177/003335491212700108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.CDC . Fourth national report of human exposure to environmental chemicals. Centers for Disease Control and Prevention; Atlanta, GA: Feb, 2015. 2015. Report No. [Google Scholar]
- 79.Adibi JJ, Hauser R, Williams PL, Whyatt RM, Calafat AM, Nelson H, et al. Maternal urinary metabolites of Di-(2-Ethylhexyl) phthalate in relation to the timing of labor in a US multicenter pregnancy cohort study. Am J Epidemiol. 2009;169(8):1015–24. doi: 10.1093/aje/kwp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meeker JD, Hu H, Cantonwine DE, Lamadrid-Figueroa H, Calafat AM, Ettinger AS, et al. Urinary phthalate metabolites in relation to preterm birth in Mexico city. Environ Health Perspect. 2009;117(10):1587–92. doi: 10.1289/ehp.0800522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ferguson KK, McElrath TF, Meeker JD. Environmental phthalate exposure and preterm birth. JAMA pediatrics. 2014;168(1):61–7. doi: 10.1001/jamapediatrics.2013.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ferguson KK, McElrath TF, Ko YA, Mukherjee B, Meeker JD. Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environ Int. 2014;70:118–24. doi: 10.1016/j.envint.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang Y, Li J, Garcia JM, Lin H, Wang Y, Yan P, et al. Phthalate levels in cord blood are associated with preterm delivery and fetal growth parameters in Chinese women. PLoS One. 2014;9(2):e87430. doi: 10.1371/journal.pone.0087430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cantonwine D, Meeker JD, Hu H, Sanchez BN, Lamadrid-Figueroa H, Mercado-Garcia A, et al. Bisphenol a exposure in Mexico City and risk of prematurity: a pilot nested case control study. Environ Health. 2010;9:62. doi: 10.1186/1476-069X-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cantonwine DE, Ferguson KK, Mukherjee B, McElrath TF, Meeker JD. Urinary Bisphenol A Levels during Pregnancy and Risk of Preterm Birth. Environmental health perspectives. 2015;123(9):895–901. doi: 10.1289/ehp.1408126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Behnia F, Peltier M, Getahun D, Watson C, Saade G, Menon R. High bisphenol A (BPA) concentration in the maternal, but not fetal, compartment increases the risk of spontaneous preterm delivery. J Matern Fetal Neonatal Med. 2016;29(22):3583–9. doi: 10.3109/14767058.2016.1139570. [DOI] [PubMed] [Google Scholar]
- 87.Calafat AM. Contemporary Issues in Exposure Assessment Using Biomonitoring. Current Epidemiology Reports. 2016;3(2):145–53. doi: 10.1007/s40471-016-0075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Andrews KW, Savitz DA, Hertz-Picciotto I. Prenatal lead exposure in relation to gestational age and birth weight: a review of epidemiologic studies. Am J Ind Med. 1994;26(1):13–32. doi: 10.1002/ajim.4700260103. [DOI] [PubMed] [Google Scholar]
- 89.Ahmad SA, Sayed MH, Barua S, Khan MH, Faruquee MH, Jalil A, et al. Arsenic in drinking water and pregnancy outcomes. Environ Health Perspect. 2001;109(6):629–31. doi: 10.1289/ehp.01109629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nishijo M, Nakagawa H, Honda R, Tanebe K, Saito S, Teranishi H, et al. Effects of maternal exposure to cadmium on pregnancy outcome and breast milk. Occup Environ Med. 2002;59(6):394–6. doi: 10.1136/oem.59.6.394. discussion 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cantonwine D, Hu H, Sanchez BN, Lamadrid-Figueroa H, Smith D, Ettinger AS, et al. Critical windows of fetal lead exposure: adverse impacts on length of gestation and risk of premature delivery. J Occup Environ Med. 2010;52(11):1106–11. doi: 10.1097/JOM.0b013e3181f86fee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vigeh M, Yokoyama K, Seyedaghamiri Z, Shinohara A, Matsukawa T, Chiba M, et al. Blood lead at currently acceptable levels may cause preterm labour. Occup Environ Med. 2011;68(3):231–4. doi: 10.1136/oem.2009.050419. [DOI] [PubMed] [Google Scholar]
- 93.Taylor CM, Golding J, Emond AM. Adverse effects of maternal lead levels on birth outcomes in the ALSPAC study: a prospective birth cohort study. BJOG. 2015;122(3):322–8. doi: 10.1111/1471-0528.12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang B, Xia W, Li Y, Bassig BA, Zhou A, Wang Y, et al. Prenatal exposure to lead in relation to risk of preterm low birth weight: A matched case-control study in China. Reprod Toxicol. 2015;57:190–5. doi: 10.1016/j.reprotox.2015.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brown MJ, Margolis S. Lead in drinking water and human blood lead levels in the United States. MMWR Suppl. 2012;61(4):1–9. [PubMed] [Google Scholar]
- 96.Sowers M, Jannausch M, Scholl T, Li W, Kemp FW, Bogden JD. Blood lead concentrations and pregnancy outcomes. Arch Environ Health. 2002;57(5):489–95. doi: 10.1080/00039890209601442. [DOI] [PubMed] [Google Scholar]
- 97.Falcon M, Vinas P, Luna A. Placental lead and outcome of pregnancy. Toxicology. 2003;185(1-2):59–66. doi: 10.1016/s0300-483x(02)00589-9. [DOI] [PubMed] [Google Scholar]
- 98.Perkins M, Wright RO, Amarasiriwardena CJ, Jayawardene I, Rifas-Shiman SL, Oken E. Very low maternal lead level in pregnancy and birth outcomes in an eastern Massachusetts population. Ann Epidemiol. 2014;24(12):915–9. doi: 10.1016/j.annepidem.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhu M, Fitzgerald EF, Gelberg KH, Lin S, Druschel CM. Maternal low-level lead exposure and fetal growth. Environ Health Perspect. 2010;118(10):1471–5. doi: 10.1289/ehp.0901561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Taylor CM, Golding J, Emond AM. Blood mercury levels and fish consumption in pregnancy: Risks and benefits for birth outcomes in a prospective observational birth cohort. Int J Hyg Environ Health. 2016;219(6):513–20. doi: 10.1016/j.ijheh.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bashore CJ, Geer LA, He X, Puett R, Parsons PJ, Palmer CD, et al. Maternal mercury exposure, season of conception and adverse birth outcomes in an urban immigrant community in Brooklyn, New York, U.S.A. Int J Environ Res Public Health. 2014;11(8):8414–42. doi: 10.3390/ijerph110808414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Myers SL, Lobdell DT, Liu Z, Xia Y, Ren H, Li Y, et al. Maternal drinking water arsenic exposure and perinatal outcomes in inner Mongolia, China. J Epidemiol Community Health. 2010;64(4):325–9. doi: 10.1136/jech.2008.084392. [DOI] [PubMed] [Google Scholar]
- 103.Zhang YL, Zhao YC, Wang JX, Zhu HD, Liu QF, Fan YG, et al. Effect of environmental exposure to cadmium on pregnancy outcome and fetal growth: a study on healthy pregnant women in China. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2004;39(9):2507–15. doi: 10.1081/ese-200026331. [DOI] [PubMed] [Google Scholar]
- 104.Landgren O. Environmental pollution and delivery outcome in southern Sweden: a study with central registries. Acta Paediatr. 1996;85(11):1361–4. doi: 10.1111/j.1651-2227.1996.tb13926.x. [DOI] [PubMed] [Google Scholar]
- 105.Fagher U, Laudanski T, Schutz A, Sipowicz M, Akerlund M. The relationship between cadmium and lead burdens and preterm labor. Int J Gynaecol Obstet. 1993;40(2):109–14. doi: 10.1016/0020-7292(93)90368-7. [DOI] [PubMed] [Google Scholar]
- 106.Wang H, Liu L, Hu YF, Hao JH, Chen YH, Su PY, et al. Association of maternal serum cadmium level during pregnancy with risk of preterm birth in a Chinese population. Environ Pollut. 2016;216:851–7. doi: 10.1016/j.envpol.2016.06.058. [DOI] [PubMed] [Google Scholar]
- 107.Yang J, Huo W, Zhang B, Zheng T, Li Y, Pan X, et al. Maternal urinary cadmium concentrations in relation to preterm birth in the Healthy Baby Cohort Study in China. Environ Int. 2016;94:300–6. doi: 10.1016/j.envint.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 108.Huang K, Li H, Zhang B, Zheng T, Li Y, Zhou A, et al. Prenatal cadmium exposure and preterm low birth weight in China. J Expo Sci Environ Epidemiol. 2016 doi: 10.1038/jes.2016.41. [DOI] [PubMed] [Google Scholar]
- 109.Hertz-Picciotto I, Schramm M, Watt-Morse M, Chantala K, Anderson J, Osterloh J. Patterns and determinants of blood lead during pregnancy. Am J Epidemiol. 2000;152(9):829–37. doi: 10.1093/aje/152.9.829. [DOI] [PubMed] [Google Scholar]
- 110.Stieb DM, Chen L, Eshoul M, Judek S. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res. 2012;117:100–11. doi: 10.1016/j.envres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 111.Dadvand P, Basagaña X, Figueras F, Martinez D, Beelen R, Cirach M, et al. Air pollution and preterm premature rupture of membranes: a spatiotemporal analysis. Am J Epidemiol. 2013:kwt240. doi: 10.1093/aje/kwt240. [DOI] [PubMed] [Google Scholar]
- 112.Panasevich S, Håberg SE, Aamodt G, London SJ, Stigum H, Nystad W, et al. Association between pregnancy exposure to air pollution and birth weight in selected areas of Norway. Archives of Public Health. 2016;74(1):26. doi: 10.1186/s13690-016-0138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stieb DM, Chen L, Hystad P, Beckerman BS, Jerrett M, Tjepkema M, et al. A national study of the association between traffic-related air pollution and adverse pregnancy outcomes in Canada, 1999–2008. Environ Res. 2016;148:513–26. doi: 10.1016/j.envres.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 114 •.Hyder A, Lee HJ, Ebisu K, Koutrakis P, Belanger K, Bell ML. PM2. 5 exposure and birth outcomes: use of satellite-and monitor-based data. Epidemiology (Cambridge, Mass) 2014;25(1):58. doi: 10.1097/EDE.0000000000000027. This paper utilized satellite data to estimate particulate matter exposures in small geospatial areas, and examined associations by week of exposure in pregnancy.
- 115.Kloog I, Melly SJ, Ridgway WL, Coull BA, Schwartz J. Using new satellite based exposure methods to study the association between pregnancy PM 2.5 exposure, premature birth and birth weight in Massachusetts. Environmental Health. 2012;11(1):1. doi: 10.1186/1476-069X-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lavigne E, Yasseen AS, Stieb DM, Hystad P, van Donkelaar A, Martin RV, et al. Ambient air pollution and adverse birth outcomes: Differences by maternal comorbidities. Environ Res. 2016;148:457–66. doi: 10.1016/j.envres.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 117 •.Laurent O, Hu J, Li L, Kleeman MJ, Bartell SM, Cockburn M, et al. A statewide nested case-control study of preterm birth and air pollution by source and composition: California, 2001–2008. Environ Health Perspect Published Online First. 2016:19. doi: 10.1289/ehp.1510133. This study examined source (e.g., roadways, meat cooking) and composition (e.g., nitrate, organic carbon) of particulate matter specifically in relation to preterm birth.
- 118.Pereira G, Bell ML, Lee HJ, Koutrakis P, Belanger K. Sources of fine particulate matter and risk of preterm birth in connecticut, 2000-2006: a longitudinal study. Environmental Health Perspectives (Online) 2014;122(10):1117. doi: 10.1289/ehp.1307741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119 •.Rappazzo KM, Daniels JL, Messer LC, Poole C, Lobdell DT. Exposure to elemental carbon, organic carbon, nitrate, and sulfate fractions of fine particulate matter and risk of preterm birth in New Jersey, Ohio, and Pennsylvania (2000–2005) Environ Health Perspect. 2015;123(10):1059. doi: 10.1289/ehp.1408953. This paper examined fractions of PM2.5 in relation to preterm birth.
- 120.Hao H, Chang HH, Holmes HA, Mulholland JA, Klein M, Darrow LA, et al. Air pollution and preterm birth in the US state of Georgia (2002–2006): associations with concentrations of 11 ambient air pollutants estimated by combining Community Multiscale Air Quality Model (CMAQ) simulations with stationary monitor measurements. Environ Health Perspect. 2016;124(6):875. doi: 10.1289/ehp.1409651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mendola P, Wallace M, Hwang BS, Liu D, Robledo C, Männistö T, et al. Preterm birth and air pollution: Critical windows of exposure for women with asthma. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2015.12.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122 •.Li S, Guo Y, Williams G. Acute Impact of Hourly Ambient Air Pollution on Preterm Birth. Environ Health Perspect. 2016 doi: 10.1289/EHP200. [epub ahead of print] This paper examined hourly exposures of criteria air pollutants in the window immediately preceding onset of labor.
- 123.Padula AM, Mortimer KM, Tager IB, Hammond SK, Lurmann FW, Yang W, et al. Traffic-related air pollution and risk of preterm birth in the San Joaquin Valley of California. Ann Epidemiol. 2014;24(12):888–95. e4. doi: 10.1016/j.annepidem.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rappazzo KM, Daniels JL, Messer LC, Poole C, Lobdell DT. Exposure to fine particulate matter during pregnancy and risk of preterm birth among women in New Jersey, Ohio, and Pennsylvania, 2000-2005. Environmental Health Perspectives (Online) 2014;122(9):992. doi: 10.1289/ehp.1307456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pereira G, Bell ML, Belanger K, de Klerk N. Fine particulate matter and risk of preterm birth and pre-labor rupture of membranes in Perth, Western Australia 1997–2007: A longitudinal study. Environ Int. 2014;73:143–9. doi: 10.1016/j.envint.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pereira G, Evans KA, Rich DQ, Bracken MB, Bell ML. Fine particulates, preterm birth, and membrane rupture in Rochester, NY. Epidemiology. 2016;27(1):66–73. doi: 10.1097/EDE.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 127 •.Wallace ME, Grantz KL, Liu D, Zhu Y, Kim SS, Mendola P. Exposure to Ambient Air Pollution and Premature Rupture of Membranes. Am J Epidemiol. 2016 doi: 10.1093/aje/kwv284. [epub ahead of print] This paper examined acute exposure to criteria air pollutants by focusing on the 8 days and 5 hours preceding delivery, and focused on one specific presentation of preterm birth (PPROM).
- 128.Lin Y-T, Jung C-R, Lee YL, Hwang B-F. Associations between ozone and preterm birth in women who develop gestational diabetes. Am J Epidemiol. 2015;181(4):280–7. doi: 10.1093/aje/kwu264. [DOI] [PubMed] [Google Scholar]
- 129.Bertin M, Chevrier C, Serrano T, Monfort C, Rouget F, Cordier S, et al. Association between prenatal exposure to traffic-related air pollution and preterm birth in the PELAGIE mother–child cohort, Brittany, France. Does the urban–rural context matter? Environ Res. 2015;142:17–24. doi: 10.1016/j.envres.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 130.Lamichhane DK, Leem J-H, Lee J-Y, Kim H-C. A meta-analysis of exposure to particulate matter and adverse birth outcomes. Environ Health Toxicol. 2015;30 doi: 10.5620/eht.e2015011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sapkota A, Chelikowsky AP, Nachman KE, Cohen AJ, Ritz B. Exposure to particulate matter and adverse birth outcomes: a comprehensive review and meta-analysis. Air Quality, Atmosphere & Health. 2012;5(4):369–81. [Google Scholar]
- 132.Sun X, Luo X, Zhao C, Zhang B, Tao J, Yang Z, et al. The associations between birth weight and exposure to fine particulate matter (PM 2.5) and its chemical constituents during pregnancy: A meta-analysis. Environmental Pollution. 2016;211:38–47. doi: 10.1016/j.envpol.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 133.Zhu X, Liu Y, Chen Y, Yao C, Che Z, Cao J. Maternal exposure to fine particulate matter (PM2. 5) and pregnancy outcomes: a meta-analysis. Environmental Science and Pollution Research. 2015;22(5):3383–96. doi: 10.1007/s11356-014-3458-7. [DOI] [PubMed] [Google Scholar]
- 134.Gray SC, Edwards SE, Schultz BD, Miranda ML. Assessing the impact of race, social factors and air pollution on birth outcomes: a population-based study. Environmental Health. 2014;13(1):1. doi: 10.1186/1476-069X-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Johnson S, Bobb JF, Ito K, Savitz DA, Elston B, Shmool J, et al. Ambient Fine Particulate Matter, Nitrogen Dioxide, and Preterm Birth in New York City. Environ Health Perspect. 2016 doi: 10.1289/ehp.1510266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Symanski E, Davila M, McHugh MK, Waller DK, Zhang X, Lai D. Maternal exposure to fine particulate pollution during narrow gestational periods and newborn health in Harris County, Texas. Maternal and child health journal. 2014;18(8):2003–12. doi: 10.1007/s10995-014-1446-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fleischer Outdoor Air Pollution, Preterm Birth, and Low Birth Weight: Analysis of the World Health Organization Global Survey on Maternal and Perinatal Health (vol 122, pg 425, 2014) Environ Health Perspect. 2014;122(6):A151–A. doi: 10.1289/ehp.1306837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ha S, Hu H, Roth J, Kan H, Xu X. Associations Between Residential Proximity to Power Plants and Adverse Birth Outcomes. Am J Epidemiol. 2015;182(3):215–24. doi: 10.1093/aje/kwv042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Balsa AI, Caffera M, Bloomfield J. Exposures to Particulate Matter from the Eruptions of the Puyehue Volcano and Birth Outcomes in Montevideo, Uruguay. Environ Health Perspect. 2016 doi: 10.1289/EHP235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Huang C, Nichols C, Liu Y, Zhang Y, Liu X, Gao S, et al. Ambient air pollution and adverse birth outcomes: a natural experiment study. Population health metrics. 2015;13(1):1. doi: 10.1186/s12963-015-0050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Candela S, Ranzi A, Bonvicini L, Baldacchini F, Marzaroli P, Evangelista A, et al. Air pollution from incinerators and reproductive outcomes: a multisite study. Epidemiology. 2013;24(6):863–70. doi: 10.1097/EDE.0b013e3182a712f1. [DOI] [PubMed] [Google Scholar]
- 142.Capobussi M, Tettamanti R, Marcolin L, Piovesan L, Bronzin S, Gattoni ME, et al. Air Pollution Impact on Pregnancy Outcomes in Como, Italy. J Occup Environ Med. 2016;58(1):47–52. doi: 10.1097/JOM.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 143.Estarlich M, Ballester F, Davdand P, Llop S, Esplugues A, Fernández-Somoano A, et al. Exposure to ambient air pollution during pregnancy and preterm birth: A Spanish multicenter birth cohort study. Environ Res. 2016;147:50–8. doi: 10.1016/j.envres.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 144.Olsson D, Ekström M, Forsberg B. Temporal variation in air pollution concentrations and preterm birth—a population based epidemiological study. Int J Environ Res Public Health. 2012;9(1):272–85. doi: 10.3390/ijerph9010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Trasande L, Wong K, Roy A, Savitz DA, Thurston G. Exploring prenatal outdoor air pollution, birth outcomes and neonatal health care utilization in a nationally representative sample. Journal of Exposure Science and Environmental Epidemiology. 2013;23(3):315–21. doi: 10.1038/jes.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Arroyo V, Díaz J, Ortiz C, Carmona R, Sáez M, Linares C. Short term effect of air pollution, noise and heat waves on preterm births in Madrid (Spain) Environ Res. 2016;145:162–8. doi: 10.1016/j.envres.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 147.Baker P, Agius R. Air pollution exposure and adverse pregnancy outcomes in a large UK birth cohort: use of a novel spatio-temporal modelling technique. Scand J Work Environ Health. 2014;40(5):518. doi: 10.5271/sjweh.3423. [DOI] [PubMed] [Google Scholar]
- 148.Dibben C, Clemens T. Place of work and residential exposure to ambient air pollution and birth outcomes in Scotland, using geographically fine pollution climate mapping estimates. Environ Res. 2015;140:535–41. doi: 10.1016/j.envres.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Olsson D, Mogren I, Eneroth K, Forsberg B. Traffic pollution at the home address and pregnancy outcomes in Stockholm, Sweden. BMJ open. 2015;5(8):e007034. doi: 10.1136/bmjopen-2014-007034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Olsson D, Mogren I, Forsberg B. Air pollution exposure in early pregnancy and adverse pregnancy outcomes: a register-based cohort study. BMJ open. 2013;3(2):e001955. doi: 10.1136/bmjopen-2012-001955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Poirier A, Dodds L, Dummer T, Rainham D, Maguire B, Johnson M. Maternal Exposure to Air Pollution and Adverse Birth Outcomes in Halifax, Nova Scotia. J Occup Environ Med. 2015;57(12):1291–8. doi: 10.1097/JOM.0000000000000604. [DOI] [PubMed] [Google Scholar]
- 152.Qian Z, Liang S, Yang S, Trevathan E, Huang Z, Yang R, et al. Ambient air pollution and preterm birth: A prospective birth cohort study in Wuhan, China. Int J Hyg Environ Health. 2016;219(2):195–203. doi: 10.1016/j.ijheh.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 153.Schifano P, Lallo A, Asta F, De Sario M, Davoli M, Michelozzi P. Effect of ambient temperature and air pollutants on the risk of preterm birth, Rome 2001–2010. Environ Int. 2013;61:77–87. doi: 10.1016/j.envint.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 154.van den Hooven EH, Pierik FH, de Kluizenaar Y, Willemsen SP, Hofman A, van Ratingen SW, et al. Air pollution exposure during pregnancy, ultrasound measures of fetal growth, and adverse birth outcomes: a prospective cohort study. Environ Health Perspect. 2012;120(1):150. doi: 10.1289/ehp.1003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.ATSDR [09/26/2014];Toxicological Profile for Polycyclic Aromatic Hydrocarbons Atlanta: Agency for Toxic Substances and Disease Registry. 1995 Available from: http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=122&tid=25. [PubMed]
- 156.Padula AM, Noth EM, Hammond SK, Lurmann FW, Yang W, Tager IB, et al. Exposure to airborne polycyclic aromatic hydrocarbons during pregnancy and risk of preterm birth. Environ Res. 2014;135:221–6. doi: 10.1016/j.envres.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wilhelm M, Ghosh JK, Su J, Cockburn M, Jerrett M, Ritz B. Traffic-related air toxics and preterm birth: a population-based case-control study in Los Angeles County, California. Environ Health. 2011;10:89. doi: 10.1186/1476-069X-10-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Guo Y, Huo X, Wu K, Liu J, Zhang Y, Xu X. Carcinogenic polycyclic aromatic hydrocarbons in umbilical cord blood of human neonates from Guiyu, China. Sci Total Environ. 2012;427-428:35–40. doi: 10.1016/j.scitotenv.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 159.Forand SP, Lewis-Michl EL, Gomez MI. Adverse birth outcomes and maternal exposure to trichloroethylene and tetrachloroethylene through soil vapor intrusion in New York State. Environ Health Perspect. 2012;120(4):616. doi: 10.1289/ehp.1103884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Porter TR, Kent ST, Su W, Beck HM, Gohlke JM. Spatiotemporal association between birth outcomes and coke production and steel making facilities in Alabama, USA: a cross-sectional study. Environmental Health. 2014;13(1):1. doi: 10.1186/1476-069X-13-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ghosh JKC, Wilhelm M, Ritz B. Effects of residential indoor air quality and household ventilation on preterm birth and term low birth weight in Los Angeles County, California. Am J Public Health. 2013;103(4):686–94. doi: 10.2105/AJPH.2012.300987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ion R, Bernal AL. Smoking and Preterm Birth. Reprod Sci. 2014 doi: 10.1177/1933719114556486. [DOI] [PubMed] [Google Scholar]
- 163.Salmasi G, Grady R, Jones J, McDonald SD. Environmental tobacco smoke exposure and perinatal outcomes: A systematic review and meta-analyses. Acta Obstet Gynecol Scand. 2010;89(4):423–41. doi: 10.3109/00016340903505748. [DOI] [PubMed] [Google Scholar]
- 164.Cui H, Gong T-T, Liu C-X, Wu Q-J. Associations between Passive Maternal Smoking during Pregnancy and Preterm Birth: Evidence from a Meta-Analysis of Observational Studies. PLoS One. 2016;11(1):e0147848. doi: 10.1371/journal.pone.0147848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Crane J, Keough M, Murphy P, Burrage L, Hutchens D. Effects of environmental tobacco smoke on perinatal outcomes: a retrospective cohort study. BJOG. 2011;118(7):865–71. doi: 10.1111/j.1471-0528.2011.02941.x. [DOI] [PubMed] [Google Scholar]
- 166.Qiu J, He X, Cui H, Zhang C, Zhang H, Dang Y, et al. Passive smoking and preterm birth in urban China. Am J Epidemiol. 2014;180(1):94–102. doi: 10.1093/aje/kwu092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ion RC, Wills AK, Bernal AL. Environmental tobacco smoke exposure in pregnancy is associated with earlier delivery and reduced birth weight. Reprod Sci. 2015 doi: 10.1177/1933719115612135. 1933719115612135. [DOI] [PubMed] [Google Scholar]
- 168.Slama R, Darrow L, Parker J, Woodruff TJ, Strickland M, Nieuwenhuijsen M, et al. Meeting report: Atmospheric pollution and human reproduction. Environ Health Persp. 2008;116(6):791–8. doi: 10.1289/ehp.11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zeger SL. Invited commentary: epidemiologic studies of the health associations of environmental exposures with preterm birth. Am J Epidemiol. 2011:kwr405. doi: 10.1093/aje/kwr405. [DOI] [PubMed] [Google Scholar]
- 170.Pereira G, Belanger K, Ebisu K, Bell ML. Fine particulate matter and risk of preterm birth in Connecticut in 2000–2006: a longitudinal study. Am J Epidemiol. 2013:kwt216. doi: 10.1093/aje/kwt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Parker JD, Rich DQ, Glinianaia SV, Leem JH, Wartenberg D, Bell ML, et al. The international collaboration on air pollution and pregnancy outcomes: initial results. University of British Columbia; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172 •.Ferguson KK, McElrath TF, Ko Y-A, Mukherjee B, Meeker JD. Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environ Int. 2014;70:118–24. doi: 10.1016/j.envint.2014.05.016. Adds to the literature characterizing variability in phthalate levels across pregnancy. Serves as the first study to examine utilize longitudinally collected exposure data to explore differences in the association of phthalates and preterm birth at different times of exposure during pregnancy. the association between phthalate metabolites and preterm birth using multiple measures of exposure collected longitudinally across pregnancy.
- 173 •.Cantonwine DE, Ferguson KK, Mukherjee B, McElrath TF, Meeker JD. Urinary bisphenol A levels during pregnancy and risk of preterm birth. Environ Health Perspect. 2015;123(9):895. doi: 10.1289/ehp.1408126. Serves as the first study to examine the association between BPA exposure and preterm birth using multiple measures of exposure collected longitudinally across pregnancy. Further explored the association with BPA and preterm birth by assessing differences by infant sex.
- 174.Ferguson KK, McElrath TF, Meeker JD. Environmental phthalate exposure and preterm birth. JAMA pediatrics. 2014;168(1):61–8. doi: 10.1001/jamapediatrics.2013.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ferguson KK, McElrath TF, Chen Y-H, Mukherjee B, Meeker JD. Urinary phthalate metabolites and biomarkers of oxidative stress in pregnant women: a repeated measures analysis. 2014 doi: 10.1289/ehp.1307996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ferguson KK, McElrath TF, Chen Y-H, Loch-Caruso R, Mukherjee B, Meeker JD. Repeated measures of urinary oxidative stress biomarkers during pregnancy and preterm birth. Am J Obstet Gynecol. 2015;212(2):208, e1–e8. doi: 10.1016/j.ajog.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177 ••.Ferguson KK, Chen Y-H, VanderWeele TJ, McElrath TF, Meeker JD, Mukherjee B. Mediation of the relationship between maternal phthalate exposure and preterm birth by oxidative stress with repeated measurements across pregnancy. Environ Health Perspect. 2016 doi: 10.1289/EHP282. [epub ahead of print] This study examined windows of vulnerability to exposure during pregnancy, spontaneous preterm birth specifically, and examined 8-isoprostane as a biomarker of oxidative stress to demonstrate statistical mediation of the phthalate-preterm birth association.
- 178.Darrow LA, Stein CR, Steenland K. Serum perfluorooctanoic acid and perfluorooctane sulfonate concentrations in relation to birth outcomes in the Mid-Ohio Valley, 2005–2010. Environ Health Perspect. 2013;121(10):1207. doi: 10.1289/ehp.1206372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Albouy-Llaty M, Limousi F, Carles C, Dupuis A, Rabouan S, Migeot V. Association between Exposure to Endocrine Disruptors in Drinking Water and Preterm Birth, Taking Neighborhood Deprivation into Account: A Historic Cohort Study. Int J Environ Res Public Health. 2016;13(8) doi: 10.3390/ijerph13080796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cantonwine D, Meeker JD, Hu H, Sanchez BN, Lamadrid-Figueroa H, Mercado-Garcia A, et al. Bisphenol a exposure in Mexico City and risk of prematurity: a pilot nested case control study. Environ Health. 2010b;9:62. doi: 10.1186/1476-069X-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ruckart PZ, Bove FJ, Maslia M. Evaluation of contaminated drinking water and preterm birth, small for gestational age, and birth weight at Marine Corps Base Camp Lejeune, North Carolina: a cross-sectional study. Environmental Health. 2014;13(1):1. doi: 10.1186/1476-069X-13-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cantonwine D, Hu H, Sanchez BN, Lamadrid-Figueroa H, Smith D, Ettinger AS, et al. Critical windows of fetal lead exposure: Adverse impacts on length of gestation and risk of premature delivery. J Occup Environ Med. 2010a;52(11):1106–11. doi: 10.1097/JOM.0b013e3181f86fee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Shi X, Ayotte JD, Onda A, Miller S, Rees J, Gilbert-Diamond D, et al. Geospatial association between adverse birth outcomes and arsenic in groundwater in New Hampshire, USA. Environ Geochem Health. 2015;37(2):333–51. doi: 10.1007/s10653-014-9651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Le HQ, Batterman SA, Wirth JJ, Wahl RL, Hoggatt KJ, Sadeghnejad A, et al. Air pollutant exposure and preterm and term small-for-gestational-age births in Detroit, Michigan: long-term trends and associations. Environ Int. 2012;44:7–17. doi: 10.1016/j.envint.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lee P-C, Roberts JM, Catov JM, Talbott EO, Ritz B. First trimester exposure to ambient air pollution, pregnancy complications and adverse birth outcomes in Allegheny County, PA. Maternal and child health journal. 2013;17(3):545–55. doi: 10.1007/s10995-012-1028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pereira G, Bracken MB, Bell ML. Particulate air pollution, fetal growth and gestational length: The influence of residential mobility in pregnancy. Environ Res. 2016;147:269–74. doi: 10.1016/j.envres.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Schifano P, Asta F, Dadvand P, Davoli M, Basagana X, Michelozzi P. Heat and air pollution exposure as triggers of delivery: A survival analysis of population-based pregnancy cohorts in Rome and Barcelona. Environ Int. 2016;88:153–9. doi: 10.1016/j.envint.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 188.Symanski E, McHugh MK, Zhang X, Craft ES, Lai D. Evaluating narrow windows of maternal exposure to ozone and preterm birth in a large urban area in Southeast Texas. Journal of Exposure Science and Environmental Epidemiology. 2015 doi: 10.1038/jes.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chang HH, Reich BJ, Miranda ML. Time-to-event analysis of fine particle air pollution and preterm birth: results from North Carolina, 2001–2005. Am J Epidemiol. 2012;175(2):91–8. doi: 10.1093/aje/kwr403. [DOI] [PubMed] [Google Scholar]
- 190.DeFranco E, Moravec W, Xu F, Hall E, Hossain M, Haynes EN, et al. Exposure to airborne particulate matter during pregnancy is associated with preterm birth: a population-based cohort study. Environmental Health. 2016;15(1):1. doi: 10.1186/s12940-016-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Arffin F, AL-Bayaty FH, Hassan J. Environmental tobacco smoke and stress as risk factors for miscarriage and preterm births. Arch Gynecol Obstet. 2012;286(5):1187–91. doi: 10.1007/s00404-012-2417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Luo Y-J, Wen X-Z, Ding P, He Y-H, Xie C-B, Liu T, et al. Interaction between maternal passive smoking during pregnancy and CYP1A1 and GSTs polymorphisms on spontaneous preterm delivery. PLoS One. 2012;7(11):e49155. doi: 10.1371/journal.pone.0049155. [DOI] [PMC free article] [PubMed] [Google Scholar]