Abstract
Persons aged over 65 years account for over the vast majority of healthcare expenditures and deaths attributable to cardiovascular disease (CVD). Accordingly, reducing CVD risk among older adults is an important public health priority. Structured physical activity (i.e. exercise) is a well-documented method of decreasing CVD risk, but recent large-scale trials suggest that exercise alone is insufficient to reduce CVD events in high-risk populations of older adults. Thus adjuvant strategies appear necessary to reduce CVD risk. Accumulating evidence indicates that prolonged sedentary behavior (e.g. sitting) has detrimental health effects that are independent of engagement in recommended levels of moderate-intensity exercise. Yet clinical trials in this area are lacking. We hypothesize that exercise, when combined with a novel technology based intervention specifically designed to reduce sedentary behavior will reduce CVD risk among sedentary older adults. The purpose of this study is to evaluate the feasibility and efficacy of combining a traditional, structured exercise intervention with an innovative intervention designed to decrease sedentary behavior and increase non-exercise physical activity (NEPA). This study will provide us with critical data necessary to design and implement a full-scale trial to test our central hypothesis. Participants aged ≥60 years with moderate to high risk of coronary heart disease (CHD) events are randomly assigned to either the exercise and technology intervention (EX + NEPA) or exercise alone (EX) groups. Study dependent outcomes include changes in 1) daily activity patterns, 2) blood pressure, 3) exercise capacity, 4) waist circumference, and 5) circulating indices of cardiovascular function. This study will provide critical information for designing a fully-powered clinical trial, which could have health implications for the ever increasing population of older adults.
Keywords: Aging, Cardiovascular, Exercise, Physical activity, Activity monitor, RCT protocol
1. Introduction
Cardiovascular disease (CVD) is the leading cause of death in the U.S. and worldwide, and persons over age 65 account for nearly 80% of cardiovascular-related deaths [1]. Structured physical activity (i.e. exercise) is a well-documented method of decreasing CVD in a variety of populations [2], [3], but recent evidence from large, multicenter clinical trials suggests that exercise alone is insufficient in reducing CVD-related deaths in some high-risk populations of older adults [4], [5]. Accordingly, adjuvant strategies are needed to reduce CVD risk among the rapidly growing population of older adults.
Accumulating evidence suggests that persistent sedentary behavior (e.g. sitting) has detrimental health effects independent of participation in recommended levels of moderate-intensity exercise [6], [7], [8]. Chronic sedentary behavior is associated with increased risk of CVD, obesity, diabetes, cancer, and mortality [9], [10], [11], [12], [13]. High levels of sedentary behavior have also been linked to numerous CVD risk factors including elevated waist circumference, BMI, systolic blood pressure, as well as fasting triglycerides, HDL cholesterol, and insulin [10], [14], [15]. Evidence suggests that older adults spend 60–80% of their waking hours (>11 h/day) engaged in sedentary behavior [16], [17], [18]. Thus, reducing sedentary behavior among older adults represents a novel but important objective given the detrimental effects of high levels of sedentary behavior on CVD risk.
Despite growing concern about the health risks of sedentary behavior independent of exercise, limited data exists on the effects of interventions designed to modify sedentary behavior. Randomized controlled trials are thus needed to identify efficacious interventions for decreasing sedentary behavior and determine the utility of such interventions in decreasing CVD risk. One emerging approach with potential to reduce sedentary activity is the use of wearable, physical activity monitoring technologies. For example, a recent study found that wearing an activity monitor increased activity levels among sedentary adults by more than 2000 steps per day compared to adults without an activity monitor [19]. However, little is presently known about the efficacy of wearing an activity monitor among older adults as well as when combined with a structured exercise intervention.
Therefore, this study was designed to evaluate the feasibility and efficacy of combining a traditional, structured exercise intervention with an innovative, technology based intervention designed to decrease sedentary behavior and increase non-exercise physical activity (NEPA). Specifically, this intervention combines traditional behavior modification strategies with wearable monitors to promote NEPA among older adults with elevated CVD profiles. Our central hypothesis is that the intervention will reduce sedentary behavior and concomitant CVD risk compared to a standard intervention consisting of center-based exercise only. This study will provide us with critical data necessary to design and implement a full-scale trial to test this hypothesis.
2. Methods
2.1. Overview
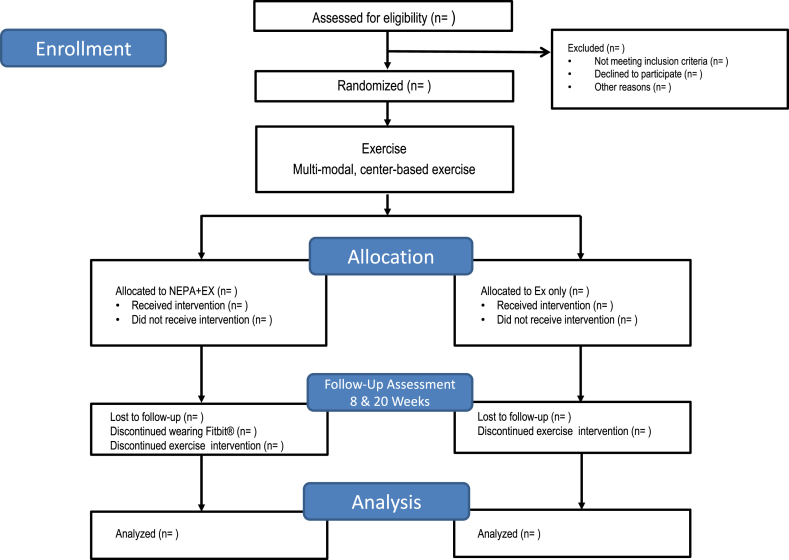
The study is a two-arm randomized, single-masked, pilot trial to evaluate the feasibility and efficacy of utilizing wearable technology to reduce sedentary behavior and CVD risk among older adults. Participants are randomly assigned to either the exercise and technology intervention (EX + NEPA) or the exercise only (EX) group. Both groups undergo an eight-week, twice-weekly, center-based exercise intervention. Participants are then assessed for changes in daily NEPA and cardiovascular risk factors at eight and 20 weeks post-randomization (Fig. 1). A comprehensive study team oversees participant safety including: the principal investigator, study physician, study staff, and an appointed Data and Safety Monitoring Board. The study was registered at www.clinicaltrials.gov prior to participant recruitment (NCT02632487), and all participants provide written informed consent based on documents approved by the University of Florida Institutional Review Board.
Fig. 1.
Overview of study design according to CONSORT format.
2.2. Participants
The study is recruiting up to 40 (n = 20/group) sedentary older adult men and women. Eligible participants are ≥60 years old and have moderate to high risk of coronary heart disease (CHD) events according to the National Cholesterol Education Program's Adults Treatment Panel (ATP-III) risk classification algorithm [20]. Persons with absolute contraindication to exercise [21], resting blood pressure of >180 systolic or >110 diastolic, or with other medical conditions that would prohibit safe participation are excluded. We anticipate the study to last approximately 18 months from first recruited participant until last recruited participant completes the study.
2.3. Screening and randomization
Interested individuals initially complete a telephone pre-screening prior to in-person screening visit. Following the informed consent, initial screening procedures include a review of medical history and medication use, short physical exam including blood pressure, the Community Health Activities Model Program for Seniors (CHAMPS) [22] to ensure participants are sedentary (<150 min moderate-intensity physical activity/week), and the Mini-mental State Exam [23] to ensure no significant cognitive impairment (MMSE ≥ 24).
If all eligibility criteria are met, participants return to the clinic for initial baseline assessments prior to randomization. The study design is an accepted procedure for exercise-based studies as indicated by the Consolidated Standards of Reporting Trials (CONSORT) Group [24], [25], with the study staff being blinded to the intervention assignment. To ensure masking of the assessment staff to intervention assignment, staff not involved in the assessments perform the randomization procedures. To enhance masking of the assessor, participants are asked not to disclose their assigned group and not to talk about their interventions during the testing sessions. Maintenance of staff blinding is facilitated by the fact that study interventions and assessments occur in separate physical locations. Intervention groups are also conducted at different times to prevent contamination bias between groups. A schedule of study assessments is provided in Table 1.
Table 1.
Data collection summary by study visit.
| Study Phase |
Pre-randomization |
Randomization |
||
|---|---|---|---|---|
| Visit description (FU = follow-up, CO = close-out) | Screen | Baseline | FU | CO |
| Visit number | 1 | 2 | 3 | 4 |
| Visit week | −2 | 0 | 8 | 20 |
| Informed consent, review inclusion/exclusion criteria | x | |||
| Personal interveiw, medical history, medication use | x | |||
| Office blood pressure (sitting + standing), vital signs | x | x | x | x |
| Physical exam, height/weight, ECG | x | |||
| Randomization | x | |||
| Waist circumference, 6 min walk, blood collection | x | x | x | |
| Accelerometry, assess adverse experiences | x | x | x | |
| Diet recall, 4 m walk, grip strength | x | x | x | |
| Short Phsyical Performance Battery | x | x | ||
2.4. Assessments
2.4.1. Daily activity patterns
A hip worn, solid-state triaxial accelerometer (Actigraph GT3X) [17], [18] is used to compare daily activity patterns between the two groups. Participants are asked to wear the accelerometer for seven days surrounding each assessment visit. The accelerometers provide no feedback to the participants regarding their activity patterns.
2.4.2. Blood pressure
Both systolic and diastolic blood pressures are evaluated during assessment visits using standard clinical procedures. Sitting and standing blood pressures are assessed to identify potential safety issues related to orthostatic hypotension. The seated pressures will be utilized in study outcome analysis.
2.4.3. Exercise capacity
To assess exercise capacity, the 6-min walk test is performed. This test is a safe and reliable test of aerobic endurance in older adults and people with cardiovascular disease [26], [27]. The test has strong reproducibility, with intra-subject coefficients of variation averaging <10%, and a modest correlation with peak ventilator oxygen (VO2) [28]. Participants are asked to walk as far and fast as safely possible for 6-min on a 100-foot course. Total distance is measured at the end of the 6-min time.
2.4.4. Waist circumference
Elevated waist circumference is a known risk factor for CVD [21]. Waist circumference is measured according to the National Health and Nutrition Examination Survey (NHANES) Anthropometry Procedures Manual protocol [29].
2.4.5. Circulating indices of CV risk
Fasting blood samples will be collected during the baseline, follow-up, and close-out visits. Fasting blood samples (serum or plasma as appropriate) are taken to measure blood lipids, glucose, and hemoglobin A(1c) levels. In addition, samples will be assayed for markers of inflammation and oxidative stress, including oxidized low-density lipoprotein (LDL) and myeloperoxidase (MPO).
2.4.6. Supportive outcome measures
A select group of inexpensive supporting measures are being evaluated to aid in the interpretation of study outcomes and inform the future trial. Because of the importance of diet to cardiovascular risk, dietary habits are assessed via 3-day dietary recalls and analyzed using commercial software (ESHA, Salem, OR). Additionally, usual-paced walking speed (via 4 m walk), grip strength (Jamar Hydraulic Hand Dynamometer, Fred Sammons, Inc. Burr Ridge, IL) and lower-extremity function via the Short Physical Performance Battery [26] are measured because of strong associations between physical function and cardiovascular risk among older adults [27], [28], [29], [30]. Diet recall, usual paced walking speed, and grip strength are assessed at the baseline, follow-up, and close-out visits, while the lower-extremity function is assessed at baseline and close-out.
2.5. Exercise intervention
All participants participate in a structured exercise program twice weekly for the first eight weeks of the study. The exercise program is designed to meet exercise and physical activity guidelines for older adults from the American College of Sports Medicine (ACSM) and American Heart Association (AHA) [31]. Exercise sessions begin with a brief warm-up followed by 30 min of moderate-intensity walking, 30 min of light upper and lower body resistance training, balance, and flexibility exercises. Exercise intensity is individually monitored using a subjective 0–10 scale for physical exertion (Borg CR10 scale) [32]. Participants are initially instructed to walk at a moderate intensity, equivalent to a 5–6 on the CR10 scale and encouraged to incorporate periods of vigorous walking (7–8 on CR10) as possible. Following the 8-week center based exercise intervention the subjects in both the EX and EX + NEPA groups are given instructions to achieve 150 min of moderate to vigorous activity per week, per the exercise prescription guidelines recommended by ACSM for the remainder of the study (12 weeks).
Participants in both groups are provided with cognitive-behavioral counseling focused on reducing sedentary behavior and increasing NEPA during their daily routines. A behavioral specialist assists with goal setting of NEPA minutes and includes considerable attention placed on the process of how NEPA might be increased given the daily demands and environmental constraints of the individual. For the EX + NEPA group, we monitor NEPA using the Fitbit® Zip activity monitor. The EX + NEPA participants are then provided with an activity monitor and asked to wear it during all waking hours. Adherence to monitor wearing is tracked by participants via a wear log to record all times the device is not worn. In order to encourage NEPA, the study team monitors participants' daily NEPA and communicates as necessary to provide motivation to participants in support of meeting their daily goals. Individual solutions are devised to overcome barriers to meeting their daily goals.
2.6. Safety
Numerous safety procedures are put in place to ensure participant safety. For example, during the informed consent process trained study personnel explain potential adverse events for study related activities and interventions to each participant. Participants are encouraged to notify study staff immediately if they have any adverse experiences that could be related to the study interventions. Study coordinators monitor adverse experiences at each study visit and as reported. Interventionists also monitor adverse events as they are reported as well as any potential events that occur during performance of the exercise intervention. Clinical blood tests (i.e. CVC metabolic panel, coagulation markers) are performed at each clinic assessment visit and are utilized to monitor potential hematologic and metabolic abnormalities in response to the interventions.
2.7. Statistical analysis
The primary analysis will follow an “intent-to-treat” model in which participants are grouped according to randomization assignment. Constrained mixed effects models using an unstructured correlation structure and random intercept will be used to determine intervention effects. Fixed effects included in the model will include baseline level of the outcome, age, gender, visit, and interaction between group assignment and visit. Hypothesis tests for intervention effects at assessment visits will be performed using contrasts of the 8- and 20-week intervention means. Contrasts will be used to determine group effects at specific visits and overall comparisons across follow-up visits for main outcome measure. For missing data, baseline characteristics of participants who do and do not have follow-up measures will be compared. Maximum likelihood will be used to obtain tests for fixed effects to account for the possibility that missing outcomes are dependent on observed covariates or previously observed outcomes [33]. Sensitivity of results to missing outcomes using multiple imputation [34] or propensity scores [35]. Sensitivity analyses will also be conducted to explore the potential impact of other relevant medical conditions (e.g. hypertension, type 2 diabetes, etc) on study outcomes. Caution will be taken in the interpretation of hypothesis tests as the relatively small sample size may create an imbalance in pre-randomization covariates [36]. However, this sample size will provide for nominal estimation (using a 95% confidence interval) of the mean changes in dependent variables within each arm of the study, which will facilitate planning of a larger, fully-powered trial.
3. Discussion
Our long-term goal is to identify efficacious interventions for reducing sedentary behavior and determine if the intervention aids in reducing CVD risk among older adults. Recent evidence from large scale trials suggests that exercise alone may be insufficient to reduce the risk of cardiovascular events in some high-risk individuals, including older adults [4], [5]. One potential explanation is persistant sedentary behavior regardless of participation in regular exercise. Among individuals who achieve the recommended moderate to vigorous activity minutes per week the detrimental effects of sedentary behavior persist [8], [37]. High levels of sedentary activities, such as watching television, have been found to increase all-cause risk independent of gender, age, education, smoking, alcohol, medication, family history of CVD, body size, and energy expenditure [38]. Indeed, cross-sectional studies show that time spent engaged in sedentary activities are independently associated with cardio-metabolic risk and are related to suppression of skeletal muscle metabolic activity [10], [39], [40], [41], [42], [43]. A recent cross-sectional study our group conducted on older adults found for every 25–30 min spent sedentary there is a 1% increase in predicted risk of a cardiovascular event (i.e. myocardial infarction or coronary death) and for every 20 min spent engaged in low-intensity activities there is an associated 1% decrease in predicted cardiovascular event risk [17]. Thus, reducing the amount of time older adults spend in sedentary behavior holds promise as a potential avenue to improving cardiovascular health among older adults.
Accordingly, we hypothesize that interventions which are efficacious in increasing NEPA may hold significant promise for mitigating the negative effects of sedentary activity on older adults' CVD risk. One prior large cohort study (N = 4232) among older adults (>60 years) found that reporting a high baseline NEPA level, compared with low, on an ordinal physical activity questionannaire scale was associated with a lower risk of a first CVD event (HR = 0.73; 95% CI 0.57 to 0.94) and lower all-cause mortality (0.70; 0.53 to 0.98) among older adults [44]. Higher levels of NEPA were also associated with reduction of known CVD-risk factors such as improved waist circumference, insulin level, HDL, triglycerides, and glucose.44Moreover, one recent prospective intervention study from rural, middle-aged adults reported that decreasing sedentary activity by 30 min a day reduced primary CVD risk factors including body weight and BMI [45]. These findings provide some promise that interventions designed to promote NEPA among older adults may be efficacious in reducing CVD risk. However, evidence is lacking from prospective trials to confirm this hypothesis. The present study will help to fill this gap in the literature while capitalizing on the growing popularity of wearable techonologies to address the need.
In summary, the current study aims to address the need to reduce the growing risk of CVD by promoting NEPA with a wearable activity monitor in addition to a structured exercise program. This study will provide critical data for designing a fully-powered clinical trial, which could have health implications for the ever increasing population of older adults. In addition, a key objective of the trial is also to refine study protocols and procedures prior to conducting a fully-powered trial. Furthermore, it should be noted that the study protocol might be modified during the trial to optimize study procedures (recruitment, safety, etc.) based upon the information obtained during the course of the trial. Because of this and other limitations (e.g. relatively small sample size; single site design) the results of this pilot study should not be over-interpreted. However, these data will inform the efficient and definitive full-scale trial to determine if exercise and NEPA monitoring decreases the risk of cardiovascular events. If our hypothesis is correct, the findings could provide evidence for the use of activity tracking in older adults to decrease sedentary behavior, thus cardiovascular disease.
Acknowledgements
We would like to thank all study participants and staff devoting their time and effort to the study. The study is funded by a grant from the American Heart Association (16IRG27250237) with support from the University of Florida Claude D. Pepper Older Americans Independence Center (1P30AG028740).
References
- 1.Mozaffarian D., Benjamin E.J., Go A.S. Heart disease and stroke statistics–2015 update: a report from the American heart association. Circulation. 2015;131(4):e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Pollock M.L., Franklin B.A., Balady G.J. AHA science advisory. resistance exercise in individuals with and without cardiovascular disease: benefits, rationale, safety, and prescription: an advisory from the committee on exercise, rehabilitation, and prevention, council on clinical cardiology, american heart association; position paper endorsed by the American college of sports medicine. Circulation. 2000;101(7):828–833. doi: 10.1161/01.cir.101.7.828. [DOI] [PubMed] [Google Scholar]
- 3.Pescatello L.S., Franklin B.A., Fagard R. American college of sports medicine position stand. exercise and hypertension. Med. Sci. Sports Exerc. 2004;36(3):533–553. doi: 10.1249/01.mss.0000115224.88514.3a. doi: 00005768-200403000-00025 [pii] [DOI] [PubMed] [Google Scholar]
- 4.Look AHEAD Research Group. Wing R.R., Bolin P. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N. Engl. J. Med. 2013;369(2):145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pahor M., Guralnik J.M., Ambrosius W.T. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311(23):2387–2396. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thyfault J.P., Booth F.W. Lack of regular physical exercise or too much inactivity. Curr. Opin. Clin. Nutr. Metab. Care. 2011;14(4):374–378. doi: 10.1097/MCO.0b013e3283468e69. [DOI] [PubMed] [Google Scholar]
- 7.Dunstan D.W., Thorp A.A., Healy G.N. Prolonged sitting: is it a distinct coronary heart disease risk factor? Curr. Opin. Cardiol. 2011;26(5):412–419. doi: 10.1097/HCO.0b013e3283496605. [DOI] [PubMed] [Google Scholar]
- 8.Owen N., Healy G.N., Matthews C.E., Dunstan D.W. Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci. Rev. 2010;38(3):105–113. doi: 10.1097/JES.0b013e3181e373a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthews C.E., George S.M., Moore S.C. Amount of time spent in sedentary behaviors and cause-specific mortality in US adults. Am. J. Clin. Nutr. 2012;95(2):437–445. doi: 10.3945/ajcn.111.019620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thorp A.A., Healy G.N., Owen N. Deleterious associations of sitting time and television viewing time with cardiometabolic risk biomarkers: australian diabetes, obesity and lifestyle (AusDiab) study 2004-2005. Diabetes Care. 2010;33(2):327–334. doi: 10.2337/dc09-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sisson S.B., Camhi S.M., Church T.S. Leisure time sedentary behavior, occupational/domestic physical activity, and metabolic syndrome in U.S. men and women. Metab. Syndr. Relat. Disord. 2009;7(6):529–536. doi: 10.1089/met.2009.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stamatakis E., Hamer M., Dunstan D.W. Screen-based entertainment time, all-cause mortality, and cardiovascular events: population-based study with ongoing mortality and hospital events follow-up. J. Am. Coll. Cardiol. 2011;57(3):292–299. doi: 10.1016/j.jacc.2010.05.065. [DOI] [PubMed] [Google Scholar]
- 13.Katzmarzyk P.T., Church T.S., Craig C.L., Bouchard C. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med. Sci. Sports Exerc. 2009;41(5):998–1005. doi: 10.1249/MSS.0b013e3181930355. [DOI] [PubMed] [Google Scholar]
- 14.Saunders T.J., Larouche R., Colley R.C., Tremblay M.S. Acute sedentary behaviour and markers of cardiometabolic risk: a systematic review of intervention studies. J. Nutr. Metab. 2012;2012:712435. doi: 10.1155/2012/712435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peddie M.C., Bone J.L., Rehrer N.J., Skeaff C.M., Gray A.R., Perry T.L. Breaking prolonged sitting reduces postprandial glycemia in healthy, normal-weight adults: a randomized crossover trial. Am. J. Clin. Nutr. 2013;98(2):358–366. doi: 10.3945/ajcn.112.051763. [DOI] [PubMed] [Google Scholar]
- 16.Matthews C.E., Chen K.Y., Freedson P.S. Amount of time spent in sedentary behaviors in the United States, 2003-2004. Am. J. Epidemiol. 2008;167(7):875–881. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitzgerald J.D., Johnson L., Hire D.G. Association of objectively measured physical activity with cardiovascular risk in mobility-limited older adults. J. Am. Heart Assoc. 2015;4(2) doi: 10.1161/JAHA.114.001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mankowski R.T., Aubertin-Leheudre M., Beavers D.P. Sedentary time is associated with the metabolic syndrome in older adults with mobility limitations–the LIFE study. Exp. Gerontol. 2015;70:32–36. doi: 10.1016/j.exger.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bravata D.M., Smith-Spangler C., Sundaram V. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298(19):2296–2304. doi: 10.1001/jama.298.19.2296. doi: 298/19/2296 [pii] [DOI] [PubMed] [Google Scholar]
- 20.Grundy S.M., Cleeman J.I., Merz C.N. Implications of recent clinical trials for the national cholesterol education program adult treatment panel III guidelines. Circulation. 2004;110(2):227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 21.American College of Sports Medicine . Lippincott Williams & Wilkins; 2013. ACSM's Guidelines for Exercise Testing and Prescription. [DOI] [PubMed] [Google Scholar]
- 22.Stewart A.L., Mills K.M., King A.C., Haskell W.L., Gillis D., Ritter P.L. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med. Sci. Sports Exerc. 2001;33(7):1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. 0022-3956(75)90026-6 [pii] [DOI] [PubMed] [Google Scholar]
- 24.Boutron I., Moher D., Altman D.G., Schulz K.F., Ravaud P., CONSORT Group Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann. Intern. Med. 2008;148(4):295–309. doi: 10.7326/0003-4819-148-4-200802190-00008. doi: 148/4/295 [pii] [DOI] [PubMed] [Google Scholar]
- 25.Boutron I., Moher D., Altman D.G., Schulz K.F., Ravaud P., CONSORT Group Methods and processes of the CONSORT group: example of an extension for trials assessing nonpharmacologic treatments. Ann. Intern. Med. 2008;148(4):W60–W66. doi: 10.7326/0003-4819-148-4-200802190-00008-w1. doi: 148/4/W-60 [pii] [DOI] [PubMed] [Google Scholar]
- 26.Guralnik J.M., Simonsick E.M., Ferrucci L. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 27.McDermott M.M., Applegate W.B., Bonds D.E. Ankle brachial index values, leg symptoms, and functional performance among community-dwelling older men and women in the lifestyle interventions and independence for elders study. J. Am. Heart Assoc. 2013;2(6):e000257. doi: 10.1161/JAHA.113.000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newman A.B., Simonsick E.M., Naydeck B.L. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295(17):2018–2026. doi: 10.1001/jama.295.17.2018. doi: 295/17/2018 [pii] [DOI] [PubMed] [Google Scholar]
- 29.Lumley T., Kronmal R.A., Cushman M., Manolio T.A., Goldstein S. A stroke prediction score in the elderly: validation and web-based application. J. Clin. Epidemiol. 2002;55(2):129–136. doi: 10.1016/s0895-4356(01)00434-6. doi: S0895435601004346 [pii] [DOI] [PubMed] [Google Scholar]
- 30.Cleveland J.C., Jr. Frailty, aging, and cardiac surgery outcomes: the stopwatch tells the story. J. Am. Coll. Cardiol. 2010;56(20):1677–1678. doi: 10.1016/j.jacc.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 31.American College of Sports Medicine. Chodzko-Zajko W.J., Proctor D.N. American college of sports medicine position stand. exercise and physical activity for older adults. Med. Sci. Sports Exerc. 2009;41(7):1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 32.Borg G. Human kinetics; 1998. Borg's Perceived Exertion and Pain Scales. [Google Scholar]
- 33.Espeland M.A., Craven T.E., Miller M.E., D'Agostino R., Jr. 1996 remington lecture: modeling multivariate longitudinal data that are incomplete. Ann. Epidemiol. 1999;9(3):196–205. doi: 10.1016/s1047-2797(98)00069-6. doi: S1047279798000696 [pii] [DOI] [PubMed] [Google Scholar]
- 34.Rubin Donald B. 1987. Multiple Imputation for Nonresponse in Surveys. [Google Scholar]
- 35.D'Agostino R.B., Jr., Rubin D.B. Estimating and using propensity scores with partially missing data. J. Am. Stat. Assoc. 2000;95(451):749–759. [Google Scholar]
- 36.Lancaster G.A., Dodd S., Williamson P.R. Design and analysis of pilot studies: recommendations for good practice. J. Eval. Clin. Pract. 2004;10(2):307–312. doi: 10.1111/j..2002.384.doc.x. [DOI] [PubMed] [Google Scholar]
- 37.Inoue S., Sugiyama T., Takamiya T., Oka K., Owen N., Shimomitsu T. Television viewing time is associated with overweight/obesity among older adults, independent of meeting physical activity and health guidelines. J. Epidemiol. 2012;22(1):50–56. doi: 10.2188/jea.JE20110054. doi: JST.JSTAGE/jea/JE20110054 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wijndaele K., Brage S., Besson H. Television viewing time independently predicts all-cause and cardiovascular mortality: the EPIC norfolk study. Int. J. Epidemiol. 2011;40(1):150–159. doi: 10.1093/ije/dyq105. [DOI] [PubMed] [Google Scholar]
- 39.Dunstan D.W., Kingwell B.A., Larsen R. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care. 2012;35(5):976–983. doi: 10.2337/dc11-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Latouche C., Jowett J.B., Carey A.L. Effects of breaking up prolonged sitting on skeletal muscle gene expression. J. Appl. Physiol. 1985;114(4):453–460. doi: 10.1152/japplphysiol.00978.2012. 2013. [DOI] [PubMed] [Google Scholar]
- 41.Hamilton M.T., Hamilton D.G., Zderic T.W. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007;56(11):2655–2667. doi: 10.2337/db07-0882. doi: db07-0882 [pii] [DOI] [PubMed] [Google Scholar]
- 42.Bey L., Hamilton M.T. Suppression of skeletal muscle lipoprotein lipase activity during physical inactivity: a molecular reason to maintain daily low-intensity activity. J. Physiol. 2003;551(Pt 2):673–682. doi: 10.1113/jphysiol.2003.045591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Safdar A., Hamadeh M.J., Kaczor J.J., Raha S., Debeer J., Tarnopolsky M.A. Aberrant mitochondrial homeostasis in the skeletal muscle of sedentary older adults. PLoS One. 2010;5(5):e10778. doi: 10.1371/journal.pone.0010778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ekblom-Bak E., Ekblom B., Vikstrom M., de Faire U., Hellenius M.L. The importance of non-exercise physical activity for cardiovascular health and longevity. Br. J. Sports Med. 2014;48(3):233–238. doi: 10.1136/bjsports-2012-092038. [DOI] [PubMed] [Google Scholar]
- 45.Saleh Z.T., Lennie T.A., Mudd-Martin G. Decreasing sedentary behavior by 30 minutes per day reduces cardiovascular disease risk factors in rural americans. Heart Lung. 2015;44(5):382–386. doi: 10.1016/j.hrtlng.2015.06.008. [DOI] [PubMed] [Google Scholar]