Introduction
The role of the stratum corneum (SC) and several associated epidermal barrier (EB) functions in both healthy and compromised skin have gained increased recognition over more recent years. This is most evident based on the work of research that has defined correlations between EB impairments and compromised skin, including those related to specific skin disorders.1-3 Although atopic skin and eczematous dermatitis have been the primary focus, the importance of SC impairments and EB dysfunction in other skin diseases has also gained increased recognition.3-7 In addition, the importance of developing topical vehicle formulations that are “barrier friendly” is well recognized.
The main goal of this supplement is to encourage clinicians to understand the importance of addressing EB function in maintaining healthy skin and to appreciate its role in the overall management of many skin disorders. Objectives to support this goal are to provide summaries of physiologic SC and EB barrier functions, outline SC self-repair mechanisms, define compromised skin and SC impairments, evaluate potential differences in the EB among different skin types and ethnicities, and review the roles of moisturization and barrier repair in the management of specific skin disorders.
What is the Epidermal Barrier?
It is important to recognize that the EB represents a collection of specific diverse functions, many of which occur primarily within the SC. These include maintenance of water content and balance (permeability barrier), prevention and responses to invasion by microbial organisms and antigens (antimicrobial barrier and immune response barrier), reduction of the effects of ultraviolet (UV) light exposure (photoprotection barrier), and mitigation of the effects of oxidative stresses (antioxidant barrier).3 Many of the activities of the EB occur within the SC, which is why the terms SC impairment and EB impairment are often used interchangeably. In fact, the entire epidermis contributes to the EB, although many of the major activities of barrier maintenance and repair occur within the SC. The major components of the SC and EB function are depicted in Table 1. These EB functions are dynamic and work collectively to maintain healthy skin, characterized by invisible desquamation, smooth texture, elasticity, and ability to respond to shearing forces without rigidity and microfissuring.3
TABLE 1.
The stratum corneum and simultaneous multitasking: Individual epidermal barrier functions
| BARRIER FUNCTION | MAJOR COMPONENTS |
|---|---|
| PERMEABILITY BARRIER | Formation of stratum corneum lipids in specific ratio from precursor lipids |
| Production of lamellar bodies packaging precursor lipids and some antimicrobial peptides | |
| Formation of natural moisturizing factor from filaggrin (converted from profillagrin) | |
| Formation of cornified envelope and the corneocyte-lipid envelope | |
| Maintenance of water gradient, calcium gradient, acid mantle (acidic pH) | |
| Response of primary proinflammatory cytokines to impairment of permeability barrier | |
| ANTIMICROBIAL BARRIER | Maintenance of an acidic skin pH decreases skin colonization by pathogenic bacteria and yeasts |
| Antibacterial activity of stratum corneum lipids (e.g., free fatty acids, sphingosine, others) | |
| Genetically encoded primary antimicrobial peptides (defensins, cathelicidins, dermcidins) synthesized in SC, present in sebum and in sweat (dermicidin-derived) | |
| Multiple agents with antimicrobial activity as alternative function (some chemokines, some neuropeptides, others) | |
| ANTIOXIDANT BARRIER | Network of enzymatic and nonenzymatic antioxidant systems to counter oxidative stress |
| Antioxidants present in epidermis (stratum corneum, skin surface lipids) and dermis | |
| Hydrophilic nonenzymatic antioxidants include ascorbic acid (vitamin C) and uric acid | |
| Major lipid-soluble nonenzymatic antioxidant is alpha-tocopherol (vitamin E) | |
| Co-antioxidants (ascorbic acid, ubiquinol [coenzyme Q10]) allow tocopherol regeneration | |
| Gradients in stratum corneum for ascorbic acid and tocopherol (lowest near surface) | |
| Interceptive antioxidant enzymes (catalase, superoxide dismutases, glutathione peroxidases) | |
| Antioxidant repair enzymes (e.g., methionine sulfoxide reductase) | |
| High concentration of alpha-tocopherol in sebum accounts for high levels in facial sebaceous gland stratum corneum (sebum serves as a physiological delivery pathway) | |
| IMMUNE RESPONSE BARRIER | Dendritic cells involved in immune surveillance and antigen recognition (e.g., plasmacytoid dendritic cells, myeloid dendritic cells, Langerhans cells) |
| Toll-like receptors involved in recognition of microbial pathogens and other agonists | |
| Antimicrobial peptides and some of their enzymatic conversion products (e.g., LL-37) | |
| Innate and acquired immune response pathways and balance with T regulatory cell system | |
| PHOTOPROTECTION BARRIER | Epidermal melanin barrier (degree of protection related to Fitzpatrick skin ttype) |
| Stratum corneum protein barrier | |
| Antioxidants within stratum corneum (protection against photo-oxidative stress) | |
| Optical reflective properties of the stratum corneum (stratum corneum thickness more important than epidermal thickness for ptrotection against ultraviolet/solar radiation) |
Reprinted with permission from: Del Rosso JQ, Levin J. J Clin Aesthet Dermatol. 2011;4(9): 22-42. © 2011 Matrix Medical Communications.
The structural and functional activities of the SC have been well described in the literature and will only be summarized in this supplement in order to elucidate clinical relevance.1-3,7-9 Overall, the EB functions to physiologically maintain the integrity of the skin. By maintaining proper cutaneous water balance and mitigating exogenous environmental and microbial stresses, the SC sustains normal desquamation and skin elasticity.1,3,7-11
Sustaining an intact and noncompromised SC and maintenance of physiologic EB function are dependent on the continuous replenishment of specific structural components of the epidermis. As the lower epidermis gives rise to the SC at the transition zone of the granular layer, the formation of the intercellular lipid membrane between corneocytes, and its direct relationship to the establishment of a proper water gradient provide the foundation for optimal enzymatic functioning and establishment of the proper acidic pH within the SC. The end results of both optimal functioning of SC enzymes and a proper pH are the continuous building of stable epidermal structures via corneocyte envelopment and adhesion, formation of an intercellular lipid membrane that is specified in composition and lamellar structure, and the reparative ability to increase production of filaggrin, the precursor of natural moisturizing factors (NMF) which provide intracellular humectancy within the SC (Figures 1 and 2).1-3,7-14 Enzymatic activity also contributes to formation of the physiologic SC lipids, which comprise approximately 20 percent of the volume of the SC and are composed of ceramides (40-50%), cholesterols (25%), and free fatty acids (10-15%), most of which are present within the intercellular lipid membrane.1,3,8
Figure 1.
This figure depicts the “bricks and mortar” structure of the stratum corneum. The corneocytes represent the bricks and the intercellular lamellar lipid membrane represents the mortar. Corneocytes comprise primarily keratin macrofibrils, are protected externally by a cornified cell envelope, and are held together by corneodesmosomes. The intercellular lamellar lipid membrane is primarily composed of ceramides, cholesterol, and fatty acids. A mixture of multiple small hygroscopic compounds present within corneocytes, referred to collectively as natural moisturizing factor (NMF), plays a vital role in the physiological maintenance of stratum corneum hydration.
(Reprinted with permission from Harding CR. Dermatol Ther. 2004;17:6-15.)
Figure 2.
The “Epidermal Factory”: Progressive layers and corresponding production steps.
Reprinted with permission from: Del Rosso JQ, Levin J. J Clin Aesthet Dermatol. 2011 ;4(9): 22-42. © 2011 Matrix Medical Communications.
What is “Compromised Skin?”
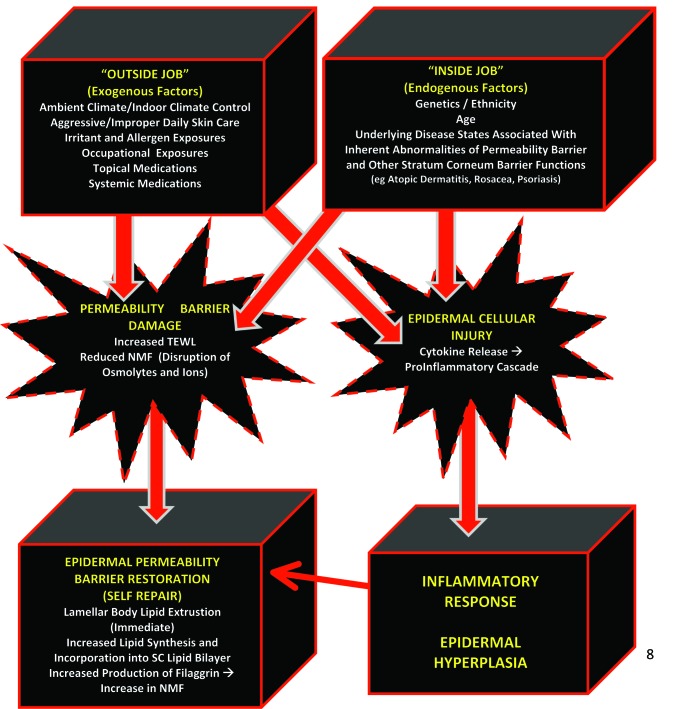
Exogenous factors that can alter the integrity of the SC cause an increase in transepidermal water loss (TEWL) and alterations of SC proteins and lipids, progressively leading to compromised skin.1,3,15,16 Unless these factors are adequately countered by SC self-repair mechanisms and/or moisturization, the SC becomes overstressed, with continued increased TEWL leading to incomplete desquamation, loss of skin elasticity, increased skin rigidity, and epidermal proliferation.1,3 Exogenous factors that lead to increased TEWL and SC/EB dysfunctions include improper skin care, exposure to cutaneous irritants, occupational exposures, application of certain topical agents, and low ambient humidity (Figure 3).3,15,16
Figure 3.
Physiological adaptions of the stratum corneum In response to factors promoting desiccation (e.g., increase in transepidermal water loss, decrease in natural moisturizing factor, damage or reduction in stratum corneum lipids, damage to stratum corneum proteins).
Reprinted with permission from: Del Rosso JQ, Levin J. J Clin Aesthet Dermatol. 2011;4(9): 22-42. © 2011 Matrix Medical Communications.
In some individuals, underlying skin status and disease states, such as atopic dermatitis, innate xerosis, ichthyosis, psoriasis, diabetes, and increased age, exhibit inherent SC impairments, which predispose to increased TEWL, and when adversely affected by the aforementioned exogenous factors, the magnitude of EB permeability barrier compromise is further compounded.1-3,6,17,18 ln such cases, it is more difficult for SC self-repair mechanisms to normalize SC functional integrity and EB function as both exogenous and endogenous factors are contributing to EB impairment. For example, indivuduals with atopic skin are unable to produce adequate amounts of certain ceramides, and many exhibit filaggrin gene mutations, thus leading to a baseline increase in TEWL within their normal-appearing skin that increases during eczematous flares.2,3
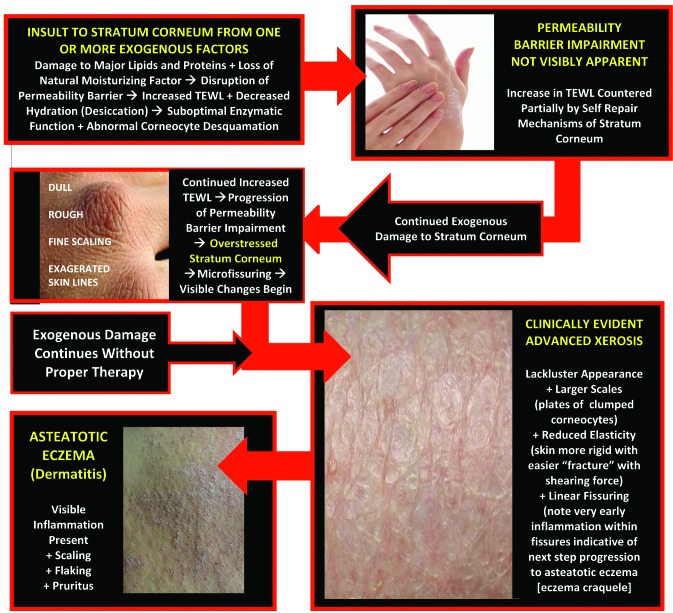
Figure 3 shows the exogenous and endogenous factors that contribute to compromised skin and depicts the physiologic adaptations of the SC in response to these factors. When compromise of the SC permeability barrier is allowed to persist without adequate repair, the underlying pathophysiologic aberrations lead to clinically apparent changes in the skin.1,3 Abnormal desquamation leads to clumping of corneocytes, which presents visibly as scaling and flaking; loss of elasticity and increased rigidity leads to microfissuring and macrofissuring; and epidermal proliferation can lead to hyperkeratosis.3 The stepwise progression of changes secondary to continued SC permeability barrier damage are demonstrated in Figure 4, depicting alterations induced by increased TEWL over time within visibly normal skin, xerotic skin, and eczematous skin.
Figure 4.
Stepwise progression from damage to permeability barrier → xerosis → eczematous dermatitis.
Reprinted with permission from: Del Rosso JQ, Levin J. J Clin Aesthet Dermatol. 2011;4(9): 22-42. © 2011 Matrix Medical Communications.
What are the Physiologic Stratum Corneum Self-repair Mechanisms?
As skin is exposed to multiple exogenous factors that may lead to EB impairments, the SC is continuously active in maintaining a functional physiologic state by utilizing a variety of self-repair mechanisms. Once TEWL is increased beyond the normal homeostatic level, multiple self-repair mechanisms are induced within the SC and at the granular zone transition layer of the epidermis.3,8,10,19,20-22 These self-mechanisms are:
Immediate release of precursor lipids into the SC from lamellar bodies within the granular layer that are immediately converted to physiologic lipids (such as ceramides), providing recovery of approximately 20 percent of overall permeability barrier function.
Increase in synthesis of physiologic lipid precursors and conversion to SC lipids for replenishment of the intercellular lipid membrane.
Increased conversion of prefilaggrin to fillagrin with degradation of filaggrin to several NMFs. These NMFs, primarily amino acids, maintain humectancy within the SC, which decreases TEWL by inhibiting loss into the ambient atmosphere.
Increased TEWL initiates an inflammatory cascade through release of tumor necrosis factor (TNF), interleukin (IL)-1, and IL-6. This cascade promotes keratinocyte hyperproliferation as increasing epidermal thickness serves to decrease TEWL.
When microbial organisms gain access to the SC, specific antimicrobial peptides may be activated by certain microbes, thus providing an innate immune response to deter the development of infection.
On a daily basis, the SC is dynamic and utilizes self-repair mechanisms to maintain a normal physiologic balance within skin, and to contribute to overall homeostatic water balance. When exogeneous and/or exogenous factors create EB impairment that is not correctable by self-repair alone (overstressed SC), visible xerotic, eczematous, and specific disease state manifestations emerge.1,3,8
This underscores the need to incorporate proper daily skin care utilizing a gentle cleanser and well-formulated moisturizer to assist the SC in maintaining EB integrity. The use of gentle skin cleansing and moisturization/barrier repair becomes more evident clinically in patients with underlying disease states that are characterized by asteatotic skin changes and/or sensitive skin due to the disease state itself and/or topical therapies used for treatment.1,4,23-30
Are there Differences in the Epidermal Barrier Related to Race or Ethnicity?
Although the structural and functional components of the SC and EB barrier have been reasonably defined, there is more recent interest in ethnic and racial differences that may translate to differences in clinical presentations of xerotic skin changes and skin sensitivity.31-34 Although conflicting evidence exists in some areas, some observations have been made that may assist in targeting certain groups with specific skin care formulations.31-37 The following observations have been gleaned from studies assessing potential differences in SC/EB barrier structure and functions among different races and ethnicities:
The major structural and physiochemical differences in African American skin when compared to Caucasian skin in the SC and parameters that assess EB function are 1) increased number of cell layers and resistance to tape stripping despite equal thickness, 2) increased SC lipid content with a decrease in ceramides, 3) increased electrical resistance, 4) increased desquamation, and 5) more rapid barrier recovering after SC tape stripping.33,37
Using an imaging technique for skin capacitance, xerosis was greater on sun-exposed forearm sites from women with lighter skin tones (Chinese, Caucasian) than on primarily sun-protected sites; no differences are seen among sites from women with darker skin (African American, Mexican). Xerosis did not change as a function of ethnicity for the younger group regardless of forearm sites tested. With increasing age, xerosis was higher for African American and Caucasian women than for Chinese and Mexican women, with a higher percentage increase in Caucasian women.34
In healthy Asian (n=25), African (n=18), and Caucasian skin (n=28), ceramide/cholesterol ratios were statistically significantly different between groups. Asians had the highest ratio as compared with both white and African skin (P<0.001); African skin exhibited the lowest ratio values. No statistically significant differences were noted between any of the ceramide subgroups.35
In a study evaluating SC pH, permeability barrier homeostasis, and SC integrity in three geographically disparate populations comparing outcomes with Fitzpatrick Skin Type (FST) I-II versus FST IV-V skin, FST IV-V subjects showed 1) lower surface pH, 2) enhanced SC integrity (based on TEWL changes with sequential tape strippings), and 3) more rapid barrier recovery than FST I-II subjects. Enhanced barrier function could be ascribed to increased epidermal lipid content, increased lamellar body production, and reduced acidity, leading to enhanced lipid processing. Compromised SC integrity in FST I-II subjects could be ascribed to increased serine protease activity leading to greater corneo-desmosome degradation. Adjustment of SC pH in FST I-II SC to levels seen with FST IV-V SC improved EB function. These outcomes support marked pigmenttype differences in epidermal structure and function that are pH driven.38
SC impairment in Caucasian skin initially appeared to be more severely affected by SLS-induced epidermal damage than African American skin. However, after 24 hours, the TEWL level of Caucasian skin did recover to levels comparable with those of African American skin. A limitation of this study noted by the authors is small sample size.39
More data are needed on differences in SC/EB structure and function among individuals of different races, ethnicities, and different magnitudes of skin pigmentation (FST). A summary of findings gleaned to date depicts relative differences based on an overall consensus from available literature.31 Racial variability in physiological properties of the SC and EB can directly impact SC water content and sensitivity to exogenously applied agents. Given the large number of moisturizers presently available in the marketplace, several different moisturizer/barrier repair formulations with individual ingredients may be employed to promote skin hydration. More data are needed to determine if certain moisturizers/barrier repair formulations may be more or less effective than others to maintain SC/EB structure and function and to manage xerotic skin disorders.31
Is the Use of Moisturizer/Barrier Repair Formulations Relevant in Clinical Practice?
There is a body of evidence to show that optimal management of many skin diseases, including eczematous dermatitis, acne vulgaris, rosacea, psoriasis, and xerosis, includes appropriate skin care.33,23-30 Incorporation of a gentle (nonirritating) skin cleanser and a well-designed moisturizer/barrier repair formulation can contribute to improvement of disease-associated signs and symptoms and can mitigate cutaneous irritation caused by certain topical medications.3,23-27 The distinction between moisturizers and topical barrier repair products is not clearly defined. Conventional moisturizers, available over-the-counter (OTC), can be purely occlusive (i.e., petrolatum, lanolin), or can contain both occlusive agents (i.e., petrolatum, occlusive/protective emollients) and humectant ingredients (i.e., glycerin, hyaluronic acid), designed primarily to reduce TEWL and increase SC hydration.25,26,40 Barrier repair formulations, usually made available as prescription products, contain the fundamental ingredients of a conventional moisturizer along with specific “physiologic” ingredients (i.e., ceramides, essential fatty acids) and formulation design characteristics that directly target barrier repair (i.e., replenishment of the SC intercellular lipid membrane).26 However, there are also OTC moisturizer formulations that contain physiologic lipids (i.e., ceramides, ceramide precursors, fatty acids) and other special additives (i.e., niacinamide) that can assist in barrier repair.26 The following are examples of clinical use of moisturizers and barrier repair formulations, which contributed to favorable therapeutic outcomes:
The effectiveness of a moisturizer used as an adjunct to topical corticosteroid therapy for the treatment of mild-to-moderate atopic dermatitis was studied in comparison to low-potency topical corticosteroid alone for three weeks in children 3 to 15 years of age. Adjunctive moisturizer therapy provided a steroid-sparing alternative to topical corticosteroids alone in the treatment of mild-to-moderate atopic dermatitis.41
A six-week cohort study evaluated the effectiveness of a twice-daily regimen of a ceramide-containing cleanser and moisturizer in children and adults with atopic dermatitis (N=151). The results showed that the ceramide-containing cleanser and moisturizer regimen substantially improved clinical outcomes with reduction in disease severity and improvement in quality-of-life parameters.42
A ceramide-dominant, physiologic lipid-based emollient, substituted for currently used moisturizers, was studied in children undergoing topical therapy with a calcineurin inhibitor or corticosteroid for recalcitrant atopic dermatitis. Severity scoring of atopic dermatitis (SCORAD) markedly improved significantly in 22 of 24 patients by three weeks, with further progressive improvement in all patients between 6 and 20 or 21 weeks. TEWL decreased in direct correlation with SCORAD scores and continued to decline even after SCORAD scores plateaued. SC cohesion and hydration also improved progressively over the course of therapy. Ultrastructure of the SC treated with ceramide-dominant emollient revealed defined intercellular lamellar lipid membranes, which were mostly absent in baseline SC skin samples.43
The use of a moisturizer alone was studied over four weeks duration in mild-to-moderate plaque psoriasis (5-10% body surface area) in patients who either were not being treated or had discontinued the use of all topical psoriasis medications and all previous moisturizers (N=30). Results from objective evaluations showed stabilization of TEWL and an increase in skin hydration over the course of the study. Desquamation measurements showed a significant percentage of participants with skin improvements from very dry to dry or normal (P<0.0001 for all time points). The investigator concluded that moisturizer use is appropriate and can be helpful in the management of plaque psoriasis.44
The efficacy of a ceramide-dominant, triple-lipid barrier repair topical emulsion (3:1:1 ceramides to cholesterol to fatty acids ratio) was compared to fluticasone propionate cream in a five-center, investigator-blinded, randomized trial in patients with moderate-to-severe atopic dermatitis (N=121). The 3:1:1 barrier repair formulation reduced clinical disease severity, decreased pruritus, and improved sleep habits both 14 and 28 days after initiation of therapy. The onset of efficacy in the fluticasone-treated group was more rapid, showing significantly greater improvement at 14 days; SCORAD, pruritus, and sleep habit scores did not differ significantly among both study groups by 28 days.45
Footnotes
Supplement disclosure:This supplement was supported by educational grants from Valeant and PuraCap. The content and preparation of this supplement was completed solely by the authors. No individuals from any company or agency was involved in content development, preparation, review, or submission of this manuscript.
References
- 1.Harding CR. The stratum corneum: structure and function in health and disease. Dermatol Ther. 2004;17:6–15. doi: 10.1111/j.1396-0296.2004.04s1001.x. [DOI] [PubMed] [Google Scholar]
- 2.Proksch E, Elias PM. Epidermal barrier in atopic dermatitis. In: Bieber T, Leung DYM, editors. Atopic Dermatitis. New York: Marcel Dekker; 2002. pp. 123–143. [Google Scholar]
- 3.Del Rosso JQ, Levin J. The clinical relevance of maintaining the functional integrity of the stratum corneum in both healthy and disease-affected skin. J Clin Aesthet Dermatol. 2011;4(9):22–42. [PMC free article] [PubMed] [Google Scholar]
- 4.Thiboutot D, Del Rosso JQ. Acne vulgaris and the epidermal barrier: is acne vulgaris associated with inherent epidermal abnormalities that cause impairment of barrier functions? Do any topical acne therapies alter the structural and/or functional integrity of the epidermal barrier? J Clin Aesthet Dermatol. 2013;6(2):18–24. [PMC free article] [PubMed] [Google Scholar]
- 5.Dirschka T, Tronnier H, Folster-Holst R. Epithelial barrier function and atopic diathesis in rosacea and perioral dermatitis. Br J Dermatol. 2004;150:1136–1141. doi: 10.1111/j.1365-2133.2004.05985.x. [DOI] [PubMed] [Google Scholar]
- 6.Ghadially R. Psoriasis and ichthyosis. In: Leyden JJ, Rawlings AV, editors. Skin Moisturization. New York: Marcel-Dekker; 2002. pp. 165–178. 1st ed. [Google Scholar]
- 7.Dasgupta BR, Bajor J, Mazzati DJ, Manoj M. Cosmeceuticals: function and the skin barrier. In: Draelos ZD, editor. Cosmeceuticals. Elsevier: Philadelphia; 2016. pp. 3–10. 3rd ed. [Google Scholar]
- 8.Elias PM. The epidermal permeability barrier: from Saran Wrap to biosensor. In: Elias PM, Feingold KR, editors. Skin Barrier. New York: Taylor Francis; 2006. pp. 25–32. [Google Scholar]
- 9.Menon GK, Norlen L. Skin Moisturization, New York: Marcel-Dekker; 2002. Stratum corneum ceramides and their role in skin barrier function. In: Leyden JJ, Rawlings AV, eds; pp. 165–178. 1st ed. [Google Scholar]
- 10.Elias P. Stratum corneum defensive functions: an integrated view. J Invest Dermatol. 2005;5:183–200. doi: 10.1111/j.0022-202X.2005.23668.x. [DOI] [PubMed] [Google Scholar]
- 11.Sato J. Desquamation and the role of stratum corneum enzymes. In: Leyden JJ, Rawlings AV, editors. Skin Moisturization. New York: Marcel-Dekker; 2002. pp. 81–94. 1st ed. [Google Scholar]
- 12.Watkinson A, Harding CR, Rawlings AV. The cornified envelope: its role in stratum corneum structure and maturation. In: Leyden JJ, Rawlings AV, editors. Skin Moisturization, New York: Marcel-Dekker; 2002. pp. 95–118. 1st ed. [Google Scholar]
- 13.Behne MJ, Epidermal pH. In: Skin Moisturization, Rawlings AV, Leyden JJ, editors. New York: Informa Healthcare; 2009. pp. 163–180. 2nd ed. [Google Scholar]
- 14.Uitto J, McGrath JA. The role of filaggrin in skin diseases. In: Rawlings AV, Leyden JJ, editors. Skin Moisturization. New York: Informa Healthcare; 2009. pp. 57–68. 2nd ed. [Google Scholar]
- 15.Misra M, Ananthapadmanaban KP, Hoyberg K, et al. Correlation between surfactant-induced ultrastructural changes in epidermis and transepidermal water loss. J Soc Cosmet Chem. 1997;48:219–234. [Google Scholar]
- 16.Rawlings AW, Watkinson A, Rogers J, et al. Abnormalities in stratum corneum structure, lipid composition, and desmosome degradation in soap-induced winter xerosis. J Soc Cosmet Chem. 1994;45:203–220. [Google Scholar]
- 17.Cork MJ, Moustafa M, Danby S, et al. Skin barrier dysfunction in atopic dermatitis. In: Rawlings AV, Leyden JJ, editors. Skin Moisturization. New York: Informa Healthcare; 2009. pp. 211–240. 2nd ed. [Google Scholar]
- 18.Sakai S, Tagami H. Rawlings AV, Leyden JJ. Skin Moisturization. New York: Informa Healthcare; 2009. Xerotic skin conditions and SC properties: diabetic dry skin; pp. 197–210. 2nd ed. [Google Scholar]
- 19.Bouwstra JA. Lipid organization of the skin barrier. In: Rawlings AV, Leyden JJ, editors. Skin Moisturization. New York: Informa Healthcare; 2009. pp. 17–39. 2nd ed. [Google Scholar]
- 20.Elias PM, Ansel JC, Woods LD, et al. Signaling networks in barrier homeostasis: the mystery widens. Arch Dermatol. 1996;132:1505–1506. [PubMed] [Google Scholar]
- 21.Mao-Qiang M, Brown PE, Wu-Pong S, et al. Exogenous nonphysiologic lipids vs. physiologic lipids: divergent mechanisms for correction of permeability barrier dysfunction. Arch Dermatol. 1995;131:809–816. doi: 10.1001/archderm.131.7.809. [DOI] [PubMed] [Google Scholar]
- 22.Menon GK, Feingold KR, Elias PM. Lamellar body secretory response to barrier disruption. J Invest Dermatol. 1992;98:279–289. doi: 10.1111/1523-1747.ep12497866. [DOI] [PubMed] [Google Scholar]
- 23.Draelos ZD. Concepts in skin care maintenance. Cutis. 2005;76(6 Suppl):19–25. [PubMed] [Google Scholar]
- 24.Subramanyan K. Role of mild cleansing in the management of patient skin. Dermatol Ther. 2004;17(Suppl 1):26–34. doi: 10.1111/j.1396-0296.2004.04s1003.x. [DOI] [PubMed] [Google Scholar]
- 25.Loden M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am J Clin Dermatol. 2003;4(11):771–778. doi: 10.2165/00128071-200304110-00005. [DOI] [PubMed] [Google Scholar]
- 26.Del Rosso JQ. Moisturizer and barrier repair formulations. In: Draelos ZD, editor. Cosmeceuticals. Philadelphia: Elsevier; 2016. pp. 81–89. 3rd ed. [Google Scholar]
- 27.Del Rosso JQ. The use of moisturizers as an integral component of topical therapy for rosacea: clinical results based on assessment of skin characteristics. Cutis. 2009;84:72–76. [PubMed] [Google Scholar]
- 28.Chamlin S, Kao J, Friedlander IJ, et al. Ceramide-dominant barrier repair lipids alleviate childhood atopic dermatitis: changes in barrier repair function provide a sensitive indicator of disease activity. J Am Acad Dermatol. 2002;47:198–208. doi: 10.1067/mjd.2002.124617. [DOI] [PubMed] [Google Scholar]
- 29.Del Rosso JQ, Brandt S. The role of skin care as an integral component in the management of acne vulgaris. part 2: tolerability and performance of a designated skin care regimen using a foam wash and moisturizer SPF 30 in patients with acne vulgaris undergoing active treatment. J Clin Aesthet Dermatol. 2013;6:28–36. [PMC free article] [PubMed] [Google Scholar]
- 30.Del Rosso JQ, Cask K. Topical corticosteroid application and the structural and functional integrity of the epidermal barrier. J Clin Aesthet Dermatol. 2013;6:20–27. [PMC free article] [PubMed] [Google Scholar]
- 31.Wan DC, Wong VW, Longaker MT, et al. Moisturizing different racial skin types. J Clin Aesthet Dermatol. 2014;7(6):25–32. [PMC free article] [PubMed] [Google Scholar]
- 32.Wesley NO, Maibach HI. Racial (ethnic) differences in skin properties: the objective data. Am J Clin Dermatol. 2003;4(12):843–860. doi: 10.2165/00128071-200304120-00004. [DOI] [PubMed] [Google Scholar]
- 33.Fluhr JW, Darlenski R, Berardesca E. Ethnic groups and sensitive skin: two examples of special populations in dermatology. Drug Discovery Today. 2008;5(2):249–263. [Google Scholar]
- 34.Diridollou S, de Rigal J, Querleux B, et al. Comparative study of the hydration of the stratum corneum between four ethnic groups: influence of age. Int J Dermatol. 2007;46(Suppl1):11–14. doi: 10.1111/j.1365-4632.2007.03455.x. [DOI] [PubMed] [Google Scholar]
- 35.Jungersted JM, Høgh JK, L.I. Hellgren LI, et al. Ethnicity and stratum corneum ceramides. Br J Dermatol. 2010;163:1169–1173. doi: 10.1111/j.1365-2133.2010.10080.x. [DOI] [PubMed] [Google Scholar]
- 36.Rawlings AV. Ethnic skin types: are there differences in skin structure and function. Int J Cosmet Sci. 2006;28:79–93. doi: 10.1111/j.1467-2494.2006.00302.x. [DOI] [PubMed] [Google Scholar]
- 37.Berardesca E, Maibach H. Ethnic skin: overview of structure and function. J Am Acad Dermatol. 2003;48:S139–S142. doi: 10.1067/mjd.2003.273. [DOI] [PubMed] [Google Scholar]
- 38.Gunathilake R, Schurer NY, Shoo BA, et al. pH-Regulated mechanisms account for pigment-type differences in epidermal barrier function. J Invest Dermatol. 2009;129:1719–1729. doi: 10.1038/jid.2008.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grimes P, Edison BL, Green BA, et al. Evaluation of inherent differences between African American and White skin surface properties using subjective and objective measures. Cutis. 2004;73:392–396. [PubMed] [Google Scholar]
- 40.Barton S. Formulation of moisturizers. In: Leyden JJ, Rawlings AV, editors. Skin Moisturization. New York: Marcel-Dekker; 2002. pp. 547–584. 1st ed. [Google Scholar]
- 41.Lucky AW, Leach AD, Laskarzewski P, et al. Use of an emollient as a steroid-sparing agent in the treatment of mild to moderate atopic dermatitis in children. Pediatr Dermatol. 1997;14(4):321–324. doi: 10.1111/j.1525-1470.1997.tb00968.x. [DOI] [PubMed] [Google Scholar]
- 42.Lynde CW, Andriessen A. A cohort study on a ceramide-containing cleanser and moisturizer used for atopic dermatitis. Cutis. 2014;93(4):207–213. [PubMed] [Google Scholar]
- 43.Chamlin SL, Kao J, Frieden IJ, et al. Ceramide-dominant barrier repair lipids alleviate childhood atopic dermatitis: changes in barrier function provide a sensitive indicator of disease activity. J Am Acad Dermatol. 2002;47:198–208. doi: 10.1067/mjd.2002.124617. [DOI] [PubMed] [Google Scholar]
- 44.Draelos ZD. Moisturizing cream ameliorates dryness and desquamation in participants not receiving topical psoriasis treatment. Cutis. 2008;82(3):211–216. [PubMed] [Google Scholar]
- 45.Sugarman JL, Parish LC. Efficacy of a lipid-based barrier repair formulation in moderate-to-severe pediatric atopic dermatitis. J Drugs Dermatol. 2009;8(12):1106–1111. [PubMed] [Google Scholar]