Abstract
Background
The present study aimed to validate the pooled cohort risk (PCR) equations in a Chinese ischemic stroke population and to explore its prognostic value in predicting stroke recurrence, coronary heart disease, and vascular death.
Material/Method
Patients were selected from the China National Stroke Registry. The C statistic was used to examine the clinical prediction of the scores. To analyze the relevant risk factors, univariate and multivariate logistic regressions were performed.
Results
Out of a total of 22 216 patients, 8287 patients (including 7652 acute ischemic stroke [AIS] and 635 transient ischemic attack [TIA] patients) were selected and enrolled in the study. At 1-year follow-up, for stroke recurrence rate, the C statistic value was 0.584 in AIS patients and 0.573 in all patients. For non-fatal myocardial infarction, the C statistic value was 0.533 in AIS patients and 0.493 in all patients. For vascular death, the C statistic value was 0.592 in AIS patients and 0.592 in all patients. For all events, the C statistic value was 0.582 in AIS patients and 0.575 in all patients. For AIS patients, the 12-month cumulative rates for recurrent stroke, vascular death, and combined vascular events were higher in the high-PCR group (PCR ≥20%).
Conclusions
Pooled cohort risk equations may serve as potential tools to predict and stratify the 1-year risk of recurrent stroke and combined vascular events in AIS/TIA patients in China.
MeSH Keywords: Acute Ischemic Stroke, Pooled Cohort Risk Equations, Recurrence, Transient Ischemic Attack
Background
Stroke is the leading cause of disability and mortality worldwide, resulting in an enormous socioeconomic burden. There has been a greater that 100% increase in stroke incidence rates in low-to-middle income countries over the past 4 decades [1]. Readmissions were reported to be 31–53% in the first year after stroke, frequently from recurrent stroke or cardiovascular diseases [2–4]. About half of the patients experienced readmission or death during the first year after stroke and carried substantial risk and burden [5]. The ability to accurately estimate the 1-year clinical outcome of stroke is very important and may help healthcare providers and family make decisions on a treatment plan, discharge arrangement, and resource use. The risk assessment would aid practitioners in identifying high-risk patients and developing a targeted and cost-effective preventive strategy.
In 2013, the American College of Cardiology/American Heart Association (ACC/AHA) task force developed the Guideline on the Assessment of Cardiovascular Risk and introduced the pooled cohort risk (PCR) equations, which were designed to predict 10-year risk for a first atherosclerotic cardiovascular disease (ASCVD) event [6]. The ASCVD event includes non-fatal myocardial infarction (MI), coronary heart disease (CHD) death, as well as non-fatal and fatal stroke. The PCR equations were derived from 4 major population-based cohort studies in the United States involving white and black Americans. The inclusion of stroke as an endpoint is a major strength. It is recommended as a guide to make decision on initiating statin therapy for primary prevention in adults without clinical ASCVD. When the PCR score is ≥7.5%, statin use is recommended for non-diabetic patients. The PCR equations have been further validated by different external cohorts [7,8].
The present study aimed to validate the performance of the PCR equations in acute ischemic stroke (AIS) and transient ischemic attack (TIA) patients in China. We investigated whether the PCR equations can predict the clinical outcomes, including stroke recurrence, CHD, and vascular death, during 1-year follow-up. We also compared the predictive validity between the PCR equations and the ESSEN score, which has been widely validated internally and externally [9–11].
Material and Methods
The cohort was derived from the China National Stroke Registry (CNSR) [12], which is the largest, nationwide, multicenter, and prospective registry of consecutive patients with acute cerebrovascular diseases corresponding to World Health Organization diagnostic standards [13]. The study of CNSR was approved by the Central Institutional Review Board at Beijing Tiantan Hospital. All patients or their designated relatives signed the informed consent forms and their data were kept confidential and protected. The cohort, comprising AIS and TIA patients, was followed up for 1 year. The inclusion criteria according to the PCR equations were: age: 40–79 years; total cholesterol: 130–320 mg/L; HDL: 20–100 mg/L; and BP: 90–200 mmHg. Patients diagnosed with hemorrhagic strokes, or missing baseline characteristics, or without follow-up outcomes, or did not meet the PCR standards were excluded. Among the 22 216 acute stroke patients who were hospitalized from 132 participating hospitals in the CNSR, a total of 8287 patients, including 7652 AIS and 635 TIA patients, fulfilled the inclusion criteria, finished the follow-up, and were thus enrolled in the study (Figure 1).
Figure 1.
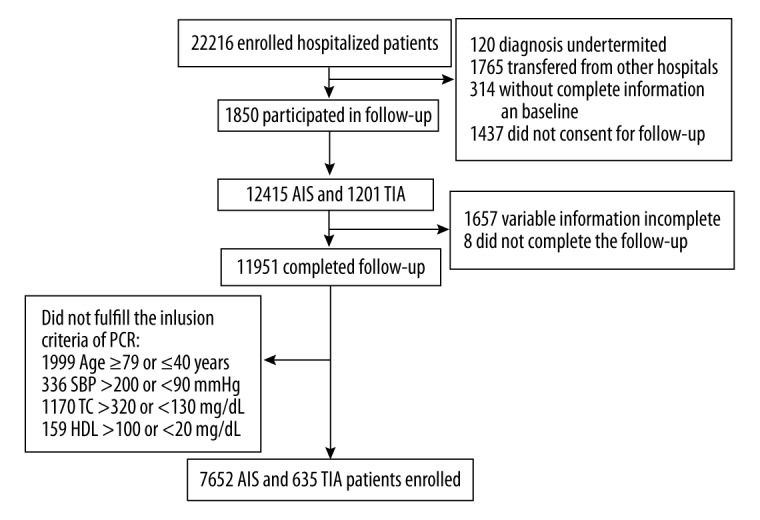
Schematic illustration of study population. AIS – acute ischemic stroke; TIA – transient ischemic attack; SBP – systolic blood pressure; TC – triglyceride; HDL – high-density lipoprotein.
For the present study, the following baseline variables were analyzed: (1) demographics (age and gender); (2) stroke risk factors: body mass index (BMI), current smoking, and heavy alcohol consumption (≥2 standard alcohol beverages per day), hypertension, diabetes mellitus, dyslipidemia, heart failure, atrial fibrillation, coronary heart disease, peripheral artery disease, and history of stroke/TIA; (3) admission stroke severity based on the National Institutes of Health Stroke Scale score (NIHSS); and (4) pre-admission medications: antihypertensive treatment, hypoglycemic treatment, statins usage, and antiplatelet treatment.
The primary endpoint was stroke recurrence during 1-year follow-up, referring to a new or worsened neurological deficit (defined as NIHSS worsened ≥4), or readmission due to cerebrovascular diseases including ischemic stroke, intracerebral hemorrhage, or subarachnoid hemorrhage. The secondary endpoints were CHD (including MI, coronary revascularization, and cardiac resuscitation) and all-cause vascular death during 1-year follow-up. Blinded trained interviewers obtained the outcome data by using standardized questionnaires via phone calls.
Statistical analysis
Data were summarized as mean values and SD for continuous parameters, or absolute count and percentage for categorical endpoints. Comparisons across the groups were examined using the chi-square test for categorical variables. Student’s t-test was used for continuous/score variables. To analyze relationship between the risk factors and the outcomes, univariate and multivariate logistic regression were utilized. To validate the discrimination of the PCR equations and the ESSEN score, the area under the receiver operating curve (AUC) by C statistic was assessed. All tests were 2-tailed, and P<0.05 was considered statistically significant. Statistical analysis was performed using SAS software version 9.3 (SAS Institute Inc, Cary, NC).
Results
Baseline characteristics of the selected 8287 patients are shown in Table 1. The clinical features, including demographic characteristics, risk factors, and drug treatments, were compared between the patients with and without recurrences. The cumulative 1-year stroke recurrence was 11.27%. Older patients and patients with smoking, heavy drinking, hypertension, diabetes mellitus, dyslipidemia, heart failure, coronary heart disease, atrial fibrillation, history of stroke/TIA, and high admission NIHSS score were more likely to be recurrent (P<0.05). On the other hand, patients with lipid-lowering agents, antihypertensive agents, hypoglycemic agents, and antiplatelet agents were less likely to be recurrent (P<0.05).
Table 1.
Baseline characteristics of patients with and without recurrence.
| Characteristics | Overall | With recurrence | Without recurrence | P-value |
|---|---|---|---|---|
| Sample size | 8287 | 934 | 7353 | |
| Sex (male), n,% | ||||
| Female | 3054/8287 (36.9%) | 349 (37.4%) | 2705 (36.8%) | |
| Male | 5233/8287 (63.1%) | 585/934 (62.6%) | 4648/7353 (63.2%) | 0.7299 |
| Age, y (mean±SD) | 63.24±9.65 | 64.86±9.05 | 63.03±9.71 | 0 |
| Age, median (Q1–3), y | 64 (56–72) | 66 (58–73) | 64 (55–71) | 0 |
| BMI (kg/m2) (mean ±SD) | 24.62±3.83 | 24.68±3.38 | 24.61±3.88 | 0.6594 |
| BMI (kg/m2) median (Q1–Q3) | 24.34 (22.49–26.45) | 24.46 (22.49–26.67) | 24.3 (22.48–26.42) | 0.1786 |
| BMI <25 | 4442/7534 (59%) | 483/838 (57.6%) | 3959/6696 (59.1%) | 0.5463 |
| BMI 25–30 | 2698/7534 (35.8%) | 314/838 (37.5%) | 2384/6696 (35.6%) | |
| BMI ≥30 | 394/7534 (5.2%) | 41 (4.9%) | 353 (5.3%) | |
| Vascular risk factor, n,% | ||||
| Smoking | 5664/8287 (68.3%) | 683/934 (73.1%) | 4981/7353 (67.7%) | 0.0009 |
| Heavy drinking | 2395/8287 (28.9%) | 237/934 (25.4%) | 2158/7353 (29.3%) | 0.0116 |
| Hypertension | 5285/8287 (63.8%) | 671/934 (71.8%) | 4614/7353 (62.7%) | 0 |
| Diabetes mellitus | 1794/8287 (21.6%) | 228/934 (24.4%) | 1566/7353 (21.3%) | 0.0295 |
| Dyslipidemia | 1018/8287 (12.3%) | 148/934 (15.8%) | 870/7353 (11.8%) | 0.0004 |
| Heart failure | 112/8287 (1.4%) | 22/934 (2.4%) | 90/7353 (1.2%) | 0.0048 |
| Coronary heart disease | 1086/8287 (13.1%) | 201/934 (21.5%) | 885/7353 (12.0%) | 0 |
| Peripheral artery disease | 40/8287 (0.5%) | 4/934 (0.4%) | 36/7353 (0.5%) | 0.7989 |
| Atrial fibrillation | 434/8287 (5.2%) | 97/934 (10.4%) | 337/7353 (4.6%) | 0 |
| History of stroke/TIA | 2586/8287 (31.2%) | 429/934 (45.9%) | 2157/7353 (29.3%) | 0 |
| Admission NIHSS score | 5.53+5.93 (8287) | 6.09+6.69 (934) | 5.46+5.83 (7353) | 0.0021 |
| NIHSS, median (Q1–Q3) | 4 (2–8) (8287) | 4 (1–8) (934) | 4 (2–7) (7353) | 0.247 |
| Drugs | ||||
| Lipid-lowering agents | 223/8218 (2.7%) | 44/919 (4.8%) | 179/7299 (2.5%) | 0 |
| Antihypertensive agents | 3887/8287 (46.9%) | 507/934 (54.3%) | 3380/7353 (46.0%) | 0 |
| Hypoglycemic agents | 1983/8287 (23.9%) | 269/934 (28.8%) | 1714/7353 (23.3%) | 0.0002 |
| Antiplatelet agents | 1359/8287 (16.4%) | 223/934 (23.9%) | 1136/7353 (15.4%) | 0 |
BMI – body mass index; NIHSS – the National Institutes of Health Stroke Scale. Heavy drinking indicates ≥2 standard alcohol intake/day.
Table 2 shows the univariate logistic regression analysis results of the risk factors. Older patients (OR 1.250, 95% CI: 1.130–1382), continual smoking (OR 1.296, 95% CI: 1.112–1.510), heart failure (OR 1.947, 95% CI: 1.215–3.119), coronary heart disease (OR 2.004, 95% CI: 1.689–2.378), hypertension (OR 1.514, 95% CI: 1.303–1.759), diabetes mellitus (OR 1.193, 95% CI: 1.018–1.400), dyslipidemia (OR 1.404, 95% CI: 1.162–1.696), history of stroke/TIA (OR 2.046, 95% CI: 1.782–2.350), and high admission NIHSS score (OR 1.017, 95% CI: 1.006–1.028), were associated with higher risk of stroke recurrence. The multivariate logistic regression analysis (Table 3) found that stoke recurrence was higher in patients with atrial fibrillation and history of stroke/TIA. The adjusted odds ratio was 1.958 (95% CI: 1.508–2.542) and 1.736 (95% CI: 1.496–2.014), respectively.
Table 2.
Risk factors for stroke recurrence by univariate logistic regression.
| Risk factors | OR (95% CI) | P-value |
|---|---|---|
| Age | 1.020 (1.013–1.028) | <0.001 |
| Age_c | 1.250 (1.130–1.382) | <0.001 |
| Gender | 1.025 (0.891–1.180) | 0.729 |
| BMI | 1.004 (0.986–1.023) | 0.659 |
| BMI-cat | 1.032 (0.915–1.164) | 0.608 |
| Smoking | 1.296 (1.112–1.510) | <0.001 |
| Heavy drinking | 0.819 (0.701–0.956) | 0.011 |
| Heart failure | 1.947 (1.215–3.119) | 0.005 |
| Coronary heart disease | 2.004 (1.689–2.378) | <0.001 |
| Hypertension | 1.514 (1.303–1.759) | <0.001 |
| Diabetes mellitus | 1.193 (1.018–1.400) | 0.029 |
| Dyslipidemia | 1.404 (1.162–1.696) | <0.001 |
| Peripheral artery disease | 0.874 (0.310–2.462) | 0.799 |
| History of stroke/TIA | 2.046 (1.782–2.350) | <0.001 |
| Admission NIHSS score | 1.017 (1.006–1.028) | 0.002 |
| Hypoglycemic agents | 1.331 (1.144–1.549) | <0.001 |
| Lipid-lowering agents | 2.000 (1.428–2.802) | <0.001 |
| Antihypertensive agents | 1.396 (1.217–1.600) | <0.001 |
| Antiplatelet agents | 1.716 (1.458–2.021) | <0.001 |
OR – odds ratio; CI – confidence interval; BMI – body mass index; NIHSS – the National Institutes of Health Stroke Scale. Heavy drinking indicates the standard alcohol intake per day ≥2; OR represents the standard alcohol intake per day ≥2 vs. <2.
Table 3.
Risk factors for stroke recurrence by multivariate logistic regression.
| Risk factors | OR (95% CI) |
|---|---|
| Age | 1.010 (1.002–1.018) |
| Admission NIHSS score | 1.009 (0.997–1.020) |
| Gender | 0.889 (0.746–1.060) |
| Smoking | 0.960 (0.803–1.148) |
| Heavy drinking | 0.925 (0.769–1.114) |
| Heart failure | 1.026 (0.620–1.698) |
| Coronary heart disease | 1.515 (1.258–1.826) |
| Hypertension | 1.367 (1.107–1.689) |
| Diabetes mellitus | 0.820 (0.659–1.021) |
| Dyslipidemia | 1.060 (0.857–1.311) |
| Atrial fibrillation | 1.958 (1.508–2.542) |
| History of stroke/TIA | 1.736 (1.496–2.014) |
| Hypoglycemic agents | 1.397 (1.135–1.720) |
| Lipid-lowering agents | 1.351 (0.929–1.966) |
| Antihypertensive agents | 0.923 (0.759–1.123) |
| Antiplatelet agents | 1.159 (0.966–1.390) |
OR – odds ratio; CI – confidence interval; NIHSS – the National Institutes of Health Stroke Scale. Reference for age was <65-years-old. Reference for NI-HSS score was 3. Reference for female sex was male sex. Adjusted for gender, ethnicity, educational background, smoking, heavy drinking, adiposity, and history of disease including heart failure, hypertension, diabetes mellitus, hyperlipidemia, vascular disease, and drug intervention such as antihypertensive agents use, hypoglycemic agents use, lipid-lowering agents use, antiplatelet agents and anticoagulants use.
At 1-year follow-up, the C statistic values were as follows: (1) For the stroke recurrence rate, 0.584 for PCR equations and 0.565 for ESSEN score in AIS patients and 0.573 for PCR equations and 0.558 for ESSEN score in all patients. (2) For non-fatal MI, 0.533 for PCR equations and 0.512 for ESSEN score in AIS patients and 0.493 for PCR equations and 0.520 for ESSEN score in all patients. (3) For vascular death, 0.592 for PCR equations and 0.612 for ESSEN score in AIS patients and 0.592 for PCR equations and 0.609 for ESSEN score in all patients. (4) For all events, 0.582 for PCR equations and 0.579 for ESSEN score in AIS patients and 0.575 for PCR equations and 0.572 for ESSEN score in all patients (Table 4).
Table 4.
Predictive accuracy of PCR equations and ESSEN Score.
| Recurrence stroke | Non-fatal MI | Vascular death | RS+MI+VD | |||||
|---|---|---|---|---|---|---|---|---|
| AUC | 95% CI | AUC | 95% CI | AUC | 95% CI | AUC | 95% CI | |
| PCR | ||||||||
| AIS | 0.584 | 4.927 (83.302, 7.353) | 0.533 | 0.825 (0.164, 4.138) | 0.592 | 4.927 (3.221, 7.537) | 0.582 | 4.725 (3.422, 6.525) |
| TIA | 0.499 | 0.753 (0.206, 2.754) | 0.490 | 0.776 (0.004, 140.407) | 0.588 | 9.654 (1.023, 91.116) | 0.499 | 0.872 (0.267, 2.856) |
| AIS and TIA patients | 0.573 | 3.945 (2.697, 5.771) | 0.493 | 0.811 (0.174, 3.776) | 0.592 | 5.375 (3.547, 8.145) | 0.575 | 4.174 (3.062, 5.690) |
| ESSEN | ||||||||
| AIS | 0.565 | 1.095 (1.065, 1.125) | 0.512 | 0.978 (0.881, 1.085) | 0.612 | 1.173 (1.138, 1.210) | 0.579 | 1.117 (1.093, 1.142) |
| TIA | 0.495 | 0.998 (0.922, 1.080) | 0.594 | 0.862 (0.605, 1.227) | 0.526 | 1.028 (0.871, 1.213) | 0.503 | 0.988 (0.918, 1.063) |
| AIS and TIA patients | 0.558 | 1.083 (1.055, 1.112) | 0.520 | 0.967 (0.875, 1.068) | 0.609 | 1.169 (1.134, 1.204) | 0.572 | 1.106 (1.083, 1.129) |
The study subjects were categorized into 2 groups: a low-PCR group (PCR<20%) and a high-PCR group (PCR ≥20%). For AIS patients, the 12-month cumulative rates of recurrent stroke, vascular death, and combined vascular events were higher in the high-PCR group (P<0.001); however, the rates for non-fatal MI did not differ between the 2 groups (Table 5). For TIA patients, there were no significant differences in the 12-month cumulative rates for recurrent stroke, non-fatal MI, vascular death, and combined vascular events between the 2 groups (Table 6).
Table 5.
12-month cumulative rates for recurrent stroke and combined vascular events stratified by PCR in AIS patients.
| Recurrence stroke | Non-fatal MI | Vascular death | RS+MI+VD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Percent (95% CI) | P | N | Percent (95% CI) | P | N | Percent (95% CI) | P | N | Percent (95% CI) | P | |
| PCR <20% | 259 | 8.38% | 19 | 0.59% | 218 | 6.83% | 471 | 14.75% | ||||
| PCR ≥20% | 564 | 12.72% | 35 | 0.76% | 478 | 10.37% | 961 | 20.85% | ||||
| Total | 823 | 10.94% | <0.001 | 54 | 0.69% | 0.3891 | 696 | 8.92% | <0.001 | 1432 | 18.35% | <0.001 |
Table 6.
12-month cumulative rates for recurrent stroke and combined vascular events stratified by PCR in TIA patients.
| Recurrence stroke | Non-fatal MI | Vascular death | RS+MI+VD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Percent (95% CI) | P | N | Percent (95% CI) | P | N | Percent (95% CI) | P | N | Percent (95% CI) | P | |
| PCR <20% | 62 | 15.82% | 3 | 0.74% | 10 | 2.48% | 74 | 18.36% | ||||
| PCR ≥20% | 49 | 13.17% | 3 | 0.79% | 12 | 3.16% | 60 | 15.79% | ||||
| Total | 111 | 14.53% | 0.2999 | 6 | 0.77% | 0.9424 | 22 | 2.81% | 0.5670 | 134 | 17.11% | 0.3394 |
Discussion
The present study was primarily conducted to determine whether the PCR equations could predict the 1-year clinical outcome in AIS/TIA patients in China. A previous study showed that the ESSEN score can predict and stratify the risk of recurrent stroke and combined vascular events in Chinese AIS/TIA patients [14]. The present results showed that the PCR equations and the ESSEN score had similar predictive validity for either recurrent stroke or cumulative vascular events. In our study results, the C statistic values were approximately 0.6, indicating a moderate predictive value of the 2 scores in stroke/TIA patients in China. We also found that the AUC values of the PCR equations were analogous to those of a recent study wherein the patients were included from the Vitamin Intervention for Stroke Prevention (VISP) trial [15]. In the VISP cohort, comprising 3680 patients with an average of 20 months of follow-up, the C statistics of the PCR were 0.56 for stroke and 0.62 for major vascular events.
In the REGARDS study [7], the PCR model yielded better discrimination, with the C value 0.72 (95% CI, 0.70–0.75). Although the participants have not been followed up for 10 years, Muntner et al. modified the PCR model into 5-year risk version and the participants were not taking statins at baseline, for which the PCR model was specifically designed to be used. In our study, the PCR model was modified to 1-year and 46.2% had vascular comorbidities, including known-ASCVD and heart failure. Furthermore, in our cohort, there was 2.7% lipid modifier use and the secondary preventions included antithrombotic medication (16.4%), antihypertensive medication (46.9%), and hypoglycemic medication (23.9%). All these may have attenuated the discrimination power of vascular outcome events. Another potential factor was racial difference.
We used 20% as the threshold value to discriminate low and high risk, since a score of ≥20% in 10 years is known to predict increased CVD risk that requires the modification of driving risk factors [16]. Our study confirmed that the AIS patients with PCR score ≥20% faced a higher risk of recurrent stroke and combined vascular events, which could not be applied to TIA patients. Other studies also found that high PCR (PCR score ≥20%) predicts a 1.8-fold increase in risk of stroke and a 2.1-fold increase in risk of stroke/CHD/vascular death over a 2-year period [15]. The PCR equations may be a potentially useful and easy-to-use risk stratification tool to aid clinicians in identifying patients at high risk of recurrent stroke and vascular events, thereby raising awareness of secondary prevention.
The strengths of this study are the prospective and multicenter design of the CNSR and the large sample size of consecutive stroke patients. Nevertheless, our study also has several limitations. First, selection bias cannot be excluded. All the participating hospitals involved in the CNSR were located in the urban regions because of the selection for intensive care and follow-up, although they represent nationwide areas. The urban areas have more resources and expertise than their rural counterparts. Thus, we might have underestimated the unfavorable events rate. Moreover, our study selected the hospitalized patients, and exclusion of non-hospitalized patients in a study of survival after stroke may introduce significant bias [17]. Second, our study did not classify the subtypes of acute ischemic stroke, which might influence the functional outcome, survival, and recurrence of patients [18,19]. Third, the PCR equations do not involve severity of stroke and information on imaging or laboratory studies, which may affect the outcomes of patients [20].
The present report provides the first external validation of the PCR equations in China. In Chinese patients with AIS or TIA, the PCR equations may be a potentially useful tool for predicting the risk of recurrent stroke and combined vascular events.
Conclusions
The current study shows that the PCR equations and the ESSEN score have similar predictive validity for either recurrent stroke or cumulative vascular events. Our study confirms that AIS patients with PCR score ≥20% face a high risk of recurrent stroke and combined vascular events.
The pooled cohort risk equations may serve as potential tools to predict and stratify the 1-year risk of recurrent stroke and combined vascular events in AIS/TIA patients in China. The PCR equations may be a useful and easy-to-use risk tool which can raise awareness of secondary prevention.
Footnotes
Conflict of interests
The authors declare that they have no conflict of interests.
Source of support: This study was funded by the Ministry of Science and Technology and the Ministry of Health of the People’s Republic of China, the National Science and Technology Major Project of China (2008ZX09312-008), and the State Key Development Program for Basic Research of China (2009CB521905)
References
- 1.Feigin VL, Lawes CM, Bennett DA, et al. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: A systematic review. Lancet Neurol. 2009;8:355–69. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 2.Bravata DM, Ho SY, Meehan TP, et al. Readmission and death after hospitalization for acute ischemic stroke: 5-year follow-up in the medicare population. Stroke. 2007;38:1899–904. doi: 10.1161/STROKEAHA.106.481465. [DOI] [PubMed] [Google Scholar]
- 3.Tseng MC, Lin HJ. Readmission after hospitalization for stroke in Taiwan: Results from a national sample. J Neurol Sci. 2009;284:52–55. doi: 10.1016/j.jns.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Lin HJ, Chang WL, Tseng MC. Readmission after stroke in a hospital-based registry: Risk, etiologies, and risk factors. Neurology. 2011;76:438–43. doi: 10.1212/WNL.0b013e31820a0cd8. [DOI] [PubMed] [Google Scholar]
- 5.Lee HC, Chang KC, Huang YC, et al. Readmission, mortality, and first-year medical costs after stroke. J Chin Med Assoc. 2013;76:703–14. doi: 10.1016/j.jcma.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935–59. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muntner P, Colantonio LD, Cushman M, et al. Validation of the atherosclerotic cardiovascular disease Pooled Cohort risk equations. JAMA. 2014;311:1406–15. doi: 10.1001/jama.2014.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chia YC, Lim HM, Ching SM. Validation of the pooled cohort risk score in an Asian population – a retrospective cohort study. BMC Cardiovasc Disord. 2014;14:163. doi: 10.1186/1471-2261-14-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weimar C, Diener HC, Alberts MJ, et al. The Essen stroke risk score predicts recurrent cardiovascular events: A validation within the REduction of Atherothrombosis for Continued Health (REACH) registry. Stroke. 2009;40:350–54. doi: 10.1161/STROKEAHA.108.521419. [DOI] [PubMed] [Google Scholar]
- 10.Andersen SD, Gorst-Rasmussen A, Lip GY, et al. Recurrent stroke: The value of the CHA2DS2VASc Score and the Essen Stroke Risk Score in a Nationwide Stroke Cohort. Stroke. 2015;46:2491–97. doi: 10.1161/STROKEAHA.115.009912. [DOI] [PubMed] [Google Scholar]
- 11.Chen P, Liu Y, Wang Y, et al. A validation of the essen stroke risk score in outpatients with ischemic stroke. J Stroke Cerebrovasc Dis. 2016;25:2189–95. doi: 10.1016/j.jstrokecerebrovasdis.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Cui L, Ji X, et al. The China National Stroke Registry for patients with acute cerebrovascular events: Design, rationale, and baseline patient characteristics. Int J Stroke. 2011;6:355–61. doi: 10.1111/j.1747-4949.2011.00584.x. [DOI] [PubMed] [Google Scholar]
- 13.Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke — 1989. Recommendations on stroke prevention, diagnosis, and therapy. Stroke. 1989;20:1407–31. doi: 10.1161/01.str.20.10.1407. [DOI] [PubMed] [Google Scholar]
- 14.Meng X, Wang Y, Zhao X, et al. Validation of the Essen Stroke Risk Score and the Stroke Prognosis Instrument II in Chinese patients. Stroke. 2011;42:3619–20. doi: 10.1161/STROKEAHA.111.624148. [DOI] [PubMed] [Google Scholar]
- 15.Park JH, Kwon HM, Ovbiagele B. New pooled cohort risk equations: Application to a recent stroke patient population. J Neurol Sci. 2015;348:160–65. doi: 10.1016/j.jns.2014.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation. 2008;117:743–53. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 17.Bonita R, Beaglehole R. Explaining stroke mortality trends. Lancet. 1993;341:1510–11. doi: 10.1016/0140-6736(93)90640-3. [DOI] [PubMed] [Google Scholar]
- 18.Petty GW, Brown RD, Whisnant JP, et al. Ischemic stroke subtypes: A population-based study of functional outcome, survival, and recurrence. Stroke. 2000;31:1062–68. doi: 10.1161/01.str.31.5.1062. [DOI] [PubMed] [Google Scholar]
- 19.Petty GW, Brown RD, Whisnant JP, et al. Survival and recurrence after first cerebral infarction: A population-based study in Rochester, Minnesota, 1975 through 1989. Neurology. 1998;50:208–16. doi: 10.1212/wnl.50.1.208. [DOI] [PubMed] [Google Scholar]
- 20.Johnston KC, Wagner DP, Haley EC, et al. Combined clinical and imaging information as an early stroke outcome measure. Stroke. 2002;33:466–72. doi: 10.1161/hs0202.102881. [DOI] [PMC free article] [PubMed] [Google Scholar]


