Abstract
Gaze conveys emotional information, and humans present sensitivity to its direction from the earliest days of life. Bipolar disorder is a disease characterized by fluctuating states of emotional and cognitive dysregulation. To explore the role of attentional control on face processing in bipolar patients (BP) we used gaze direction as an emotion modulation parameter in a two-back Working Memory (WM) task while high-density EEG data were acquired. Since gaze direction influences emotional attributions to faces with neutral expressions as well, we presented neutral faces with direct and averted gaze. Nineteen euthymic BP and a sample of age- and gender-matched controls were examined.
In BP we observed diminished P200 and augmented P300 evoked responses, differentially modulated by non-repeated or repeated faces, as well as by gaze direction. BP showed a reduced P200 amplitude, significantly stronger for faces with direct gaze than averted gaze. Source localization of P200 indicated decreased activity in sensory-motor regions and frontal areas suggestive of abnormal affective processing of neutral faces.
The present study provides neurophysiological evidence for abnormal gaze processing in BP and suggests dysfunctional processing of direct eye contact as a prominent characteristic of bipolar disorder.
Keywords: Bipolar disorder, Gaze processing, Face recognition, Memory, ERP, EEG source imaging
Highlights
-
•
This ERP study identified abnormalities in gaze processing in bipolar patients.
-
•
We observed functional anomalies in the P200 and P300 evoked responses.
-
•
BP showed a strong suppression of the P200 for faces with direct gaze.
-
•
Source localization indicated decreased activity in sensory-motor regions.
1. Introduction
Early life experiences affect the way we learn to express and think about emotions (Frick and Morris, 2004, Graziano et al., 2010, Morris et al., 2007). Bipolar disorder is a disease typically appearing early in life, during late adolescence or young adulthood (A.P.A., DSM I-V TR, 1994), with genetic and environmental factors contributing to its development and outcome (Barnett and Smoller, 2009, Etain et al., 2008).
Emotion regulation may involve attentional and cognitive strategies (Gross and Thompson, 2007). Bipolar disorder is associated with dysfunctional attentional and cognitive regulatory processes, such as, suppression, and avoidance of thoughts/feelings and rumination (Aldao et al., 2010). It has been proposed that emotion dysregulation in bipolar patients (BP) could be explained by specific impairments of ventral and dorsal prefrontal regions involved in regulating subcortical regions (Phillips et al., 2008).
Working memory (WM) paradigms are voluntary attention control paradigms that have been used to investigate emotion regulation in BP (Bertocci et al., 2012, Frangou et al., 2008; and see Phillips et al., 2008). Functional neuroimaging studies have demonstrated that WM processing of faces induces reduced prefrontal activity (Passarotti et al., 2012, Pavuluri et al., 2010, Vizueta et al., 2012).
When viewing emotional faces, BP perform worse in emotional face labeling (Favre et al., 2015, Kohler et al., 2011), and show hyperactivity in limbic regions (Surguladze et al., 2010). Even with neutral faces, BP show increased amygdala activation (Kim et al., 2012, Rich et al., 2006) and tend to perceive these faces as more hostile than healthy controls (Rich et al., 2006).
The eye region conveys emotional information (Itier and Batty, 2009): direct gaze augments the perception of approach-related affective states (i.e. anger, joy) while averted gaze increases the perception of avoidance-related affective states (i.e. fear, sadness) (Adams et al., 2003, Adams and Kleck, 2005). Additionally, gaze direction activates brain regions associated with emotional face processing, such as the amygdala and the fusiform gyrus (Adams et al., 2003, George et al., 2001; and see Itier and Batty, 2009). Humans present a sensitivity to gaze direction from the earliest days of life (Farroni et al., 2002), and eye gaze interaction offers cognitive and affective learning opportunities (Lotzin et al., 2016, Lotzin et al., 2015, Stern, 1974, Tronick and Reck, 2009), and influences the development of emotion-regulation strategies (Aktar et al., 2016, Luoma et al., 2013, Möller et al., 2014). To the best of our knowledge, no previous work has investigated the neural correlates of gaze perception in BP.
Event-related Potential (ERP) studies mainly focused on differences in BP and controls in the processing of emotional face expression. An ERP component that is sensitive to face perception is the N170 (Bentin et al., 1996). Degabriele et al. (2011) reported significantly lower N170 amplitudes in patients with bipolar disorder compared to controls, but this reduction was independent of the emotional facial expression. On the other hand, a work by Sokhadze (Sokhadze et al., 2011) demonstrated that BP have decreased N170 amplitudes to emotional positive faces. Controversially, Wynn et al. (2013) found intact N170 responses to emotional faces. Ibanez et al. (2012) found that while in healthy controls happy faces elicited larger N170 amplitudes than angry faces, BD patients did not show valence differences in the N170, and suggested that BP might have a reduced affective detection threshold. Taken together, these data suggest that, while face encoding is overall preserved in BP, task instructions and affective requests may affect the N170 evoked responses in BP. Importantly, the N170 component is also sensitive to the encoding of gaze direction (Berchio et al., 2016, Conty et al., 2007; and for a review see Itier and Batty, 2009), that, as explained above, conveys emotional information. The question we thus asked in this study is whether gaze might influence the N170 component differently in BP than healthy controls due to altered emotional judgement of gaze.
A later ERP component, the P200 is related to attentional control and emotional processing in general, and is not specific to faces (Carretié et al., 2001, Correll et al., 2006). It has been shown that negative stimuli, such as threatening images, enhance its amplitude (Carretié et al., 2001, Correll et al., 2006, Schutter et al., 2004). Furthermore, the P200 amplitude is correlated with reduced WM performance (Judah et al., 2016). Therefore, the P200 appears to be another relevant component to explore attentional deployment and emotion processing in BP.
Anxiety and stress responses are potential confounding variables that must be taken into account when investigating gaze evoked responses and WM processing. Anxious individuals have an attentional bias for gaze direction (Schulze et al., 2013), and anxiety influences P200 evoked responses (Judah et al., 2016, Schmitz et al., 2012). Previous data have documented that stress responses affect behavior (for an exhaustive review on this topic, see Sandi and Haller, 2015), prefrontal attentional control (Liston et al., 2009), and the interpretation of another person's gaze (Rimmele and Lobmaier, 2012).
In the present study, we aimed to use gaze direction as an emotion modulation parameter in a WM task in order to explore the role of attentional control on face processing in BP. To this aim, we used a two-back WM paradigm in which we presented neutral faces with direct and averted gaze without explicit instruction about gaze direction. High-density EEG, a powerful neuro-imaging tool for describing brain network dynamics with high temporal resolution (Michel and Murray, 2012), was recorded while subjects performed the task.
Because we assumed that patients with bipolar disorder could be more susceptible to external stressor (Cohen et al., 2004, Dienes et al., 2006, Monroe and Harkness, 2005) compared to control subjects, we monitored stress differences between patients and controls by measuring heart rate variability, and self-perception of stress.
We hypothesized that BP would display increased activities in face-responsive brain regions, and decreased activation in dorsolateral prefrontal regions associated with WM for faces. Since the N170 is a face-sensitive component, and the P200 is modulated by attentional control and emotional processing, we expected augmented N170 and reduced P200 responses. We expected that altered neural responses would be also reflected in lower accuracy and increased reaction times. Finally, since BP tend to identify stimuli with neutral value as emotionally negative, we hypothesized that direct gaze would reinforce these effects compared to indirect gaze.
2. Material and methods
2.1. Participants
Euthymic BP type I and II were recruited from the Mood Disorders Unit at the University Hospital of Geneva. Control subjects were recruited by advertisement. A snowball convenience sampling was used for the selection of the BD group. Control participants were matched by gender, age (± 3 years), educational level, handedness (Edinburgh inventory, Oldfield, 1971) (see Table 1). Exclusion criteria included a history of head injury, current alcohol or drug abuse, and a history of psychiatric illness. Informed written consent was obtained from all subjects and this study was approved by the Ethical Committee for Human Research of the Geneva University Hospital, Switzerland.
Table 1.
Demographic and clinical features of the two study groups.
| Characteristics | Control participants (n = 19) | Bipolar patients (n = 19) | t value | p value |
|---|---|---|---|---|
| Age: mean, SD | 34.11 (10.69) | 34.95 (10.49) | 0.22 | 0.841 |
| Gender: male, n | 11 | 11 | ||
| Handedness: right, n | 16 | 16 | ||
| Educationa: mean, SD | 2.27 (0.81) | 2.28 (0.89) | 0.052 | 0.749 |
| IQ: mean, SD | ||||
| WM | 11.44 (2.55) | 10.41 (2.93) | − 1.376 | 0.595 |
| Arithmetic | 12.53 (2.29) | 12.33 (2.35) | 0.942 | 0.352 |
| YMRS: mean, SD | 0.72 (1.24) | 0.69 (1.53) | − 0.084 | 0.379 |
| MADRS: mean, SD | 1.41 (1.53) | 3.12 (3.39) | 2.082 | 0.001 |
| STAI-state: mean, SD | 25.97 (3.49) | 37.87 (12.27) | 4.661 | 0 |
| STAI-trait: mean, SD | 30.39 (5.85) | 42.53 (9.72) | 4.053 | 0.037 |
Education levels were classified into three groups: 3 = university studies; 2 = high school; 1 = no high school.
Three BP and one control subject were excluded from ERP analysis because of an excessive number of EEG artifacts, which resulted in 19 patients and 19 controls finally included in this study (see Table 1). All the patients were medicated, receiving pharmacological therapy including antipsychotics, antidepressants and mood stabilizers.
2.2. Clinical assessment
In order to confirm bipolar disorder diagnosis and check for comorbidities in BP patients, and to exclude psychiatric diagnosis in the controls, all participants underwent a clinical structured interview (DIGS: Diagnostic for Genetic Studies, (Nurnberger et al., 1994) by a trained collaborator [P.C.] Consensus diagnoses were determined in consultation with psychiatrists [J-M.A.; C.P.] and psychologists [P.C.; Anne-Lise Kung]).
Euthymia was defined as the absence of major depression, hypomania, or mania. Symptoms of mania and depression were evaluated using the Young Mania Rating Scale (YMRS, Young et al., 1978), and the Hamilton Depression Rating Scale (HDRS, Hamilton, 1960), respectively. Participants were considered euthymic if they scored < 6 on YMRS and < 12 on HDRS. Both BP type I (n = 9), and type II (n = 10) were recruited. Moreover, to compare WM capacity between groups, two subtests of the WAIS-R (Wechsler, 1981) were evaluated: arithmetic, as well as forward and backward digit span.
All subjects were also assessed prior to electrophysiological recordings with the State-Trait Anxiety Inventory (STAI; state and trait; Spielberger et al., 1970).
2.3. Paradigm
During high-density EEG recordings, all subjects completed a two-back WM task (Stimuli and the experimental paradigm are described in detail in Berchio et al., 2016). Stimuli were neutral faces (size: 14.11 × 20.99 cm) with either direct or averted gaze (Radboud Faces Database, (Langner et al., 2010); the database provided by Dr. Nathalie George, (George et al., 2001); the NIMH-chEFS Picture Set, (Egger et al., 2011); and the Amsterdam Dynamic Facial Expression Set, (van der Schalk et al., 2011)).2
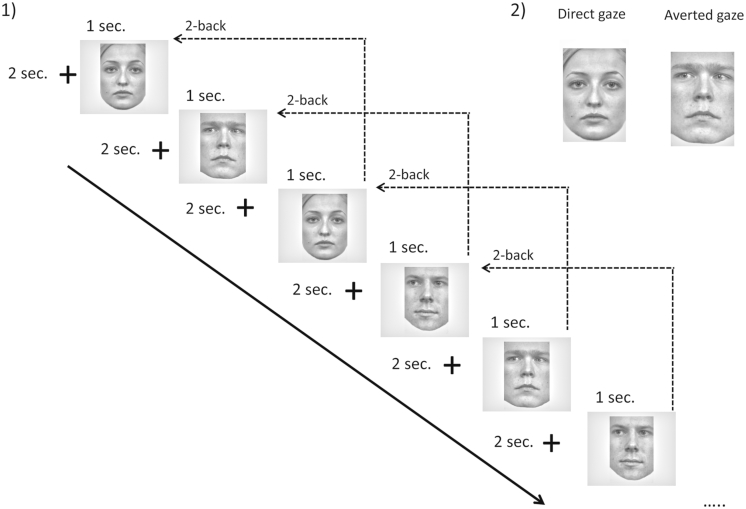
Faces were presented for 1000 ms, followed by an interval of 2000 ms. Subjects were asked to press a down arrow if the face was exactly the same as the face presented two faces before, and an up arrow if the face was different from the face presented two faces before (Fig. 1). A two-back match trial always referred to a match both in face identity and gaze direction. No explicit indication about gaze direction was given to the participants. Furthermore, participants were instructed to respond as quickly as possible, and to fixate the center of the screen.
Fig. 1.
a) Experimental task and b) stimuli: neutral faces with direct or averted gaze.
The task consisted of four conditions: non-repeated faces with direct gaze and averted gaze, -repeated faces with direct gaze and averted gaze (two-back target). Three six-minute blocks were presented in a pseudo-randomized order. Faces were presented with a ratio of 40% target faces to 60% non-target faces (number of target stimuli per condition = 60).
Participants then performed a rating on the perceived affective states of the neutral faces. For each participant, a sample of ten faces was randomly selected from the two-back task (five with averted gaze, five with direct gaze). Two questions were presented: a) “How hostile is the face?”; b) “How fearful is the face?”. Subjects were required to respond using 7-point Likert scales (0 = not at all; 7 = extremely) as quickly and accurately as possible. A fixation cross appeared for 500 ms, and stimuli were presented for 3000 ms.
The two-back task and the emotional rating were presented using E-prime (2.0), with a 60-cm distance between the screen and viewer. The total duration of the session was 90 min.
2.4. EEG recordings and pre-processing
High-density EEG was recorded with a 256-channel system (EGI System 200; Electrical Geodesic Inc., OR, USA), sampling rate of 1000 Hz, electrode impedance below 30 k-ohms, and CZ as acquisition reference.
ERP analyses were performed using the freely available Cartool Software 3.60, programmed by Denis Brunet (http://www.fbmlab.com/cartool-software/). Data were band-pass filtered between 0.3–40 Hz. EEG artifacts were identified by visual inspection, and contaminated epochs were rejected. ERP were baseline corrected over the pre-stimulus interval (− 100 to 0 ms). Bad channels were interpolated using a 3D spline interpolation method. The ERPs were down-sampled to 250 Hz, and the data were re-referenced to the average reference. The raw recordings were segmented and averaged into epochs of 700 ms, including 100 ms before stimulus onset. For subsequent analyses, to remove muscular artifacts located in the neck and face, the EEG data was reduced from 256 to 204 channels (see Berchio et al., 2014).
2.5. Data analysis
2.5.1. Behavioral analysis: accuracy, reaction time, rating
Behavioral performance was assessed in terms of accuracy and reaction time.
A repeated measures ANOVA was used to examine Accuracy, with Gaze (‘direct’ vs. ‘averted’) and memory Load (‘non-repeated’ vs. ‘repeated’) as within subject factors, and group (BP vs. control participants) as a between subject factor.
Median reaction times (RTs) were analyzed for the trials where the participants responded correctly. A repeated measures ANOVA was performed on RTs, with Gaze (direct vs. averted) and memory Load (‘non-repeated’ vs. ‘repeated’) as within subject factors, and group (BP vs. control participants) as between subject factor.
A repeated measures ANOVA was also used to compare the behavioral ratings between groups, with Gaze (direct vs. averted) and Emotion (‘hostile’ vs. ‘fearful’) as within subject factors, and group (BP vs. control participants) as a between subject factor.
Alpha levels were set to p < 0.05 on all ANOVAs, and Bonferroni corrections were applied for all comparisons.
2.5.2. ERP analysis
2.5.2.1. Analysis on the scalp level
We investigated modulations in the amplitude responses between groups and conditions by computing a resampling permutation test. This test was performed for each electrode and each time point, from − 100 to 400 ms post-stimulus. We opted for permutation statistics to reduce the risk of false positive effects due to multiple tests (Maris and Oostenveld, 2007). Moreover, effects were considered statistically significant only when they lasted for consecutive time frames of at least 20 ms with a threshold of p < 0.01 (Michel, 2009, Murray et al., 2008).
Furthermore, two global tests across all electrodes were applied, one to test for global strength difference of the electric field and the other to test for differences in the topography of the potential distribution (see Michel and Murray, 2012).
Difference in map strength was evaluated using the Global Field Power (GFP), which indicates the total amount of neuronal synchronization (Skrandies, 1990). The GFP is defined by the sum of all squared potential differences and is equivalent to the standard deviation of the potentials (Lehmann and Skrandies, 1980). Crucially, the mean potential of all electrodes (the average reference) is subtracted from each potential before the square-sum is computed. GFP differences were evaluated in a 2 × 2 × 2 design with permutation tests, with Gaze (direct vs. averted) and Load (‘non-repeated’ vs. ‘repeated’) as within subject factors, and Group (BP vs. control participants) as a between subject factor (Koenig et al., 2011). Effects were considered statistically significant only if they lasted for consecutive time frames of at least 10 ms and with a p value set to < 0.05.
Differences in map topography between groups and conditions were assessed by a non-parametric permutation test called ‘topographic ANOVA’ or TANOVA (for technical details see Koenig et al., 2011, Michel and Murray, 2012, Murray et al., 2008). It is based on the calculation of the global map dissimilarity (GMD) between two maps (Karniski et al., 1994, Srebro, 1996). The GMD is a reference-independent measure of topographic differences of two scalp potential maps. It is defined as the square root of the mean of the squared differences between the potentials measured at each electrode (vs. the average reference) after scaling them to unitary strength by dividing them by the Global Field Power (Koenig et al., 2011, Lehmann and Skrandies, 1980, Michel and Murray, 2012). Because the maps have unitary strength, only topographic differences are considered. If two maps differ in topography, independent of their strength, this directly indicates that the two maps were generated by a different configuration of sources in the brain (Srebro, 1996, Vaughan, 1982). The test for statistical significant topographic differences is done by assigning the maps of each single subject randomly to one of the conditions (i.e. permutations of the data) and recalculating the group-average ERPs. This procedure is repeated many times and the probability that the GMD of the real data lies significantly outside of the distribution of the randomized data is calculated for each time point (see Koenig et al., 2011). We used 1000 permutations a threshold of p < 0.05, and a time constraint of ≥ 10 ms of successive significant tests. As in the test for GFP differences, we applied the TANOVA to test the data for main effects, and interactions, i.e. a 2 × 2 × 2 design, with Gaze (direct vs. averted) and Load (‘non-repeated’ vs. ‘repeated’) as within subject factors and Group (BP vs. control participants) as a between subjects factor.
2.5.2.2. Analysis in the source space
We performed analyses in the source space using a linear distributed inverse solution capable of dealing with multiple active sources (LAURA, Grave de Peralta Menendez et al., 2001). We used an anatomically constrained head model (L-SMAC model, Birot et al., 2014, Brunet et al., 2011, Spinelli et al., 2000), and the average brain of the Montreal Neurological Institute as a template head (http://www.bic.mni.mcgill.ca/brainweb). 5018 solution points were distributed equally in the grey matter of this template brain. We then divided the solution space into 84 regions of interest over occipital, parietal, temporal, central, frontal regions and the limbic lobe (Automated Anatomical Labeling template, Tzourio-Mazoyer et al., 2002). Seven subcortical structures and the cerebellum were excluded.
Since the purpose of this study was to explore differences between groups, we performed contrast analysis between groups for each condition. To solve the multiple comparisons problem, resampling tests were conducted (see Maris and Oostenveld, 2007). The current density values of each ROI were averaged and then permuted (10,000 runs, p-values < 0.05). For each significant difference, contrast directions were assessed by a paired t-test (p < 0.05).
3. Results
3.1. Sample characteristics
χ2 analysis showed that there were no significant differences between the groups in terms of age, education, or gender (see Table 1). The clinical variables of the two groups were compared with two-tailed unpaired t-tests. As shown in Table 1, BP participants had higher scores on state and trait scales of the STAI, and participant groups also differed in depression scores.
Healthy controls and BP were screened for social anxiety using the DIGS clinical interview. According to the DIGS, only one BP patient had social phobia.
Performance on the arithmetic and forward and backward digits span of the WAIS-R did not differ between groups. Furthermore, despite very low mean scores, patients showed statistically more depression symptoms than controls.
3.2. Behavioral results
Accuracy was significantly modulated by Gaze (F(1,36) = 122.79, p < 0.001) and Load (F(1,36) = 6.62, p = 0.014). Post hoc analyses revealed that accuracy was significantly higher for faces with averted gaze (M = 84.86, SD = 11.02) than with direct gaze (M = 77.14, SD = 11.12) (df = 36, p < 0.001), as well as for repeated faces (M = 83.22, SD = 8.86) than non-repeated faces (M = 78.78, SD = 11.43) (p = 0.014). There was no effect of group and no significant interaction.
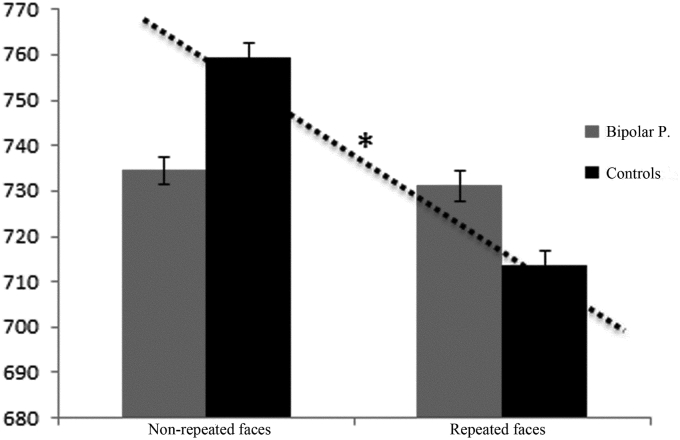
RT analysis revealed a main effect of Load (F(1,36) = 6.94, p = 0.012) and significant interaction effect Load ∗ Group (F(1,36) = 5.23, p = 0.028). Participants had significantly faster RTs when categorizing repeated faces (M = 722.36, SD = 89.48) than non-repeated faces (M = 746.94, SD = 78.13) (p < 0.007), but this was the case only for the control participants, while the performance of the patients was not modulated by face repetition. There was also a significant interaction effect Load ∗ Gaze (F(1,36) = 12.92, p < 0.001): for averted gaze stimuli only, repeated faces (M = 715.13, SD = 88.3) were recognized faster than non-repeated faces (M = 752.31, SD = 87.58) (p < 0.001).
A repeated-measures ANOVA including Emotion (‘hostile’ vs. ‘fearful’) × Gaze (direct vs averted) compared the rating scores between the two groups. The internal consistence of the five-items questionnaire was verified measuring the Cronbach's alpha: the alpha coefficient was 0.81 for hostility-items, and 0.89 for the afraid-items. ANOVA results indicated that groups did not differ on post-task ratings of the faces: there was no significant Group (F(1,36) = 1.64, p > 0.05) main effect or Group interactions (Emotion × Group: F(1,36) = 0.74, Gaze × Group: F(1,36) = 0.19, Emotion × Gaze × Group: F(1,36) = 0.24; all ps > 0.05).
In summary, the behavioral results indicate that in both participant groups, faces with averted gaze were discriminated better than faces with direct gaze. However, BP did not show decreased reaction time for repeated faces as did the controls (Fig. 2).
Fig. 2.
a) Median reaction times (in milliseconds) BP scores are plotted in red, control's scores in black. Asterisks (*) indicate significant effects, error bars represent standard errors.
3.3. ERP results
3.3.1. Amplitude results
For both groups of participants, ERPs were analyzed only for correct trials (total number of trials accepted: non-repeated faces with direct gaze, BP: M = 37.78, SD = 5.33, control participants: M = 38.21, SD = 7.90; non-repeated faces with averted gaze, BP: M = 38.5, SD = 5.78, control participants: M = 39.36, SD = 7.74; repeated faces with direct gaze: BP: M = 36.89, SD = 7.72, control participants: M = 36.73, SD = 6.83; repeated faces with averted gaze: BP M = 36.11; SD = 7.40, control participants: M = 38.94, SD = 8.9).
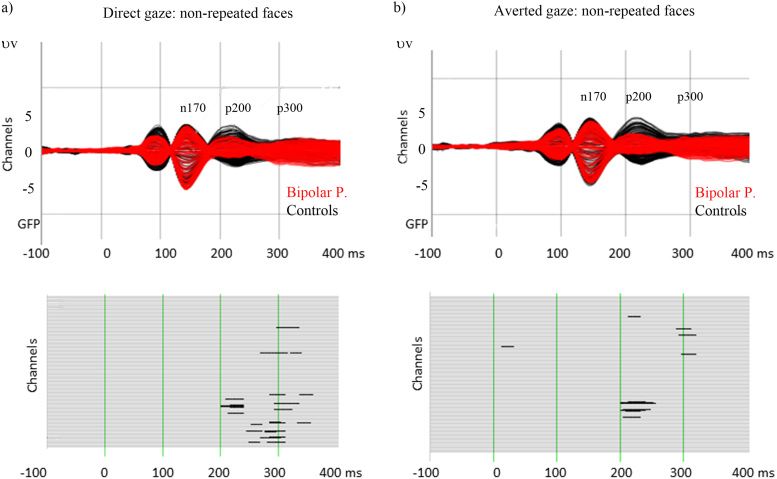
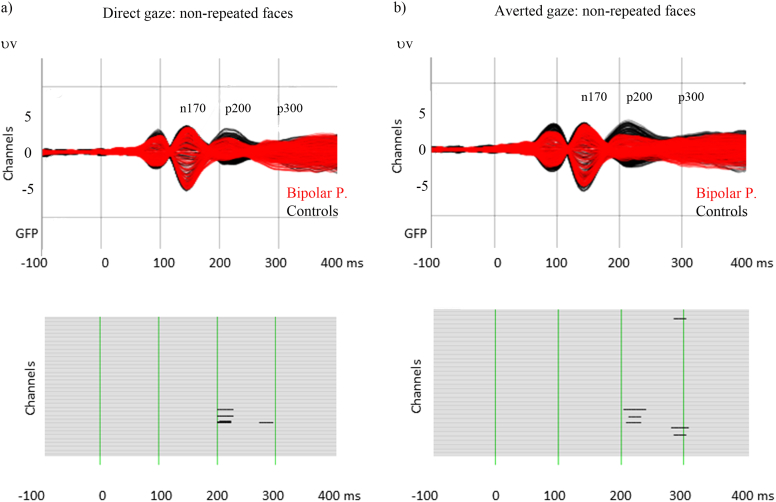
Visual inspection of the grand-mean evoked responses evidences four main ERP components elicited by the two-back WM task in both groups: the P100, the N170, the P200, and the P300 (Fig. 3, Fig. 4). Their mean latencies at the GFP-peaks are summarized in Table 2.
Fig. 3.
Grand average waveforms (butterfly montage): non-repeated faces with direct gaze (a) and averted gaze (b). Amplitude analysis results are shown in the lower part of the figure; black lines correspond to the time course of significant differences between groups (p ≤ 0.05).
Fig. 4.
Grand average waveforms: repeated faces with direct gaze (a) and averted gaze (b). Amplitude analysis results (lower part of the figure); black lines indicate significant differences between groups (p ≤ 0.05).
Table 2.
Global Field Power peaks latencies (ms).
| Controls | BP | |
|---|---|---|
| Non-repeated faces | ||
| Direct gaze | ||
| P100 | 95 (SD:12.898) | 92 (SD:12.682) |
| N170 | 144 (SD:11.735) | 140 (SD:13.056) |
| P200 | 204 (SD:16.517) | 196 (SD:20.667) |
| P300 | 280 (SD:37.798) | 276 (SD:50.383) |
| Averted gaze | ||
| P100 | 96 (SD:10.498) | 96 (SD:11.193) |
| N170 | 143 (SD:8.801) | 144 (SD:8.743) |
| P200 | 214 (SD:14.988) | 209 (SD:14.486) |
| P300 | 286 (SD:37.365) | 284 (SD:35.492) |
| Repeated faces | ||
| Direct gaze | ||
| P100 | 96 (SD:13.199) | 92 (SD:13.662) |
| N170 | 146 (SD:20.836) | 144 (SD:19.251) |
| P200 | 213 (SD:16.102) | 199 (SD:29.726) |
| P300 | 292 (SD:38.087) | 271 (SD:59.715) |
| Averted gaze | ||
| P100 | 94 (SD:9.817) | 94 (SD:10.857) |
| N170 | 143 (SD:10.145) | 141 (SD:12.189) |
| P200 | 212 (SD:14.875) | 202 (SD:24.198) |
| P300 | 287 (SD:36.86) | 268 (SD:50.885) |
For non-repeated faces with direct gaze (see Fig. 3a), the amplitude analysis showed differences between groups from 200 to 225 ms (right parietal electrodes: BP: M = + 0.84, SD = 3.56, control participants: M = + 1.35, SD = 1.12, p = 0.005), and from 245 to 360 ms (right frontal electrodes: BP: M = + 0.61, SD = 1.68, control participants: M = − 1.33, SD = 1.53; central electrodes: BP: M = + 0.78, SD = 1.63, control participants: M = − 1.26, SD = 2.10; right occipital electrodes: BP: M = − 1.22, SD = 3.79 control participants: M = + 0.79, SD = 2.73, all ps ≤ 0.007). For non-repeated faces with averted gaze (Fig. 3b), differences between BP and healthy controls were detected at 200–250 ms (left frontal electrodes: BP: M = − 0.65, SD = 2.40, control participants: M = − 2.25, SD = 2.63, all ps ≤ 0.01; right parietal electrodes: BP: M = − 0.05, SD = 1.46; control participants: M = + 1.45, SD = 1.15) and at 290–320 ms (left central electrodes: BP: M = − 0.58, SD = 2.77, control participants: M = − 1.63, SD = 1.82, p = 0.002).
Analysis of the repeated faces with direct gaze revealed differences between groups at 200–225 ms (right parietal electrodes: BP: M = + 0.61, SD = 1.75, control participants: M = + 2.47, SD = 1.82, p = 0.003) and at 270–290 ms (right parietal electrodes: BP: M = − 0.25, SD = 1.99, control participants: M = + 1.45, SD = 1.40, p = 0.007) (Fig. 4a). Finally, for repeated faces with averted gaze, the resampling test showed significant differences between groups at 200–235 ms (right parietal electrodes: BP: M = + 0.88, SD = 2.08, control participants: M = + 2.35, SD = 1.80, p = 0.004) and 280–300 ms (central frontal electrodes: BP: M = − 0.61, SD = 2.36, control participants: M = + 0.95 SD = 2.06; right temporal electrodes: BP: M = + 0.18, SD = 2.20, control participants: M = - 1.42+ SD = 2.08, all ps ≤ 0.007) (Fig. 4b).
In summary, the amplitude analysis highlighted lower amplitudes at the latency of the P200 component, for all conditions, in the BP group. For non-repeated faces with direct gaze and averted gaze, an increase in amplitude at the rising phase of the P300 peak was also observed in the BP.
For all the analyses performed, the p values are summarized in Table 1 in the Supplementary Appendix.
3.3.2. Global topographic measures
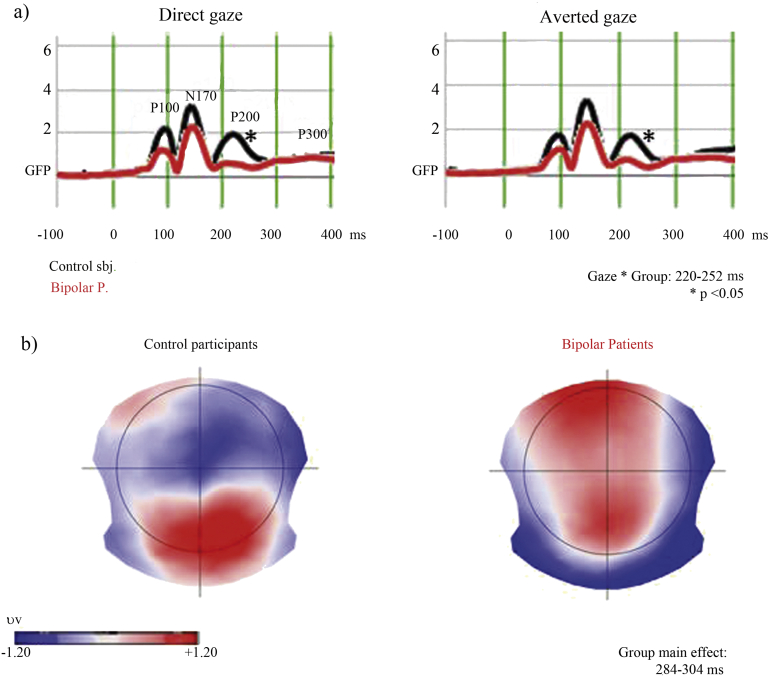
The permutation analysis on the GFP revealed a significant main effect of Load in the following time windows: 20–40 ms (p = 0.016), 176–192 ms (p = 0.010), and 352–396 ms (p = 0.002); a significant main effect of Gaze: from 80 to 92 ms (p = 0.002). Furthermore, the GFP analysis showed an interaction effect Gaze ∗ Group from 220 to 252 ms (p = 0.003) (Fig. 5a). A comparison within the BP Group, showed a stronger GFP amplitude reduction for direct gaze than averted gaze BP (p = 0.001).
Fig. 5.
a) Global Field Power measures for each experimental condition and group. Asterisks (*) indicate significant group differences. b) Topographic ERP maps. Significant differences between groups were found from 284 to 304 ms (TANOVA analysis).
Concerning scalp topographies, the TANOVA revealed a significant main effect of Load (28–40 ms, p = 0.022; 292–400 ms, p = 0.001), of Gaze (80–112 ms, p = 0.002), and Group (284–304 ms, p = 0.045). Finally, a significant interaction between Gaze ∗ Group was detected from 356 to 376 ms (p = 0.029) (Fig. 5b).
To test if the effects found over these time periods were stable, we performed post hoc analysis (for technical details see Koenig et al., 2011). Because the first aim of this study was to explore ERP differences between groups, planned comparisons were performed only on significant group main effects and interactions. Post hoc analysis confirmed that the results found with the TANOVA and GFP analysis were stable and consistent (TANOVA: Group main effect (284–304 ms): p = 0.045; interaction Gaze ∗ Group (356–376 ms): p = 0.029; interaction GFP: Gaze ∗ Group (220–252): p = 0.003).
3.3.3. Analysis in the source space
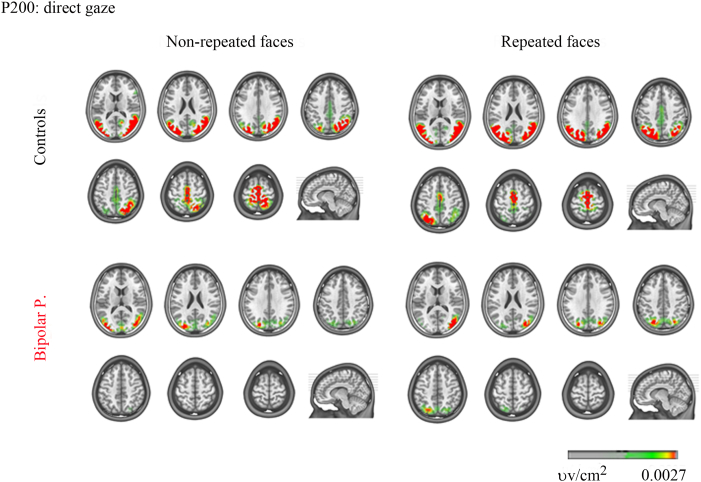
To test between group effects, source space analyses were conducted in the time windows where the amplitude analyses at the sensor level revealed significant differences between the patients and the healthy participants, and where these effects were confirmed by the global measures analyses. We performed analyses in two consecutive time windows that correspond to the P200 component (180–250 ms) and at the rising phase of the P300 component (250–300 ms) (For all the contrast analyses performed, positive t values indicate higher current density for the control group, negative t values indicate higher current density for the BP).
In response to non-repeated faces with direct gaze, localization of P200 sources revealed lower current source density in the patients in the left supplementary motor cortex, right postcentral gyrus, and the bilateral paracentral lobule (see Table 2 in the Supplementary Appendix, Fig. 6). Additionally, at the latency of the P300 maximum for repeated faces, lower current source density in BP was detected in the left medial superior frontal cortex.
Fig. 6.
Source localization of the P200: direct gaze conditions. Group source space maps at the time points of the P200 GFP maximum. Yellow to red colors indicate current source density activity. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
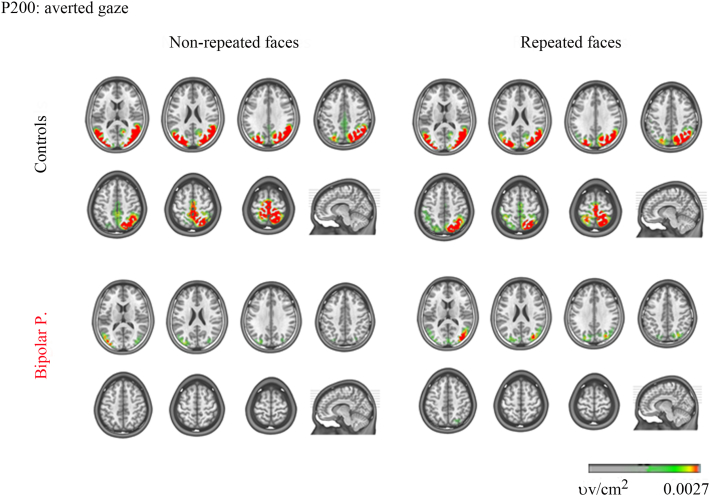
BP also displayed significantly less current source density in response to non-repeated faces with averted gaze (see Table 3 in the Supplementary Appendix, Fig. 7). Localization of P200 sources showed significant differences in the right superior frontal gyrus, left medial frontal cortex, bilateral supplementary motor cortex, right supramarginal gyrus, and bilateral paracentral lobule. At the P300, significant differences and less current source density in the BP were found in the left middle frontal gyrus.
Fig. 7.
EEG source imaging of the P200: faces with averted gaze. Group source space distribution at the GFP peaks. Yellow to red colors indicate current source density activity. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Comparison of activation, for repeated faces with direct gaze, revealed lower values of current source density for the patients in the right medial frontal gyrus, left supplementary motor cortex, left precentral gyrus, right postcentral gyrus, and bilateral paracentral lobule (Fig. 6). At the P300, patients demonstrated higher values in the left precentral gyrus (see Table 4 in the Supplementary Appendix).
Finally, a direct group comparison of responses to repeated faces with averted gaze revealed lower current source density in the BP in the left superior frontal gyrus, right rolandic operculum, left supplementary motor cortex, and bilateral paracentral lobule during a WM memory task (Fig. 7). At the P300, no significant differences between groups were found (see Table 5 in the Supplementary Appendix).
3.3.4. Supplementary post hoc analyses
3.3.4.1. Effect of anxiety
To examine the effects of anxiety on the evoked responses, we conducted an analysis of covariance (ANCOVA). This analysis was performed to determine whether anxiety scores predicted the ERP amplitude measures. For each condition and participant, the GFP maximum was selected at the latency of the P200. We used the GFP because it is a global quantitative, and reference-independent measure of the amount of neuronal synchronization (see Skrandies, 1990). Anxiety scores (STAI-state or STAI-trait) were the predictor, Group (BP vs. control participants) was the categorical factor, and the dependent variables were GFP for faces with direct gaze (‘non-repeated’ vs. ‘repeated’) and averted gaze (‘non-repeated’ vs. ‘repeated’).
This analysis revealed that in all of the models tested, neither predictor, STAI-trait (Main effects: all Fs(1,34) < 8.70, Interactions effects: all Fs(1,34) < 3.46), or STAI-state (Main effects: all Fs(1,34) < 8.47, Interactions effects: all Fs(1,34) < 1.50), had significant influence on the GFP maximum (all ps > 0.05).
We also investigated the influence of anxiety scores on the emotional rating. We performed an ANCOVA with Emotion (‘hostile’, ‘fearful’) and Gaze (direct vs. averted) as dependent variables, Group (BP vs. control participants) as categorical factor, and anxiety scores (STAI-state or STAI-trait) as predictor. This analysis only showed that STAI-state scores positively predicts ‘hostile’ emotion for faces with direct gaze (F = 2.91, df model = 2, R2 = 0.204, p = 0.043). No other effects were significant (all Fs(1,34) < 0.79, ps > 0.05).
3.3.4.2. Effect of depressive scores
In our sample, BP showed statistically more depression symptoms than controls (see Table 1). Depression is associated with deficit in attentional control (De Raedt and Koster, 2010) and difficulties inhibiting negative emotions (Joormann and Gotlib, 2010). To examine the extent to which depressive scores predict P200 evoked responses (amplitude), we performed ANCOVA analysis. P200 GFP maximum for faces with direct gaze (‘non-repeated’ vs. ‘repeated’) and averted gaze (‘non-repeated’ vs. ‘repeated’) were used as dependent variables, Group (BP vs control participants) was the categorical factor, and depressive scores were the predictor.
This analysis highlighted a positive correlation between depressive scores and P200 GFP maximum for repeated face with direct gaze (F = 4.27, df model = 2 R2 = + 0.150, p = 0.021). No other effects were significant (all Fs(1,35) < 0.79, all ps > 0.05).
4. Discussion
Our study investigated the neural substrate of gaze processing during WM memory task in BP. BP showed diminished P200 and augmented P300 evoked responses to neutral faces differentially modulated by direct and averted gaze, as well as non-repeated and repeated faces. At these latencies, the BP group showed reduced activation in prefrontal, premotor and parietal regions. On the other hand, behavioral data showed that face repetition doesn't facilitate face recognition in the BP, regardless of gaze direction.
The key discovery of this work is the general reduced amplitude of P200 in BP compared with controls, modulated by gaze direction and WM processing. Previous work has shown that this component is sensitive to negative emotional stimuli and attentional control (Correll et al., 2006), functions that are thought to be impaired in BP (see Green et al., 2007). Our results indeed suggest a primary dysfunction in both attentional control and gaze processing, but also that the two systems may affect each other because the P200 was differentially modulated by gaze direction (see GFP results). Interestingly, the P200 amplitude reduction was particularly pronounced for faces with direct gaze. Perceived direct gaze enhances cognitive processing and brain responses (for a review see, Itier and Batty, 2009, Senju and Johnson, 2009), a processing advantage that has been defined as the eye contact effect (Senju and Johnson, 2009). In this sense, our ERP results suggest that a dysfunctional appreciation of gaze direction is a prominent impairment in bipolar disorder.
Anxiety is highly associated with bipolar disorder (Simon et al., 2004), and several studies have shown that anxiety affects gaze perception (Gamer et al., 2011, Horley et al., 2003, Moukheiber et al., 2010, Schneier et al., 2011), and that the P200 is affected by anxiety responses (Judah et al., 2016). Our results indicate that modulation of the P200 was not affected by anxiety scores. Moreover, our results seem to indicate that anxiety as measured by STAI might not be a central characteristic in our clinical sample.
Stress measures were also investigated as possible confounding variables. However, no differences between groups were found in terms of heart-rate variability and self-reported stress (see Supplementary Appendix).
Interestingly, the analysis of covariance showed that depressive symptoms predict the P200 GFP maximum for repeated faces with direct gaze. This finding is not surprising considering that people who suffer from depression are unable to inhibit neutral materials during WM processing (Gohier et al., 2009) and have rumination thoughts (Donaldson et al., 2007). Our results suggest that direct gaze is particularly salient in mood disorders.
We have also shown that there were no group differences for the N170 evoked responses. This result suggests that the ability to encode the structural properties of faces were preserved in our clinical cohort. Only few ERP studies have been conducted on face processing in BP, and the effects concerning the N170 are controversial (Feuerriegel et al., 2015). Though our patients showed typical N170 responses, the P200 was affected by gaze perception. These results, taken together, may be seen as consistent with the idea that bipolar disorder is associated with atypical processing of the eye region and its gaze rather than the processing of the face per se.
In contrast to the reduced P200 component, BP showed increased P300 amplitude to non-repeated faces with direct and averted gaze. Increased P300 amplitudes have been proposed to be an index of attention allocation to novel and unattended stimuli (Polich, 2007, Polich and Kok, 1995). There is also evidence that unattended face features enhance P300 evoked responses (Campanella et al., 2002, Mueller et al., 2017). Moreover, some studies have shown that perceptual categorization and task difficulty increase the evoked responses of this component (Hagen et al., 2006, Polich and Kok, 1995). In our BP sample, the enhanced P300 amplitudes could indicate that novel faces were perceived as more unusual and also that recognizing novel faces was a demanding task.
Independent of face repetition, in the P300 time window, the TANOVA analysis also revealed a significant Group × Gaze interaction effect. To some extent, this finding also supports the hypothesis of a face-specific deficit at the latency of the P300 in BP.
Another unique contribution made by the current ERP study on bipolar disorder was the examination of the brain sources underlying the ERP components. Given different neuropsychological correlates for P200 and P300 (see above), different networks may be disrupted in BP. For non-repeated faces viewed by BP, P200 source localization revealed decreased current source density in the primary somato-sensory cortex and adjacent parietal regions, the premotor cortex, and the middle frontal gyrus. Largely overlapping networks, including the premotor cortex and parietal regions, were also characterized by showing less current density in BP by repeated faces. The somato-sensory cortex is a region involved in the recognition of affective facial expressions (Adolphs et al., 2000). The somato-sensory response found in BP may thus be suggestive of reduced affective processing of neutral faces. BP may be expected to have an emotional bias in the perception of neutral stimuli (M'bailara et al., 2009, Rich et al., 2006). Gaze reinforces this bias since direct gaze promotes the perception of approach-oriented emotions, and averted gaze induces the perception of avoidance emotions (Adams and Kleck, 2005). Thus, our results suggest that faces were processed differently by BP because of the different emotional somato-sensorial experience of them (for a review see Adolphs, 2002). However, we did not detect any aberrant cognitive interpretation in BP with the current paradigm. Taken together, the EEG data and the behavioral ratings, may suggest functional deficits in the processing of face with neutral expressions, though, not mediated by cognitive mechanisms.
Furthermore, source localization of the P300 showed differential responses between the BP and the controls in the middle frontal gyrus, a region related to high-level executive functions, and regulatory processes (Ochsner and Gross, 2005, Ridderinkhof et al., 2004). Several fMRI studies report reduced medial prefrontal cortex activity in BP compared to healthy subjects (for a review see Strakowski et al., 2012). In our study, the reduced medial frontal activation may be interpreted as a lack of top-down control of attention for face encoding, and this could explain the augmented P300 amplitudes. This assumption may be consistent with evidences that healthy subjects have reduced P300 amplitudes during WM encoding for faces (Morgan et al., 2008, Polich, 2007, Polich and Kok, 1995).
It is also important to note that there were no significant gaze direction effects in the behavioral performance between groups, which could be an indication of compensatory brain mechanism engaged in the BP group. Based on our ERP results and evidence from previous studies (Bertocci et al., 2012, Frangou et al., 2008), it seems likely that a WM task exceeding a certain threshold (i.e. 3, 4…-back WM task) would be needed to augment impairments in BP.
Moreover, the premotor cortex lower current source density in the BP group may indicate deficits in visual spatial attention to neutral faces. WM spatial attention monitoring is associated with enhanced activation in premotor cortex (Owen et al., 2005).
Nevertheless, repeated faces with averted gaze, the most salient condition in our task (highest accuracy), showed less current source density in the patient group in the superior frontal gyrus. This region is also known to be involved in WM spatial attention and maintenance (du Boisgueheneuc et al., 2006), and this result is again consistent with a model that suggests a specific impairment in BP gaze attentional allocation resources and encoding.
What implication do these atypical stages of gaze processing have for our understanding of bipolar disorder? Our environmental experiences influence the development of emotion-regulation strategies (Koulomzin et al., 2002, Morales et al., 2005, Morris et al., 2007). Several behavioral studies have shown dysfunctional parental-infant gaze coordination in caregivers with post-partum depression (Lovejoy et al., 2000) and mood disorders (Lotzin et al., 2016, Lotzin et al., 2015, Tronick and Reck, 2009). In this sense, our early gaze experiences might also be considered an environmental risk factor, that might remain as a vulnerability trait in BP. Few studies have investigated gaze processing patterns in BP (Kim et al., 2009), and as far as we know, no studies have examined the neural correlates of gaze processing in mood disorders. Although we have no information on our patients' relationship with their parents shortly after birth, the present study not only provides biological evidence for abnormal gaze processing in adults with BP, but it may also lead to understand the consequences of early dysfunctional emotional experience and learning in BP.
It is important to note that our data are subject to potential limitations. First, to not affect WM processing and face storing the inter-trial interval was not randomly varied (see Fig. 1). This could have induced anticipatory responses that potentially affected the behavioral and the ERP responses. Second, to examine the extent to which medications affect WM performance and ERP responses, BP would have been compared to themselves; however, our low sample size did not allow such analysis. Effects of medications on our results can thus not be excluded. The small sample size is an eminent problem in clinical studies and replication studies are needed to exclude a selection bias. Third, EEG spatial resolution didn't allow us to investigate deep brain structures which might have been involved in the processing of the stimuli. However, we are confident that the localization precision of HD EEG source imaging for cortical sources allows one to conclude on brain activity differences described in this study. Several studies compared source localization of EEG or evoked potentials with fMRI (Klamer et al., 2015, Lascano et al., 2016, Liu and He, 2008, Plomp et al., 2010), electrocortical stimulation (Lascano et al., 2014), intracranial recordings (Mégevand et al., 2014, Nahum et al., 2011) or postsurgical outcome (Brodbeck et al., 2011, Lascano et al., 2016, Mégevand et al., 2014) and demonstrated high localization accuracy and localization precision in the range of around 15 mm.
5. Conclusion
In conclusion, the present study describes the basic properties of face perception in BP. Our results suggest altered neutral face processing, potentially reinforced by emotional attribution of direct gaze, as a characteristic of bipolar disorder. Moreover, our data suggest that brain attentional control in BP is influenced by rapid and automatic aspects of gaze processing, although very early processing stages seem untouched. However, further evidence on the interaction between ecological properties of face processing, emotional dysregulation, and the symptoms presented in bipolar disorders are still needed.
Acknowledgments
Acknowledgments
The study is supported by the Swiss National Center of Competence in Research; “Synapsy: the Synaptic Basis of Mental Diseases” financed by the Swiss National Science Foundation [grant number 51NF40-158776], as well as a grant of the Swiss National Science Foundation to C.M. [grant number 320030_159705] and to J.M.A. [grant number 32003B_156914]. The Cartool software is freely available academic software that has been programmed by Denis Brunet (http://www.unige.ch/medecine/neuf/en/research/christoph-michel1/cartool-software/) and is supported by the Center for Biomedical Imaging (CIBM) of Geneva and Lausanne. Special thanks go to Samika Kumar for her valuable contribution in editing and reviewing the manuscript, and to Anne-Lise Kung (Psychologist). The authors declare no conflict of interest.
Footnotes
Neutral faces have been ascertained to be neutral for the Radboud Faces Database (Langner et al., 2010), and in the NIMH-chEFS Picture Set (Egger et al., 2011). To examine emotion attribution on the remaining stimuli, an online survey was administered to a group of 23 adults (M age: 34.27, SD: 4.77; 13 females, 10 males). Twenty-five stimuli were presented (22 faces taken from the ADFES, 3 from the database provided by Dr. George). Subjects were asked to classify each face according to six specific emotions: happy, sad, neutral, fear, anger or surprise. For each stimulus, we calculated the proportion of participants who selected each emotion. The overall percentages for each choice were (%): 66.38% for neutral (SD = 0.30), 5.55% for happy (SD = 0.05), 12.04% for sad (SD = 0.10), 3.51% for fear (SD = 0.04), 9.53% for anger (SD = 0.10) and 3.01% for surprise (SD = 0.06). A repeated measures ANOVA with factor of Emotion (6 emotions) revealed a significant effect of Emotion (F(5,110) = 58.15, p < 0.001), and showed that the highest score was attributed to neutral emotion (Bonferroni post hoc analyses: all ps < 0.001).
Supplementary materials accompanying this work can be found in the online version, at http://dx.doi.org/10.1016/j.nicl.2017.09.006.
Contributor Information
Cristina Berchio, Email: Cristina.Berchio@unige.ch.
Camille Piguet, Email: Camille.Piguet@unige.ch.
Christoph M. Michel, Email: Christoph.Michel@unige.ch.
Tonia A. Rihs, Email: Tonia.Rihs@unige.ch.
Alexandre G. Dayer, Email: Alexander.Dayer@unige.ch.
Jean-Michel Aubry, Email: Jean-Michel.Aubry@hcuge.ch.
Appendix A. Supplementary data
Supplementary material
Table 1. Analysis of ERP amplitude
Table 2. Source analysis: direct gaze condition, non-repeated faces.
Table 3. Source analysis: averted gaze condition, non-repeated faces.
Table 4. Source analysis: direct gaze condition, repeated faces.
Table 5. Source analysis: averted gaze condition, repeated faces.
References
- Adams R.B., Kleck R.E. Effects of direct and averted gaze on the perception of facially communicated emotion. Emotion. 2005;5:3–11. doi: 10.1037/1528-3542.5.1.3. [DOI] [PubMed] [Google Scholar]
- Adams R.B., Gordon H.L., Baird A.A., Ambady N., Kleck R.E. Effects of gaze on amygdala sensitivity to anger and fear faces. Science. 2003;300:1536. doi: 10.1126/science.1082244. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Neural systems for recognizing emotion. Curr. Opin. Neurobiol. 2002;12:169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Adolphs R., Damasio H., Tranel D., Cooper G., Damasio A.R. A role for somatosensory cortices in the visual recognition of emotion as revealed by three-dimensional lesion mapping. J. Neurosci. 2000;20:2683–2690. doi: 10.1523/JNEUROSCI.20-07-02683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aktar E., Mandell D.J., de Vente W., Majdandžić M., Raijmakers M.E.J., Bögels S.M. Infants' temperament and mothers', and fathers' depression predict infants' attention to objects paired with emotional faces. J. Abnorm. Child Psychol. 2016;44:975–990. doi: 10.1007/s10802-015-0085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldao A., Nolen-Hoeksema S., Schweizer S. Emotion-regulation strategies across psychopathology: a meta-analytic review. Clin. Psychol. Rev. 2010;30:217–237. doi: 10.1016/j.cpr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . American Psychiatric Association<; Washington DG: 1994. Diagnostic criteria from DSM-IV (No. 616.89 A43) [Google Scholar]
- Barnett J.H., Smoller J.W. The genetics of bipolar disorder. Neuroscience. 2009;164:331–343. doi: 10.1016/j.neuroscience.2009.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentin S., Allison T., Puce A., Perez E., McCarthy G. Electrophysiological studies of face perception in humans. J. Cogn. Neurosci. 1996;8:551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchio C., Rihs T.A., Michel C.M., Brunet D., Apicella F., Muratori F., Gallese V., Umiltà M.A. Parieto-frontal circuits during observation of hidden and visible motor acts in children. A high-density EEG source imaging study. Brain Topogr. 2014;27:258–270. doi: 10.1007/s10548-013-0314-x. [DOI] [PubMed] [Google Scholar]
- Berchio C., Rihs T.A., Piguet C., Dayer A.G., Aubry J.-M., Michel C.M. Early averted gaze processing in the right fusiform gyrus: an EEG source imaging study. Biol. Psychol. 2016;119:156–170. doi: 10.1016/j.biopsycho.2016.06.008. [DOI] [PubMed] [Google Scholar]
- Bertocci M.A., Bebko G.M., Mullin B.C., Langenecker S.A., Ladouceur C.D., Almeida J.R.C., Phillips M.L. Abnormal anterior cingulate cortical activity during emotional n-back task performance distinguishes bipolar from unipolar depressed females. Psychol. Med. 2012;42:1417–1428. doi: 10.1017/S003329171100242X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birot G., Spinelli L., Vulliémoz S., Mégevand P., Brunet D., Seeck M., Michel C.M. Head model and electrical source imaging: a study of 38 epileptic patients. NeuroImage Clin. 2014;5:77–83. doi: 10.1016/j.nicl.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Boisgueheneuc F., Levy R., Volle E., Seassau M., Duffau H., Kinkingnehun S., Samson Y., Zhang S., Dubois B. Functions of the left superior frontal gyrus in humans: a lesion study. Brain. 2006;129:3315–3328. doi: 10.1093/brain/awl244. [DOI] [PubMed] [Google Scholar]
- Brodbeck V., Spinelli L., Lascano A.M., Wissmeier M., Vargas M.-I., Vulliemoz S., Pollo C., Schaller K., Michel C.M., Seeck M. Electroencephalographic source imaging: a prospective study of 152 operated epileptic patients. Brain. 2011;134:2887–2897. doi: 10.1093/brain/awr243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet D., Murray M.M., Michel C.M. Spatiotemporal analysis of multichannel EEG: CARTOOL. Comput. Intell. Neurosci. 2011;2011:813870. doi: 10.1155/2011/813870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella S., Quinet P., Bruyer R., Crommelinck M., Guerit J.-M. Categorical perception of happiness and fear facial expressions: an ERP study. J. Cogn. Neurosci. 2002;14:210–227. doi: 10.1162/089892902317236858. [DOI] [PubMed] [Google Scholar]
- Carretié L., Mercado F., Tapia M., Hinojosa J.A. Emotion, attention, and the “negativity bias”, studied through event-related potentials. Int. J. Psychophysiol. 2001;41:75–85. doi: 10.1016/s0167-8760(00)00195-1. [DOI] [PubMed] [Google Scholar]
- Cohen A.N., Hammen C., Henry R.M., Daley S.E. Effects of stress and social support on recurrence in bipolar disorder. J. Affect. Disord. 2004;82:143–147. doi: 10.1016/j.jad.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Conty L., N'Diaye K., Tijus C., George N. When eye creates the contact! ERP evidence for early dissociation between direct and averted gaze motion processing. Neuropsychologia. 2007;45:3024–3037. doi: 10.1016/j.neuropsychologia.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Correll J., Urland G.R., Ito T.A. Event-related potentials and the decision to shoot: the role of threat perception and cognitive control. J. Exp. Soc. Psychol. 2006;42:120–128. [Google Scholar]
- De Raedt R., Koster E.H.W. Understanding vulnerability for depression from a cognitive neuroscience perspective: a reappraisal of attentional factors and a new conceptual framework. Cogn. Affect. Behav. Neurosci. 2010;10:50–70. doi: 10.3758/CABN.10.1.50. [DOI] [PubMed] [Google Scholar]
- Degabriele R., Lagopoulos J., Malhi G. Neural correlates of emotional face processing in bipolar disorder: an event-related potential study. J. Affect. Disord. 2011;133:212–220. doi: 10.1016/j.jad.2011.03.033. [DOI] [PubMed] [Google Scholar]
- Dienes K.A., Hammen C., Henry R.M., Cohen A.N., Daley S.E. The stress sensitization hypothesis: understanding the course of bipolar disorder. J. Affect. Disord. 2006;95:43–49. doi: 10.1016/j.jad.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Donaldson C., Lam D., Mathews A. Rumination and attention in major depression. Behav. Res. Ther. 2007;45:2664–2678. doi: 10.1016/j.brat.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Egger H.L., Pine D.S., Nelson E., Leibenluft E., Ernst M., Towbin K.E., Angold A. The NIMH Child Emotional Faces Picture Set (NIMH-ChEFS): a new set of children's facial emotion stimuli. Int. J. Methods Psychiatr. Res. 2011;20:145–156. doi: 10.1002/mpr.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etain B., Henry C., Bellivier F., Mathieu F., Leboyer M. Beyond genetics: childhood affective trauma in bipolar disorder. Bipolar Disord. 2008;10:867–876. doi: 10.1111/j.1399-5618.2008.00635.x. [DOI] [PubMed] [Google Scholar]
- Farroni T., Csibra G., Simion F., Johnson M.H. Eye contact detection in humans from birth. Proc. Natl. Acad. Sci. U. S. A. 2002;99:9602–9605. doi: 10.1073/pnas.152159999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre P., Polosan M., Pichat C., Bougerol T., Baciu M. Cerebral correlates of abnormal emotion conflict processing in euthymic bipolar patients: a functional MRI study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0134961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerriegel D., Churches O., Hofmann J., Keage H.A.D. The N170 and face perception in psychiatric and neurological disorders: a systematic review. Clin. Neurophysiol. 2015;126:1141–1158. doi: 10.1016/j.clinph.2014.09.015. [DOI] [PubMed] [Google Scholar]
- Frangou S., Kington J., Raymont V., Shergill S.S. Examining ventral and dorsal prefrontal function in bipolar disorder: a functional magnetic resonance imaging study. Eur. Psychiatry. 2008;23:300–308. doi: 10.1016/j.eurpsy.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Frick P.J., Morris A.S. Temperament and developmental pathways to conduct problems. J. Clin. Child Adolesc. Psychol. 2004;33:54–68. doi: 10.1207/S15374424JCCP3301_6. [DOI] [PubMed] [Google Scholar]
- Gamer M., Hecht H., Seipp N., Hiller W. Who is looking at me? The cone of gaze widens in social phobia. Cogn. Emot. 2011;25:756–764. doi: 10.1080/02699931.2010.503117. [DOI] [PubMed] [Google Scholar]
- George N., Driver J., Dolan R.J. Seen gaze-direction modulates fusiform activity and its coupling with other brain areas during face processing. NeuroImage. 2001;13:1102–1112. doi: 10.1006/nimg.2001.0769. [DOI] [PubMed] [Google Scholar]
- Gohier B., Ferracci L., Surguladze S.A., Lawrence E., El Hage W., Kefi M.Z., Allain P., Garre J.-B., Le Gall D. Cognitive inhibition and working memory in unipolar depression. J. Affect. Disord. 2009;116:100–105. doi: 10.1016/j.jad.2008.10.028. [DOI] [PubMed] [Google Scholar]
- Grave de Peralta Menendez R., Gonzalez Andino S., Lantz G., Michel C.M., Landis T. Noninvasive localization of electromagnetic epileptic activity. I. Method descriptions and simulations. Brain Topogr. 2001;14:131–137. doi: 10.1023/a:1012944913650. [DOI] [PubMed] [Google Scholar]
- Graziano P.A., Keane S.P., Calkins S.D. Maternal behavior and children's early emotion regulation skills differentially predict development of children's reactive control and later effortful control. Infant Child Dev. 2010;19:333–353. doi: 10.1002/icd.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M.J., Cahill C.M., Malhi G.S. The cognitive and neurophysiological basis of emotion dysregulation in bipolar disorder. J. Affect. Disord. 2007;103:29–42. doi: 10.1016/j.jad.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Gross J., Thompson R. Emotion regulation: conceptual foundations. In: Gross J.J., editor. Handbook of Emotion Regulation. Guilford Press; New York: 2007. pp. 3–24. [Google Scholar]
- Hagen G.F., Gatherwright J.R., Lopez B.A., Polich J. P3a from visual stimuli: task difficulty effects. Int. J. Psychophysiol. 2006;59:8–14. doi: 10.1016/j.ijpsycho.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horley K., Williams L.M., Gonsalvez C., Gordon E. Social phobics do not see eye to eye: a visual scanpath study of emotional expression processing. J. Anxiety Disord. 2003;17:33–44. doi: 10.1016/s0887-6185(02)00180-9. [DOI] [PubMed] [Google Scholar]
- Ibanez A., Urquina H., Petroni A., Baez S., Lopez V., do Nascimento M., Herrera E., Guex R., Hurtado E., Blenkmann A., Beltrachini L., Gelormini C., Sigman M., Lischinsky A., Torralva T., Torrente F., Cetkovich M., Manes F. Neural processing of emotional facial and semantic expressions in euthymic bipolar disorder (BD) and its association with theory of mind (ToM) PLoS One. 2012;7 doi: 10.1371/journal.pone.0046877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itier R.J., Batty M. Neural bases of eye and gaze processing: the core of social cognition. Neurosci. Biobehav. Rev. 2009;33:843–863. doi: 10.1016/j.neubiorev.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J., Gotlib I.H. Emotion regulation in depression: relation to cognitive inhibition. Cognition and Emotion. 2010;24(2):281–298. doi: 10.1080/02699930903407948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judah M.R., Grant D.M., Carlisle N.B. The effects of self-focus on attentional biases in social anxiety: an ERP study. Cogn. Affect. Behav. Neurosci. 2016;16:393–405. doi: 10.3758/s13415-015-0398-8. [DOI] [PubMed] [Google Scholar]
- Karniski W., Blair R.C., Snider A.D. An exact statistical method for comparing topographic maps, with any number of subjects and electrodes. Brain Topogr. 1994;6:203–210. doi: 10.1007/BF01187710. [DOI] [PubMed] [Google Scholar]
- Kim E., Ku J., Kim J.-J., Lee H., Han K., Kim S.I., Cho H.-S. Nonverbal social behaviors of patients with bipolar mania during interactions with virtual humans. J. Nerv. Ment. Dis. 2009;197:412–418. doi: 10.1097/NMD.0b013e3181a61c3d. [DOI] [PubMed] [Google Scholar]
- Kim P., Thomas L.A., Rosen B.H., Moscicki A.M., Brotman M.A., Zarate C.A., Blair R.J.R., Pine D.S., Leibenluft E. Differing amygdala responses to facial expressions in children and adults with bipolar disorder. Am. J. Psychiatry. 2012;169:642–649. doi: 10.1176/appi.ajp.2012.11081245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klamer S., Elshahabi A., Lerche H., Braun C., Erb M., Scheffler K., Focke N.K. Differences between MEG and High-density EEG source localizations using a distributed source model in comparison to fMRI. Brain Topogr. 2015;28:87–94. doi: 10.1007/s10548-014-0405-3. [DOI] [PubMed] [Google Scholar]
- Koenig T., Kottlow M., Stein M., Melie-García L. Ragu: a free tool for the analysis of EEG and MEG event-related scalp field data using global randomization statistics. Comput. Intell. Neurosci. 2011;938925 doi: 10.1155/2011/938925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler C.G., Hoffman L.J., Eastman L.B., Healey K., Moberg P.J. Facial emotion perception in depression and bipolar disorder: a quantitative review. Psychiatry Res. 2011;188:303–309. doi: 10.1016/j.psychres.2011.04.019. [DOI] [PubMed] [Google Scholar]
- Koulomzin M., Beebe B., Anderson S., Jaffe J., Feldstein S., Crown C. Infant gaze, head, face and self-touch at 4 months differentiate secure vs. avoidant attachment at 1 year: a microanalytic approach. Attach Hum. Dev. 2002;4:3–24. doi: 10.1080/14616730210123120. [DOI] [PubMed] [Google Scholar]
- Langner O., Dotsch R., Bijlstra G., Wigboldus D.H.J., Hawk S.T., van Knippenberg A. Presentation and validation of the Radboud faces database. Cogn. Emot. 2010;24:1377–1388. [Google Scholar]
- Lascano A.M., Grouiller F., Genetti M., Spinelli L., Seeck M., Schaller K., Michel C.M. Surgically relevant localization of the central sulcus with high-density somatosensory-evoked potentials compared with functional magnetic resonance imaging. Neurosurgery. 2014;74:517–526. doi: 10.1227/NEU.0000000000000298. [DOI] [PubMed] [Google Scholar]
- Lascano A.M., Perneger T., Vulliemoz S., Spinelli L., Garibotto V., Korff C.M., Vargas M.I., Michel C.M., Seeck M. Yield of MRI, high-density electric source imaging (HD-ESI), SPECT and PET in epilepsy surgery candidates. Clin. Neurophysiol. 2016;127:150–155. doi: 10.1016/j.clinph.2015.03.025. [DOI] [PubMed] [Google Scholar]
- Lehmann D., Skrandies W. Reference-free identification of components of checkerboard-evoked multichannel potential fields. Electroencephalogr. Clin. Neurophysiol. 1980;48:609–621. doi: 10.1016/0013-4694(80)90419-8. [DOI] [PubMed] [Google Scholar]
- Liston C., McEwen B.S., Casey B.J. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc. Natl. Acad. Sci. 2009;106:912–917. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., He B. fMRI-EEG integrated cortical source imaging by use of time-variant spatial constraints. NeuroImage. 2008;39:1198–1214. doi: 10.1016/j.neuroimage.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotzin A., Romer G., Schiborr J., Noga B., Schulte-Markwort M., Ramsauer B. Gaze synchrony between mothers with mood disorders and their infants: maternal emotion dysregulation matters. PLoS One. 2015;10 doi: 10.1371/journal.pone.0144417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotzin A., Schiborr J., Barkmann C., Romer G., Ramsauer B. Maternal emotion dysregulation is related to heightened mother-infant synchrony of facial affect. Dev. Psychopathol. 2016;28:327–339. doi: 10.1017/S0954579415000516. [DOI] [PubMed] [Google Scholar]
- Lovejoy M.C., Graczyk P.A., O'Hare E., Neuman G. Maternal depression and parenting behavior: a meta-analytic review. Clin. Psychol. Rev. 2000;20:561–592. doi: 10.1016/s0272-7358(98)00100-7. [DOI] [PubMed] [Google Scholar]
- Luoma I., Puura K., Mäntymaa M., Latva R., Salmelin R., Tamminen T. Fathers' postnatal depressive and anxiety symptoms: an exploration of links with paternal, maternal, infant and family factors. Nord. J. Psychiatry. 2013;67:407–413. doi: 10.3109/08039488.2012.752034. [DOI] [PubMed] [Google Scholar]
- Maris E., Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- M'bailara K., Demotes-Mainard J., Swendsen J., Mathieu F., Leboyer M., Henry C. Emotional hyper-reactivity in normothymic bipolar patients. Bipolar Disord. 2009;11:63–69. doi: 10.1111/j.1399-5618.2008.00656.x. [DOI] [PubMed] [Google Scholar]
- Mégevand P., Spinelli L., Genetti M., Brodbeck V., Momjian S., Schaller K., Michel C.M., Vulliemoz S., Seeck M. Electric source imaging of interictal activity accurately localises the seizure onset zone. J. Neurol. Neurosurg. Psychiatry. 2014;85:38–43. doi: 10.1136/jnnp-2013-305515. [DOI] [PubMed] [Google Scholar]
- Michel C.M. Cambridge University Press; 2009. Electrical Neuroimaging. [Google Scholar]
- Michel C.M., Murray M.M. Towards the utilization of EEG as a brain imaging tool. NeuroImage. 2012;61:371–385. doi: 10.1016/j.neuroimage.2011.12.039. [DOI] [PubMed] [Google Scholar]
- Möller E.L., Majdandžić M., Bögels S.M. Fathers' versus mothers' social referencing signals in relation to infant anxiety and avoidance: a visual cliff experiment. Dev. Sci. 2014;17:1012–1028. doi: 10.1111/desc.12194. [DOI] [PubMed] [Google Scholar]
- Monroe S.M., Harkness K.L. Life stress, the “kindling” hypothesis, and the recurrence of depression: considerations from a life stress perspective. Psychol. Rev. 2005;112:417–445. doi: 10.1037/0033-295X.112.2.417. [DOI] [PubMed] [Google Scholar]
- Morales M., Mundy P., Crowson M.M., Neal A.R., Delgado C.E.F. Individual differences in infant attention skills, joint attention, and emotion regulation behaviour. Int. J. Behav. Dev. 2005;29:259–263. [Google Scholar]
- Morgan H.M., Klein C., Boehm S.G., Shapiro K.L., Linden D.E.J. Working memory load for faces modulates P300, N170, and N250r. J. Cogn. Neurosci. 2008;20:989–1002. doi: 10.1162/jocn.2008.20072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A.S., Silk J.S., Steinberg L., Myers S.S., Robinson L.R. The role of the family context in the development of emotion regulation. Soc. Dev. 2007;16:361–388. doi: 10.1111/j.1467-9507.2007.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moukheiber A., Rautureau G., Perez-Diaz F., Soussignan R., Phanie Dubal S., Jouvent R., Pelissolo A. Gaze avoidance in social phobia: objective measure and correlates. Behav. Res. Ther. 2010;48:147–151. doi: 10.1016/j.brat.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Mueller C.J., Fritsch N., Hofmann M.J., Kuchinke L. Differences in the dynamics of affective and cognitive processing – an ERP study. Brain Res. 2017;1655:41–47. doi: 10.1016/j.brainres.2016.11.018. [DOI] [PubMed] [Google Scholar]
- Murray M.M., Brunet D., Michel C.M. Topographic ERP analyses: a step-by-step tutorial review. Brain Topogr. 2008;20:249–264. doi: 10.1007/s10548-008-0054-5. [DOI] [PubMed] [Google Scholar]
- Nahum L., Gabriel D., Spinelli L., Momjian S., Seeck M., Michel C.M., Schnider A. Rapid consolidation and the human hippocampus: intracranial recordings confirm surface EEG. Hippocampus. 2011;21:689–693. doi: 10.1002/hipo.20819. [DOI] [PubMed] [Google Scholar]
- Nurnberger J.I., Blehar M.C., Kaufmann C.A., York-Cooler C., Simpson S.G., Harkavy-Friedman J., Severe J.B., Malaspina D., Reich T. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH genetics initiative. Arch. Gen. Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Ochsner K., Gross J. The cognitive control of emotion. Trends Cogn. Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Owen A.M., McMillan K.M., Laird A.R., Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti A.M., Ellis J., Wegbreit E., Stevens M.C., Pavuluri M.N. Reduced functional connectivity of prefrontal regions and amygdala within affect and working memory networks in pediatric bipolar disorder. Brain Connect. 2012;2:320–334. doi: 10.1089/brain.2012.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri M.N., Passarotti A.M., Harral E.M., Sweeney J.A. Enhanced prefrontal function with pharmacotherapy on a response inhibition task in adolescent bipolar disorder. J. Clin. Psychiatry. 2010;71:1526–1534. doi: 10.4088/JCP.09m05504yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.L., Ladouceur C.D., Drevets W.C. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol. Psychiatry. 2008;13(829):833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomp G., Michel C.M., Herzog M.H. Electrical source dynamics in three functional localizer paradigms. NeuroImage. 2010;53:257–267. doi: 10.1016/j.neuroimage.2010.06.037. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin. Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J., Kok A. Cognitive and biological determinants of P300: an integrative review. Biol. Psychol. 1995;41:103–146. doi: 10.1016/0301-0511(95)05130-9. [DOI] [PubMed] [Google Scholar]
- Rich B.A., Vinton D.T., Roberson-Nay R., Hommer R.E., Berghorst L.H., McClure E.B., Fromm S.J., Pine D.S., Leibenluft E. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc. Natl. Acad. Sci. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof K.R., Ullsperger M., Crone E.A., Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rimmele U., Lobmaier J.S. Stress increases the feeling of being looked at. Psychoneuroendocrinology. 2012;37:292–298. doi: 10.1016/j.psyneuen.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Sandi C., Haller J. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat. Rev. Neurosci. 2015;16:290–304. doi: 10.1038/nrn3918. [DOI] [PubMed] [Google Scholar]
- van der Schalk J., Hawk S.T., Fischer A.H., Doosje B. Moving faces, looking places: validation of the Amsterdam Dynamic Facial Expression Set (ADFES) Emotion. 2011;11:907–920. doi: 10.1037/a0023853. [DOI] [PubMed] [Google Scholar]
- Schmitz J., Scheel C.N., Rigon A., Gross J.J., Blechert J. You don't like me, do you? Enhanced ERP responses to averted eye gaze in social anxiety. Biol. Psychol. 2012;91:263–269. doi: 10.1016/j.biopsycho.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Schneier F.R., Rodebaugh T.L., Blanco C., Lewin H., Liebowitz M.R. Fear and avoidance of eye contact in social anxiety disorder. Compr. Psychiatry. 2011;52:81–87. doi: 10.1016/j.comppsych.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze L., Renneberg B., Lobmaier J.S. Gaze perception in social anxiety and social anxiety disorder. Front. Hum. Neurosci. 2013;7:872. doi: 10.3389/fnhum.2013.00872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutter D.J.L.G., de Haan E.H.F., van Honk J. Functionally dissociated aspects in anterior and posterior electrocortical processing of facial threat. Int. J. Psychophysiol. 2004;53:29–36. doi: 10.1016/j.ijpsycho.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Senju A., Johnson M.H. The eye contact effect: mechanisms and development. Trends Cogn. Sci. 2009;13:127–134. doi: 10.1016/j.tics.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Simon N.M., Otto M.W., Wisniewski S.R., Fossey M., Sagduyu K., Frank E., Sachs G.S., Nierenberg A.A., Thase M.E., Pollack M.H. Anxiety disorder comorbidity in bipolar disorder patients: data from the first 500 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Am. J. Psychiatry. 2004;161:2222–2229. doi: 10.1176/appi.ajp.161.12.2222. [DOI] [PubMed] [Google Scholar]
- Skrandies W. Global field power and topographic similarity. Brain Topogr. 1990;3:137–141. doi: 10.1007/BF01128870. [DOI] [PubMed] [Google Scholar]
- Sokhadze E.M., Tasman A., Tamas R., El-Mallakh R.S. Event-related potential study of the effects of emotional facial expressions on task performance in euthymic bipolar patients. Appl. Psychophysiol. Biofeedback. 2011;36:1–13. doi: 10.1007/s10484-010-9140-z. [DOI] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R.E. 1970. Manual for the State-Trait Anxiety Inventory. [Google Scholar]
- Spinelli L., Andino S.G., Lantz G., Seeck M., Michel C.M. Electromagnetic inverse solutions in anatomically constrained spherical head models. Brain Topogr. 2000;13:115–125. doi: 10.1023/a:1026607118642. [DOI] [PubMed] [Google Scholar]
- Srebro R. A bootstrap method to compare the shapes of two scalp fields. Electroencephalogr. Clin. Neurophysiol. 1996;100:25–32. doi: 10.1016/0168-5597(95)00205-7. [DOI] [PubMed] [Google Scholar]
- Stern D.N. Wiley-Interscience; Oxford, England: 1974. Mother and Infant at Play: The Dyadic Interaction Involving Facial, Vocal, and Gaze Behaviors. [Google Scholar]
- Strakowski S.M., Adler C.M., Almeida J., Altshuler L.L., Blumberg H.P., Chang K.D., DelBello M.P., Frangou S., McIntosh A., Phillips M.L., Sussman J.E., Townsend J.D. The functional neuroanatomy of bipolar disorder: a consensus model. Bipolar Disord. 2012;14:313–325. doi: 10.1111/j.1399-5618.2012.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surguladze S.A., Marshall N., Schulze K., Hall M.-H., Walshe M., Bramon E., Phillips M.L., Murray R.M., McDonald C. Exaggerated neural response to emotional faces in patients with bipolar disorder and their first-degree relatives. NeuroImage. 2010;53:58–64. doi: 10.1016/j.neuroimage.2010.05.069. [DOI] [PubMed] [Google Scholar]
- Tronick E., Reck C. Infants of depressed mothers. Harv. Rev. Psychiatry. 2009;17:147–156. doi: 10.1080/10673220902899714. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vaughan H.G. The neural origins of human event-related potentials. Ann. N. Y. Acad. Sci. 1982;388:125–138. doi: 10.1111/j.1749-6632.1982.tb50788.x. [DOI] [PubMed] [Google Scholar]
- Vizueta N., Rudie J.D., Townsend J.D., Torrisi S., Moody T.D., Bookheimer S.Y., Altshuler L.L. Regional fMRI hypoactivation and altered functional connectivity during emotion processing in nonmedicated depressed patients with bipolar II disorder. Am. J. Psychiatry. 2012;169:831–840. doi: 10.1176/appi.ajp.2012.11030349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. 1981. WAIS-R Manual: Wechsler Adult Intelligence Scale-Revised. [Google Scholar]
- Wynn J.K., Jahshan C., Altshuler L.L., Glahn D.C., Green M.F. Event-related potential examination of facial affect processing in bipolar disorder and schizophrenia. Psychol. Med. 2013;43:109–117. doi: 10.1017/S0033291712001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R.C., Biggs J.T., Ziegler V.E., Meyer D.A. A rating scale for mania: reliability, validity and sensitivity. Br. J. Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Table 1. Analysis of ERP amplitude
Table 2. Source analysis: direct gaze condition, non-repeated faces.
Table 3. Source analysis: averted gaze condition, non-repeated faces.
Table 4. Source analysis: direct gaze condition, repeated faces.
Table 5. Source analysis: averted gaze condition, repeated faces.