Abstract
Phlebotomus perniciosus is one of the major vectors of Leishmania infantum in the Mediterranean basin. The aim of this work was (i) to provide information about abundance and temporal dynamics of this Larroussius species in a hot spot area of visceral leishmaniasis in Tunisia, (ii) to detect L. infantum DNA in wild caught female sandflies and (iii) to measure Phlebotomus perniciosus infection rate throughout the active season. Sandflies were collected monthly during one year using CDC miniature light-traps in house and in animal shelters. Male specimens were identified at species level according to morphological characters. Female specimens were conserved individually for molecular study. Leishmania infection was tested by kinetoplast DNA real-time PCR and ITS-1 PCR-sequencing. Subsequent sandfly species identification of infected specimens was done by mitochondrial cytochrome b sequencing. In one year period, overall 4,441 specimens (2230 males and 2211 females) were collected. Sandfly activity started in end-April and ended in early-November. Mean sandfly density in house was significantly lower than in animal shelters (51 ± 50 versus 504 ± 460 sandflies /CDC night, p<0.05). However, a higher proportion of females was found in house (58.4% versus 49.2%, p<0.001). Based on species identification of male specimens, Phlebotomus perniciosus was the dominant species (56% of the whole male sandfly fauna, p<0.0001). It showed two peaks of density in the active season, a sharp one in early May and a higher long lasting one from end-July to end-September. DNA was extracted from 190 female specimens randomly sampled and corresponding to 96 specimens from house and 94 from animal shelters. Twenty four female sandfly were infected by Leishmania infantum. All infected specimens were recognized as Phlebotomus perniciosus. Leishmania infantum infection rate in female sandflies was 2.3 fold higher in house than in animal shelters (17.7% versus 7.4%, p<0.05). In house, estimated number of infected specimens was the highest at the end of the active season. Abundance, dynamics of density and Leishmania infantum infection prevalence of Phlebotomus perniciosus in Tunisian hot spot of visceral leishmaniasis highlight the major role of this Phlebotominae species in L. infantum transmission.
Introduction
Zoonotic visceral leishmaniasis (VL) caused by Leishmania (L.) infantum is endemic in almost all countries of the Mediterranean basin [1]. The parasite is transmitted by the bite of infected female sandflies belonging to the sub-genus Larroussius and Phlebotomus (P.) perniciosus is one of the major vectors of L. infantum in the Mediterranean [2–4]. Reservoir hosts are mainly represented by dogs. However, other animals as wild canids, rabbits or hares were also incriminated in L. infantum transmision [4–7].
In Tunisia, VL remains primarily a pediatric disease that occurs in children less than five years of age [8]. An incidence rate of about 10 VL cases/100,000 children per year is reported for the whole country [9]. However, this incidence rate varies according to geographical area with presence of hot spot region in the Northern part of the country where the disease is homogeneously observed with high frequency [9]. In this interesting location, common ecologic characteristics may have led to uniform high level of disease prevalence.
In his various studies, Rioux conceptualized how ecological and epidemiological concepts and methods could be combined to argument Leishmania transmission [10]. Bioclimatic maps were considered as indicators of ecological conditions and were the elective support to identify vector areas and to refer transmission systems to spatial scales [10]. In previous study, we have shown that Tunisian VL hot spot area is located in the semi-arid bioclimatic zone with warm winters and semi-continental climate [9]. We have also reported that P. perniciosus is the most abundant Larroussius species in human leishmaniasis sites located in semi-arid bioclimate [11]. The aim of this work was (i) to provide information about abundance and temporal dynamics of Larroussius species in anthropic biotopes of VL hot spot region (ii) to detect Leishmania DNA in wild caught female sandflies and (iii) to measure L. infantum infection rate throughout the active season.
Materials and methods
Study area
The field study was performed in Eastern-North Tunisia, in Zagouan governorate, on the “dorsale” upland (Fig 1A). The region is locatedin a hot spot area of VL where weather conditions are those typical of the Mediterranean semi-arid climate characterized by typical vegetation series of Pinus halpensis and Tetraclinus articulata. The sampling station was El khadhra (36°11’05.80”N / 10°02’57.14”E) (Fig 1A). It is a rural locality characterized by the presence of a variety of domestic animals that are potential sandfly hosts with close contact with humans. Animals were either kept in semi-open air shelters built with small tree trunks (mostly goats and sheep) (Fig 1B and 1C) or kept free in at most radius of 100 meters between shelters and houses (mainly dogs, chickens and cats). The study was carried out on private land. Land’s owner gave permission to conduct the study on the site.
Fig 1. Geographical situation and landscape of sample collection sites.
A: The map of Tunisia shows the governorate of Zaghouan (shaded grey) with a focus on the study site location ("El Khadhra"). B and C: two photographs show the house with close semi-open air shelters where are kept animals (mainly sheep). CDC traps were set in house and in animal shelters.
Entomological survey
Sandflies were collected using CDC miniature light traps (John W. Hock Co. FL, U.S.A.) each month, from 26 June 2010 to 12 June 2011 (one year). Two CDC were set up, one inside house (IH) and one in animal shelters (AS) and were operated between 6:00 pm and 08:00 am for one night/month. Over one year, a total of 26 CDC were set up during 13 nights of trapping. No specific permission was required for these activities. The field study did not involve endangered or protected species. Captured specimens were conserved in ethanol 70°. Male (M) specimens were identified at species level according to morphological characters described by Croset et al. (1978) [12]. Female (F) specimens were conserved individually for study of Leishmania infection.
Study of Leishmania infection
Study of Leishmania infection concerned randomly sampled female specimens collected from June to October either in house or animal shelters. Whole bodies of female sandflies were washed twice with distilled water before DNA extraction. DNA was extracted using the method described by Ready et al., 1991 [13] with minor modifications. DNA was eluted in 50 μL TE and stored at -20°C until use.
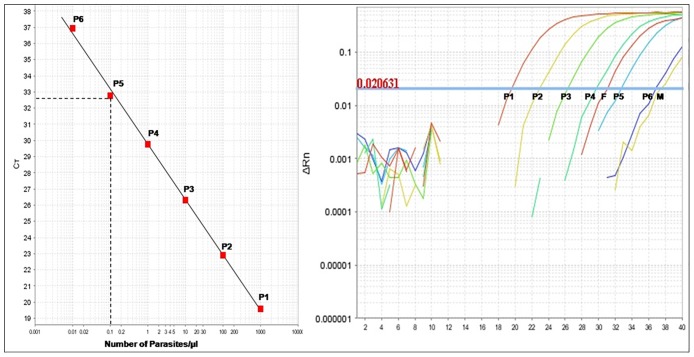
All DNA samples were screened for Leishmania infection by kinetoplast DNA (kDNA) real-time PCR (qPCR) [14]. Briefly, qPCR was conducted in a final volume of 25 μL by using a TaqMan universal master mixture (Applied Biosystems, USA) containing 100mM of each primer (5’-CTTTTCTGGTCCTCCGGGTAGG-3’) and (5’-CCACCCGGCCCTATTTTACACCAA-3’), 50Mm of probe (FAM 5’-TTTTCGCAGAACGCCCCTACCCGC-3’) and 1 μL of DNA extract. The DNA was amplified in an Applied Biosystems® (Applied Biosystems, Foster City, CA) for 40 cycles at 95 and 60°C. Each sample was tested in duplicate, and each run included both positive and negative controls. The sensitivity of the qPCR reaction was tested by using serial dilutions of parasite DNA extracted from a known number of parasites. Detection of the kinetoplast DNA of L. infantum reached the level of 0.01 parasites per reaction tube with a dynamic range of 105 (Fig 2A). Real time PCR was considered positive for Leishmania when the threshold cycle (Ct) was ≤ to 32.8 which corresponded to 0.1 parasite per reaction tube. Taking into account the amount of biological sample (1 μl of sample DNA) and the elution volume of the extracted DNA (50 μl), selected qPCR threshold corresponded to 5 parasites DNA per sandfly specimen. DNA from 10 male specimens were tested for amplification. Their Ct values were > 36 (Fig 2B). All female specimens that showed a Ct ≤ 32.8 were subsequently assayed by ITS1 PCR.
Fig 2. Leishmania identification by kDNA real time PCR.
A: Standard curve obtained from serial dilutions of Leishmania DNA expressed as the number of parasites per reaction tube. The standard curve was established from Leishmania DNA extracted from 106 L. infantum promastigotes. One μl of serial dilutions, ranging from 1000 (P1) to 0.01 parasites (P6) was introduced into reaction tubes. P5 (0.01 parasites per μl) showed a Ct = 32.8. B: Real time PCR amplification curves showing qPCR positive sandfly female specimen (F) as well as negative sandfly male specimen (M). Amplification plots obtained from the serial dilution of Leishmania DNA (P1 to P6) are also shown.
All positive kDNA qPCR specimens were systematically amplified by ITS1-PCR as previously described [15] using LITSR (5′-CTGGATCATTTTCCGATG-3′) and L5.8S (5′-TGATACCACTTATCGCACTTA-3′) primers. Analysis on a 2% agarose gel was used to verify the amplified product size. ITS1 PCR products were purified by using ExoSAP (ThermoScientific, EU) and Leishmania species identification was done by DNA sequencing using an ABI Prism® Big Dye™ Terminator, Cycle Sequencing Ready Reaction Kit and AB1 3130 sequencing system (ABI, PE Applied Biosystems), with the same primers used for PCR. DNA sequences from both strands were aligned and edited using Staden software package (http://staden.sourceforge.net/). MEGA version 7 software (www.megasoftware.net) was used to conduct multiple sequence alignments (ClustalW option) and to construct phylogenic tree. Relationships between specimens and reference isolates (L. infantum isolates from Mediterranean countries as well as Tunisian isolates of L. tropica and L. major) were inferred based on genetic distances using the Neighbor Joining (NJ) method using the Kimura 2 parameters model. Statistical support for tree distances was evaluated using bootstrapping (2000 replicates).
Sandfly molecular typing
Positive female specimens were molecularly typed based on the cytochrome b method. Amplicons were generated by PCR using (5′-CAT/CATTCAA CCA/TGAATGATA-3′) and N1N-PDR (5′-GGTAC/TA/TTTGCCTCGAT/ATTCG T/ATATGA-3′) primers [16]. PCR products were purified and cycle-sequenced as described previously. DNA sequences from both strands were aligned and edited using Staden software package (http://staden.sourceforge.net/). MEGA version 7 software (www.megasoftware.net) was used to conduct multiple sequence alignments (ClustalW option) and to construct phylogenic tree. Sequences were aligned with Phlebotomus perniciosus sequences already obtained from Tunisia and other countries [17–19]. Neighbor Joining (NJ) tree was performed using the Kimura 2 parameters model with homologous sequences from Phlebotomus longicuspis as the least ambiguous out-group. Statistical support for tree distances was evaluated using bootstrapping (2000 replicates).
Statistical analysis
Statistical analysis was done using the MedCalc Statistical software (version 11.4.4.0). The t-test was used to compare mean sandfly densities. Chi-squared test was used for comparison of proportions. A test was considered significant if p-value was less than 0.05.
Results
Sandfly fauna and seasonal dynamics of male specimens
In one year period, overall 4,441 specimens (2230 males and 2211 females) were collected. Sandfly activity started in end-April and ended in early-November with a mean density of 278 sandflies by CDC night in the active season. Throughout this period mean density in AS (504 ± 460 sandflies / CDC night) was significantly higher than IH (51 ± 50 sandflies / CDC night) (p<0.05). Furthermore, sex-ratio of IH sandflies (0.79) was different from sex-ratio of AS specimens (1.03) with a significant higher proportion of females in house than in animal shelters (58.4% versus 49.2%, p = 0.0001) (S1 Table).
Based on species identification of male specimens, P. perniciosus was the dominant species corresponding to 56% of the whole male sandfly fauna (S1 Table). It was significantly more abundant than other Phlebotominae species namely P. papatasi (32,8%), Sergentomyia fallax (3,3%), Sergentomyia minuta (2,7%), P. longicuspis (2,6%), P. antennata (2.2%) and P. perfiliewi (0.3%) (p<0.0001).
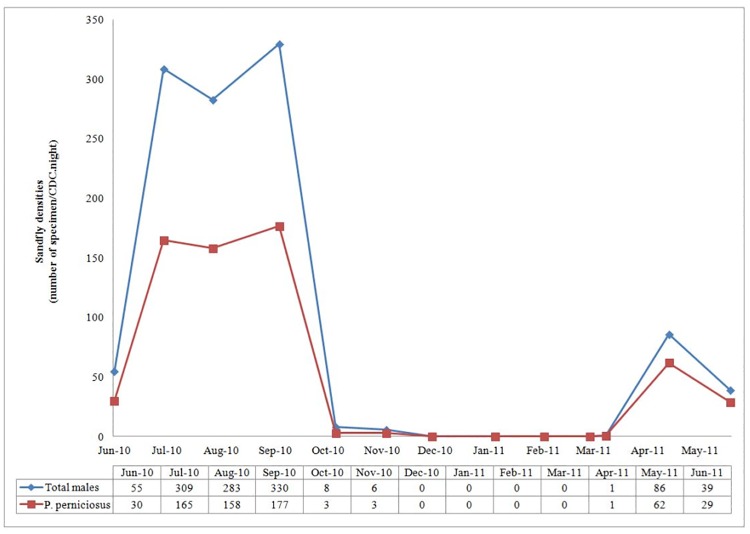
Seasonal density patterns of male specimens were reported in Fig 3. The whole male sandfly fauna as well as the male P. perniciosus population showed 2 peaks: a sharp one in early May and a higher long lasting one from end-July to end-September (Fig 3).
Fig 3. Densities of total sandfly and Phlebotomus perniciosus male specimens according to period of capture.
Leishmania infection in phlebotomine sandfly female specimens
From end-June to end-October, 1715 female specimens (208 from IH and 1507 from AS) were collected and stored individually. According to the period of capture, a dozen to thirty specimens from each monthly IH and AS collection were randomly sampled for DNA extraction (Table 1).
Table 1. Leishmania infection in phlebotomine female specimens.
| Number of female specimens caught in house | Number female specimens caught in animal shelters | |||||
|---|---|---|---|---|---|---|
| Number of collected specimens | Number of DNA extraction | Number of positive ITS1 PCR (infection rate) | Number of collected specimens | Number of DNA extraction | Number of positive ITS1 PCR (infection rate) | |
| June 26th | 47 | 23 | 5 (21.7%) | 183 | 12 | 1 (8.3%) |
| July 24th | 20 | 8 | 1 (12.5%) | 349 | 12 | 1 (8.3%) |
| August 21th | 33 | 33 | 6 (18.2%) | 199 | 30 | 5 (16.6%) |
| September 28th | 101 | 25 | 5 (20%) | 713 | 20 | 0 (0%) |
| October 30th | 7 | 7 | 0 (0%) | 63 | 20 | 0 (0%) |
| Total | 208 | 96 | 17 (17.7%) | 1507 | 94 | 7 (7.4%) |
Among the 190 analyzed specimens, 28 were positive by kDNA qPCR. Among them, 24 (12.6%) were confirmed by ITS1-PCR and were considered for result analysis (Table 1). ITS1 amplification showed a band of expected size (360 bp). Purified PCR products from the 24 specimens corresponded to one unique sequence identified by inspection of the input data matrix and deposited into GenBank database (http://www.ncbi.nlm.nih.gov/) under the accession numbers MF597933 and MF597934. Neighbor Joining analyses positioned our specimens' sequences in the same cluster than L. infantum sequences reported by other authors (Fig 4). Leishmania infantum was retained as the Leishmania species infecting female sandflies in the study site.
Fig 4. Neighbor-joining tree based on 305 aligned base pairs of the ITS1gene.
The sequences from sandflies collected during this study (GenBank accession numbers MF597933 and MF597934) were compared to L. infantum, L. tropica and L. major ITS1 sequences available in GenBank. The percent bootstrap values are indicated on the branches.
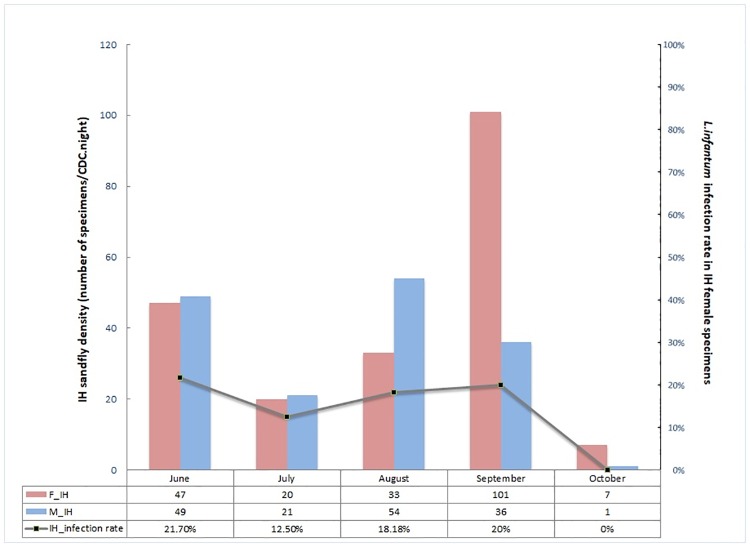
Leishmania infantum infection rate in female sandflies was 2.3 fold higher IH than in AS (17.7% versus 7.4%, p<0.05) (Table 1). Fig 5 shows how varied in house the percentage of infected females as well as the density of collected male and female specimens during the period June-October. Male density showed a drastic decrease in July followed by a marked and rapid increase during august. Female density also showed a drastic decrease in July. However, density of female sandfly increased more progressively reaching a pick at the end of September. Percentage of female infection was around 20% in June, dropped to 12.5% in July, increased slowly to about 20% in august and remained steady until late September. Thus, density of female sandfly as well as the estimated number of infected specimens was the highest at the end of the active season. During October, both female density and infection rate decreased significantly (Table 1, Fig 5). Percentage of infected females in animal sheds according to period of sampling is reported in Table 1. The drop of positive sandflies in AS in September should be interpreted with caution given the limited number of specimens explored for infection.
Fig 5. In house male and female sandfly densities and percentage of infected females according to period of capture.
Sandfly molecular typing
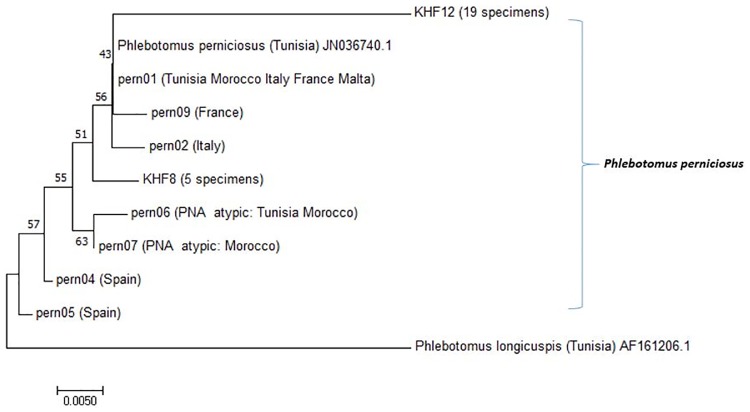
For the 24 positive female sandflies infected by L. infantum, the last 279 nucleotides of Cytochrome b (Cyt b) were sequenced. Two unique sequences (haplotypes KHF8 and KHF12) were identified by inspection of the input data matrix and deposited into GenBank database (http://www.ncbi.nlm.nih.gov/). KHF8 haplotype (accession number MF682976) was identified in 5 specimens. Whereas haplotype KHF12 (accession number MF682977) was shown in 19 specimens. Neighbor joining analyses showed that the two sandfly haplotypes fell in the P. perniciosus branch. KHF12 which was the predominant haplotype in our study fell in the P. perniciosus branch that included haplotype pern01 whereas the second haplotye KHF8 defined a new divergent branch (Fig 6). According to place of capture, the 5 infected specimens caught in house in June belonged to the same P. perniciosus haplotype-KHF8 whereas all positive specimens identified in house since July belonged to the same haplotype-KHF12. In AS, all infected specimens were identified as P. perniciosus haplotype- KHF12.
Fig 6. Neighbor-joining tree based on 279 aligned base pairs of the cytochrome b mitochondrial DNA (mtDNA).
The sequences from sandflies collected during this study (KHF8 and KHF12) were compared to Mediterranean haplotypes of P. perniciosus and P. longicuspis available in GenBank. The percent bootstrap values are indicated on the branches.
Discussion
Sandflies were monthly collected in anthroponized sites favorable to L. infantum transmission, where vertebrate hosts including dogs were in close contact to humans. CDC miniature light-traps were used for sandfly collection. They are known to catch active sandflies and then they are useful to determine temporal activity of species [20]. Moreover, they allow to capture phototropic species as P. perniciosus [21,22].
Seasonal sandfly activity in the study region began in late April and extended to early November. This is in concordance with other entomological studies performed in temperate zones of Mediterranean region, [2,23–25]. In the study site, sandfly density seemed strongly influenced by host abundance and availability [20]. In fact, it was significantly higher in AS then in IH. Moreover, predominance of males in AS may indicate that it can be considered as a sandfly breeding site [26]. Indeed, organic matter produced by domestic animals constitutes suitable conditions for sandfly development [27]. Significant predominance of females in IH may be explained either by their important capacity to disperse [28,29] or by the endophilic behavior of several species present in the site and preferring more closed habitat as houses [30]. Seven species out of the 16 recorded in Tunisia [12,31] were collected. Phlebotomus perniciosus was the dominant species as previously described in semi-arid bioclimatic zone [11]. Moreover, this species is known by its ecological plasticity which allows colonization of domestic environment [32].
Phenology of P. perniciosus showed a typical bimodal distribution as described by previous studies in Tunisia and North Africa [33–35]. The first peak, in early May, is formed by spring generation that emerged from larvae who spent winter in diapauses. Whereas the second long-lasting pick (end-July to end-September) may correspond to more than one wave of emergence in summer [12,34]. On the other hand, P. perniciosus peaked earlier than described in the same bioclimatic zone [34]. However, surveying population abundance during only one year don’t give reliable picture of abundance patterns of sandfly species and shift of activity can be explained by local climatic events during capturing days [2].
Currently, molecular methods are extensively used to study Leishmania infection in wild-caught sandflies [36–39]. kDNA-real time PCR is known to be highly sensitive [40,41] whereas the ITS1 sequencing allows Leishmania species identification with high specificity [42]. Using these techniques, we found that P. perniciosus, the main vector of L. infantum in Mediterranean basin, was the only infected species by L. infantum [37,43,44]. Infected P. perniciosus specimens were already reported in Tunisia [45]. However, L. infantum transmission in Tunisian foci may involve other Larroussius species according to bioclimatic position of the endemic area [46,47].
In the study site, percentage of infected sandfly females was relatively high (12.6%). However, our results correspond to a sandfly collection in very favorable conditions of L. infantum transmission. In fact, sandflies were caught in a semi-arid bioclimatic zone in peri-domiciliary environment where both P. perniciosus (the major L. infantum vector) and dogs (main reservoir hosts) were abundant. In this context, endophilic behavior of P. perniciosus [30] associated to its high infection rate inside the house support the risk of transmission to human population and may explain the high VL incidence rate in the area.
In house, two picks of sandfly infection were observed, in June and August-September respectively. Each pick of infection concerned a specific P. perniciosus haplotype and may be linked to a different wave of adult sandfly emergence. In fact, decrease of infection rate observed during July was concomitant to a drop of both male and female sandfly abundance during the same period and was followed by a sharp increase of sandfly density during august suggesting a new wave of emergence (Croset et al, 1970). Furthermore, density of female sandfly as well as the estimated number of infected specimens was the highest at the end of the active season. During this period, females are more likely to be infectious [48].
Conclusions
In VL hot spot region, P. perniciosus the main vector of VL in Mediterraneen region was the dominant species. Its population dynamics, endophilic behavior and high infection rate inside houses especially at the end of active season support the risk of transmission to human population. More investigations, especially on reservoir hosts is needed to better document the high VL incidence rate in the area.
Supporting information
(XLSX)
Acknowledgments
We would like to thank Marwa WESLATI (PhD student) and Manel ZERZERI (technician) for their kind contribution to the technical aspect of this work.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the Ministry of Higher Education and Scientific Research, Tunisia in the setting of the Research Laboratory Medical Parasitology, Biotechnologies and Biomolecules (PMBB), Institut Pasteur of Tunis, Tunisia (LR11-IPT06). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Desjeux P. Leishmaniasis: current situation and new perspectives. Comparative Immunology, Microbiology and Infectious Diseases. 2004;27(5):305–18. doi: 10.1016/j.cimid.2004.03.004 [DOI] [PubMed] [Google Scholar]
- 2.Alten B, Maia C, Afonso MO, Campino L, Jiménez M, González E, et al. Seasonal dynamics of phlebotomine sand fly species proven vectors of Mediterranean leishmaniasis caused by Leishmania infantum. PLoS Negl Trop Dis. 2016;10(2):e0004458 doi: 10.1371/journal.pntd.0004458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Killick-Kendrick R. Phlebotomine vectors of the leishmaniasis: a review. Medical and veterinary entomology. 1990;4(1):1–24. [DOI] [PubMed] [Google Scholar]
- 4.Gramiccia M, Gradoni L. The current status of zoonotic leishmaniases and approaches to disease control. Int J Parasitol. 2005; 35: 1169–1180. doi: 10.1016/j.ijpara.2005.07.001 [DOI] [PubMed] [Google Scholar]
- 5.Karayiannis S, Ntais P, Messaritakis I, Tsirigotakis N, Dokianakis E, Antoniou M et al. Detection of Leishmania Infantum in red foxes (Vulpes vulpes) in Central Greece. Parasitology. 2015; 142: 1574–1578. doi: 10.1017/S0031182015001158 [DOI] [PubMed] [Google Scholar]
- 6.Garcia N, Moreno I, Alvarez J, de la Cruz ML, Navarro A, Perez-Sancho M et al. Evidence of Leishmania infantum infection in rabbits (Oryctolagus cuniculus) in a natural area in Madrid, Spain. Biomed Res Int. 2014: 318254 doi: 10.1155/2014/318254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carrillo E, Moreno J, Cruz I. What is responsible for a large and unusual outbreak of leishmaniasis in Madrid? Trends Parasitol. 2013; 29: 579–580. doi: 10.1016/j.pt.2013.10.007 [DOI] [PubMed] [Google Scholar]
- 8.Aoun K, Jeddi F, Amri F, Ghrab J, Bouratbine A. Current epidemiological data on visceral leishmaniasis in Tunisia. Medecine et maladies infectieuses. 2009;39(10):775–9. doi: 10.1016/j.medmal.2009.08.010 [DOI] [PubMed] [Google Scholar]
- 9.Ben-Ahmed K, Aoun K, Jeddi F, Ghrab J, El-Aroui M-A, Bouratbine A. Visceral leishmaniasis in Tunisia: spatial distribution and association with climatic factors. The American journal of tropical medicine and hygiene. 2009;81(1):40–5. [PubMed] [Google Scholar]
- 10.Rioux J, Lanotte G, Serres E, Pratlong F, Bastien P, Perieres J. Taxonomy of Leishmania. Use of isoenzymes. Suggestions for a new classification. Ann Parasitol Hum Comp. 1990;65(3):111–25. doi: 10.1051/parasite/1990653111 [DOI] [PubMed] [Google Scholar]
- 11.Ghrab J, Rhim A, Bach-Hamba D, Chahed M, Aoun K, Nouira S, et al. Phlebotominae (Diptera: Psychodidae) of human leishmaniosis sites in Tunisia. Parasite. 2006;13(1):23–33. doi: 10.1051/parasite/2006131023 [DOI] [PubMed] [Google Scholar]
- 12.Croset H, Rioux J, Maistre M, Bayar N. phlébotomes de Tunisie (Diptera, Phlebotomidae). Mise au point systematique, chorologique et éthologique. Annales de parasitologie humaine et comparée. 1978. [PubMed] [Google Scholar]
- 13.Ready P, Lainson R, Shaw J, Souza A. DNA probes for distinguishing Psychodopygus wellcomei from Psychodopygus complexus (Diptera: Psychodidae). Memorias do Instituto Oswaldo Cruz. 1991;86(1):41–9. [DOI] [PubMed] [Google Scholar]
- 14.Mary C, Faraut F, Lascombe L, Dumon H. Quantification of Leishmania infantum DNA by a real-time PCR assay with high sensitivity. Journal of Clinical Microbiology. 2004;42(11):5249–55. doi: 10.1128/JCM.42.11.5249-5255.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schönian G, Nasereddin A, Dinse N, Schweynoch C, Schallig HD, Presber W, et al. PCR diagnosis and characterization of Leishmania in local and imported clinical samples. Diagnostic microbiology and infectious disease. 2003;47(1):349–58. [DOI] [PubMed] [Google Scholar]
- 16.Esseghir S, Ready P, Killick-Kendrick R, Ben-Ismail R. Mitochondrial haplotypes and phylogeography of Phlebotomus vectors of Leishmania major. Insect molecular biology. 1997;6(3):211–25. [DOI] [PubMed] [Google Scholar]
- 17.Pesson B, Ready JS, Benabdennbi I, Martin-Sanchez J, Esseghir S, Cadi-Soussi M et al. Sandflies of the Phlebotomus perniciosus complex: mitochondrial introgression and a new sibling species of P. longicuspis in the Moroccan Rif. Med Vet Entomol. 2004; 18: 25–37. [DOI] [PubMed] [Google Scholar]
- 18.Peyrefitte CN, Grandadam M, Bessaud M, Andry PE, Fouque F, Caro V et al. Diversity of Phlebotomus perniciosus in Provence, southeastern France: Detection of two putative new phlebovirus sequences. Vector Borne Zoonotic Dis. 2013; 13: 630–636. doi: 10.1089/vbz.2012.1169 [DOI] [PubMed] [Google Scholar]
- 19.Boudabous R, Jaouadi K, Bounamous A, Babba H. Morphological and molecular investigations of population structure of Phlebotomus perniciosus and Phlebotomus longicuspis (Diptera: Psychodidae) in Tunisia. J Med Entomol. 2012; 49: 787–793. [DOI] [PubMed] [Google Scholar]
- 20.Prudhomme J, Rahola N, Toty C, Cassan C, Roiz D, Vergnes B, et al. Ecology and spatiotemporal dynamics of sandflies in the Mediterranean Languedoc region (Roquedur area, Gard, France). Parasites & vectors. 2015;8(1):642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dolmatova A, NA-Les Phlébotomes D. les maladies qu’ils transmettent. ORSTOM ed. 1971.
- 22.Abonnenc E. Les phlébotomes de la région éthiopienne (Diptera, Psychodidae). 1972.
- 23.Gálvez R, Descalzo M, Miró G, Jiménez M, Martín O, Dos Santos-Brandao F, et al. Seasonal trends and spatial relations between environmental/meteorological factors and leishmaniosis sand fly vector abundances in Central Spain. Acta tropica. 2010;115(1):95–102. [DOI] [PubMed] [Google Scholar]
- 24.Lisi O, D’Urso V, Vaccalluzzo V, Bongiorno G, Khoury C, Severini F, et al. Persistence of phlebotomine Leishmania vectors in urban sites of Catania (Sicily, Italy). Parasites & vectors. 2014;7(1):560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Branco S, Alves-Pires C, Maia C, Cortes S, Cristovão J, Gonçalves L, et al. Entomological and ecological studies in a new potential zoonotic leishmaniasis focus in Torres Novas municipality, Central Region, Portugal. Acta tropica. 2013;125(3):339–48. doi: 10.1016/j.actatropica.2012.12.008 [DOI] [PubMed] [Google Scholar]
- 26.Casanova C. A soil emergence trap for collections of phlebotomine sand flies. Memórias do Instituto Oswaldo Cruz. 2001;96(2):273–5. [DOI] [PubMed] [Google Scholar]
- 27.Amóra SS, Bevilaqua CM, Feijó F, Alves ND, Maciel MdV. Control of phlebotomine (Diptera: Psychodidae) leishmaniasis vectors. Neotropical entomology. 2009;38(3):303–10. [DOI] [PubMed] [Google Scholar]
- 28.Killick-Kendrick R. Some epidemiological consequences of the evolutionary fit between Leishmaniae and their phlebotomine vectors. Bulletin de la Societe de Pathologie Exotique et de ses Filiales. 1984;78(5 Pt 2):747–55. [PubMed] [Google Scholar]
- 29.De Oliveira CI, de Carvalho AM, Oliveira F. Sand-fly saliva-Leishmania-man: the trigger trio. Frontiers in immunology. 2013;4:375 doi: 10.3389/fimmu.2013.00375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rioux J, Crozet H, Corre J-J, Simonneau P, Gras G. Les bases phyto-écologiques de la lutte anticulicidienne. Cartographie des biotopes larvaires, ses applications opérationnelles dans le Midi méditerranéen. Annales de parasitologie humaine et comparée. 1967;42(6):665–80. [PubMed] [Google Scholar]
- 31.Chelbi I, Zhioua E. Confirmation de la présence en Tunisie de Sergentomyia (Sintonius) clydei (Sinton, 1928). Bulletin de la Société de pathologie exotique. 2012:1–3. [DOI] [PubMed] [Google Scholar]
- 32.Rossi E, Bongiorno G, Ciolli E, Di Muccio T, Scalone A, Gramiccia M, et al. Seasonal phenology, host-blood feeding preferences and natural Leishmania infection of Phlebotomus perniciosus (Diptera, Psychodidae) in a high-endemic focus of canine leishmaniasis in Rome province, Italy. Acta tropica. 2008;105(2):158–65. doi: 10.1016/j.actatropica.2007.10.005 [DOI] [PubMed] [Google Scholar]
- 33.Talbi FZ, El Ouali Lalami A, Janati Idrissi A, Sebti F, Faraj C. Leishmaniasis in central Morocco: seasonal fluctuations of phlebotomine sand fly in Aichoun locality, from Sefrou province. Pathology research international. 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Croset H, Abonnenc E, Rioux J. Phlebotomus (Paraphlebotomus) chabaudi n. sp.(Díptera, Psychodidae). Ann Parasit Humaine et Comparée. 1970;45(6):863–73. [DOI] [PubMed] [Google Scholar]
- 35.Morillas MF, Sanchís MM, Martín SJ, Acedo SC. On Phlebotomus perniciosus Newstead, 1911 (Diptera, Phlebotomidae) in the Province of Almeria in southeastern Spain. Parassitologia. 1991;33:437–44. [PubMed] [Google Scholar]
- 36.Testa J, Montoya-Lerma J, Cadena H, Oviedo M, Ready P. Molecular identification of vectors of Leishmania in Colombia: mitochondrial introgression in the Lutzomyia townsendi series. Acta tropica. 2002;84(3):205–18. [DOI] [PubMed] [Google Scholar]
- 37.Jiménez M, González E, Iriso A, Marco E, Alegret A, Fúster F, et al. Detection of Leishmania infantum and identification of blood meals in Phlebotomus perniciosus from a focus of human leishmaniasis in Madrid, Spain. Parasitology research. 2013;112(7):2453–9. doi: 10.1007/s00436-013-3406-3 [DOI] [PubMed] [Google Scholar]
- 38.Ranasinghe S, Rogers ME, Hamilton JGC, Bates PA, Maingon RDC. A real-time PCR assay to estimate Leishmania chagasi load in its natural sand fly vector Lutzomyia longipalpis. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2008;102(9):875–82. doi: 10.1016/j.trstmh.2008.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aghaei AA, Rassi Y, Sharifi I, Vatandoost H, Mollaie H, Oshaghi M, et al. First report on natural Leishmania infection of Phlebotomus sergenti due Leishmania tropica by high resolution melting curve method in South-eastern Iran. Asian Pacific journal of tropical medicine. 2014;7(2):93–6. doi: 10.1016/S1995-7645(14)60002-X [DOI] [PubMed] [Google Scholar]
- 40.Myskova J, Votypka J, Volf P. Leishmania in sand flies: comparison of quantitative polymerase chain reaction with other techniques to determine the intensity of infection. Journal of medical entomology. 2008;45(1):133–8. [DOI] [PubMed] [Google Scholar]
- 41.González E, Álvarez A, Ruiz S, Molina R, Jiménez M. Detection of high Leishmania infantum loads in Phlebotomus perniciosus captured in the leishmaniasis focus of southwestern Madrid region (Spain) by real time PCR. Acta Tropica. 2017. [DOI] [PubMed] [Google Scholar]
- 42.Van der Auwera G, Dujardin J-C. Species typing in dermal leishmaniasis. Clinical microbiology reviews. 2015;28(2):265–94. doi: 10.1128/CMR.00104-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Izri M, Belazzoug S, Boudjebla Y, Dereure J, Pratlong S, Delalbre-Belmonte A, et al. Leishmania infantum MON-1 isolé de Phlebotomus perniciosus, en Kabylie (Algérie). Annales de parasitologie humaine et comparée. 1990; 65: 150–150. [PubMed] [Google Scholar]
- 44.Maia C, Dionisio L, Afonso MO, Neto L, Cristovao JM, Campino L. Leishmania infection and host-blood feeding preferences of phlebotomine sandflies and canine leishmaniasis in an endemic European area, the Algarve Region in Portugal. Memórias do Instituto Oswaldo Cruz. 2013;108(4):481–7. doi: 10.1590/S0074-0276108042013014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chargui N, Haouas N, Slama D, Gorcii M, Jaouadi K, Essabbah-Aguir N, et al. Transmission of visceral leishmaniasis in a previously non-endemic region of Tunisia: Detection of Leishmania DNA in Phlebotomus perniciosus. Journal of Vector Ecology. 2013;38(1):1–5. doi: 10.1111/j.1948-7134.2013.12000.x [DOI] [PubMed] [Google Scholar]
- 46.Zoghlami Z, Chouihi E, Barhoumi W, Dachraoui K, Massoudi N, Helel KB, et al. Interaction between canine and human visceral leishmaniases in a holoendemic focus of Central Tunisia. Acta tropica. 2014;139:32–8. doi: 10.1016/j.actatropica.2014.06.012 [DOI] [PubMed] [Google Scholar]
- 47.Barhoumi W, Qualls WA, Archer RS, Fuller DO, Chelbi I, Cherni S, et al. Irrigation in the arid regions of Tunisia impacts the abundance and apparent density of sand fly vectors of Leishmania infantum. Acta tropica. 2015;141:73–8. doi: 10.1016/j.actatropica.2014.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bettini S, Gramiccia M, Gradoni L, Atzeni M. Leishmaniasis in Sardinia: II. Natural infection of Phlebotomus perniciosus Newstead 1911, by Leishmania infantum Nicolle 1908, in the province of Cagliari. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1986;80(3):458–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.