Abstract
Sensitization to grass pollen imposes a global risk for allergic airway diseases. Although prevention relies on local investigation of the pollen allergens, data on this topic are limited in southern China. Any available data were obtained by self-report questionnaires, skin prick tests, and total or specific IgE tests using crude extracts. For many reasons, these methods are unreliable. Serum sIgE reactivity to Bermuda grass, Timothy grass, and Humulus scandens allergens in a cohort of patients from Greater Guangzhou (southern China's largest city and its outskirts) with allergic rhinitis and/or asthma were examined using a fully-automated immunoassay analyzer as a component-resolved diagnostics (CRD) tool.
For the first time, a considerably high prevalence of Bermuda grass sIgE positivity was demonstrated in Chinese southerners with allergic rhinitis and/or asthma. In these patients, a subtle prevalence of sensitization to Timothy grass and Humulus scandens was also noted, which may arise from cross-reactivity, as the latter two are not common in the region. This was also supported by the detection of allergen components.
Fully-automated immunoassay analyzers may offer satisfactory consistency between regions, laboratories, and institutions and over time. The automaticity of the instrument may enable a standardized detection that would not have been readily revealed before the advent of CRD.
This is a study that uses a CRD approach to investigate sensitization to grass pollen allergens in southern China. It adds to current evidence in the literature. Future studies are needed to validate these findings. However, although CRD is a useful tool, the findings made with the fully-automated immunoassay analyzer should not substitute for other laboratory investigations, clinical evaluations, and physician expertise.
Keywords: Medicine, Issue 124, Allergy, Grass pollen, Respiratory disease, Asthma, Allergic rhinitis, Immunoassay analyzer, full-automated, Component-resolve diagnostics
Introduction
Grass pollen accounts for nearly 50% of immunoglobulin E (IgE)-related allergies and, because of its airborne nature, imposes a global risk of allergic airway diseases, particularly asthma and allergic rhinitis1,2,3,4. Prevention against grass pollen allergies in a region relies on information about the local distribution of the pollen allergens. Unfortunately, the spectrum of the sensitizing allergens varies from place to place—an observation generally known as regional specificity.
In China, a vast East-Asian territory, regional specificity of grass pollen sensitization is further complicated by limited data currently available from the southern part of this country over the past decades5. Such a paucity in data has led to a difficulty in showing the overall grass pollen sensitization in this large country6. Additionally, the very limited data on Chinese southerners were largely obtained from self-report questionnaires, skin prick tests (rather than laboratory serum tests), total IgE measurements (rather than allergen-specific IgE (sIgE)), and sIgE detection to allergens (rather than to allergen component molecules).
The self-reporting of allergic symptoms can be unreliable when specific allergens are concerned. Skin prick tests, although widely accessible, involve the use of non-standardized, crude allergen extracts, which themselves may contain other sensitizing components that confound the outcome interpretation. The reliability of the skin prick test also critically depends on skillful manipulation by allergists or specialist nurses, recent histamine use, and patient compliance (sometimes very difficult for young children). Total IgE is useful for screening an allergy but does not sensibly determine the offending allergens in a patient. Moreover, almost all allergens contain a number of allergen component molecules. Sensitization to the major components (i.e., Der p 1 and Der p 2 from house dust mites) may suggest a "genuine" allergy (allergy to a specific allergen, rather than false positivity due to cross-reactions with other allergens), but sensitization to certain ones (i.e., the cross-reactive Der p 10, a tropomyosin) may not7.
Before component-resolved diagnostics (CRD) was introduced, these disappointing setbacks impeded allergen studies in China. CRD precisely detects the components of an allergen by using recombinant or purified allergen molecules, thereby making the measurement easily quantifiable and characterizable8. In past decades, allergy studies frequently employed crude extracts of allergens that inevitably contain a number of irrelevant components. Many of these components are inert, but occasionally, some could lead to confounding positive reactions. The use of recombinant or purified allergen molecules in CRD can circumvent this and therefore provide better test accuracy. When processed in microarrays, a CRD approach rapidly screens or identifies sensitization to hundreds of allergen molecules in individual subjects. A well-designed CRD study on grass pollen sensitization in southern China is currently lacking. Ideally, such a study should include a local population of considerable geographic coverage, account for grass pollens commonly reported in China, and address the technical aspects mentioned above. To reach a practical level of clinical relevance and to facilitate the recruitment of serum samples, early investigation on sensitization to grass pollens as inhalant allergens may as well be focused on patients with allergic respiratory disorders, rather than on the general population.
This work describes a method used to test serum sIgE reactivity to Bermuda grass, Timothy grass, and Humulus scandens in patients from Greater Guangzhou (southern China's largest city and its outskirts) with allergic rhinitis and/or asthma using the CRD approach5. These findings on sensitization to these subtropical and temperate grass pollens in southern China add important evidence to that currently in the literature.
Protocol
The study protocol, including human serum sample use, was approved by the Ethics Committee, First Affiliated Hospital of Guangzhou Medical University. All participants offered written informed consent, either independently or via their parents (in the case of children). The protocol has been registered online (Chinese Clinical Trial Registry, Reg No.: ChiCTR-DCC-13004003, refer to http://www.chictr.org/cn/).
1. Identification of the Study Population
NOTE: The Allergy Information Repository of State Key Laboratory of Respiratory Disease (AIR-SKLRD) is the primary source of patient recruitment, because AIR is the most well-documented large database of patients for allergen tests in southern China9,10. AIR has been storing serum samples of patients for nearly a decade. Briefly, these stored samples were previously prepared by centrifuging 5 mL of phlebotomized venous blood for 10 min at 3,000 x g and recovering the supernatant. The serum samples were placed in -80 °C refrigerators for long-term storage. Samples that met patient inclusion criteria were ready to use for all testing in the present study.
From the AIR database, retrieve data about patients with allergic rhinitis and/or asthma who underwent serum tests for grass allergen sIgE between January 2013 and June 2015, the pre-designed study period.
Select patients with mild to moderate allergic rhinitis and/or asthma according to the Allergic Rhinitis and Its Impact on Asthma (ARIA)11 and the Global Initiative for Asthma (GINA)12 guidelines. Assign them as the "study group."
Exclude patients with incomplete medical records, those lost to follow up, those who refuse to give informed consent regarding the use of their serum samples for scientific purposes, those with identified immunodeficiency, those currently on immunotherapy or immunomodulatory agents, or those found to have parasitic infections.
Select a contemporary cohort of patients with non-respiratory allergies (i.e., eczema, allergic dermatitis, and food allergy) according to the same exclusion criteria and assign them as the "control group."
Make sure that the serum samples were collected before any prescriptions or treatments were given to minimize confounding effects on the laboratory findings. Exclude any serum sample not fulfilling this requirement.
Make sure that all subjects are permanent residents from all parts of Greater Guangzhou (i.e., born or have lived there for more than 10 years).
2. Study Flow and Measurements of Interest
Retrieve the serum samples of eligible patients from the bio-bank. To test an allergen/allergen component, use 140 µL of serum (including 100 µL to fill up the dead space). NOTE: The maximum total serum volume needed for each patient in this study is: 3 specific allergens and 8 allergen components, sIgE = 140 µL * 11 = 1,540 µL.
When using this instrument, primarily test the serum samples for sIgEs to whole allergens of Bermuda grass, Timothy grass, and Humulus scandens. Follow the instructions in Section 3. NOTE: These pollens were selected for the measurements because Bermuda grass13,14,15 and Humulus scandens16 have been widely reported in China, while Timothy grass17,18,19 is a typical plant species that lives in environments ranging from tropical to temperate zones and is most-studied worldwide.
Secondarily, test the serum samples identified in step 2.3 to have sIgEs to whole allergens for the presence of sIgEs to allergen components of Bermuda [Cyn d 1 (g216)], Timothy [Phlp 1 (g205), Phl p 4 (g208), Phl p 5 (g215), Phl p 6 (g209), Phl p 7 (g210), Phl p 11 (g211), and Phl p 12 (g212)], and cross-reactive carbohydrate determinants [CCD (o214)]. Follow the instructions in Section 3.
3. Fully-automated Test Procedure using the Immunoassay Analyzing System
NOTE: The whole procedure of allergen sIgE testing, which is fully automated and has been described elsewhere7,8,9,10, follows the manufacturer's protocol.
Start the fully-automated immunoassay analyzer (e.g., ImmunoCAP1000) and use it to perform the immunoassay throughout the study. Turn on the built-in Information Data Management (IDM) computer. NOTE: The immunoassay analyzer is routinely in stand-by mode.
With the "Primary Power" on, switch on the "System Power" and wait 3 min until the built-in software starts. Click on "Load Rinse Solution" and "Load Washing Solution."
Add 140 µL of serum to a vial for each allergen/allergen component test. Label each vial containing 140 µL of serum with an identification number unique to each patient.
- From the IDM interface, execute the following steps by clicking on the menu.
- Check the "Request List" window on the IDM to make sure that "sIgE" is the test method of choice.
- Load the sample tubes into the sample racks and the quality-control tubes into the quality-control racks, with bar codes. NOTE: For each patient, testing was initially planned for sIgE to 3 specific allergens and 8 allergen components, or 346*11 (3,806) sample tubes. For each sample tube, 3 quality-control tubes that contain low, medium, and high levels of sIgE control (see the Table of Materials for details) were matched. Eventually, only 58 patients who tested positive in the primary test (step 2.3) were further examined in the secondary test (step 2.3). The loading of tubes can be automatically set on the computer console.
- Select "Load Reagents" in the "Assay Processing" screen. Complete the loading of the sample and quality-control racks, development solution, conjugate, calibrators, carrier, pipette tips, stop solution, and washing solution according to the "Loadlist" (see the Table of Materials for details).
- In "Load and Start," press "OK." NOTE: At the end of the measurement, the results appear on the IDM.
- From the IDM interface, select the data to be exported. Click on "Menu," "Approve," and "Save As" and export the sIgE measurement results as a spreadsheet file.
- Import the spreadsheet file to a statistical software (e.g., SPSS)10. Using a specific module for Spearman's coefficients from the SPSS menu, analyze the correlations in sensitization between these grass pollens (positive sIgE to whole allergen) and pollen components (positive sIgE to allergen molecules).
4. Definition of sIgE Reactivity
NOTE: For an undiluted serum specimen, the range of specific IgE measurement is 0.35 to 100 kU/L.
Dilute the samples with sIgE values >100 kU/L 1:5 and re-assay. Where the value remains higher than 100 kU/L, proceed with further dilutions until an end-point level up to 1,000 kU/L is reached.
Based on the threshold value of 0.35 kU/L, consider an sIgE level exceeding 0.35 kU/L to be positive5,7,10. Rate the reactivity of the sIgE tests as: class 1 (≥0.35 and <0.70 kU/L), class 2 (≥0.70 and <3.50 kU/L), class 3 (≥3.50 and <17.50 kU/L), class 4 (≥17.50 and <50.00 kU/L), class 5 (≥50.00 and <100.00 kU/L), and class 6 (≥100.00 kU/L).
5. Statistical Analysis
Analyze the data with statistical software (e.g., SPSS)10. Report the quantitative data as the mean and standard deviation, the qualitative data as the median and interquartile ranges (IQR), and the categorical data as the proportion of positive results.
Use a chi-square test for the between-group comparison of proportions, an unpaired t-test for quantitative data, and a rank-sum test for qualitative data.
Use Pearson's test for correlation analyses between parametric data, with the correlation coefficients expressed as "r," and Spearman's test for non-parametric values, with the correlation coefficients expressed as "rs." Consider statistical significance to be when P-values <0.05.
Representative Results
The fully-automated immunoassay analyzer used in this study has a solid-phase structure covered with covalently coupling allergens or allergen components that react with the molecules of interest (sIgE) in serum specimens from the patient. With an automatic program, the analyzer washes off all non-specific IgEs with 150 µL of rinse solution and repeats this 5 times. Then it adds enzyme-labeled anti-IgE antibodies (50 µL) to generate a complex. The enzyme-labeled anti-IgE antibody (conjugate) is a secondary antibody that binds to a specific IgE. Unbound anti-IgE antibodies are eliminated by washing with 150 µL of washing solution (repeated 3 times). The bound complex is treated with a developing agent (50 µL) for 9 min at 37 °C. The developing agent is an enzyme-digesting agent that cuts off all bound anti-IgE antibodies. The latter emits fluorescence under alkaline condition. The reaction is stopped with the addition of alkaline stop solution. After this, the instrument compresses the solid-phase structure to squeeze out the eluate and to measure the fluorescence of the resultant eluate at a wavelength of 470 nm. The fluorescence is linearly correlated to the absolute level of the analyte molecules of interest in the eluate. A higher fluorescence value means more sIgE in the serum specimen. To interpret the test results, the system automatically transforms the responses for samples to concentrations using a calibration curve. The measurement data are electronically transferred to the IDM software, which integrates functions for the calculation of analytical results, the calculation of statistics, and result reporting.
Following the inclusion and exclusion criteria, the present study investigated serum from 346 patients. The study group included 78 subjects with allergic rhinitis alone, 92 with asthma alone, and 88 concomitantly with asthma and rhinitis. The control group included 88 patients with non-respiratory allergies. These patients were aged 1-87 years old and were confirmed to be permanent residents from all parts of Greater Guangzhou, the target region of study. All study groups and the control group were comparable in demographical and social characteristics, including gender, age, and family history. Of the patients in the study group, 22.5% tested positive to Bermuda grass, 13.6% to Timothy grass, and 7.0% to Humulus scandens. In the study group, the rate of sensitization to any grass pollen was much higher than in the control group (Table 1). All Bermuda grass sIgE-negative patients were also negative to Timothy grass and Humulus scandens. It is of interest to note that patients with allergic rhinitis and/or asthma demonstrated a remarkably higher rate of sensitization to Bermuda grass (n = 58, 22.5%) than those with other allergies (n = 4, 4.5%).
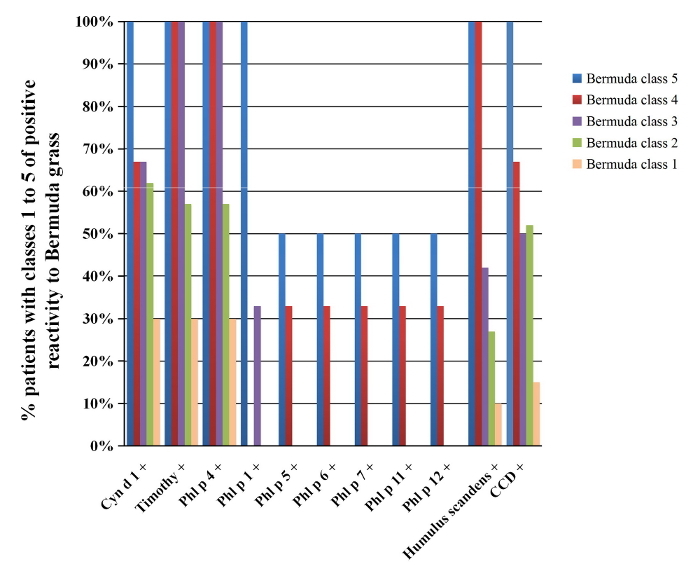
There were no patients with class-6 sIgE reactivity in this study. Among the patients with Bermuda grass class-1 sIgE reactivity, the likelihood of positive tests for Cyn d 1, Timothy grass allergens, Humulus scandens, or CCD was below 30%. In contrast, patients with class-4 Bermuda grass sIgE reactivity had a 67-100% chance of concomitant positivity to Cyn d 1, Timothy grass allergens, Humulus scandens, or CCD (Figure 1). Bermuda grass sIgE reactivity levels demonstrated a significant correlation, with chances of positivity to other grass pollen IgEs (rs = 0.669, P <0.001).
Timothy grass sensitization was further assessed by sIgE to Timothy grass components. Among the 35 Timothy grass-positive patients, the most frequently detected allergen component was Phl p 4 (100%, 35/35), followed by Phl p 1 (17.1%, 6/35). Three patients each (3/35, 8.6%) were positive for any one of Phl p 5, 6, 7, 11, and 12 (Table 2). Serum Phl p 4 sIgE levels correlated strongly with total Timothy grass allergen and CCD sIgE levels (rs = 0.941 and 0.928, respectively, P <0.001; Table 3). 41.4% (24/58) of Bermuda grass-positive patients and 68.6% (24/35) of Timothy grass-sensitized patients were sIgE positive to CCD. Spearman's rank correlation analysis showed a significant association in sensitization between CCD and other grass allergens (Table 3).
Figure 1: Co-sensitization Among Patients Positive to Bermuda Grass sIgE. This figure shows the percentage of patients with different classes of sIgE reactivity to Bermuda grass (n = 58) and a concomitant sensitization to Timothy grass, Humulus scandens, CCD, and other grass allergen components. Please click here to view a larger version of this figure.
| Rate of positive tests [% (n/patients)] | |||
| Bermuda grass | Timothy grass | Humulus scandens | |
| Patients with AR and/or AS | 22.5% (58/258) ††‡ | 13.6% (35/258) † | 7.0% (18/258) |
| Patients with AR | 21.8% (17/78) †† | 10.2% (8/78) | 7.7% (6/78) |
| Patients with AS | 22.8% (21/92) †† | 14.1% (13/92) † | 5.4% (5/92) |
| Patients with AR and AS | 22.7% (20/88) †† | 15.9% (14/88) | 8.0% (7/88) |
| Age | |||
| <14 years or younger | 18.6% (8/43) | 14.0% (6/43) | 7.0% (3/43) |
| > 4 years | 23.3% (50/215) | 13.5% (29/215) | 7.0% (15/215) |
| Sex | |||
| Male | 25.7% (35/136) | 13.9% (19/136) | 7.4% (10/136) |
| Female | 18.8% (23/122) | 13.1% (16/122) | 6.6% (8/122) |
| Patients with other allergy | 4.5% (4/88) ** | 1.1% (1/88) ** | 0* |
Table 1: Patients with sIgE-positive Tests for Bermuda Grass, Timothy Grass, and Humulus scandens Based on Disease Entity and Demographic Details. A higher proportion of patients with allergic respiratory diseases were positive for Bermuda grass (χ2 = 14.35, P <0.001), Timothy grass (χ2 = 10.88, P <0.001), and Humulus scandens (χ2 = 5.14, P = 0.02) versus the other allergy controls (**P <0.01, *P <0.05). The proportion of patients with a positive Bermuda grass test was remarkably higher than the proportion for Timothy grass (‡P <0.05) and Humulus scandens (††P <0.01). The proportion of patients with a positive Timothy grass test was higher than that of Humulus scandens (†P <0.05). The proportion of positive tests to Bermuda grass, Timothy grass, and Humulus scandens sIgE did not vary significantly between the minor and adult groups (χ2 = 0.07, P = 0.79; χ2 = 0.06, P = 0.80; χ2 = 0.11, P = 0.74; respectively). Rates of grass pollen sensitivities were not statistically different between males and females (χ2 = 1.338, P =0.25; χ2 = 0.063, P = 0.80; χ2 = 0.38, P = 0.54; respectively). Similarly, no significant differences in sensitivity rates were indicated between the rhinitis and asthma patients (χ2 = 0.030, P = 0.985; χ2 = 1.165, P = 0.558; χ2 = 0.528, P = 0.768; respectively).
| Patients (%) | Phl p 4 | Phl p 1 | Phl p 5 | Phl p 6 | Phl p 11 | Phl p 7 | Phl p 12 | |
| 28 (80.0%) | + | |||||||
| 4 (11.4%) | + | + | ||||||
| 1 (2.9%) | + | + | + | + | + | + | ||
| 2 (5.7%) | + | + | + | + | + | + | + | |
| Total | 35 (100%) | 6 (17.1%) | 3 (8.6%) | 3 (8.6%) | 3 (8.6%) | 3 (8.6%) | 3 (8.6%) | |
| sIgEa (kU/L) | 0.52, 0.06-2.02 | 0.02, 0.01-0.10 | 0.04, 0.03-0.24 | 0.12, 0.03-0.34 | 0.05, 0.02-0.27 | 0.03, 0.02-0.36 | 0.10, 0.04-0.28 |
Table 2: Timothy Grass Sensitization According to sIgE Positivity and Levels of Timothy Grass Components (N = 35). +, positive tests; a, sIgE levels (kU/L) are shown as the median and IQR. Of the 35 Timothy grass sIgE-positive patients, 80.0% were solely Phl p 4-positive, while 11.4% were positive to Phl p 4 or Phl p 1.
| Allergen | Bermuda | Timothy | Humulus scandens | CCD |
| Bermuda | - | 0.709** | 0.682** | 0.565** |
| Cyn d 1 | 0.583** | 0.787** | 0.751** | 0.865** |
| Timothy | 0.709** | - | 0.921** | 0.856** |
| Phl p 4 | 0.645** | 0.941** | 0.886* | 0.928** |
| Phl p 1 | 0.616** | 0.681** | 0.712** | 0.507** |
| Phl p 5 | 0.46 | 0.273 | 0.590* | 0.624* |
| Phl p 6 | 0.445 | 0.306 | 0.583* | 0.569 |
| Phl p 7 | 0.681* | 0.481 | 0.632* | 0.531 |
| Phl p 11 | 0.589* | 0.372 | 0.620* | 0.568 |
| Phl p 12 | 0.671* | 0.411 | 0.565 | 0.446 |
| Humulus scandens | 0.682** | 0.921** | - | 0.853** |
Table 3: Correlation coefficients† for positivity between CCD and the grass pollen allergens. † is presented as the correlation coefficient rs; Spearman's correlation analyses were performed for this non-parametric dataset. * P <0.05, ** P <0.01.
Discussion
Fully-automated immunoassay analyzers may offer satisfactory consistency between regions, laboratories, and institutions and over time. Such an automatic procedure has been found to substantially support bench research and clinical studies, enable the standardized detection of many allergens and/or allergen components, and identify probable allergen cross-reactivity that would not have been readily revealed before the advent of CRDs. Moreover, the whole testing procedure can be completed with strict quality control, allowing for high-throughput methods, as required in a survey of a large geographical region. Although the testing procedure is mostly run by a computer console, several critical issues should be brought to attention. It is mandatory that the instrument is used in rigorous compliance with the user manual; any aberration could result in unnecessary test errors, mechanical damage, or personal casualty from laser beam or high electric voltage. Always ensure that a proper and correct calibration curve has been stored in the system, as this is linked to the accuracy of the test. Otherwise, the sIgE concentration of a specified calibrator should be measured prior to the serum specimen tests. Afterwards, the system may automatically compute and store the calibration curve for subsequent use. As with other laboratory techniques, it is also important to observe the safety of human serum manipulation to protect technicians from infections and to avoid contaminating the environment.
For the first time, by using a fully-automated immunoassay analyzer as a CRD approach, this study showed a considerable prevalence of Bermuda grass sIgE positivity (22.5%) in patients with allergic rhinitis and/or asthma in Guangzhou, southern China. Since Timothy grass and Humulus scandens are not common, even non-existant, in Guangzhou, the positivity rates of Timothy grass (13.6%) and Humulus scandens sIgE (7.0%) may be attributed to cross-reactivity among grass pollens.
By analyzing grass pollen allergens, it was clearly shown that all Timothy grass IgE-positive patients were also positive to the specific Timothy grass antigen Phl p 4. Very few (17.1%) tested positive to Phl p 1, which was surprisingly different from data in Europe, where the rate of positivity to Phl p 1 is much higher than that of Phl p 418,20,21. Since Phl p 1 is the major component of Timothy grass, the predominant rate of Phl p 4 positivity over Phl p 1 in this study population may also suggest probable cross-reactivity other than "genuine" sensitization, because Phl p 4 shares a structural similarity with CCD and a number of other allergens, including trees, vegetables, and tropical fruits19,22,23,24. The clinical relevance of this observation could be that the majority of Timothy grass-positive patients in this study should never have been advised to receive Timothy grass immunotherapy, which currently uses Phl p 125.
Several limitations of the study methods and techniques must be mentioned. First, since up to 3 different calibrator strips can be placed in a pipette/reagent Tray in the immunoassay analyzer, at the maximum, three test batches can be run at the same time. When a heavy workload is required, like in a greater regional survey, it still takes a significant amount of time to process. Second, the cross-reactivity with human IgA, IgD, IgM, and IgG is nonexistent at physiological concentrations when using the analyzer from this study, so other investigational approaches are needed when cross-reactivity with these immunoglobulins are concerned. Third, despite the sophistication of the device, the findings made with the fully-automated immunoassay analyzer should not substitute for other laboratory investigations, clinical evaluations, and physician expertise when a definitive diagnosis is being made. Fourth, due to the late introduction of allergen CRD in China, several commercial products of subtropical grass pollen components are unavailable or have not been approved for research until recently. Therefore, using this technique as a CRD approach for allergens substantially relies on the commercial availability of product supplies from the manufacturer for specific use in a region. In tropical and subtropical regions with a ubiquitous complexity of plant pollens, a CRD study can be insufficient and difficult due to a lack of corresponding allergen products for the tests. Such a dilemma could greatly compromise the efficiency of this technique.
Nevertheless, to the latest of our knowledge, this is a pioneering study that uses the CRD approach to examine sensitization to grass pollen allergens in southern China. Further studies can continue in this vein and validate these findings.
So far, techniques using the fully-automatic immunoassay analyzer have been accepted as the gold standard for in vitro allergen sIgE tests26,27. Given the very limited data on allergen sensitization within the vast territory of China, large-sample studies using the fully-automated immunoassay as a CRD approach can fill the gaps and add to the evidence available in this field. It is important to note that such studies, usually multi-center and nationwide collaborations, may necessitate the networking of immunoassays based on a protocol for standardization.
Disclosures
The authors have nothing to disclose.
Acknowledgments
We thank Yueming Su for his assistance in preparing the figure. This study was funded by the Guangdong Foundation of Science and Technology (Project No.: 2014A020212352), the Guangzhou Education Bureau (1201630044; 1201630393) and the National Natural Science Foundation of China (NSFC 81572063; NSFC 81601394).
References
- Scadding GK. BSACI guidelines for the management of allergic and non-allergic rhinitis. Clin Exp Allergy. 2007;38(1):19–42. doi: 10.1111/j.1365-2222.2007.02888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollart SM, Reid MJ, Fling JA, Chapman MD, Platts-Mills TA. Epidemiology of emergency room asthma in northern California: association with IgE antibody to rye grass pollen. J Allergy Clin Immunol. 1988;82(2):224–230. doi: 10.1016/0091-6749(88)91003-2. [DOI] [PubMed] [Google Scholar]
- Wang Q. Lower airway inflammation and hyperresponsiveness in non-asthmatic patients with non-allergic rhinitis. J Thorac Dis. 2015;7(10):1756–1764. doi: 10.3978/j.issn.2072-1439.2015.10.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y. The link between mold sensitivity and asthma severity in a cohort of northern Chinese patients. J Thorac Dis. 2015;7(4):585–590. doi: 10.3978/j.issn.2072-1439.2015.01.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W. Major grass pollen allergens and components detected in a southern Chinese cohort of patients with allergic rhinitis and/or asthma. Mol Immunol. 2016;78:105–112. doi: 10.1016/j.molimm.2016.08.013. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang L. Prevalence of allergic rhinitis in china. Allergy Asthma Immunol Res. 2014;6(2):105–113. doi: 10.4168/aair.2014.6.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng G, Zheng P, Luo W, Huang H, Wei N, Sun B. Longitudinal profiles of serum specific IgE and IgG4 to Dermatophagoides pteronyssinus allergen and its major components during allergen immunotherapy in a cohort of southern Chinese children. Mol Immunol. 2016;74:1–9. doi: 10.1016/j.molimm.2016.04.005. [DOI] [PubMed] [Google Scholar]
- Kleine-Tebbe J, Matricardi PM, Hamilton RG. Allergy work-up including component-resolved diagnosis: how to make allergen-specific immunotherapy more specific. Immunol Allergy Clin North Am. 2016;36(1):191–203. doi: 10.1016/j.iac.2015.08.012. [DOI] [PubMed] [Google Scholar]
- Sun B, Zheng P, Wei N, Huang H, Zeng G. Co-sensitization to silkworm moth (Bombyx mori) and 9 inhalant allergens among allergic patients in Guangzhou, southern China. PLoS One. 2014;9(5):e94776. doi: 10.1371/journal.pone.0094776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng G. Component-resolved diagnostic study of Dermatophagoides pteronyssinus major allergen molecules in a southern Chinese cohort. J Investig Allergol Clin Immunol. 2015;25(5):343–351. [PubMed] [Google Scholar]
- Allergic Rhinitis and Its Impact on Asthma (ARIA) Management of Allergic Rhinitis and Its Impact on Asthma Pocket Guide. 2015. Available from: http://www.whiar.org/docs/ARIA_PG_08_View_WM.pdf.
- Global Initiative for Asthma (GINA) Pocket Guide for Asthma Management and Prevention. 2015. Available from: http://ginasthma.org/wp-content/uploads/2016/01/GINA_Pocket_2015.pdf.
- Liu J, Guo A, Guo H. Morphological variation and types of Cynodon dactylon. Acta Prataculture Sinica. 2003;12(6):99–104. [Google Scholar]
- Zhang H, Xi J. Study on the germplasm resource and ecological characteristics of wild carpet grass (Axonopus compressus) in Guangdong. Grassland and Turf. 2005;13(1):25–28. [Google Scholar]
- Kao SH, Su SN, Huang SW. Sub-proteome analysis of novel IgE-binding proteins from Bermuda grass pollen. Proteomics. 2005;5(14):3805–3813. doi: 10.1002/pmic.200401229. [DOI] [PubMed] [Google Scholar]
- Ouyang YH, Zhang DS, Fan EZ, Li Y, Zhang L. Correlation between symptoms of pollen allergic rhinitis and pollen grain spreading in summer and autumn. Zhonghua Er Bi Yan Hou Tou Jing Waike Zazhi. 2012;47(8):623–627. [PubMed] [Google Scholar]
- Westritschnig K. Analysis of the sensitization profile towards allergens in central Africa. Clin Exp Allergy. 2003;33(1):22–27. doi: 10.1046/j.1365-2222.2003.01540.x. [DOI] [PubMed] [Google Scholar]
- Rossi RE, Monasterolo G, Monasterolo S. Measurement of IgE antibodies against purified grass-pollen allergens (Phl p 1, 2, 3, 4, 5, 6, 7, 11, and 12) in sera of patients allergic to grass pollen. Allergy. 2001;52(12):1180–1185. doi: 10.1034/j.1398-9995.2001.00258.x. [DOI] [PubMed] [Google Scholar]
- Zafred D, Nandy A, Pump L, Kahlert H, Keller W. Crystal structure and immunologic characterization of the major grass pollen allergen Phl p 4. J Allergy Clin Immunol. 2013;132(3):696–703. doi: 10.1016/j.jaci.2013.03.021. [DOI] [PubMed] [Google Scholar]
- Chabre H. Molecular variability of group 1 and 5grass pollen allergens between Pooideae species: implications for immunotherapy. Clin Exp Allergy. 2010;40(3):505–519. doi: 10.1111/j.1365-2222.2009.03380.x. [DOI] [PubMed] [Google Scholar]
- Mari A. Skin test with a timothy grass (Phleum pratense) pollen extract vs.IgE to a timothy extract vs. IgE to rPhl p 1 rPhl p 2, nPhl p 4, rPhl p 5, rPhl p 6, rPhl p 7, rPhl p 11, and rPhl p 12: epidemiological and diagnostic data. Clin Exp Allergy. 2003;33(1):43–51. doi: 10.1046/j.1365-2222.2003.01569.x. [DOI] [PubMed] [Google Scholar]
- Grote M, Stumvoll S, Reichelt R, Lidholm J, Rudolf V. Identification of an allergen related to Phl p 4, a major timothy grass pollen allergen, in pollens, vegetables, and fruits by immunogold electron microscopy. Biol Chem. 2002;383(9):1441–1445. doi: 10.1515/BC.2002.163. [DOI] [PubMed] [Google Scholar]
- Andersson K, Lidholm J. Characteristics and immunobiology of grass pollen allergens. Int Arch Allergy Immunol. 2003;130(2):87–107. doi: 10.1159/000069013. [DOI] [PubMed] [Google Scholar]
- Tripodi S. Molecular profiles of IgE to Phleum pratense in children with grass pollen allergy: implications for specific immunotherapy. J Allergy Clin Immunol. 2012;129(3):834–839. doi: 10.1016/j.jaci.2011.10.045. [DOI] [PubMed] [Google Scholar]
- Stumvoll S, et al. Purification, structural and immunological characterization of a timothy grass (Phleum pratense) pollen allergen Phl p 4, with cross-reactive potential. Biol Chem. 2002;383(9):1383–1396. doi: 10.1515/BC.2002.157. [DOI] [PubMed] [Google Scholar]
- Wood RA, Segall N, Ahlstedt S, Williams PB. Accuracy of IgE antibody laboratory results. Ann Allergy Asthma Immunol. 2007;99(1):34–41. doi: 10.1016/S1081-1206(10)60618-7. [DOI] [PubMed] [Google Scholar]
- Wang J, Godbold JH, Sampson HA. Correlation of serum allergy (IgE) tests performed by different assay systems. J Allergy Clin Immunol. 2008;121(5):1219–1224. doi: 10.1016/j.jaci.2007.12.1150. [DOI] [PubMed] [Google Scholar]


