Abstract
Central dopaminergic (DAergic) pathways have an important role in a wide range of functions, such as attention, motivation, and movement. Dopamine (DA) is implicated in diseases and disorders including attention deficit hyperactivity disorder, Parkinson's disease, and traumatic brain injury. Thus, DA neurotransmission and the methods to study it are of intense scientific interest. In vivo fast-scan cyclic voltammetry (FSCV) is a method that allows for selectively monitoring DA concentration changes with fine temporal and spatial resolution. This technique is commonly used in conjunction with electrical stimulations of ascending DAergic pathways to control the impulse flow of dopamine neurotransmission. Although the stimulated DA neurotransmission paradigm can produce robust DA responses with clear morphologies, making them amenable for kinetic analysis, there is still much debate on how to interpret the responses in terms of their DA release and clearance components. To address this concern, a quantitative neurobiological (QN) framework of stimulated DA neurotransmission was recently developed to realistically model the dynamics of DA release and reuptake over the course of a stimulated DA response. The foundations of this model are based on experimental data from stimulated DA neurotransmission and on principles of neurotransmission adopted from various lines of research. The QN model implements 12 parameters related to stimulated DA release and reuptake dynamics to model DA responses. This work describes how to simulate DA responses using QNsim1.0 and also details principles that have been implemented to systematically discern alterations in the stimulated dopamine release and reuptake dynamics.
Keywords: Neuroscience, Issue 124, dopamine, fast-scan cyclic voltammetry, synaptic transmission, psychostimulants, methylphenidate, quantitative neurobiological model
Introduction
Dopamine (DA) neurotransmission plays an essential role in various cognitive and behavioral functions, and its dysfunction is implicated in several common diseases and disorders. As such, it is critical to develop accurate methods of quantitatively studying DA neurotransmission in vivo to evaluate how DA neurotransmission is altered in the contexts of disease models and drug pharmacology. Fast-scan cyclic voltammetry (FSCV) allows for monitoring in vivo DA neurotransmission with fine spatial and temporal resolution. While it is possible to monitor physiological DA neurotransmission in awake, freely behaving animals, the electrical stimulation of ascending dopaminergic pathways in anesthetized animals can produce robust DA responses that are amenable to the enhanced kinetic analysis of DA neurotransmission.
Electrically stimulated DA responses reflect a dynamic interplay of DA release and reuptake, and interpretations of these responses have predominantly used a simple model of stimulated DA neurotransmission called the Michaelis-Menten (M-M) model12. The M-M model consists of 3 variables to describe DA responses in terms of a constant DA release rate and a constant reuptake efficiency (i.e., the relationship between the DA reuptake rate and extracellular DA concentrations), as described by Equation 1: 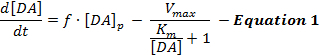 (DA release) (DA reuptake)
(DA release) (DA reuptake)
In Equation 1, f is the frequency of stimulation; [DA]p is the estimated DA concentration increase per pulse of stimulation; Vmax represents the estimated maximal reuptake rate; and Km is the estimated M-M constant, which is theoretically equivalent to the extracellular DA concentration that saturates 50% of DAT, leading to a half-maximal reuptake rate. This differential equation can be integrated to simulate experimental DA responses by estimating the [DA]p, Vmax, and Km parameters.
Although the M-M model has facilitated significant advances in the understanding of DA neurotransmission kinetics in various experimental contexts, the M-M model makes simplistic fundamental assumptions that limit its applicability when modeling DA responses elicited by supraphysiological stimulations2,13. For instance, the M-M model can only approximate DA response shapes if they rise in a convex manner, but it cannot account for the gradual (concave) rising responses found in dorsal striatal regions12. Thus, the M-M model assumptions do not accurately capture the dynamic release and reuptake processes of stimulated DA neurotransmission.
To model stimulated DA responses according to a realistic quantitative framework, the quantitative neurobiological (QN) framework was developed based on principles of stimulated neurotransmission kinetics derived from complementary research and experimentation2. Various lines of neurotransmission research demonstrate that (1) stimulated neurotransmitter release is a dynamic process that decreases in rate over the course of stimulation14, (2) release continues in the post-stimulation phase with biphasic decay kinetics15, and (3) DA reuptake efficiency is progressively inhibited during the duration of the stimulation itself2,16. These three concepts serve as the foundation of the QN framework, and the three equations consisting of 12 parameters describing the dynamics of DA release and reuptake (Table 1). The QN framework can closely simulate heterogeneous experimental DA response types, as well as the predicted effects of experimental manipulations of stimulation parameters and drug administration2,6. Although further research is necessary to refine the data modeling approach, future experiments can greatly benefit from this neurobiologically grounded modeling approach, which significantly adds to the inferences drawn from the stimulated DA neurotransmission paradigm.
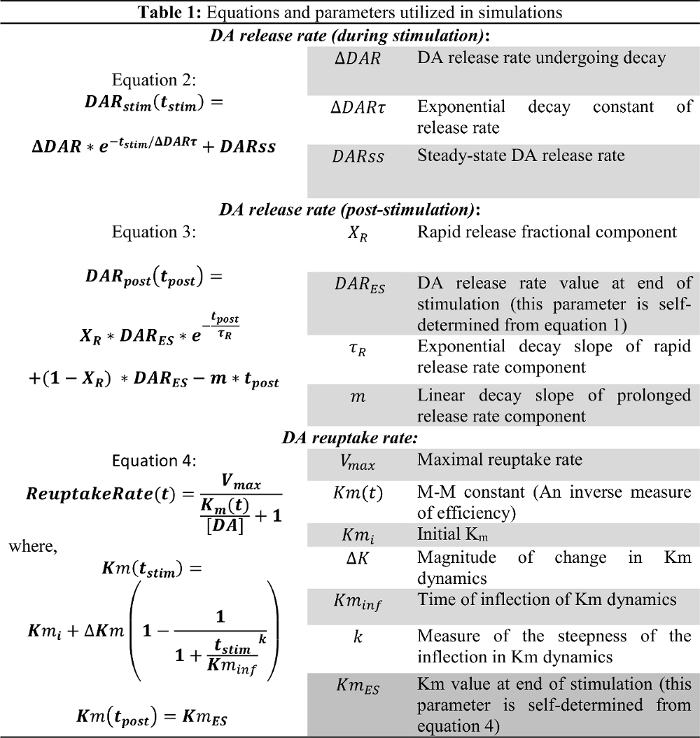 Table 1: Modeling Equations and Parameters. Please click here to view a larger version of this figure.
Table 1: Modeling Equations and Parameters. Please click here to view a larger version of this figure.
This tutorial describes how to model stimulated DA response data to estimate DA release and reuptake kinetics using QNsim 1.0. The actual experimental data collection and processing is not described here and only requires temporal DA concentration data. The theoretical support and foundations of the QN framework have been extensively described previously2, but a practical perspective on applying the QN framework to model DA response data is described below.
The QN framework models the dynamic interplay between: 1) dynamic DA release, 2) DA reuptake, and 3) the effects of supraphysiological stimulations on these processes to extract meaningful kinetic information from DA response data. The QN framework is best suited for modeling FSCV data acquired using highly supraphysiological stimulations of long duration (e.g., 60 Hz, 10 s stimulations), which produce robust DA responses that are amenable for kinetic analysis. Following the accurate modeling of the underlying release and reuptake processes, the model parameters can be used to simulate a DA response that should approximate the shape of the experimental DA response.
The equations of the QN framework describe the rates of DA release and reuptake over the course of the stimulated DA responses. The QN framework describes the stimulated DA release rate as a function of time from the start of stimulation (tstim), when the DA release rate exponentially decreases over the course of stimulation. This is consistent with the depletion of a readily releasable pool, with an added steady-state DA release rate (DARss) to account for vesicle replenishment, similar to other reports (Equation 2)14,17.
![]()
Manipulations that increases the DA release rate, such as increasing ΔDAR, ΔDARτ, or DARss, lead to increased response amplitudes on DA versus time plots. Each parameter contributes differentially to DA response shapes. Increasing DARss and ΔDARτ both make the rising phase of responses more linear (less convex). Decreasing ΔDARτ promotes convexity, which is controlled by the magnitude of ΔDAR. Based on modeling experience, DARss is generally less than 1/5th of ΔDAR; thus, ΔDAR is the release parameter that primarily determines the overall response amplitude of a DA response.
The post-stimulation DA release rate is modeled by Equation 3 as a continuation of the stimulated DA release rate from the end of stimulation (DARES) as a function of time after stimulation (tpost). The post-stimulation DA release rate follows a biphasic decay pattern, as previously described15, with a rapid exponential decay phase and a prolonged linear decay phase to model two calcium-dependent neurotransmitter release processes.
![]()
(Rapid exponential decay) (Prolonged linear decay)
It is not currently possible to determine how much post-stimulation DA release occurs. This limitation can be addressed by systematically minimizing estimates of post-stimulation DA release and validating model parameters across a set of experimental DA responses collected from the same recording site using varying stimulation durations. This minimization allows users to make conservative estimates of release and reuptake. Because electrical stimulations lead to the calcium accumulation that promotes post-stimulation neurotransmitter release, the duration of stimulation influences the post-stimulation neurotransmitter release parameters18,19. Based on modeling experience, it was found that as the stimulation duration increases, τR increases and XR decreases, consistent with the anticipated effects of a greater calcium accumulation20.
Equation 4 describes the DA reuptake rate as an extension of the M-M framework and incorporates a dynamic Km term, which increases during stimulation to model a progressively decreasing reuptake efficiency caused by the supraphysiological stimulations2,16. The Km after stimulation is held constant at the Km value at the end of stimulation (KmES).
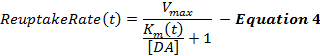
where,
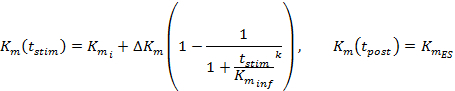
(During stimulation) (After stimulation)
Stimulated DA responses, especially from ventral striatal regions, are often insensitive to changes in the initial Km value (Kmi), which makes defining a Kmi value problematic. Thus, like the original M-M framework, Kmi is approximated at 0.1-0.4 µM for DA responses collected from control untreated animals12. The ΔKm term determines the extent of reuptake efficiency change during stimulation, which from our experience is about 20 µM over the course of a 60-Hz, 10-s stimulation. The k and Kminf values determine how Km changes over time, and increasing either of these terms promotes the concavity of the rising phase. Vmax is the maximal reuptake rate that partly relates to local DA transporter density, which exhibits a ventromedial to dorsolateral gradient21. Accordingly, Vmax values in the dorsal striatum (D-Str) are generally greater than 30 µM/s but generally less than 30 µM/s in the ventral regions, like the nucleus accumbens (NAc)6.
The general guidelines above can aid in modeling experimental DA response data, but generating a simulation that approximates the experimental DA response requires iteratively adjusting model parameters. The accuracy of the model parameters can be improved by obtaining DA responses to supraphysiological stimulations that provide a robust substrate for simulation, as well as by obtaining and modeling multiple DA responses to stimulations of varying durations at the same recording site (e.g., 60-Hz, 5-s and 10-s stimulations) to validate the accuracy of the parameters (see the sample data). To demonstrate, a dataset is included with the software package containing regiospecific stimulated DA responses collected in the nucleus accumbens and dorsal striatum, before and after a pharmacological challenge that was already modeled using the QN framework. By extension, users will find this methodology can similarly be applied to characterize the kinetics of DA neurotransmission in various disease contexts and pharmacological manipulations.
Protocol
1. Installation, Data Preparation, and Launching QNsim1.0
Download "QNsim1.0.zip" (provided as a supplement) and extract it to a desired directory.
Prepare stimulated DA response data for modeling with the software program by organizing a spreadsheet in which each column contains a temporal DA response converted to µM DA concentrations. Save this (.xlsx) file to the same directory as the program files. Note: The spreadsheet can contain multiple responses in an individual experiment or multiple studies containing a fixed number of responses (see "Sample.xlsx" for an example).
Open the programming environment software, navigate to the "QNSim1.0" directory in the "Current Folder" window, and open the filed entitled "Initialization.m." Click "Run" to launch the initialization screen (Figure 1).
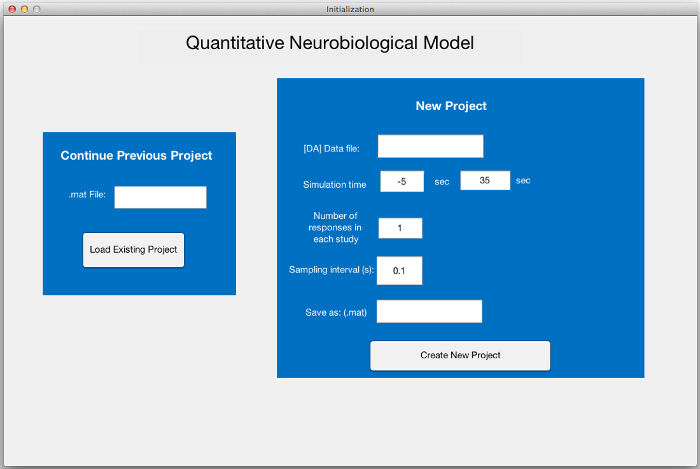
Figure 1: Initialization Screen. This screen allows users to continue an existing project, by entering a previous *.mat project file (left), or to begin a new project from stored data of stimulated DA neurotransmission (right). Textboxes are provided to input the *.xlsx filename containing the data, a few essential descriptors of the data, and the *.mat project filename that will contain all the data relating to the project. Please click here to view a larger version of this figure.
2. Initialization of the Simulation Environment
Note: The initialization screen allows users to begin a new project (step 2.1) or continue with a previously saved project (step 2.2).
- Option 1: Beginning a new project
- Under the New Project section, type in the name of the spreadsheet file that contains the DA response data for the project (e.g., Sample.xlsx) into the [DA] Data file textbox. Note: The file must be in the same folder as the program files.
- Next to Simulation time, input the time points corresponding to the start and end of data collection (in seconds) relative to the start of the stimulation. For example, if the data collection begins 5 s before the stimulation and ends 35 s after, as in the Sample.xlsx, input "-5" and "35."
- Input the number of responses in each study next to the corresponding text box.
- Next to sampling interval, input the experimental data sampling interval in seconds.
- Next to Save as (.mat), designate the filename, including the file type (.mat) where the project is to be saved. Note: This is the file that will be accessed to create the simulations, and all work can be saved to this project file.
- Click Create New Project to launch the Simulator window (Figure 2).
- Option 2: Continuing a previous project
- In the Continue Previous Project section of the Initialization window, enter the.mat filename of a previously begun project (e.g., Sample.mat). Note: This file must be contained within the same folder as the software program files.
- Click Load Existing Project to launch the Simulator window (Figure 2).
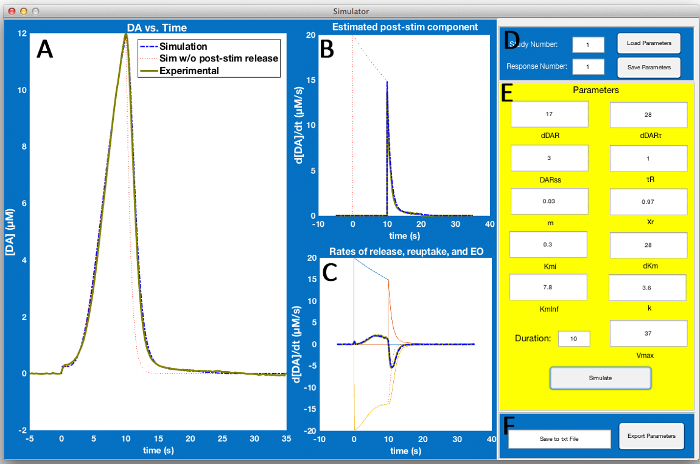
Figure 2: Simulator Screen. The simulator screen allows users to select the experimental data in D and adjust simulation parameters in E to model the experimental data. Visual inspection of (A, B, and C) can then aid in the refinement of simulation parameters so that the simulation (blue dashed lines) models the experimental data (thick green lines). Here, (A) contains experimental and simulated DA concentration versus time data. (B) is a graph that aids in estimating post-stimulation DA release. (C) is a plot of the experimental and simulated first derivatives of DA concentration versus time data from A or the rate of evoked overflow (EO). The rate of EO is theoretically a balance of release-reuptake rates, which is superimposed on this graph. Please click here to view a larger version of this figure.
3. Using the Simulator Window
Note: The Simulator screen allows for selecting the experimental data to model (step 3.1), adjust parameters to simulate experimental DA response data (step 3.2), save/load modeling parameters for each DA response (step 3.3), and export the saved parameters (step 3.4).
- Selecting the experimental DA response to simulate.
- Select the experimental DA response to simulate by inputting the study number, response number, and duration of the stimulation in the corresponding textboxes. Press the "enter" key or click simulate to begin the simulation process, which creates 3 graphs containing experimental data (thick green lines), simulated data (blue dashed lines), and simulated data that does not account for post-stimulation DA release (red dotted lines), as in Figure 2.
- Modeling experimental DA responses. Note: The objective of modeling is to adjust the model parameters relating to stimulated DA release (step 3.2.1), DA reuptake (step 3.2.2), and post-stimulation DA release (step 3.2.3) to accurately model experimental DA responses. Modeling is an iterative process whereby the starting parameters are refined until the parameters yield a simulated DA response that approximates the experimental data in the DA versus Time graph (Panel A), which can be aided by fitting the simulated data to the experimentally derived data in the Estimated post-stimulation component graph(Panel B)and the Rates of release, reuptake, and EO graph (Panel C).
- Adjust the ΔDAR, ΔDARτ, and DARss parameters associated with DA release (Equation 2) to match the amplitude of the stimulation. Note: These parameters are only starting estimates that will get refined, but ΔDARτ=25 and a DARss value that is 1/5th of ΔDAR are satisfactory starting conditions. Increasing any of these parameters will increase the amplitude in the DA versus Time graph and increase the DA release rate in the Estimated post-stimulation release component and Rates of release, reuptake, and EO graphs.
- Adjust the Vmax, Kmi, ΔKm, Kminf, and k parameters associated with to DA reuptake (Equation 4), such that in Panel A, the simulated data approximates the shape of the rising phase of the experimental data (thick green line) and such that the simulation w/o post-stim release trace (red dotted line) is less than the experimental data trace for all post-stimulation time points. Note: This step likely requires readjusting DA release parameters (step 3.2.1).
- Adjust the XR, τR, and m parameters associated with post-stimulation DA release (Equation 3), such that the simulation approximates the experimental data in the DA versus Time graph. Note: XR should take on a value between 0 and 1 and should generally be greater than 0.7.
- Saving/loading the modeling parameters.
- Once a set of parameters closely models the experimental data, click Save Parameters, which will save that set of parameters for the given response to the.mat file for the project.
- If required, load previously saved parameters for a particular response by clicking Load Parameters. Ensure that the appropriate study number and response number are inputted in the corresponding text boxes.
- Exporting saved parameters.
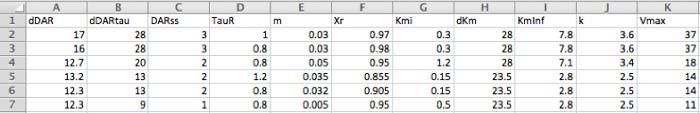
- In the textbox next to the Export Parameters button, type in the filename (e.g., Sample.txt) and click Export Parameters to export a text file with all the parameters of the simulations. Delimit the text file using spaces to generate a spreadsheet of parameters, as in Figure 4 of the Representative Results section below.
Representative Results
Included with the software program are sample DA neurotransmission data obtained from the rat dorsal striatum (Study 1) and the nucleus accumbens (Study 2) that are compiled into "Sample.xlsx." The spreadsheet contains the DA concentration data of the baseline responses to 60 Hz, 10 s and 5 s stimulations and a response to a 60 Hz, 5 s stimulation 35 min after the administration of the DA transport inhibitor methylphenidate (MPH) (10 mg/kg, i.p.). The stimulated DA response data is organized into columns, as demonstrated in Figure 3, which serves as a template for organizing data to be modeled using the software program.
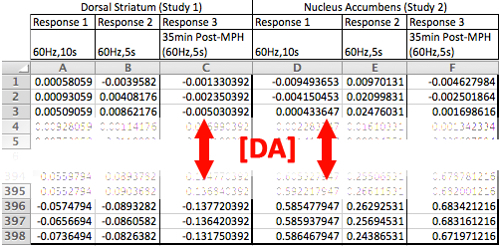
Figure 3:Structuring the Data into a Readable Format for Modeling. The stimulated DA response data from Sample.xlsx is organized into columns, as above, with each column containing an individual stimulated DA response. Note that there are no columns relating to the time domain in this spreadsheet. This is accounted for in the initialization screen.
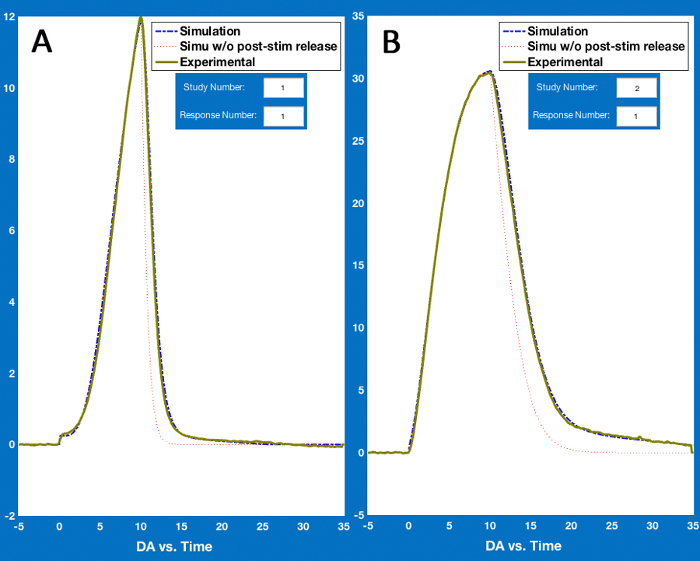
The sample data was modeled as described in step 3.2 (modeling experimental DA responses) using the parameters in Figure 4. This yielded simulations that approximated the experimental data well in both the D-Str (Figure 5A) and the NAc (Figure 5B).
Figure 4: Exported Simulation Parameters for Modeling Sample Experimental Data. Each row contains simulation parameters correlating to an individual stimulated DA response. Here, rows 2-4 and 5-7 correspond to simulation parameters for the dorsal striatum and nucleus accumbens data (see Figure 3 above). Please click here to view a larger version of this figure.
FSCV experiments involving the collection of multiple DA responses generally incorporate sufficient inter-stimulation intervals to allow the system to restore itself to produce reproducible stimulated DA responses25. In an ideal scenario that produced reproducible responses, stimulated DA release parameters (ΔDAR, ΔDATτ, and DARss) and reuptake parameters (Vmax, Kmi, ΔKm, Kminf, and k) would be constant for baseline responses to the same frequency of stimulation. However, in practice, there are small changes in DA response shapes and amplitudes over time that translate to decreases in DA release metrics, like ΔDAR in this dataset (compare row 2 to 3 and 5 to 6 in Figure 4), or to decreases in Vmax.
In contrast to stimulated DA release, post-stimulation DA release parameters change based upon the duration of stimulation. This is likely due to a stimulation-induced accumulation of intracellular Ca2+ that prolongs the post-stimulation release and increases the relative contribution of the slower linear decay component of post-stimulation DA release20,26. These stimulation duration-dependent effects can be seen in the representative modeling parameters, with the 10 s stimulation exhibiting larger τR values and smaller XR values than the 5 s stimulations (compare row 2 to 3 and 5 to 6 in Figure 4).
Figure 5: Simulations of Sample Data. Simulations demonstrate the close fit of simulated data (blue dashed lines) to the sample experimental data (thick green lines). Here, (A and B) depict simulations and experimental data collected in the dorsal striatum and nucleus accumbens, respectively. See Figure 4 for the parameters used to model the experimental data. Please click here to view a larger version of this figure.
Stimulated DA responses from the two regions produced very different response shapes, with concave rising shapes in the D-Str (Figure 5A) and convex rising shapes in the NAc (Figure 5B). Both response shapes could be modeled with a few notable differences in parameterizations. Although there is variability in response shapes and amplitudes even within a given region, Kminf is generally much lower in in the NAc compared to the D-Str. Moreover, Vmax, ΔDAR, and ΔDARτ tend to be lower in the NAc as well, as is the case in this sample dataset (compare rows 2 and 5 in Figure 4).
Included in the sample dataset are responses to an MPH challenge. Although primarily a DAT inhibitor, MPH is known to have secondary effects on DA release22,27 and may alter the plasmalemmal expression of DAT like other DAT inhibitors28,29. Experimental responses were modeled after a 10 mg/kg MPH by holding the rapid component of post-stimulation release, τR, constant pre- and post-MPH administration, making the arguable assumption that MPH-induced changes in stimulated DA neurotransmission kinetics are not due to changes in post-stimulation DA release. This allowed for the examination of changes in stimulated release and reuptake kinetics. In this example, MPH induced an increase in simulated Kmi, values as is expected for a competitive DAT inhibitor, but also a decrease in ΔDARτ and Vmax.
Supplemental File QNSim1.0.zip: Please click here to download this file.
Discussion
The use of FSCV to study in vivo stimulated DA neurotransmission originated in the 1980s30 and still continues to be a rich source of in vivo neurotransmission data with unparalleled spatial and temporal resolution. Stimulated DA responses reflect a complex balance of DA release and reuptake that are modulated by the electrical stimulations themselves. The QN model incorporates principles from contemporary neurotransmission research to model in vivo stimulated DA neurotransmission data in terms of dynamic release and reuptake assumptions2. Moreover, the QN framework extends the possible regions of analysis with FSCV to sites that do not produce “convex” responses, like the dorsal striatum. These advancements allow for the regional characterization of in vivo DAergic pharmacodynamics6 and DAergic alterations in CNS disease models. The regional characterization of DA neurotransmission is especially important because the dorsal and ventral striatum have different functional implications, receive different DAergic innervation from different neuronal populations, and are differentially susceptible in disease-states like Parkinson’s31,32.
As Figure 5 demonstrates, the QN framework is capable of closely modeling experimental data; however, there are limitations with the modeling methods. With 12 adjustable parameters in the QN framework, multiple sets of parameterizations can closely simulate an experimental DA response, and it is important to determine which set of parameterizations most precisely and accurately reflects the underlying kinetics of DA release and reuptake. While it is not possible to extract model parameters that accurately reflect the underlying neurobiology with certainty, it is possible to systematically determine minimal estimates of release and reuptake parameters that fit the assumptions of the QN framework. Thus, the model parameters should be interpreted as conservative estimates of release and reuptake kinetics. To hone the accuracy of the model parameters, multiple DA responses elicited by different durations of stimulation should be obtained from the same recording site. This generates multiple substrates for modeling that can constrain simulation parameters to enhance their accuracy. Other constraints can systematically be placed on model parameters based on literature data (e.g., Kmi ≈ 0.1-0.4 µM and τR ≈ 1.2 for 60 Hz,10 s stimulations) to facilitate data modeling, especially for study designs where modeling relative temporal changes is more important than the absolute accuracy of model parameters. Strategies to enhance the precision and accuracy of model parameters are an ongoing effort.
The QN framework has strong theoretical foundations for its assumptions on how stimulation affects DA neurotransmission kinetics. The QN framework itself does not account for the possible effects of electrode response lag and diffusional distortion in the DA responses33,34, the existence and relative importance of which are debated in the field3. For instance, the regional kinetic variability of DA responses has previously been attributed to cytoarchitectural differences, with white matter tracts acting as a mass transport barrier in the dorsal striatum35. However, variability in DA response shapes are diminished by the administration of D2 antagonists or DAT inhibitors3,13, suggesting that regional response variability is linked to underlying differences in release and reuptake kinetics. If users see fit, DA responses can be deconvoluted to remove diffusional distortions according to previously published methods33,34,36, and this processed DA response data can still be modeled using the software program as usual.
Disclosures
The authors have nothing to disclose.
Acknowledgments
We acknowledge the UPMC Rehabilitation Institute for supporting this work.
References
- Taylor IM, et al. Kinetic diversity of dopamine transmission in the dorsal striatum. J Neurochem. 2015;133(4):522–531. doi: 10.1111/jnc.13059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harun R, Grassi CM, Munoz MJ, Torres GE, Wagner AK. Neurobiological model of stimulated dopamine neurotransmission to interpret fast-scan cyclic voltammetry data. Brain Res. 2015;1599:67–84. doi: 10.1016/j.brainres.2014.12.020. [DOI] [PubMed] [Google Scholar]
- Taylor IM, Jaquins-Gerstl A, Sesack SR, Michael AC. Domain-dependent effects of DAT inhibition in the rat dorsal striatum. Journal of neurochemistry. 2012;122(2):283–294. doi: 10.1111/j.1471-4159.2012.07774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris PA, Ciolkowski EL, Wightman RM. Heterogeneity of evoked dopamine overflow within the striatal and striatoamygdaloid regions. Neuroscience. 1994;59(2):417–427. doi: 10.1016/0306-4522(94)90606-8. [DOI] [PubMed] [Google Scholar]
- May LJ, Wightman RM. Heterogeneity of stimulated dopamine overflow within rat striatum as observed with in vivo voltammetry. Brain Res. 1989;487(2):311–320. doi: 10.1016/0006-8993(89)90835-4. [DOI] [PubMed] [Google Scholar]
- Harun R, et al. Fast-scan cyclic voltammetry demonstrates that L-DOPA produces dose-dependent regionally selective, bimodal effects on striatal dopamine kinetics in vivo. J Neurochem. 2015. [DOI] [PMC free article] [PubMed]
- Jones SR, Garris PA, Wightman RM. Different effects of cocaine and nomifensine on dopamine uptake in the caudate-putamen and nucleus accumbens. The Journal of pharmacology and experimental therapeutics. 1995;274(1):396–403. [PubMed] [Google Scholar]
- Budygin EA, John CE, Mateo Y, Jones SR. Lack of cocaine effect on dopamine clearance in the core and shell of the nucleus accumbens of dopamine transporter knock-out mice. J Neurosci. 2002;22(10):RC222. doi: 10.1523/JNEUROSCI.22-10-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, et al. Loss of autoreceptor functions in mice lacking the dopamine transporter. Nat Neurosci. 1999;2(7):649–655. doi: 10.1038/10204. [DOI] [PubMed] [Google Scholar]
- Wagner AK, et al. Chronic methylphenidate treatment enhances striatal dopamine neurotransmission after experimental traumatic brain injury. J Neurochem. 2009;108(4):986–997. doi: 10.1111/j.1471-4159.2008.05840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AK, et al. Controlled cortical impact injury influences methylphenidate-induced changes in striatal dopamine neurotransmission. J Neurochem. 2009;110(3):801–810. doi: 10.1111/j.1471-4159.2009.06155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman RM, et al. Real-time characterization of dopamine overflow and uptake in the rat striatum. Neuroscience. 1988;25(2):513–523. doi: 10.1016/0306-4522(88)90255-2. [DOI] [PubMed] [Google Scholar]
- Moquin KF, Michael AC. Tonic autoinhibition contributes to the heterogeneity of evoked dopamine release in the rat striatum. J Neurochem. 2009;110(5):1491–1501. doi: 10.1111/j.1471-4159.2009.06254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyott SJ, Rosenmund C. The effects of temperature on vesicular supply and release in autaptic cultures of rat and mouse hippocampal neurons. J Physiol. 2002;539 (Pt 2):523–535. doi: 10.1113/jphysiol.2001.013277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atluri PP, Regehr WG. Delayed release of neurotransmitter from cerebellar granule cells. J Neurosci. 1998;18(20):8214–8227. doi: 10.1523/JNEUROSCI.18-20-08214.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SR, et al. Role of vesicle pools in action potential pattern-dependent dopamine overflow in rat striatum in vivo. J Neurochem. 2011;119(2):342–353. doi: 10.1111/j.1471-4159.2011.07440.x. [DOI] [PubMed] [Google Scholar]
- Taschenberger H, von Gersdorff H. Fine-tuning an auditory synapse for speed and fidelity: developmental changes in presynaptic waveform, EPSC kinetics, and synaptic plasticity. J Neurosci. 2000;20(24):9162–9173. doi: 10.1523/JNEUROSCI.20-24-09162.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda Y, Stevens CF. Two components of transmitter release at a central synapse. Proc Nat Acad of Sci U S A. 1994;91(26):12942–12946. doi: 10.1073/pnas.91.26.12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Gaffaney JD, Kwon SE, Chapman ER. Doc2 is a Ca2+ sensor required for asynchronous neurotransmitter release. Cell. 2011;147(3):666–677. doi: 10.1016/j.cell.2011.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler DJ, Goda Y. Properties of synchronous and asynchronous release during pulse train depression in cultured hippocampal neurons. J Neurophysiol. 2001;85(6):2324–2334. doi: 10.1152/jn.2001.85.6.2324. [DOI] [PubMed] [Google Scholar]
- Ciliax BJ, et al. The dopamine transporter: immunochemical characterization and localization in brain. J Neurosci. 1995;15(3 Pt 1):1714–1723. doi: 10.1523/JNEUROSCI.15-03-01714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz TJ, Farnsworth SJ, Rowley SD, Hanson GR, Fleckenstein AE. Methylphenidate-induced increases in vesicular dopamine sequestration and dopamine release in the striatum: the role of muscarinic and dopamine D2 receptors. J Pharm Exp Ther. 2008;327(1):161–167. doi: 10.1124/jpet.108.139386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresel SH, Kung MP, Plossl K, Meegalla SK, Kung HF. Pharmacological effects of dopaminergic drugs on in vivo binding of [99mTc]TRODAT-1 to the central dopamine transporters in rats. Eur J Nucl Med. 1998;25(1):31–39. doi: 10.1007/s002590050191. [DOI] [PubMed] [Google Scholar]
- Near JA, Bigelow JC, Wightman RM. Comparison of uptake of dopamine in rat striatal chopped tissue and synaptosomes. J Pharm Exp Ther. 1988;245(3):921–927. [PubMed] [Google Scholar]
- Michael AC, Ikeda M, Justice JB., Jr Dynamics of the recovery of releasable dopamine following electrical stimulation of the medial forebrain bundle. Neurosci Lett. 1987;76(1):81–86. doi: 10.1016/0304-3940(87)90196-0. [DOI] [PubMed] [Google Scholar]
- Fierro L, DiPolo R, Llano I. Intracellular calcium clearance in Purkinje cell somata from rat cerebellar slices. The Journal of physiology. 1998;510 (Pt 2):499–512. doi: 10.1111/j.1469-7793.1998.499bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval V, Riddle EL, Hanson GR, Fleckenstein AE. Methylphenidate redistributes vesicular monoamine transporter-2: role of dopamine receptors. J Neurosci. 2002;22(19):8705–8710. doi: 10.1523/JNEUROSCI.22-19-08705.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws LC, et al. Cocaine increases dopamine uptake and cell surface expression of dopamine transporters. Biochem Biophys Res Commun. 2002;290(5):1545–1550. doi: 10.1006/bbrc.2002.6384. [DOI] [PubMed] [Google Scholar]
- Little KY, Kirkman JA, Carroll FI, Clark TB, Duncan GE. Cocaine use increases [3H]WIN 35428 binding sites in human striatum. Brain Res. 1993;628(1-2):17–25. doi: 10.1016/0006-8993(93)90932-d. [DOI] [PubMed] [Google Scholar]
- Ewing AG, Bigelow JC, Wightman RM. Direct in vivo monitoring of dopamine released from two striatal compartments in the rat. Science. 1983;221(4606):169–171. doi: 10.1126/science.6857277. [DOI] [PubMed] [Google Scholar]
- Janezic S, et al. Deficits in dopaminergic transmission precede neuron loss and dysfunction in a new Parkinson model. Proc Natl Acad Sci U S A. 2013;110(42):E4016–E4025. doi: 10.1073/pnas.1309143110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald PA, Monchi O. Differential effects of dopaminergic therapies on dorsal and ventral striatum in Parkinson's disease: implications for cognitive function. Parkinsons Dis. 2011;2011:572743. doi: 10.4061/2011/572743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kile BM, et al. Optimizing the Temporal Resolution of Fast-Scan Cyclic Voltammetry. ACS Chem Neurosci. 2012;3(4):285–292. doi: 10.1021/cn200119u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venton BJ, Troyer KP, Wightman RM. Response times of carbon fiber microelectrodes to dynamic changes in catecholamine concentration. Anal Chem. 2002;74(3):539–546. doi: 10.1021/ac010819a. [DOI] [PubMed] [Google Scholar]
- May LJ, Wightman RM. Heterogeneity of stimulated dopamine overflow within rat striatum as observed with in vivo voltammetry. Brain research. 1989;487(2):311–320. doi: 10.1016/0006-8993(89)90835-4. [DOI] [PubMed] [Google Scholar]
- Wu Q, Reith ME, Wightman RM, Kawagoe KT, Garris PA. Determination of release and uptake parameters from electrically evoked dopamine dynamics measured by real-time voltammetry. J Neurosci Methods. 2001;112(2):119–133. doi: 10.1016/s0165-0270(01)00459-9. [DOI] [PubMed] [Google Scholar]


