Abstract
Our current taxonomic perspective on Entamoeba is largely based on small-subunit ribosomal RNA genes (SSU rDNA) from Entamoeba species identified in vertebrate hosts with minor exceptions such as E. moshkovskii from sewage water and E. marina from marine sediment. Other Entamoeba species have also been morphologically identified and described from non-vertebrate species such as insects; however, their genetic diversity remains unknown. In order to further disclose the diversity of the genus, we investigated Entamoeba spp. in the intestines of three cockroach species: Periplaneta americana, Blaptica dubia, and Gromphadorhina oblongonota. We obtained 134 Entamoeba SSU rDNA sequences from 186 cockroaches by direct nested PCR using the DNA extracts of intestines from cockroaches, followed by scrutinized BLASTn screening and phylogenetic analyses. All the sequences identified in this study were distinct from those reported from known Entamoeba species, and considered as novel Entamoeba ribosomal lineages. Furthermore, they were positioned at the base of the clade of known Entamoeba species and displayed remarkable degree of genetic diversity comprising nine major groups in the three cockroach species. This is the first report of the diversity of SSU rDNA sequences from Entamoeba in non-vertebrate host species, and should help to understand the genetic diversity of the genus Entamoeba.
Introduction
The genus Entamoeba is an important taxonomic group consisting of parasitic species that reside in a variety of vertebrate and invertebrate hosts, and potentially free living species that are isolated from the environment. E. histolytica is one of the major causes of diarrheal diseases in tropical regions, which ranks fifth of DALY in 2015 [1]. Since other Entamoeba species generally lack virulence in humans, comparative biology, biochemistry, and genetics have been applied to the Entamoeba genus mainly to attempt to discover the virulence-related genes and to understand the evolution of Entamoeba pathogenicity in humans.
Genetic diversity of E. histoltyica from humans has been well investigated due to its medical importance. Clark and colleagues proposed to use “ribosomal lineages”, the nomenclature for newly discovered SSU rDNA sequences close enough to those from other Entamoeba species, but not convincingly considered to be from independent Entamoeba species [2–9]. In contrast, although quite a few Entamoeba species were identified at the molecular level from primates (e.g. E. nuttalli, and E. gingivalis), reptiles (E. invadens, E. insolita, and E. terrapinae), and environments (E. moshkovskii, E. ecuadoriensis, and E. marina [10]), the genetic diversity of the entire genus Entamoeba remains poorly understood. Other Entamoeba species have also been described, but only morphologically identified, from non-vertebrate hosts such as insects (E. apis [11], E. philippinensis [12] and E. polypodia [13]), leeches (E. aulastomi [14]), and protozoon (E. paulista [15]).
In order to better understand the genetic diversity of Entamoeba inhabiting invertebrate organisms, we investigated Entamoeba from cockroaches. Here we report SSU rDNA-based genetic diversity of Entamoeba from three cockroach species: one common house cockroach, Periplaneta americana, and two forest cockroaches, Blaptica dubia (orange-spotted cockroach, Guyana spotted cockroach, or Argentinian wood cockroach) and Gromphadorhina oblongonota (Madagascar forest hissing cockroach).
Materials and methods
Cockroach collection and isolation of intestinal contents
Three cockroach species were used in this study: Periplaneta americana (American cockroach), Blaptica dubia (Argentinian forest cockroach, Dubia cockroach) and Gromphadorhina oblongonota (Madagascar hissing cockroach). P. americana were collected from an apartment in Bangplee, located in an urban area of Samutprakarn, Thailand (13° 36' 0" N, 100° 36' 0" E) in April 21, 2016 and July 28, 2016 by manual capture (No specific permissions were required for field studies. The field studies did not involve endangered or protected species.). Individual bugs were identified as P. americana by their yellowish circular marking on the prothorax and were collected in two sampling periods. B. dubia and G. oblongonota (3–5 cm in size) were purchased from a pet shop in Tokushima, Japan (34° 4' 0" N, 134° 34' 0" E) where they were domestically bred. The cockroaches were dissected in order to isolate and excise their intestines. For the first batch of P. americana collected (Pa_01 to Pa_30), intestines isolated from 4 individual cockroaches were combined, and then ground in a sterile mortar and pestle in 2 ml of sterile normal saline; that is, sample Pa_01 contained the intestines of 4 cockroaches. For P. americana collected in the second period, B. dubia and G. oblongonota (Pa_31 to Pa_80, Bd_01 to Bd_22 and Go_01 to Go_14 respectively), the intestines were not combined and were ground separately.
DNA extraction and amplification of SSU rDNA derived from Entamoeba
DNA was extracted from approximately 500 μL of the ground intestine(s) using DNeasy Blood and Tissue kit (QIAGEN, Tokyo, Japan). A fragment corresponding to Entamoeba SSU rDNA was amplified by nested PCR using DNA extracted from the isolated cockroach intestine(s). In the first round of PCR, an approximately 1,950 bp long fragment corresponding to SSU rDNA was amplified using eukaryotic universal oligonucleotide primers specific for SSU rDNA (EukA: 5'-AACCTGGTTGATCCTGCCAGT-3' and EukB: 5'-TGATCCTTCTGCAGGTTCACCTAC-3'; [16]) by Tks Gflex DNA Polymerase (TaKaRa, Shiga, Japan). PCR conditions consisted of 30 cycles of denaturation at 94°C for 22 seconds, annealing at 42°C for 1 minute and extension at 72°C for 1 minute. One μL of PCR products were used as templates of the second round PCR. In the second round of PCR, an approximately 1,900 bp fragment of Entamoeba SSU rDNA was selectively amplified using oligonucleotide primers specific for Entamoeba SSU rDNA (01F: 5’-GCCAGTATTATATGCTGA-3’ and 01R: 5’-CCTTGTTACGACTTCTCCTT-3’). PCR conditions consisted of 30 cycles of denaturation at 94°C for 22 seconds, annealing at 52°C for 1 minute and extension at 72°C for 1 minute.
Sequencing and screening of SSU rDNA of Entamoeba from cockroaches
The amplicons obtained from the second round PCR were cloned into pCRTM-Blunt II-TOPO (Thermo Fisher Scientific, Waltham, Massachusetts, USA) and the plasmids were transfected into competent Escherichia coli DH5α cells. Five to twenty colonies were examined by PCR using the universal oligonucleotide primers M13F/R (5'-GTAAAACGACGGCCAGTG-3' and 5'-CAGGAAACAGCTATGACCATG-3') to confirm if an insert is present in the plasmids from the bacterial colonies. After purification of plasmids, an insert of each plasmid was fully sequenced in both directions with M13F, M13R, M13Mid1 (5’-TACTTTGAATAAATACGAGTGTT-3’), and M13Mid2 (5’-TCCCGTGTTGAGTCAAATTAA-3’) primers. The latter two primers correspond to 18S rRNA gene. The sequences were examined by BLASTn [17] search against non-redundant (nr) nucleotide database of NCBI with default parameters to verify whether they only show highest similarity with Entamoeba. When needed, phylogenetic analysis (described below) was also used. Sequence reads were assembled using CLC Genomics Workbench Version 8.5.1 (Qiagen Aahus A/S, Aahus C, Denmark).
Molecular phylogenetic analysis
Molecular phylogenetic analysis was performed to determine the relationship of cockroach-derived Entamoeba SSU rDNA with other eukaryotic organisms including other known Entamoeba species and Archamoebae. Analyses were performed as follows: 1) Sequences were aligned by MAFFT v7.187 [18], 2) aligned nucleotide sites were selected by Gblocks [19] and manual inspection using SeaView 4.6 [20], 3) Maximum-likelihood (ML) tree was inferred by RAxML 8.1.5 [21] with General Time-Reversible (GTR) + gamma substitution model. Statistical confidence of ML trees was evaluated with bootstrap proportions of the trees from 100 or 1,000 replicates for screening and detailed analyses, respectively. In the screening, when a sequence analyzed showed monophyly with other known Entamoeba species, it was considered to be included in the Entamoeba genus.
Results and discussion
A total of 134 Entamoeba SSU rDNA sequences were obtained from 186 cockroaches
The workflow of acquisition and screening of Entamoeba SSU rDNA genes from cockroaches is summarized in Fig 1. In brief, we isolated and purified DNA from the intestines of 186 cockroaches (150 P. americana, 22 B. dubia, and 14 G. oblongonota), and SSU rDNA was amplified by nested PCR. Nested PCR was successful for 54, 16, and 8 samples, respectively. The plasmids that contained nested PCR products (256, 50 and, 36 from each cockroach group) were obtained and sequenced. Subsequently, BLASTn search and phylogenetic analyses were performed to exclude non-Entamoeba SSU rDNA sequences. Finally, 77, 39, and 18 Entamoeba SSU rDNA sequences were subjected to further analyses (Table 1).
Fig 1. Flow diagram depicting experimental procedures and the number of analyzed samples.
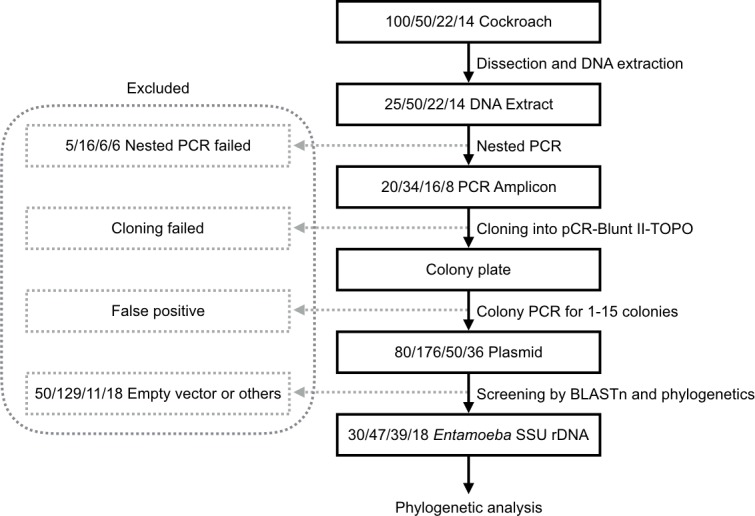
The numbers in rectangles indicate those of samples from P. americana (first sampling), P. americana (second sampling), B. dubia and G. oblongonota, respectively. For samples from the first sampling of P. americana, the intestines from 4 cockroaches were pooled.
Table 1. The list of the sequences used in this study.
| # | Sequence ID | Source | Cockroach ID | Colony ID | Accession No |
|---|---|---|---|---|---|
| 1 | Bd_06–2 | B. dubia | 6 | 2 | LC259314 |
| 2 | Bd_06–10 | B. dubia | 6 | 10 | LC259315 |
| 3 | Bd_08–1 | B. dubia | 8 | 1 | LC259316 |
| 4 | Bd_08–7 | B. dubia | 8 | 7 | LC259317 |
| 5 | Bd_09–1 | B. dubia | 9 | 1 | LC259318 |
| 6 | Bd_09–2 | B. dubia | 9 | 2 | LC259319 |
| 7 | Bd_09–3 | B. dubia | 9 | 3 | LC259320 |
| 8 | Bd_10–1 | B. dubia | 10 | 1 | LC259321 |
| 9 | Bd_10–2 | B. dubia | 10 | 2 | LC259322 |
| 10 | Bd_10-2b | B. dubia | 10 | 2b | LC259323 |
| 11 | Bd_11–1 | B. dubia | 11 | 1 | LC259324 |
| 12 | Bd_11–2 | B. dubia | 11 | 2 | LC259325 |
| 13 | Bd_11–6 | B. dubia | 11 | 6 | LC259326 |
| 14 | Bd_12–2 | B. dubia | 12 | 2 | LC259327 |
| 15 | Bd_13–1 | B. dubia | 13 | 1 | LC259328 |
| 16 | Bd_13–4 | B. dubia | 13 | 4 | LC259329 |
| 17 | Bd_13–5 | B. dubia | 13 | 5 | LC259330 |
| 18 | Bd_14–1 | B. dubia | 14 | 1 | LC259331 |
| 19 | Bd_14–2 | B. dubia | 14 | 2 | LC259332 |
| 20 | Bd_15–2 | B. dubia | 15 | 2 | LC259333 |
| 21 | Bd_15–3 | B. dubia | 15 | 3 | LC259334 |
| 22 | Bd_15–4 | B. dubia | 15 | 4 | LC259335 |
| 23 | Bd_16–1 | B. dubia | 16 | 1 | LC259336 |
| 24 | Bd_16–2 | B. dubia | 16 | 2 | LC259337 |
| 25 | Bd_16–3 | B. dubia | 16 | 3 | LC259338 |
| 26 | Bd_17–2 | B. dubia | 17 | 2 | LC259339 |
| 27 | Bd_17–3 | B. dubia | 17 | 3 | LC259340 |
| 28 | Bd_18–6 | B. dubia | 18 | 6 | LC259341 |
| 29 | Bd_18–7 | B. dubia | 18 | 7 | LC259342 |
| 30 | Bd_18–8 | B. dubia | 18 | 8 | LC259343 |
| 31 | Bd_19–5 | B. dubia | 19 | 5 | LC259344 |
| 32 | Bd_19–6 | B. dubia | 19 | 6 | LC259345 |
| 33 | Bd_20–1 | B. dubia | 20 | 1 | LC259346 |
| 34 | Bd_20–2 | B. dubia | 20 | 2 | LC259347 |
| 35 | Bd_21–2 | B. dubia | 21 | 2 | LC259348 |
| 36 | Bd_21–3 | B. dubia | 21 | 3 | LC259349 |
| 37 | Bd_22–1 | B. dubia | 22 | 1 | LC259350 |
| 38 | Bd_22–2 | B. dubia | 22 | 2 | LC259351 |
| 39 | Bd_22–3 | B. dubia | 22 | 3 | LC259352 |
| 40 | Go_06–1 | G. oblongonota | 6 | 1 | LC259353 |
| 41 | Go_06–9 | G. oblongonota | 6 | 9 | LC259354 |
| 42 | Go_07–1 | G. oblongonota | 7 | 1 | LC259355 |
| 43 | Go_07–5 | G. oblongonota | 7 | 5 | LC259356 |
| 44 | Go_07–6 | G. oblongonota | 7 | 6 | LC259357 |
| 45 | Go_07–8 | G. oblongonota | 7 | 8 | LC259358 |
| 46 | Go_08–1 | G. oblongonota | 8 | 1 | LC259359 |
| 47 | Go_09–2 | G. oblongonota | 9 | 2 | LC259360 |
| 48 | Go_09–3 | G. oblongonota | 9 | 3 | LC259361 |
| 49 | Go_09–4 | G. oblongonota | 9 | 4 | LC259362 |
| 50 | Go_10–1 | G. oblongonota | 10 | 1 | LC259363 |
| 51 | Go_10–3 | G. oblongonota | 10 | 3 | LC259364 |
| 52 | Go_11–3 | G. oblongonota | 11 | 3 | LC259365 |
| 53 | Go_11–5 | G. oblongonota | 11 | 5 | LC259366 |
| 54 | Go_13–5 | G. oblongonota | 13 | 5 | LC259367 |
| 55 | Go_14–2 | G. oblongonota | 14 | 2 | LC259368 |
| 56 | Go_14–3 | G. oblongonota | 14 | 3 | LC259369 |
| 57 | Go_14–4 | G. oblongonota | 14 | 4 | LC259370 |
| 58 | Pa_02–2 | P. americana | 2 | 2 | LC259371 |
| 59 | Pa_02–3 | P. americana | 2 | 3 | LC259372 |
| 60 | Pa_02–4 | P. americana | 2 | 4 | LC259373 |
| 61 | Pa_03–1 | P. americana | 3 | 1 | LC259374 |
| 62 | Pa_03–3 | P. americana | 3 | 3 | LC259375 |
| 63 | Pa_03–4 | P. americana | 3 | 4 | LC259376 |
| 64 | Pa_04–1 | P. americana | 4 | 1 | LC259377 |
| 65 | Pa_06–2 | P. americana | 6 | 2 | LC259378 |
| 66 | Pa_07–2 | P. americana | 7 | 2 | LC259379 |
| 67 | Pa_08–1 | P. americana | 8 | 1 | LC259380 |
| 68 | Pa_08–2 | P. americana | 8 | 2 | LC259381 |
| 69 | Pa_08–3 | P. americana | 8 | 3 | LC259382 |
| 70 | Pa_08–4 | P. americana | 8 | 4 | LC259383 |
| 71 | Pa_10–4 | P. americana | 10 | 4 | LC259384 |
| 72 | Pa_14–4 | P. americana | 14 | 4 | LC259385 |
| 73 | Pa_14–6 | P. americana | 14 | 6 | LC259386 |
| 74 | Pa_16–1 | P. americana | 16 | 1 | LC259387 |
| 75 | Pa_17–1 | P. americana | 17 | 1 | LC259388 |
| 76 | Pa_19–1 | P. americana | 19 | 1 | LC259389 |
| 77 | Pa_19–2 | P. americana | 19 | 2 | LC259390 |
| 78 | Pa_19–3 | P. americana | 19 | 3 | LC259391 |
| 79 | Pa_21–2 | P. americana | 21 | 2 | LC259392 |
| 80 | Pa_22–3 | P. americana | 22 | 3 | LC259393 |
| 81 | Pa_22–4 | P. americana | 22 | 4 | LC259394 |
| 82 | Pa_24–1 | P. americana | 24 | 1 | LC259395 |
| 83 | Pa_24–2 | P. americana | 24 | 2 | LC259396 |
| 84 | Pa_24–3 | P. americana | 24 | 3 | LC259397 |
| 85 | Pa_26–3 | P. americana | 26 | 3 | LC259398 |
| 86 | Pa_27–2 | P. americana | 27 | 2 | LC259399 |
| 87 | Pa_27–4 | P. americana | 27 | 4 | LC259400 |
| 88 | Pa_33–1 | P. americana | 33 | 1 | LC259401 |
| 89 | Pa_33–3 | P. americana | 33 | 3 | LC259402 |
| 90 | Pa_33–4 | P. americana | 33 | 4 | LC259403 |
| 91 | Pa_39–1 | P. americana | 39 | 1 | LC259404 |
| 92 | Pa_39–5 | P. americana | 39 | 5 | LC259405 |
| 93 | Pa_47–1 | P. americana | 47 | 1 | LC259406 |
| 94 | Pa_47–2 | P. americana | 47 | 2 | LC259407 |
| 95 | Pa_47–3 | P. americana | 47 | 3 | LC259408 |
| 96 | Pa_47–4 | P. americana | 47 | 4 | LC259409 |
| 97 | Pa_49–3 | P. americana | 49 | 3 | LC259410 |
| 98 | Pa_49–4 | P. americana | 49 | 4 | LC259411 |
| 99 | Pa_49–13 | P. americana | 49 | 13 | LC259412 |
| 100 | Pa_49–14 | P. americana | 49 | 14 | LC259413 |
| 101 | Pa_49–15 | P. americana | 49 | 15 | LC259414 |
| 102 | Pa_49–16 | P. americana | 49 | 16 | LC259415 |
| 103 | Pa_49–17 | P. americana | 49 | 17 | LC259416 |
| 104 | Pa_49–18 | P. americana | 49 | 18 | LC259417 |
| 105 | Pa_49–19 | P. americana | 49 | 19 | LC259418 |
| 106 | Pa_50–2 | P. americana | 50 | 2 | LC259419 |
| 107 | Pa_50–4 | P. americana | 50 | 4 | LC259420 |
| 108 | Pa_50–11 | P. americana | 50 | 11 | LC259421 |
| 109 | Pa_50–12 | P. americana | 50 | 12 | LC259422 |
| 110 | Pa_50–19 | P. americana | 50 | 19 | LC259423 |
| 111 | Pa_57–2 | P. americana | 57 | 2 | LC259424 |
| 112 | Pa_57–3 | P. americana | 57 | 3 | LC259425 |
| 113 | Pa_57–5 | P. americana | 57 | 5 | LC259426 |
| 114 | Pa_61–2 | P. americana | 61 | 2 | LC259427 |
| 115 | Pa_61–4 | P. americana | 61 | 4 | LC259428 |
| 116 | Pa_62–1 | P. americana | 62 | 1 | LC259429 |
| 117 | Pa_62–3 | P. americana | 62 | 3 | LC259430 |
| 118 | Pa_62–11 | P. americana | 62 | 11 | LC259431 |
| 119 | Pa_62–14 | P. americana | 62 | 14 | LC259432 |
| 120 | Pa_62–15 | P. americana | 62 | 15 | LC259433 |
| 121 | Pa_62–17 | P. americana | 62 | 17 | LC259434 |
| 122 | Pa_62–19 | P. americana | 62 | 19 | LC259435 |
| 123 | Pa_63–2 | P. americana | 63 | 2 | LC259436 |
| 124 | Pa_63–3 | P. americana | 63 | 3 | LC259437 |
| 125 | Pa_63–4 | P. americana | 63 | 4 | LC259438 |
| 126 | Pa_64–1 | P. americana | 64 | 1 | LC259439 |
| 127 | Pa_64–2 | P. americana | 64 | 2 | LC259440 |
| 128 | Pa_64–3 | P. americana | 64 | 3 | LC259441 |
| 129 | Pa_64–4 | P. americana | 64 | 4 | LC259442 |
| 130 | Pa_79–4 | P. americana | 79 | 4 | LC259443 |
| 131 | Pa_80–1 | P. americana | 80 | 1 | LC259444 |
| 132 | Pa_80–2 | P. americana | 80 | 2 | LC259445 |
| 133 | Pa_80–3 | P. americana | 80 | 3 | LC259446 |
| 134 | Pa_80–4 | P. americana | 80 | 4 | LC259447 |
Entamoeba SSU rDNA sequences from cockroaches are extremely heterogeneous, divergent from the reported sequences of known Entamoeba species, and composed of nine major groups
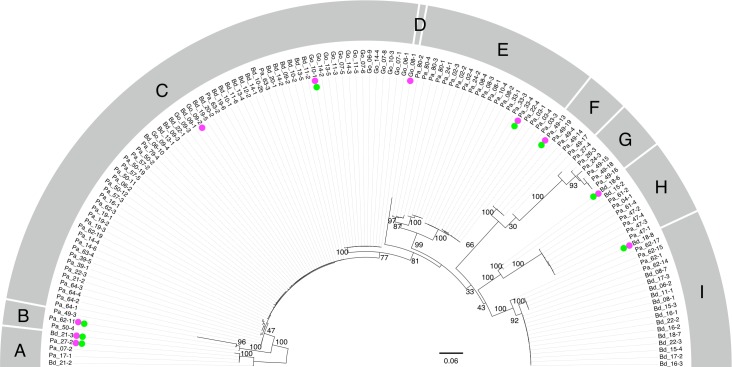
All Entamoeba SSU rDNA sequences from cockroaches are divergent from the reported sequences from known Entamoeba species. An unrooted phylogenetic tree was inferred by Maximum-likelihood (ML) method using 134 cockroach-derived Entamoeba SSU rDNA sequences (Fig 2). The 134 sequences were segregated into 9 groups (A-I), each of which was supported by good bootstrap values (> 70%), with exceptions for branching at A-B/C-I (47%), F/G (66%), H/I (43%) and F-G/H-I (33%).
Fig 2. SSU rDNA-based phylogenetic tree of 134 Entamoeba sequences from cockroaches.
SSU rDNA sequences were aligned using MAFFT v7.187. Unambiguously aligned sequences composed of 1,023 nucleotides were selected by Gblocks and manual inspection. Maximum-likelihood (ML) tree was inferred by RAxML 8.1.17 using GTRGAMMA model. The number of bootstrap pseudoreplicate trees was 1,000. ML tree was visualized using FigTree 1.4.0 and Keynote 6.6.2. Bootstrap values for major nodes are shown on each node. Nine groups (A to I) were shown to be monophyletic with high bootstrap support values. Representative sequences of each group used in Fig 3 or Fig 4 are indicated by green circles or magenta circles, respectively.
Phylogenetic position of Entamoeba SSU rDNA sequences from cockroaches in eukaryotes
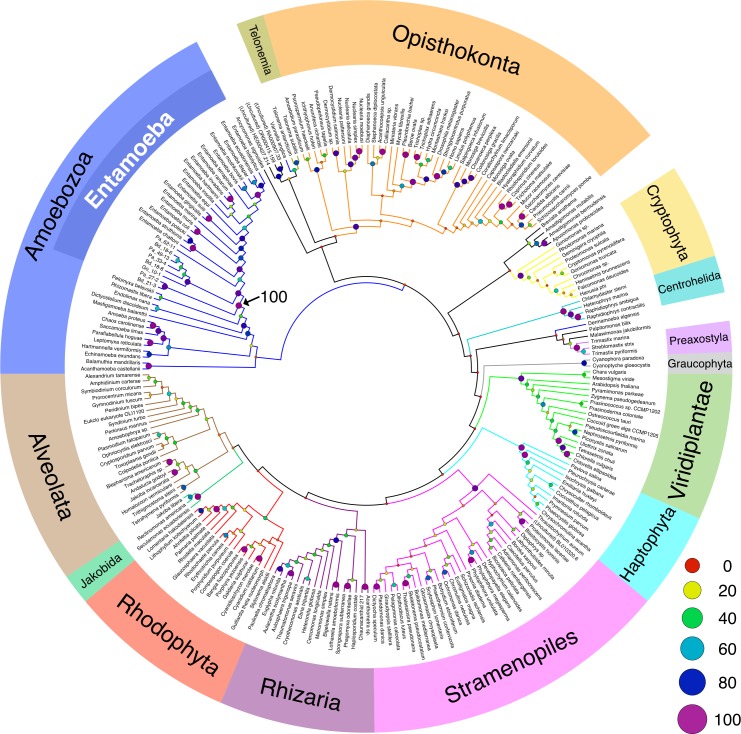
To examine the phylogenetic position of these cockroach-derived Entamoeba sequences, the cladogram was reconstructed using SSU rDNA dataset composing of major eukaryotic supergroups and eight representative sequences from Group A to I from cockroach-derived Entamoeba (Fig 3; marked with green circles in Fig 2; group D and G were omitted because of their high evolutionary rates). The monophyly of the clade comprising cockroach-derived Entamoeba (Pa_61–11, Bd_18–6, Pa_49–13, Pa_33–4, Bd_18–8, Go_10–1, Pa_27–2, and Bd_21–3) and other Entamoeba species were strongly supported (Fig 3; black arrow). This clade is nested within the node that contains other Archamoebae (Pelomyxa belevskii, Rhizomastix libera, Mastigamoeba balamuthi and Endolimax nana) and Dictyostelium discoideum, with high bootstrap support (Fig 3; black arrow). Although the monophyly of Amoebozoa was not supported by the bootstrap value, these data are consistent with the premise that the newly identified Entamoeba sequences are from novel Entamoeba ribosomal lineages.
Fig 3. SSU rDNA-based cladogram of major eukaryotic supergroups including representative cockroach-derived Entamoeba.
SSU rDNA sequences were aligned using MAFFT v7.187. Unambiguously aligned sequences composed of 914 nucleotides were selected by Gblocks and manual inspection. Maximum-likelihood (ML) tree was inferred by RAxML 8.1.17 using GTRGAMMA model. The number of bootstrap pseudoreplicate trees was 1,000. ML tree was visualized as a cladogram using FigTree 1.4.0 and Keynote 6.6.2. Note that all representative sequences of cockroach-derived Entamoeba are new Entamoeba ribosomal lineages, and their monophyly was supported by the high bootstrap value (100%; black arrow). The size and colors of circles at the nodes indicate the approximate bootstrap value.
Polymorphism of Entamoeba SSU rDNA sequences from cockroaches
As shown above, cockroach-derived Entamoeba SSU rDNA sequences were categorized into 9 groups (Fig 2). Groups A, B, D, E, H, and I were independent and well separated clades with almost maximum statistical support (bootstrap proportion: > 99%). Groups A, H and I were composed of sequences of the amoebae from both P. americana (11 of 77 P. americana-derived Entamoeba sequences) and B. dubia (4/20), whereas groups B and E were exclusively from P. americana (24/77), and group D was only from G. oblongonota (1/18).
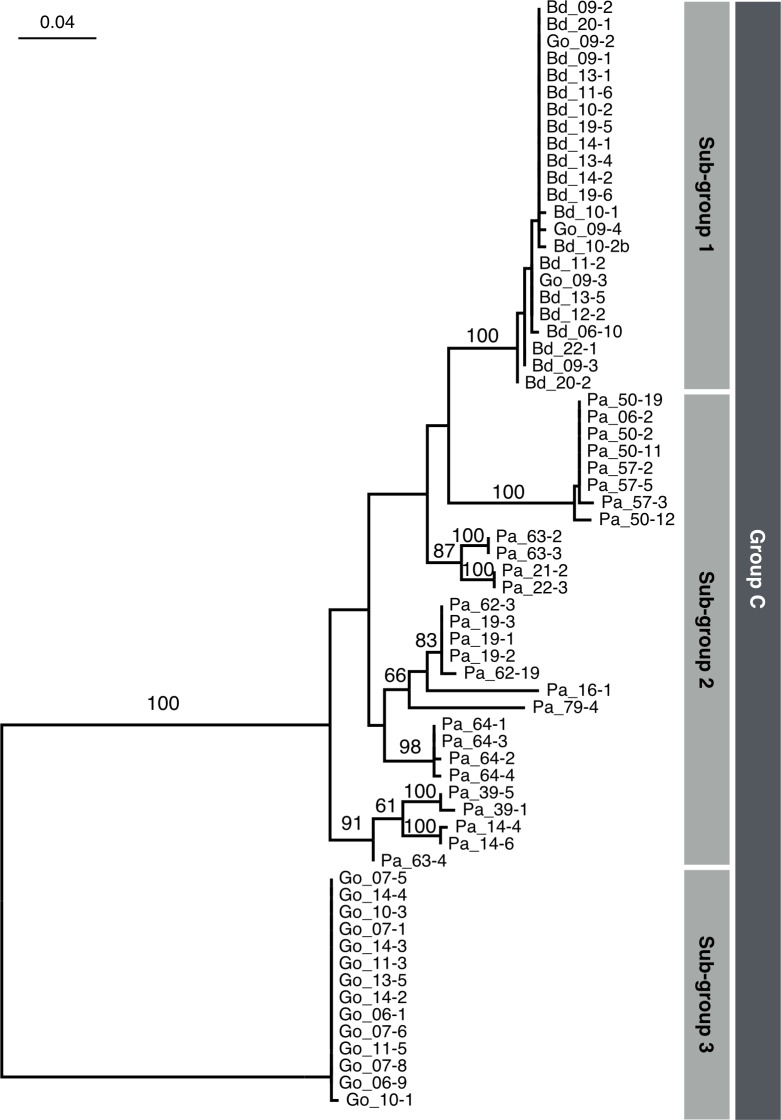
Group C represents the largest group of cockroach-derived Entamoeba and consists of 65 sequences (49% of all cockroach-derived Entamoeba sequences) from P. americana (28/77), B. dubia (20/24) and G. oblongonota (17/18). This group can be divided into three sub-groups; sub-group 1 consists of 20 sequences from B. dubia and three sequences from G. oblongonota, sub-group 2 consists of 28 sequences derived only from P. americana, and sub-group 3 consists of 14 sequences derived only from G. oblongonota (Fig 4). Note that monophyly of sub-groups 1 and 3 is well supported by the highest bootstrap proportion, while sub-group 2 does not form monophyly and may consist of multiple divergent sub-groups.
Fig 4. Phylogenetic tree of SSU rDNA of Group C sequences of cockroach-derived Entamoeba.
SSU rDNA sequences were aligned using MAFFT v7.187. Unambiguously aligned sequences composed of 1,224 nucleotides were selected by Gblocks and manual inspection. Maximum-likelihood (ML) tree was inferred by RAxML 8.1.17 using GTRGAMMA model. The number of bootstrap pseudoreplicate trees was 1,000. ML tree was visualized using FigTree 1.4.0 and Keynote 6.6.2. Bootstrap values for major nodes are shown on each node.
Groups F and G were defined by a separate analysis using amoebae only from P. americana. In the tree excluding amoebae from G. oblongonota and B. dubia, each of the groups F and G formed an independent clade with high statistical support value (S1 Fig). Whereas in the tree including amoebae from G. oblongonota and B. dubia, monophyly of group F was not reconstructed, but instead amoebae of groups F and G were shown to be monophyletic with weak statistical support value (66%). Since branch lengths leading to the amoebae of groups F and G are long, it is possible that these amoebae were attracted in the tree in Fig 2 by a long branch attraction artifact.
The genetic diversity of cockroach-derived Entamoeba among all Entamoeba and Archamoebae
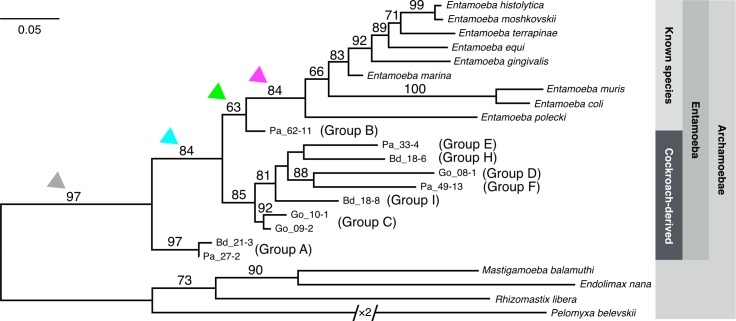
To obtain better resolution of all Entamoeba including cockroach-derived amoebae and Archamoebae species, the ML tree of the representative taxa was inferred (Fig 5). In the resulting tree, the monophyly of Entamoeba comprising representative cockroach-derived Entamoeba and 9 known Entamoeba species (E. histolytica, E. moshkovskii, E. terrapinae, E. equi, E. gingivalis, E. marina, E. muris, E. coli, and E. polecki) are strongly supported with bootstrap value (97%; gray arrow head). The monophyly of known Entamoeba is well supported (84%; magenta arrow head) and their inter-specific relationships are also unequivocally reconstructed (66% to 100% bootstrap values). The cockroach-derived Entamoeba forms three major independent clades: Group A, Group B, and the rest, Group C to I. All three clades are positioned basal to known Entamoeba species. Group A consists of the most basal ribosomal lineages of cockroach-derived Entamoeba, and the levels of observed divergence among them were relatively lower than those of other groups. On the other hand, group B comprises of members isolated exclusively from P. americana, is a sister group to known vertebrate-derived Entamoeba, although its statistical support was weak (63%; green arrow head). Group C to I forms a single largest statistically supported clade and is sister to the clade comprised of group B and known Entamoeba (84; cyan arrow head).
Fig 5. Phylogenetic tree of SSU rDNA of representative cockroach-derived Entamoeba ribosomal lineages and other Archamoebae species.
SSU rDNA sequences were aligned using MAFFT v7.187. Well-aligned 1,224 nucleotide positions were selected by Gblocks and manual operation. Maximum-likelihood (ML) tree was inferred by RAxML 8.1.17 using GTRGAMMA model. The number of bootstrap pseudoreplicate trees was 1,000. ML tree was visualized using FigTree 1.4.0 and Keynote 6.6.2. Bootstrap values (over 60%) are shown on each branch. Monophyly of Entamoeba is strongly supported with high bootstrap value (97%; gray arrow head). Commencing with Pa_27–2 and Bd_21–3, all cockroach-derived Entamoeba are positioned at the base of Entamoeba clade.
Polymorphism of Entamoeba identified in a single cockroach and presence of cockroach species-specific and common Entamoeba groups
For all the samples except for the first set of P. americana specimens (i.e., Pa_02 to Pa_27), single cockroaches were analyzed without cockroaches being pooled. Multiple groups were identified occasionally in a single P. americana (Pa_33 to Pa_80) sample (Table 2). The highest number of Entamoeba groups found in a single cockroach was 3 (Pa_49 and Pa_62), while 79% (22 of 28) of P. americana were found to harbor only a single Entamoeba group. B. dubia (31%; 5 of 16 cockroaches) had two Entamoeba groups (Table 3). In contrast, no G. oblongonota harboring multiple groups was found, although the sample size was small (8 cockroaches and 18 sequences; Table 4).
Table 2. The number of Entamoeba groups found in each P. americana.
| Source | A | B | C | D | E | F | G | H | I | |
|---|---|---|---|---|---|---|---|---|---|---|
| Pa | 02 | 3 | ||||||||
| Pa | 03 | 3 | ||||||||
| Pa | 04 | 1 | ||||||||
| Pa | 06 | 1 | ||||||||
| Pa | 07 | 1 | ||||||||
| Pa | 08 | 4 | ||||||||
| Pa | 10 | 1 | ||||||||
| Pa | 14 | 2 | ||||||||
| Pa | 16 | 1 | ||||||||
| Pa | 17 | 1 | ||||||||
| Pa | 19 | 3 | ||||||||
| Pa | 21 | 1 | ||||||||
| Pa | 22 | 1 | 1 | |||||||
| Pa | 24 | 2 | 1 | |||||||
| Pa | 26 | 1 | ||||||||
| Pa | 27 | 1 | 1 | |||||||
| Pa | 33 | 3 | ||||||||
| Pa | 39 | 2 | ||||||||
| Pa | 47 | 4 | ||||||||
| Pa | 49 | 1 | 5 | 3 | ||||||
| Pa | 50 | 1 | 4 | |||||||
| Pa | 57 | 3 | ||||||||
| Pa | 61 | 2 | ||||||||
| Pa | 62 | 1 | 2 | 4 | ||||||
| Pa | 63 | 3 | ||||||||
| Pa | 64 | 4 | ||||||||
| Pa | 79 | 1 | ||||||||
| Pa | 80 | 4 | ||||||||
| Total | 3 | 3 | 28 | 0 | 21 | 5 | 6 | 7 | 4 | |
Table 3. The number of Entamoeba groups found in each G. oblongonota.
| Source | A | B | C | D | E | F | G | H | I | |
|---|---|---|---|---|---|---|---|---|---|---|
| Go | 06 | 2 | ||||||||
| Go | 07 | 4 | ||||||||
| Go | 08 | 1 | ||||||||
| Go | 09 | 3 | ||||||||
| Go | 10 | 2 | ||||||||
| Go | 11 | 2 | ||||||||
| Go | 13 | 1 | ||||||||
| Go | 14 | 3 | ||||||||
| Total | 0 | 0 | 17 | 1 | 0 | 0 | 0 | 0 | 0 | |
Table 4. The number of Entamoeba groups found in each B. dubia.
| Source | A | B | C | D | E | F | G | H | I | |
|---|---|---|---|---|---|---|---|---|---|---|
| Bd | 06 | 1 | 1 | |||||||
| Bd | 08 | 2 | ||||||||
| Bd | 09 | 3 | ||||||||
| Bd | 10 | 3 | ||||||||
| Bd | 11 | 2 | 1 | |||||||
| Bd | 12 | 1 | ||||||||
| Bd | 13 | 3 | ||||||||
| Bd | 14 | 2 | ||||||||
| Bd | 15 | 1 | 2 | |||||||
| Bd | 16 | 3 | ||||||||
| Bd | 17 | 2 | ||||||||
| Bd | 18 | 1 | 2 | |||||||
| Bd | 19 | 2 | ||||||||
| Bd | 20 | 2 | ||||||||
| Bd | 21 | 2 | ||||||||
| Bd | 22 | 1 | 2 | |||||||
| Total | 2 | 0 | 20 | 0 | 0 | 0 | 0 | 2 | 15 | |
Group C was the most common and highly shared group discovered from three cockroach species. The 23 sequences consisting the sub-group 1 of group C were mutually very similar (> 99.5% mutual positional identity; Table 5). In other words, almost identical Entamoeba sequences that belong to group C sub-group 1 were discovered from both the forest cockroaches (B. dubia and G.oblongonota), suggestive of conservation of genetic traits of this sub-group despite distinct host species and geographic origins.
Table 5. Sequence percentage identities among representative members of the clades in group C.
| Go_09–2 | Bd_20–2 | Pa_50–19 | Pa_63–4 | Go_06–9 | |
|---|---|---|---|---|---|
| Go_09–2 | 100 | 99.5 | 89.9 | 89.6 | 86.8 |
| Bd_20–2 | 100 | 90.2 | 90.1 | 87.3 | |
| Pa_50–19 | 100 | 88.1 | 84.4 | ||
| Pa_63–4 | 100 | 88.0 | |||
| Go_06–9 | 100 |
Identities were calculated by EMBOSS Needle (http://www.ebi.ac.uk/Tools/psa/emboss_needle/).
Discovery of novel Entamoeba ribosomal lineages in cockroaches expands our understanding of genetic diversity of Entamoeba
We have demonstrated that the genetic diversity of Entamoeba derived from three cockroach species overwhelms that of previous reports which described diversity among species found in vertebrates, as well as the potential free living species (E. moshkovskii and E. marina). Despite our repeated attempts, we were unable to cultivate cockroach-derived Entamoeba and thus to get sufficient amount of genomic DNA or RNA for whole genome and transcriptome analyses. Hence, the genome of cockroach-derived Entamoeba remains to be elucidated.
Supporting information
SSU rDNA sequences were aligned using MAFFT v7.187. Unambiguously aligned sequences composed of 1069 nucleotides were selected by Gblocks and manual inspection. Maximum-likelihood (ML) tree was inferred by RAxML 8.1.17 using GTRGAMMA model. The number of bootstrap pseudoreplicate trees was 100. ML tree was visualized using FigTree 1.4.0 and Keynote 6.6.2. Bootstrap values for major nodes are shown on each node. Nine groups (A-I) were shown to be monophyletic with high bootstrap support values.
(TIF)
In order to ensure consistency of the result shown in Fig 2, the phylogenetic tree was constructed with other model. SSU rDNA sequences were aligned using MAFFT v7.187. Unambiguously aligned sequences composed of 1,023 nucleotides were selected by Gblocks and manual inspection. Maximum-likelihood (ML) tree was inferred by IQ-TREE 1.5.5 using TPM2u+I+G4 model is shown. The number of bootstrap pseudoreplicate trees was 1,000. ML tree was visualized using FigTree 1.4.0 and Keynote 6.6.2. Note that major clades supported in Fig 2 are also supported in this analysis.
(PDF)
In order to ensure consistency of the result shown in Fig 3, the phylogenetic tree was constructed with other model.m SSU rDNA sequences were aligned using MAFFT v7.187. Unambiguously aligned sequences composed of 914 nucleotides were selected by Gblocks and manual inspection. Maximum-likelihood (ML) tree was inferred by IQ-TREE 1.5.5 using TIM2+I+G4 model. The number of bootstrap pseudoreplicate trees was 1,000. ML tree was visualized as a cladogram using FigTree 1.4.0 and Keynote 6.6.2. The phylogenetic relationships of Entamoeba and cockroach amoebae in resultant tree are consistent with the tree in Fig 3, although some of Amoebozoa species are miss branched (Red rectangle).
(PDF)
SSU rDNA sequences were aligned using MAFFT v7.187. Unambiguously aligned sequences composed of 1,224 nucleotides were selected by Gblocks and manual inspection. Maximum-likelihood (ML) tree was inferred by IQ-TREE 1.5.5 using HKY+I+G4 model. The number of bootstrap pseudoreplicate trees was 1,000. ML tree was visualized using FigTree 1.4.0 and Keynote 6.6.2. Bootstrap values for major nodes are shown on each node. Major clades discovered in Fig 4 were successfully reproduced.
(PDF)
SSU rDNA sequences were aligned using MAFFT v7.187. Well-aligned 1,224 nucleotide positions were selected by Gblocks and manual operation. Maximum-likelihood (ML) tree was inferred by IQ-TREE 1.5.5 using TIM2+I+G4 model. The number of bootstrap pseudoreplicate trees was 1,000. ML tree was visualized using FigTree 1.4.0 and Keynote 6.6.2. The topology of resultant tree are consistent with the tree in Fig 5.
(PDF)
SSU rDNA sequences were aligned using MAFFT v7.187. The whole part of the alignment was visualized by SeaView4. The alignment indicates exact address of well aligned sites and variant sites.
(PDF)
Acknowledgments
We thank Dr. Shinji Izumiyama (Department of Parasitology, National Institute of Infectious Diseases, Japan) for technical support and discussion. We also thank Dr. Herbert J. Santos (Department of Parasitology, National Institute of Infectious Diseases, Japan) for copyediting.
Data Availability
Newly identified 18S rRNA gene sequences reported in this study were submitted to GenBank/EMBL/DDBJ database with accession numbers LC259314–LC259447.
Funding Statement
This work was supported in part by Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (23117001, 23117005, 23390099, 26111524) to T.N. and 15H05231 to T.H., a grant for research on emerging and re-emerging infectious diseases from Japan Agency for Medical Research and Development (AMED) to T.N., and a grant for Science and Technology Research Partnership for Sustainable Development (SATREPS) from AMED and Japan International Cooperation Agency (JICA) to T.N.. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Global Health Estimates 2015: Burden of disease by Cause, Age, Sex, by Country and by Region, 2000–2015. Geneva, World Health Organization;2016. [Google Scholar]
- 2.Clark CG. Isolation and Characterization of Polymorphic DNA from Entamoeba histolytica. Society. 2001;39(3):897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaki M, Meelu P, Sun W, Clark CG. Simultaneous differentiation and typing of Entamoeba histolytica and Entamoeba dispar. J Clin Microbiol. 2002;40(4):1271–1276. doi: 10.1128/JCM.40.4.1271-1276.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali IKM, Zaki M, Clark CG. Use of PCR Amplification of tRNA Gene-Linked Short Tandem Repeats for Genotyping Entamoeba histolytica. J Clin Microbiol. 2005;43(12):5842–5847. doi: 10.1128/JCM.43.12.5842-5847.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark CG, Ali IKM, Zaki M, Loftus BJ, Hall N. Unique organisation of tRNA genes in Entamoeba histolytica. Mol Biochem Parasitol. 2006;146(1):24–29. doi: 10.1016/j.molbiopara.2005.10.013 [DOI] [PubMed] [Google Scholar]
- 6.Tawari B, Ali IKM, Scott C, Quail MA, Berriman M, Hall N, et al. Patterns of Evolution in the Unique tRNA Gene Arrays of the Genus Entamoeba. Mol Biol Evol. 2008;25(1):187–198. doi: 10.1093/molbev/msm238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Escueta-de CA, Kobayashi S, Takeuchi T, Tachibana H, Nozaki T. Identification of an avirulent Entamoeba histolytica strain with unique tRNA-linked short tandem repeat markers. Parasitol Int. 2010;59(1):75–81. doi: 10.1016/j.parint.2009.10.010 [DOI] [PubMed] [Google Scholar]
- 8.Das K, Mukherjee AK, Chowdhury P, Sehgal R, Bhattacharya MK, Hashimoto T, et al. Multilocus sequence typing system (MLST) reveals a significant association of Entamoeba histolytica genetic patterns with disease outcome. Parasitol Int. 2014;63:308–314. doi: 10.1016/j.parint.2013.11.014 [DOI] [PubMed] [Google Scholar]
- 9.Stensvold CR, Lebbad M, Victory EL, Verweij JJ, Tannich E, Clark CG, et al. Increased Sampling Reveals Novel Lineages of Entamoeba: Consequences of Genetic Diversity and Host Specificity for Taxonomy and Molecular Detection. Protist. 2011;162:525–541. doi: 10.1016/j.protis.2010.11.002 [DOI] [PubMed] [Google Scholar]
- 10.Shiratori T, Ishida K. Entamoeba marina n. sp.; a New Species of Entamoeba Isolated from Tidal Flat Sediment of Iriomote Island, Okinawa, Japan. J Eukaryot Microbiol. 2016;63(3):280–286. doi: 10.1111/jeu.12276 [DOI] [PubMed] [Google Scholar]
- 11.Fantham H, Porter A. A bee disease due to a protozoal parasite (Nosema apis). Proc Zool Soc London. 1911;625–626. [Google Scholar]
- 12.Kidder GW. The intestinal protozoa of the wood-feeding roach Panesthia. Parasitology. 1937;29:163–205. [Google Scholar]
- 13.Kay MW. Two new amoebae from the box elder bug, Leptocoris trivittatus. Say Am Midl Nat. 1940;724–728. [Google Scholar]
- 14.Bishop A. Entamoeba aulastomi Nöller: Cultivation, Morphology, and Method of Division; and Cultivation of Hexamita sp. Parasitology. 1932;24(2), 225–232. [Google Scholar]
- 15.Carini A. Parasitisme des zellerielles par des microorganismes nouveaux (Brumptina n. g.). Ann Parasitol. 1933;11:297–300. [Google Scholar]
- 16.Terence LM, Wen-Tso L, Larry JF, Hans C. Beginning a molecular analysis of the eukaryal community in activated sludge. Wat Sci Tech. 1998;37(4):455–460. [Google Scholar]
- 17.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic Local Alignment Search Tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- 18.Katoh K, Standley DM. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol Biol Evol. 2013;30(4):772–780. doi: 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castresana J. Selection of Conserved Blocks from Multiple Alignments for Their Use in Phylogenetic Analysis. Mol Biol Evol. 2000;17(4):540–552. [DOI] [PubMed] [Google Scholar]
- 20.Gouy M, Guindon S, Gascuel O. SeaView Version 4: A Multiplatform Graphical User Interface for Sequence Alignment and Phylogenetic Tree Building. Mol Biol Evol. 2010;27(2):221–224. doi: 10.1093/molbev/msp259 [DOI] [PubMed] [Google Scholar]
- 21.Stamatakis A. RAxML Version 8: A tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics. 2014;30(9):1312–1313. doi: 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SSU rDNA sequences were aligned using MAFFT v7.187. Unambiguously aligned sequences composed of 1069 nucleotides were selected by Gblocks and manual inspection. Maximum-likelihood (ML) tree was inferred by RAxML 8.1.17 using GTRGAMMA model. The number of bootstrap pseudoreplicate trees was 100. ML tree was visualized using FigTree 1.4.0 and Keynote 6.6.2. Bootstrap values for major nodes are shown on each node. Nine groups (A-I) were shown to be monophyletic with high bootstrap support values.
(TIF)
In order to ensure consistency of the result shown in Fig 2, the phylogenetic tree was constructed with other model. SSU rDNA sequences were aligned using MAFFT v7.187. Unambiguously aligned sequences composed of 1,023 nucleotides were selected by Gblocks and manual inspection. Maximum-likelihood (ML) tree was inferred by IQ-TREE 1.5.5 using TPM2u+I+G4 model is shown. The number of bootstrap pseudoreplicate trees was 1,000. ML tree was visualized using FigTree 1.4.0 and Keynote 6.6.2. Note that major clades supported in Fig 2 are also supported in this analysis.
(PDF)
In order to ensure consistency of the result shown in Fig 3, the phylogenetic tree was constructed with other model.m SSU rDNA sequences were aligned using MAFFT v7.187. Unambiguously aligned sequences composed of 914 nucleotides were selected by Gblocks and manual inspection. Maximum-likelihood (ML) tree was inferred by IQ-TREE 1.5.5 using TIM2+I+G4 model. The number of bootstrap pseudoreplicate trees was 1,000. ML tree was visualized as a cladogram using FigTree 1.4.0 and Keynote 6.6.2. The phylogenetic relationships of Entamoeba and cockroach amoebae in resultant tree are consistent with the tree in Fig 3, although some of Amoebozoa species are miss branched (Red rectangle).
(PDF)
SSU rDNA sequences were aligned using MAFFT v7.187. Unambiguously aligned sequences composed of 1,224 nucleotides were selected by Gblocks and manual inspection. Maximum-likelihood (ML) tree was inferred by IQ-TREE 1.5.5 using HKY+I+G4 model. The number of bootstrap pseudoreplicate trees was 1,000. ML tree was visualized using FigTree 1.4.0 and Keynote 6.6.2. Bootstrap values for major nodes are shown on each node. Major clades discovered in Fig 4 were successfully reproduced.
(PDF)
SSU rDNA sequences were aligned using MAFFT v7.187. Well-aligned 1,224 nucleotide positions were selected by Gblocks and manual operation. Maximum-likelihood (ML) tree was inferred by IQ-TREE 1.5.5 using TIM2+I+G4 model. The number of bootstrap pseudoreplicate trees was 1,000. ML tree was visualized using FigTree 1.4.0 and Keynote 6.6.2. The topology of resultant tree are consistent with the tree in Fig 5.
(PDF)
SSU rDNA sequences were aligned using MAFFT v7.187. The whole part of the alignment was visualized by SeaView4. The alignment indicates exact address of well aligned sites and variant sites.
(PDF)
Data Availability Statement
Newly identified 18S rRNA gene sequences reported in this study were submitted to GenBank/EMBL/DDBJ database with accession numbers LC259314–LC259447.