Abstract
Cerebral malaria is a sign of severe malarial disease and is often a harbinger of death. While aggressive management can be life-saving, the detection of cerebral malaria can be difficult. We present an experimental mouse model of cerebral malaria that shares multiple features of the human disease, including edema and microvascular pathology. Using magnetic resonance imaging (MRI), we can detect and track the blood-brain barrier disruption, edema development, and subsequent brain swelling. We describe multiple MRI techniques that can visualize these pertinent pathological changes. Thus, we show that MRI represents a valuable tool to visualize and track pathological changes, such as edema, brain swelling, and microvascular pathology, in vivo.
Keywords: Medicine, Issue 124, Mouse, neuroscience, experimental cerebral malaria, magnetic resonance imaging, in vivo imaging, malaria
Introduction
Malaria is a significant global health problem.1 Severe malaria is characterized in part by cerebral involvement and is often a poor prognostic factor. Cerebral involvement is common in children under the age of five in areas of high malaria transmission and represents the major cause of malaria related death in that age group.1 While aggressive treatment can be life-saving, the detection of cerebral malaria, especially in early stages, can be difficult. The pathological processes involved in cerebral malaria include microvascular disruption and cerebral edema, which can lead to severe brain swelling. In this article, we present a magnetic resonance imaging (MRI) protocol that allows whole-brain in vivo imaging of experimental cerebral malaria (ECM). Whole-brain high-resolution imaging methods have been widely underutilized in this disease, even though little is known about how ECM initiates in the central nervous system or what specific mechanisms lead to the disease. In vivo MRI, covering the whole brain, represents an important research tool to gain a better understanding of ECM pathology. MRI is able to assess global cerebral brain swelling, which has recently been recognized to be an important predictor of death not only in ECM, but also in human cerebral malaria.2,3 Severe brain swelling occurs in fatal disease and represents one of several pathological features between the ECM models and human disease, a disease that is characterized by both inflammatory and microvascular alterations.4
ECM can be induced in CBA or C57BL mice through infection with lethal Plasmodium berghei ANKA.5 The onset of ECM typically occurs between days 6 and 10 post-infection and results in fitting, ataxia, respiratory distress, and coma, which lead to rapid death.4 The Rapid Murine Coma and Behavior Scale (RMCBS) is a helpful score to evaluate clinical symptoms of ECM. It consists of 10 parameters, each scored from 0 to 2, with a maximum possible score of 20.6 Recently, we showed good agreement between the severity of the RMCBS scores in ECM mice and pathological changes demonstrated by MRI.7 In this protocol, we describe ECM induction in mice and in vivo magnetic resonance imaging of mice with ECM.
Protocol
All animal experiments reported in this article were conducted according to the Federation for Laboratory Animal Science Associations (FELASA) category B and the Society of Laboratory Animal Science (GV-SOLAS) standard guidelines and were approved by the local German authorities in Karlsruhe (Regierungspräsidium Karlsruhe, Germany). Please note that biosavety level 2 applies to mosquito and Plasmodium berghei ANKA sporozoite work.
1. Infection
Infect Anopheles stephensi mosquitoes with Plasmodium berghei ANKA by feeding them for 15 min on a gametocytemic mouse. Keep the infected mosquitoes at 80% humidity and 21 °C.
Collect female mosquitoes from their cage 17 to 22 days after the blood meal. Place them on ice to anesthetize them.
Using forceps, place three to four mosquitoes on a glass slide covered with a drop of cold RPMI medium. Place the slide under a microscope.
Using forceps, carefully stretch the mosquito between the head and body. Isolate the salivary gland using a syringe and needle. Repeat this procedure with the remaining mosquitoes.
Collect the salivary glands from the glass slide by sucking them up with a glass pipette and collect them in a 1.5 mL centrifuge tube. NOTE: Depending on the infection rates, usually 8,000 to 15,000 infectious sporozoites can be obtained per salivary gland.
For approximately 3 min, smash the isolated salivary glands within the centrifuge tube with a small, plastic stick to isolate the sporozoites from the salivary gland tissue.
Centrifuge for 3 min at 1,000 x g and 4 °C to purify the sporozoites from the remaining tissue.
Pipette the supernatant, which contains the sporozoites (SPZ), to a new centrifuge tube and count the purified sporozoites in a Neubauer hemocytometer.
Adjust the concentration of purified sporozoites to 10,000/mL by adding phosphate-buffered saline.
Inject a total of 1,000 sporozoites (0.1 mL) into the tail veins of inbred C57BL/6 mice to initiate infection. To facilitate the injections, place the C57BL/6 mice in a restrainer and put the tails into warm (approximately 37 °C) water to assist with the visualization of the tail veins; NOTE: The injection itself is a short procedure that can be performed within a few seconds.
Once daily, check blood-stage parasitemia on blood smears from day 3 onwards after the SPZ infection. NOTE: Monitoring parasitemia has been previously visualized in a JoVE article by Mueller et al.8
Assess the mice once daily with the Rapid Murine Coma and Behavior Scale (RMCBS) score, starting from day 5 after the sporozoite injection. NOTE: A detailed description of this procedure, including a video demonstration, has been published by Caroll et al.6
Assess the mice with MRI imaging according to the RMCBS score and the research question to be addressed.6
2. Magnetic Resonance Imaging Setup
Perform MRI on a 9.4T small animal scanner using a volume resonator for radiofrequency transmission and a 4-channel-phased-array surface receiver coil. Turn on the temperature-controlled water bath to 42 °C in order to maintain the body temperature of the mouse.
Induce anesthesia in a chamber using 2% isoflurane and compressed air until the mouse no longer reacts to a toe pinch. Maintain the anesthesia at 1-1.5%.
Place a tail vein catheter into the tail vein of the mouse. Position the mouse for MRI by placing it prone and with a crunched back on an animal bed equipped with a headlock and tooth bar to minimize head motion. Take care not to straighten the cervical spine of the mouse.
Connect a contrast agent injection system to the tail vein catheter. Use a custom-made injection system filled with a Gd-DTPA (0.3 mmol/kg) syringe or use PE tubing connected to a Gd-DTPA (0.3 mmol/kg) syringe.
Apply dexpanthenol eye ointment to both eyes. Place the 4-channel-phased-array surface receiver head coil onto the head of the mouse. Place a breathing pad onto the back of the mouse and connect it to a respiration monitoring device.
3. Imaging Protocol
NOTE: Choose imaging sequences from the protocol listed below according to the research questions to be addressed. All listed parameters are valid for the MRI software but might need to be adjusted if other software programs are used.
Begin by performing a localizing scan to make sure that the mouse brain is in the isocenter of the magnet.
- To qualitatively assess the vasogenic edema, use 3D T2-weighted imaging by selecting a multi-slice RARE sequence.
- Enter the following parameters into the MRI software: repetition time = 2.000 ms, echo time = 22 ms, isotropic resolution = 0.1mm, field of view = 20 x 10 x 12 mm3; matrix = 200 x 100 x 120, flip angle = 90-180°; (spin-echo), and rare factor = 8. Start the sequence and wait 10 min 48 s to acquire the raw images.
- To quantitatively assess vasogenic edema perform T2 relaxometry by selecting a multi-slice, multiple spin echo sequence.
- Use the following parameters: repetition time = 3.100 ms, echo time = 8-136 ms in increments of 8 ms, number of slices = 17, slice thickness = 0.7 mm, in plane resolution = 0.116 mm x 0.116 mm, field of view 20 x 20 mm2, matrix = 172 x 172, flip angle = 90-180 degrees; (spin-echo). Start the sequence and wait 8 min 53 s to acquire the raw images.
- To quantitatively assess both vasogenic edema and cytotoxic edema, carry out diffusion-weighted imaging/apparent diffusion coefficient (ADC) mapping by selecting a spin-echo EPI diffusion sequence.
- Use the following parameters: repetition time = 3.400 ms, echo time = 20 ms, slice thickness = 0.7 mm, number of slices = 17, number of diffusion sensitized directions = 30, b value = 1.500 s/mm2, δ = 3 ms, Δ = 9 ms, partial Fourier encoding acceleration factor = 1.51, field of view = 12 x 15 mm2, matrix = 96 x 128, in-plane resolution = 0.125 mm x 0.117 mm, flip angle = 90-180 degrees, and number of saturation slices (sagittal) = 1. Start the sequence and wait 7 min 56 s until the raw images are acquired.
- To assess microhemorrhages, use 3D T2*-weighted imaging. Select a flow-compensated FLASH sequence.
- Enter the following parameters into the MRI software: repetition time = 2.000 ms, echo time = 22 ms, isotropic resolution 0.08 mm, field of view = 32 x 15 x 8 mm3, matrix size = 400 x 188 x 100, and flip angle = 12 degrees. Start the sequence and wait 15 min 40 s to acquire the raw images.
- To assess arterial patency, use time of flight angiography by selecting a 3D FLASH sequence.
- Use the following parameters in the MRI software: repetition time = 16 ms, echo time = 3.5 ms, slice thickness = 0.07 mm, in-plane resolution = 0.104 x 0.104 mm, field of view = 20 x 20 x 10 mm3, matrix = 192 x 192 x 142, and flip angle = 15 degrees. Start the sequence and wait 7 min 16 s until the images are acquired.
- To assess blood-brain barrier disruption, use 3D T1-weighted imaging before and after a contrast agent injection of 0.3 mmol/kg. Select a radio frequency-spoiled FLASH sequence with a global radio frequency excitation.
- Use the following sequence parameters in the MRI software: repetition time = 5 ms, echo time = 1.9 ms, isotropic resolution = 0.156 mm, field of view 20 x 18.7 x 18.7 mm3, matrix 128 x 120 x 120 and flip angle = 8.5°;. Start the sequence and wait 1 min 14s until the images are acquired.
4. Image Processing and Analysis
To analyze blood-brain barrier disruption, subtract 3D non-enhanced T1-weighted images from enhanced T1-weighted images with the arithmetic tool or image calculator tool in ImageJ. Evaluate the subtraction images for an increase of signal, which corresponds to blood-brain barrier disruption.9
To analyze brain volume, use native 3D T1- or 3D T2-weighted datasets. Delineate the brain from the olfactory bulb to the cerebellum using the segmentation editor.10
- Vasogenic edema
- Process the T2 relaxometry data with MRI software or use a nonlinear least-squares fit procedure.11 Process diffusion-weighted data to obtain ADC maps using MRI software or FDT toolbox (see Materials Table).
- Place regions of interest manually into the different anatomical regions. NOTE: Automatic placement of regions of interest may register incorrectly due to significant brain swelling. The T2 times and ADC values of the chosen anatomical regions are obtained in this fashion.
To analyze microhemorrhage volume, delineate microhemorrhages, which appear as ovoid, dark foci on T2*-weighted datasets, using the segmentation editor.
Representative Results
In C57BL/6 mice, the first clinical symptoms of ECM can be observed between days 6 and 10 after infection with P. berghei ANKA sporozoites. ECM develops in 60 - 80% of infected mice and rapidly progresses to coma and death within 24 to 48 h. In contrast, mice that do not develop ECM die after the second week post-infection from severe anemia due to hyperparasitemia.12
In MRI imaging, the earliest signs of ECM are visible in the olfactory bulb, which belongs to the olfactory system of the mouse and is located in the rostral part of the brain.7,13 An increase in blood-brain barrier disruption (seen on contrast-enhanced T1-weighted images) and a fluid increase (increase of T2 signal on T2-weighted images, T2 relaxometry, and diffusion-weighted imaging) indicate early vasogenic edema in the olfactory bulb that is already present in mild disease (RMCBS score 20-16) and starts to spread as the severity of the disease increases. Relative T1 signal images help to detect subtle blood-brain barrier disruption (Figure 1). Blood-brain barrier disruption and vasogenic edema progress in a specific fashion. These pathological changes spread along the rostral migratory stream, a migrating route for neuroblasts, towards the brainstem, which is reached in very severe disease (RMCBS sore 9-0). On T2 maps, the severity of vasogenic edema can be quantified by drawing regions of interest into specific anatomical regions, as demonstrated in Figure 2. For each region, a T2 relaxation time that increases with increasing edema can be obtained. Along with vasogenic edema and blood-brain barrier disruption, brain swelling starts as the ECM severity increases. Brain volume indicative of edema starts to significantly increase in moderately sick mice as compared to baseline and further increases in severely sick mice (Figure 3).
Microvascular pathology, as evidenced by microhemorrhages and an increase of vessel susceptibility contrast (corresponding to slow flow), occurs after the first signs of blood-brain barrier disruption and vasogenic edema and can be visualized with T2*-weighted imaging. Microhemorrhages predominantly occur in the olfactory bulb (Figure 4). The number of microhemorrhages increases with progressive disease. With progressive disease microhemorrhages are also evident in cortex, basal ganglia, cerebellum, white matter and brainstem.
Decreased arterial patency is seen by time-of-flight angiography as a sign of macrovascular impairment in parallel to microvascular changes. Cerebral infarction is not a key finding in ECM. In very severe disease, however, focal areas of ADC decrease can be identified, indicating cerebral infarctions.
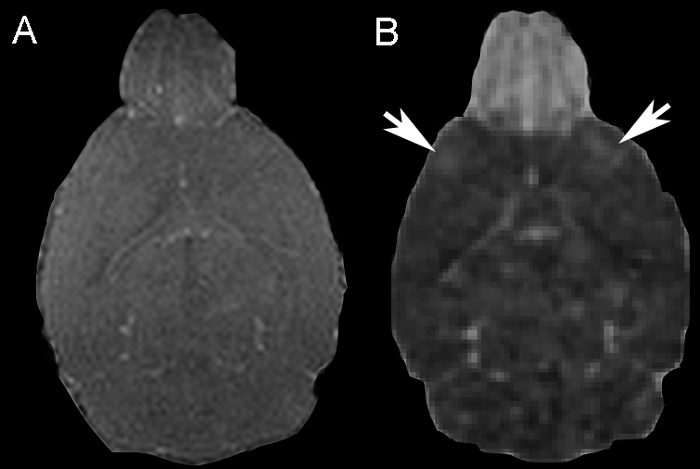 Figure 1: Blood-brain Barrier Disruption. (A) A representative coronal 3D T1-weighted image. An increase in T1 signal is visible in the central region of the olfactory bulb. (B) On the corresponding T1 relative signal image (T1 pre-contrast image subtracted from the T1 post-contrast image), contrast agent extravasation is easier to delineate. Contrast agent extravasation is visible in the frontal cortical regions (white arrows). Please click here to view a larger version of this figure.
Figure 1: Blood-brain Barrier Disruption. (A) A representative coronal 3D T1-weighted image. An increase in T1 signal is visible in the central region of the olfactory bulb. (B) On the corresponding T1 relative signal image (T1 pre-contrast image subtracted from the T1 post-contrast image), contrast agent extravasation is easier to delineate. Contrast agent extravasation is visible in the frontal cortical regions (white arrows). Please click here to view a larger version of this figure.
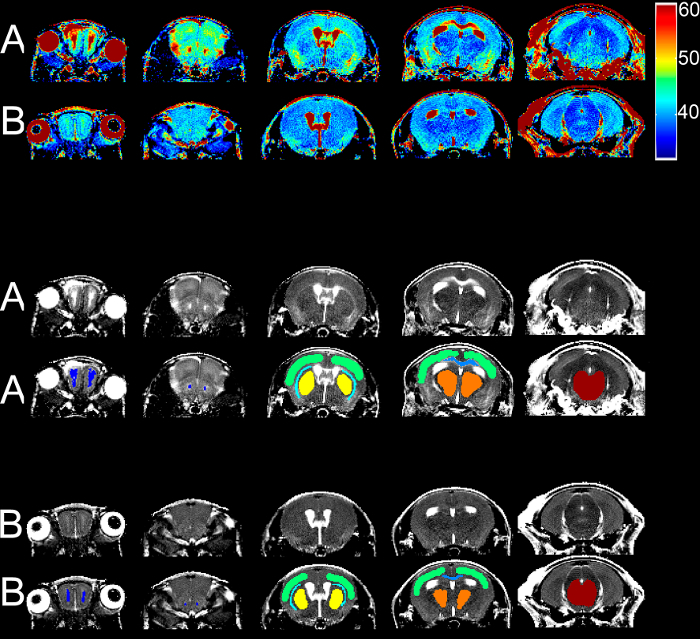 Figure 2: T2 Relaxometry. Sample T2 maps at different heights from one severely sick mouse with an RMCBS score of 5 (A) and from the baseline scan (B) are displayed in color scale, in grey scale, and in grey scale with ROIs as colored overlays. Dark blue = RMS, green = cortex, turquoise = external capsule, yellow = basal ganglia, light blue = DMS, orange = thalamus, and red = brainstem Please click here to view a larger version of this figure.
Figure 2: T2 Relaxometry. Sample T2 maps at different heights from one severely sick mouse with an RMCBS score of 5 (A) and from the baseline scan (B) are displayed in color scale, in grey scale, and in grey scale with ROIs as colored overlays. Dark blue = RMS, green = cortex, turquoise = external capsule, yellow = basal ganglia, light blue = DMS, orange = thalamus, and red = brainstem Please click here to view a larger version of this figure.
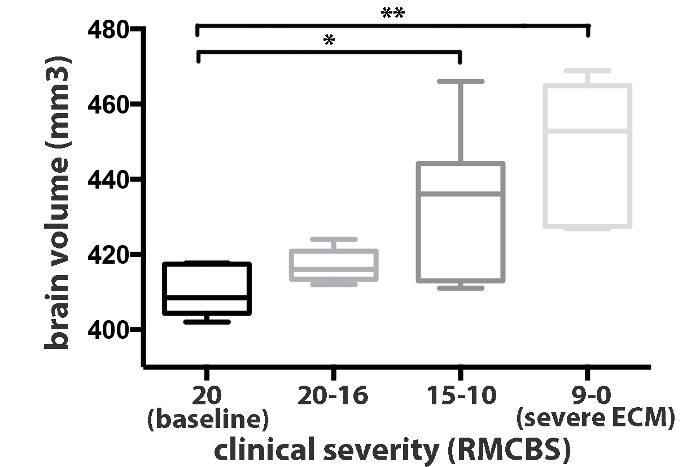 Figure 3: Brain Volume Measurements. Brain volume significantly increases in moderately sick mice (RMCBS 15-10). Representative values of 10 mice are shown. Data is presented as the mean standard deviation. A paired Student's t-test was used to analyze the differences in brain swelling between groups. In moderately/severely sick mice, the brain volume was significantly increased compared to baseline (p = 0.021/0.001). Please click here to view a larger version of this figure.
Figure 3: Brain Volume Measurements. Brain volume significantly increases in moderately sick mice (RMCBS 15-10). Representative values of 10 mice are shown. Data is presented as the mean standard deviation. A paired Student's t-test was used to analyze the differences in brain swelling between groups. In moderately/severely sick mice, the brain volume was significantly increased compared to baseline (p = 0.021/0.001). Please click here to view a larger version of this figure.
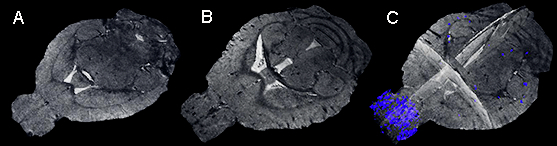 Figure 4: Microhemorrhages. Representative T2*-weighted images of a mouse before the onset of blood-stage infection (A) and on day 8 after infection with an RMCBS score of 14 (B and C) are shown. In the first image, no microhemorrhges are evident (A), while in the second and third images, several microhemorrhages are visible, with a predominance in the olfactory bulb (B: plane coronal image; C: 3D projection with segmented microhemorrhages).
Figure 4: Microhemorrhages. Representative T2*-weighted images of a mouse before the onset of blood-stage infection (A) and on day 8 after infection with an RMCBS score of 14 (B and C) are shown. In the first image, no microhemorrhges are evident (A), while in the second and third images, several microhemorrhages are visible, with a predominance in the olfactory bulb (B: plane coronal image; C: 3D projection with segmented microhemorrhages).
Discussion
In this article, we describe a whole-brain MRI protocol to delineate changes in experimental cerebral malaria. We believe that MRI has been under-utilized in malaria research to date and hope that our protocols will aid other investigators. We would like to describe some additional points that may be helpful.
If severely sick mice are imaged, positioning is crucial. Due to increased intracranial pressure, mice are susceptible to death, and thus, the cervical spine should not be stretched. Anesthesia should also be kept to a minimum. If mice with mild disease are imaged, the best time point for MRI is 12-24 h prior to the expected onset of the first clinical symptoms. Mild vasogenic edema in the olfactory bulb is visible in mice that do not exhibit any clinical signs of ECM (RMCBS score of 20).
The length of the MRI protocol itself can be adapted according to the specific research question. The minimal protocol should include a T2-weighted sequence or T2 relaxometry and a T2*-weighted sequence in order to judge the presence of vasogenic edema and microhemorrhage load/microvascular impairment. To get good-quality images, motion artefacts should be kept to a minimum. Motion will be reduced through the proper positioning of the mouse and by keeping the breathing rate at about 60-80 breaths per min.
Other in vivo imaging methods, such as intravital microscopy, show promise in visualizing pathological alterations in ECM on a cellular level.14,15,16,17 Compared to MRI, intravital microscopy can offer high spatial resolution at the cost of only covering a limited cortical area. In contrast, MRI is able to cover the whole brain at a resolution of up to 80 µm. MRI can therefore guide further cellular investigations by identifying the predilection sites-the beginning of disease-as well as the stage of the disease, which can then be assessed using further methodologies. MRI findings in ECM can be compared to pathological alterations detected with MRI in human cerebral malaria. Both human and experimental cerebral malaria show vasogenic edema in areas of neurogenesis, as well as microvascular pathology, such as secondary cerebral infarcts in watershed areas.7,18 White and grey matter are affected in a similar fashion, and brain swelling in both human and experimental cerebral malaria involves the brainstem in severe disease, explaining the comatose state.2,7
We hope that in vivo imaging will help unravel the underlying pathological mechanism of cerebral malaria, which currently remains elusive. As edema and brain swelling are barely visible ex vivo, methods to visualize this pathology in vivo are key and will help to improve the evaluation of novel therapies and vaccinations.
Disclosures
The authors declare that they have no conflicts of interest.
Acknowledgments
The expert technical assistance of Miriam Reinig is gratefully acknowledged. AH received funding from a postdoctoral stipend of the Medical Faculty of the University of Heidelberg. MP is supported by a memorial stipend from the Else-Kröner-Fresenius Foundation. AKM is a recipient of a maternity leave stipend by the DZIF Academy of the German Center for Infection Research (DZIF). JP is the recipient of a Heidelberg Research Center for Molecular Medicine (HRCMM) Career Development Fellowship. We furthermore gratefully acknowledge Julia M. Sattler and Friedrich Frischknecht for providing an exemplary movie of sporozoite movement.
References
- World Health Organization. World Malaria Report. 2014.
- Seydel KB, et al. Brain swelling and death in children with cerebral malaria. N Engl J Med. 2015;372(12):1126–1137. doi: 10.1056/NEJMoa1400116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penet MF, et al. Imaging experimental cerebral malaria in vivo: significant role of ischemic brain edema. J Neurosci. 2005;25(32):7352–7358. doi: 10.1523/JNEUROSCI.1002-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza JB, Riley EM. Cerebral malaria: the contribution of studies in animal models to our understanding of immunopathogenesis. Microbes Infect. 2002;4(3):291–300. doi: 10.1016/s1286-4579(02)01541-1. [DOI] [PubMed] [Google Scholar]
- Curfs JH, van der Meide PH, Billiau A, Meuwissen JH, Eling WM. Plasmodium berghei: recombinant interferon-gamma and the development of parasitemia and cerebral lesions in malaria-infected mice. Exp Parasitol. 1993;77(2):212–223. doi: 10.1006/expr.1993.1078. [DOI] [PubMed] [Google Scholar]
- Carroll RW, et al. A rapid murine coma and behavior scale for quantitative assessment of murine cerebral malaria. PLoS One. 2010;5(10) doi: 10.1371/journal.pone.0013124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A, et al. Experimental Cerebral Malaria Spreads along the Rostral Migratory Stream. PLoS Pathog. 2016;12(3):e1005470. doi: 10.1371/journal.ppat.1005470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller AK, Behrends J, Blank J, Schaible UE, Schneider BE. An experimental model to study tuberculosis-malaria coinfection upon natural transmission of Mycobacterium tuberculosis and Plasmodium berghei. J Vis Exp. 2014. p. e50829. [DOI] [PMC free article] [PubMed]
- Hynynen K, McDannold N, Sheikov NA, Jolesz FA, Vykhodtseva N. Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage. 2005;24(1):12–20. doi: 10.1016/j.neuroimage.2004.06.046. [DOI] [PubMed] [Google Scholar]
- Nag N, Mellott TJ, Berger-Sweeney JE. Effects of postnatal dietary choline supplementation on motor regional brain volume and growth factor expression in a mouse model of Rett syndrome. Brain Res. 2008;1237:101–109. doi: 10.1016/j.brainres.2008.08.042. [DOI] [PubMed] [Google Scholar]
- Giri S, et al. T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson. 2009;11:56. doi: 10.1186/1532-429X-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engwerda C, Belnoue E, Gruner AC, Renia L. Experimental models of cerebral malaria. Curr Top Microbiol Immunol. 2005;297:103–143. [PubMed] [Google Scholar]
- Zhao H, et al. Olfactory plays a key role in spatiotemporal pathogenesis of cerebral malaria. Cell Host Microbe. 2014;15(5):551–563. doi: 10.1016/j.chom.2014.04.008. [DOI] [PubMed] [Google Scholar]
- Nacer A, et al. Experimental cerebral malaria pathogenesis--hemodynamics at the blood brain barrier. PLoS Pathog. 2014;10(12):e1004528. doi: 10.1371/journal.ppat.1004528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacer A, et al. Neuroimmunological blood brain barrier opening in experimental cerebral malaria. PLoS Pathog. 2012;8(10):e1002982. doi: 10.1371/journal.ppat.1002982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai S, et al. Real-time imaging reveals the dynamics of leukocyte behaviour during experimental cerebral malaria pathogenesis. PLoS Pathog. 2014;10(7):e1004236. doi: 10.1371/journal.ppat.1004236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw TN, et al. Perivascular Arrest of CD8+ T Cells Is a Signature of Experimental Cerebral Malaria. PLoS Pathog. 2015;11(11):e1005210. doi: 10.1371/journal.ppat.1005210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potchen MJ, et al. Acute brain MRI findings in 120 Malawian children with cerebral malaria: new insights into an ancient disease. AJNR Am J Neuroradiol. 2012;33(9):1740–1746. doi: 10.3174/ajnr.A3035. [DOI] [PMC free article] [PubMed] [Google Scholar]


