Abstract
While there have been remarkable advances in hearing research over the past few decades, there is still no cure for Sensorineural Hearing Loss (SNHL), a condition that typically involves damage to or loss of the delicate mechanosensory structures of the inner ear. Sophisticated in vitro and ex vivo assays have emerged in recent years, enabling the screening of an increasing number of potentially therapeutic compounds while minimizing resources and accelerating efforts to develop cures for SNHL. Though homogenous cultures of certain cell types continue to play an important role in current research, many scientists now rely on more complex organotypic cultures of murine inner ears, also known as cochlear explants. The preservation of organized cellular structures within the inner ear facilitates the in situ evaluation of various components of the cochlear infrastructure, including inner and outer hair cells, spiral ganglion neurons, neurites, and supporting cells. Here we present the preparation, culture, treatment, and immunostaining of neonatal murine cochlear explants. The careful preparation of these explants facilitates the identification of mechanisms that contribute to SNHL and constitutes a valuable tool for the hearing research community.
Keywords: Medicine, Issue 124, Murine cochlear explant, organotypic culture, inner ear, outer hair cells, inner hair cells, spiral ganglion neurons, auditory synaptopathy, adeno-associated virus
Introduction
Sensorineural Hearing Loss (SNHL) reflects damage to the inner ear or ascending auditory pathway. While hearing loss is the most common sensory deficit in humans1, curative therapies do not yet exist2. Although cochlear or auditory brainstem implants can restore some degree of hearing to patients with severe to profound SNHL, the hearing provided by these devices is still very different from "natural" hearing, especially during attempts to understand speech in noise or to listen to music.
While hair cell degeneration has long been considered the primary consequence of traumatic auditory events (e.g., exposure to loud noise), there is growing evidence that the synapses transmitting information from hair cells to the auditory nerve are at least as vulnerable to acoustic trauma3,4,5,6. Since human audiometric thresholds, the current gold standard for the evaluation of hearing function, do not predict specific cellular damage in the inner ear, more refined tools are needed to detect cellular degeneration as soon as possible and to initiate adequate treatment7.
Promising pharmaceutical treatments for hearing loss are often tested on homogenous cell cultures in vitro, but such systems do not accurately model the cochlear microenvironment. Cochlear cells are known to secrete trophic factors that influence other cell types within the cochlea8,9, a crucial in vivo process that is lost when the organ of Corti10,11 or Spiral Ganglion Neurons (SGNs)12 are cultured in isolation or when molecular markers are analyzed13. However, in vivo studies that may be necessary for the validation of in vitro data to establish new, personalized treatments for hearing loss in the pursuit of "precision medicine" require significant resources and time. This is especially relevant when considering how much effort is required to perfect and perform middle ear or round window membrane injections with hearing tests and the subsequent dissection of cochlear whole-mounts. The efficient screening of promising compounds in organotypic ex vivo cultures known as cochlear explants provides an economic and reliable alternative14,15,16,17.
This article details a protocol by which to generate, maintain, and evaluate treated cochlear explants. Specific applications for this model are emphasized, including its use in the screening of potentially therapeutic compounds and the comparative evaluation of viral vectors for gene therapy. An ex vivo explant approach allows researchers to visualize the effects of a given treatment on different cell populations in situ, facilitating the identification of cell type-specific mechanisms and the subsequent refinement of targeted therapeutics.
Overall, this technique provides a model to study the cochlea ex vivo while preserving vital cross-talk between the vastly different cell types that coexist within the cochlea.
Protocol
The study protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Massachusetts Eye and Ear. Experiments were carried out according to the Code of Ethics of the World Medical Association.
1. Preparing the Dissection
- Preparing the surgical table
- Use 70% ethanol to disinfect the surgical table.
- Place two nonsterile prep pads next to the microscope.
- Prepare the instrument tray, including heat-sterilized operating scissors, a scalpel handle, a micro knife, and forceps (two each of #4 & #55). Place the instrument tray and a 50 mm, clear-walled, glass-bottomed petri dish onto one non-sterile pad.
- Obtain a sterile #15 scalpel blade; a sealable plastic bag or container (to dispose of the carcasses in line with institutional guidelines); ice in a bucket; and cold, sterile Hank's Balanced Salt Solution (HBSS). Place these items onto the other nonsterile pad.
- Transfer the HBSS into a 50 mL plastic laboratory tube and put it on ice.
- Preparing the culture
- Prepare all materials in a laminar flow hood. For every four specimens, put four autoclaved 10 mm round glass cover slips in the four inlays of the 35 mm 4-well petri dish.
- For every 4 specimens, mix a total volume of 300 µL consisting of the following in a microcentrifuge tube: 10 µL of tissue adhesive, 285 µL of NaHCO3, and 5 µL of NaOH.
- Put 45 µL of the resulting solution on every cover slip and leave it there for at least 20 min at RT; this solution can be left on the coverslip for several hours but cannot be left O/N.
- Place one or two 35 mm 4-well petri dish(es) inside a 10 cm Petri dish in order to create two barriers against contamination.
- Prepare culture medium containing 98% Dulbecco's Modified Eagle's Medium (DMEM), 1% N-2, and 1% ampicillin (65 µL per well in a (micro)centrifuge tube). Warm the solution to 25 °C prior to use.
2. Murine Cochlear Explant Dissection
- Decapitation of the neonatal mouse
- Obtain a 60 mm plastic petri dish and spray 70% ethanol in its lid so that the surface is wet.
- Pour cold HBSS from the plastic laboratory tube into the bottom part of the 60 mm plastic and the 50 mm clear-walled, glass-bottomed petri dish. Store the plastic laboratory tube and both petri dishes on ice to keep the tissue cold whenever it is not being dissected.
- Place a P3 - P5-aged neonatal mouse on ice in a cutoff finger of a latex glove and wait until the hypothermia induces loss of consciousness (approximately 1-3 min).
- Decapitate the mouse quickly with operating scissors and dispose of the body in the sealable plastic bag. Put the severed neonate head in the 70% ethanol in the lid of the 60 mm plastic petri dish.
- Macroscopic dissection of the temporal bone
- Remove the skin of the skull by making a superficial cut from the anterior to the posterior end (directly on top of the sagittal suture) using the scalpel blade.
- Cut both external auditory canals with the scalpel blade and fold the skin anteriorly to expose the whole cranium. NOTE: Although no dissection microscope is usually needed for this step, it can be used for improved visualization.
- Open the cranium along the sagittal suture using the #15 scalpel blade. Make sure to cut the cranium by gently moving the blade up and down from anterior to posterior to avoid compression of the cochleae. NOTE: The cranium should not be ossified at this age and should cut easily.
- Remove and discard the snout by making a vertical cut directly posterior to the orbits using the #15 scalpel blade. NOTE: The posterior part of the skull should still contain the brain and cochleae.
- Dispose of the forebrain, cerebellum, and brainstem through blunt dissection with #4 forceps.
- Microscopic extraction of the cochleae
- Adjust the microscope (6.5X magnification) and light source by placing forceps under the scope and optimizing the illumination and focus.
- Place the two halves of the cranium into the 60 mm plastic petri dish filled with cold HBSS.
- Completely expose the cochlea/e, identifiable as being adjacent to the surrounding stapedial artery (a tortuous artery within the temporal bone), and bluntly separate the organ from the temporal bone using #4 forceps.
- Transfer the cochlea into the 50 mm, clear-walled, glass-bottomed petri dish and position the dish under the microscope.
- Place the 60 mm plastic petri dish with the other half of the cranium on ice.
- Remove the cochlea from the vestibular system and carefully dissect the cochlear otic capsule (typically cartilaginous at this age) with #4 forceps. Use #55 forceps for all further steps.
- Final steps of tissue processing
- Continue with the processing of the inner ear tissue by carefully separating the spiral ligament adherent to the organ of Corti from the rest of the cochlea and the modiolus using the micro knife or two forceps.
- Cut into the darker, translucent area at the crevice between the spiral ligament and the hair cell region and proceed to the subsequent removal of the spiral ligament by grasping it at the most basal aspect and gently unwinding it while moving apically.
- Position the specimen to obtain a longitudinal, sagittal view of the cochlea and cut the cochlea into two to three sections using the micro knife, classifying these sections as the apical, (middle,) and basal turns. Remove the tissue superior and inferior to the actual layer of hair cells and neurites in order to achieve a cochlear explant piece that can lay horizontally on the petri dish. NOTE: An appropriate size for stable adhesion to the culture dish is approximately half of one cochlear turn.
- Remove the tectorial membrane, a very thin translucent layer immediately superior to the organ of Corti, by gently peeling it away with forceps.
- Remove Reissner's membrane, immediately superior to the SGNs, by touching it with forceps on both sides and peeling it away. NOTE: Remove any laterally-located damaged hair cell areas by cutting them away with the micro knife.
3. Transfer of Specimens to the Culture Plates
Remove the 45 µL of tissue adhesive solution from the four cover slips. Wash each cover slip with 50 µL of sterile H2O. Aspirate the water.
Pick up the 1 mL pipette. Wet the pipette tip with approximately 120 µL of DMEM to prevent the specimen from adhering to the inside of the tip.
Transfer the specimens individually using the 1 mL pipette. Pipette a maximum of 70 µL from the HBSS-filled, small, clear-walled, glass-bottomed petri dish and place the specimen along with the liquid on one of the 4 inlays (prepared with cover slips) in the 4-well petri dish.
- Check under the microscope (50X magnification) that the specimen is in the correct orientation. Ensure that the area of hair cells from which the tectorial membrane was removed faces upwards. Ensure that the basilar membrane, together with the trimmed, basal part of the modiolus, faces downwards and adheres to the coverslip.
- If the specimen is oriented upside-down upon inspection, deposit the HBSS that remains in the pipette tip into the inlay and reposition the piece until it is correctly oriented. NOTE: This will prevent hair cells from floating in culture medium without structural support from the coverslip, which could lead to degeneration.
Aspirate excess HBSS using the 1 mL pipette. Wait for 15 s.
Pipette 60 µL of the prepared warm culture medium (98% DMEM, 1% N-2, and 1% ampicillin) directly onto the specimen. Take care not to touch the specimen with the pipette. Replace the inner and outer petri dish covers and incubate O/N at 37 °C.
Put all of the stock solutions back in their proper places, dispose of carcasses and residual waste, decontaminate the surgical table according to institutional guidelines (e.g., using ethanol or hypochlorite), clean and autoclave the instruments, and discard the sharps in the appropriate container.
4. Adding Final Culture Media and Substances of Interest
NOTE: Cochlear explants will be ready for use 10-16 h later.
Prepare a mixture of 97% DMEM, 1% fetal bovine serum (FBS), 1% N-2, and 1% ampicillin (65 µL per well in a microcentrifuge tube; warm the solution to 25 °C prior to use).
Add other substances of interest to the solutions for the respective treatment groups. If fluorescent substances are added (e.g., green fluorescent protein (GFP)-expressing viruses; in a recent publication, a concentration of 1010 GC of Adeno-Associated Viruses (AAVs) was used for 48 h in 50 µL)18, protect from light.
Transfer the cochlear explant-containing petri dishes from the incubator and place them under the laminar flow hood.
Aspirate the old culture medium (w/o FBS) with the pipette from the inlays.
Add 60 µL per well of the prepared warm culture (treatment) medium (97% DMEM, 1% FBS, 1% N-2, 1% ampicillin, and the substances of interest).
Place the petri dish back into the incubator (37 °C).
View regularly under the microscope to identify signs of contamination, cellular migration, or detachment of the cochlear explant and perform staining after a maximum of 7 days.
5. Immunofluorescence - Day 1
Prepare blocking solution (94% Phosphate-Buffered Saline (PBS), 5% normal goat or horse serum (NGS/NHS, depending on the secondary antibodies), and 1% non-ionic surfactant (see the Table of Materials)) and stock antibody solution (98.6% PBS, 1% NHS, and 0.4% non-ionic surfactant with the respective antibodies). NOTE: Antibodies include rabbit anti-Myo7A at a concentration of 1:400+ (myosin 7A, for inner and outer hair cells) and mouse anti-TuJ1 1:200+ (β-tubulin, for SGNs) OR mouse(IgG1) anti-CtBP2 1:200+ (c-terminal binding protein, for presynapse), mouse(IgG2a) anti-PSD95 1:50+ (postsynaptic density, for postsynapse), and chicken anti-NF-H 1:1000+ (neurofilament, for SGNs and nerve fibers). "+" means "or higher."
Aspirate the culture medium using a pipette. Take care not to touch the specimen.
Under the laminar flow hood, rinse the cochlear explants twice with sterile PBS, placing the specimens on a shaker for 5 min after each wash.
Fix the tissue in 4% paraformaldehyde (PFA - CAUTION) for 20 min on the shaker (under the hood). Aspirate the PFA.
Apply 60 µL of PBS to the cochlear explants and place the petri dish on a shaker for 5 min. Aspirate the PBS. Repeat 3x.
Place the cochlear explants in prepared blocking solution (94% PBS, 5% NHS, and 1% non-ionic surfactant) for 30 min on shaker.
Place the cochlear explants in a prepared stock antibody solution (98.6% PBS, 1% NHS, and 0.4% non-ionic surfactant) with the respective primary antibodies (vortex before use) O/N on a counter at RT. NOTE: Wrap the petri dishes with damp paper towels enclosed in plastic wrap (or aluminum foil if the added solution contains fluorescent materials such as GFP-expressing viruses) to keep the dishes humid and to prevent evaporation of the antibody solution O/N.
6. Immunofluorescence - Day 2
Prepare the 4',6-diamidino-2-phenylindole (DAPI) stain (300 nM) and use the stock antibody solution to obtain the correct secondary antibody mixtures, complementary to the primary antibodies in step 5.1. (e.g., goat anti-rabbit 488 1:200+ and goat anti-mouse 568 1:200+ OR goat anti-mouse (IgG1) 568 1:1000+, goat anti-mouse (IgG2a) 488 1:500+, and goat anti-chicken 647 1:200+ OR Phalloidin 568 1:200+ (for inner and outer hair cells)).
Aspirate the primary antibody solution.
Apply 60 µL of PBS to the cochlear explants and place the petri dish on a shaker for 5 min. Aspirate the PBS. Repeat 3x.
Place the cochlear explants in prepared stock antibody solution (98.6% PBS, 1% NHS, and 0.4% non-ionic surfactant) with the respective secondary antibodies (vortex before use) for 1.5 h on a counter and protect from light.
Aspirate the secondary antibody solution and rinse the cochlear explants with a DAPI stain (placing on a shaker for 1 min and then aspirate).
Apply 60 µL of PBS to the cochlear explants and place the petri dish on a shaker for 5 min. Aspirate the PBS. Repeat 3x.
Use forceps to remove coverslips with specimens from the 4-well plate, dip them into a recipient filled with distilled H2O, and dry the coverslips by vertically bringing them into contact with paper towels.
Place a drop of antifade mounting medium onto the microscope slide and invert the coverslips to immerse the downward-facing tissue in the medium.
Seal the coverslip using clear nail polish circumferentially covering the edge (without crushing the specimen under the glass). Leave the slides lying flat in a covered area away from light for 15 - 20 min until dry and then place them in a box or examine under the confocal microscope. NOTE: Fluorescent staining is best preserved by storing the slides at 4 or -20 °C.
7. Confocal Imaging
Obtain an overview and zoomed-in pictures for the organ of Corti region, including neurites and SGNs. NOTE: Standard settings for the imaging process: Format of 1,024 x 1,024 pixels, speed of 400 Hz, frame average of 3, 20% overall laser intensity, and usually 20X and 63X lens with oil immersion (potentially combined with 2X digital zoom). Channel settings for the DAPI, Myo7A, and TuJ1 staining protocol are outlined above: 405 5% DAPI, "Smart Gain" 766.8V, "Smart Offset" 0.0% - 488 15% fluorescein isothiocyanate (FITC), "Smart Gain" 622.2V, "Smart Offset" 0.0% - 561 13% tetramethylrhomadine isothiocyanate (TRITC), "Smart Gain" 562.4V, "Smart Offset" 0.0%.
Merge the images in a z-stack using the software to obtain a z-axis projection.
Representative Results
While many protocols focus on organ of Corti explants, this technique attempts to preserve the anatomy of the entire cochlear turn, including the SGNs. This gives researchers the opportunity to analyze the effects of a given treatment on neurites and somata of SGNs in addition to the organ of Corti. Performing a dissection that preserves part of the modiolus, as described here, is more technically challenging than explanting the organ of Corti alone. However, the neurite and SGN area is important, because significant pathological effects were observed in this region after applying vestibular schwannoma secretions (Figure 1) and extracellular vesicles in two recent publications using the same methodology19,20. Such changes could not have been revealed by using isolated organ of Corti explants.
The quantification of damaged or GFP-expressing cells for inner and outer hair cells, supporting cells, and SGNs can be performed by manual count or with an automated process, such as that built into the ImageJ plugin NeuronJ. Representative areas for these counts can be established after confirming their correlation with total counts. 3D reconstructions are particularly useful to exclude overlay effects in the z-axis projections of SGNs. Fiber organization, number of SGNs per given area, and SGN soma area can be evaluated. The staining and counting of synapses, hair cells, and neurites is depicted in Figure 2; the same protocol can also be used for cochlear whole mounts and has been shown to be a reliable model for these purposes17,21,22. Other researchers have established elegant alternative methods that can also be incorporated into experimental paradigms23,24.
The maximum length of culture is usually limited by the start of cellular migration and depends upon the concentration of FBS in the culture medium. When the proportion of FBS was reduced from five percent to three percent or one percent, the time period for maintaining an anatomically intact cochlear explant was extended to approximately one week (Figure 3).
Figure 4 demonstrates GFP-positive cochlear explant cells after transduction with the synthetic adeno-associated virus vector Anc80 for 48 h25. Inner hair cells, outer hair cells, and supporting cells can be quantified in this view, while a 3D reconstruction can help to quantify GFP-positive cells in the SGN area. Different viral serotypes can be compared in terms of their transduction efficiency in certain cell types using the outlined methodology18.
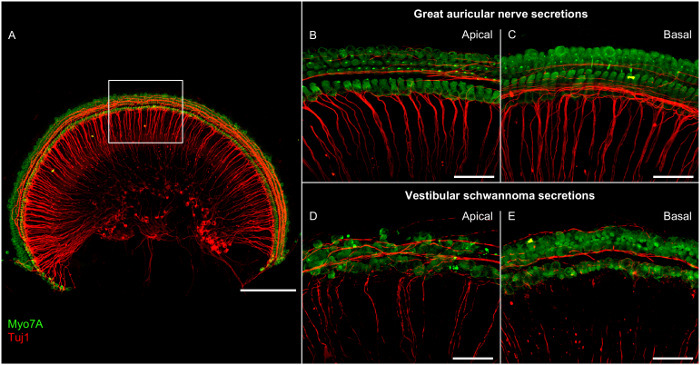 Figure 1:Representative Confocal Microscopy Images of Cochlear Explants from the Apical and Basal Regions after Incubation with Secretions from Great Auricular Nerves or Vestibular Schwannomas for 48 h. (A) Overview image of an explant. The rectangle marks the area in close-up images. Scale bar = 200 µm. (B-E) Zoomed-in views of hair cells and neurites. Scale bar = 50 µm. Green = Myosin 7A (Myo7A), stains hair cells; red = class III beta-tubulin (Tuj1), stains neuronal structures. Please click here to view a larger version of this figure.
Figure 1:Representative Confocal Microscopy Images of Cochlear Explants from the Apical and Basal Regions after Incubation with Secretions from Great Auricular Nerves or Vestibular Schwannomas for 48 h. (A) Overview image of an explant. The rectangle marks the area in close-up images. Scale bar = 200 µm. (B-E) Zoomed-in views of hair cells and neurites. Scale bar = 50 µm. Green = Myosin 7A (Myo7A), stains hair cells; red = class III beta-tubulin (Tuj1), stains neuronal structures. Please click here to view a larger version of this figure.
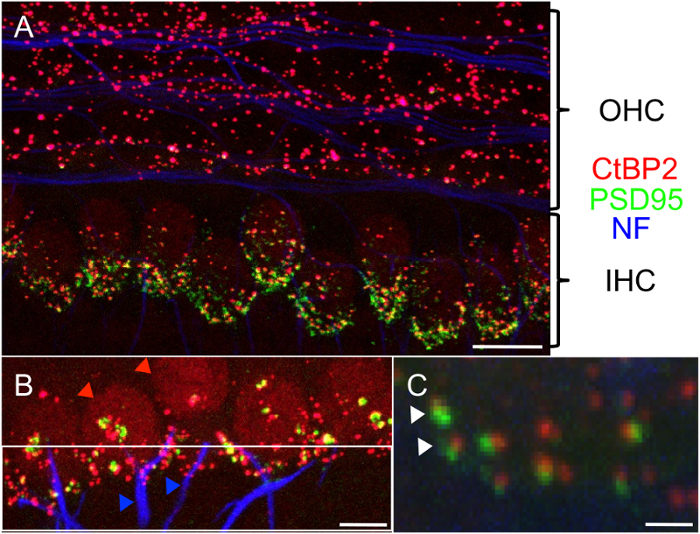 Figure 2:Confocal Microscopy Image of the Cochlear Explant Hair Cell Region. (A) Intact synapses can be assessed by labeling presynaptic (C-terminal binding protein, red) and postsynaptic (postsynaptic density-95, green) proteins together with the staining of neuronal structures (neurofilament, blue). OHC, outer hair cells; IHC, inner hair cells. Scale bar = 10 µm. (B) Focus on the inner hair cell region with the count of inner hair cells (red arrowheads) and neurites (blue arrowheads). Due to the ramification of the neuronal structures, it is important to standardize the distance from the inner hair cells during counting (highlighted with the white frame, directly connected to the synapses). Scale bar = 5 µm. (C) Close-up of separate synapses (2 pre- and postsynaptic pairs marked with white arrowheads). Scale bar = 1 µm. Please click here to view a larger version of this figure.
Figure 2:Confocal Microscopy Image of the Cochlear Explant Hair Cell Region. (A) Intact synapses can be assessed by labeling presynaptic (C-terminal binding protein, red) and postsynaptic (postsynaptic density-95, green) proteins together with the staining of neuronal structures (neurofilament, blue). OHC, outer hair cells; IHC, inner hair cells. Scale bar = 10 µm. (B) Focus on the inner hair cell region with the count of inner hair cells (red arrowheads) and neurites (blue arrowheads). Due to the ramification of the neuronal structures, it is important to standardize the distance from the inner hair cells during counting (highlighted with the white frame, directly connected to the synapses). Scale bar = 5 µm. (C) Close-up of separate synapses (2 pre- and postsynaptic pairs marked with white arrowheads). Scale bar = 1 µm. Please click here to view a larger version of this figure.
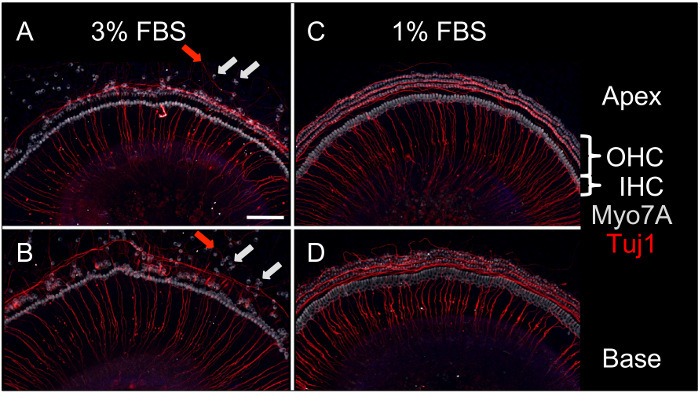 Figure 3:Representative Confocal Microscopy Images of Apical and Basal Cochlear Explants after 7 d in Culture with either 3% or 1% FBS. (A and B) Cells incubated in 3% FBS start to migrate between 4 and 5 days (light gray arrows for hair cells, red arrows for neurites), (C and D) Explants in 1% FBS maintain organizational integrity until day 7. Light gray: Myosin 7A (Myo7A), stains all hair cells; red: class III beta-tubulin (Tuj1), stains neuronal structures. OHC, outer hair cells; IHC, inner hair cells. Scale bar = 100 µm, applied to all panels. Please click here to view a larger version of this figure.
Figure 3:Representative Confocal Microscopy Images of Apical and Basal Cochlear Explants after 7 d in Culture with either 3% or 1% FBS. (A and B) Cells incubated in 3% FBS start to migrate between 4 and 5 days (light gray arrows for hair cells, red arrows for neurites), (C and D) Explants in 1% FBS maintain organizational integrity until day 7. Light gray: Myosin 7A (Myo7A), stains all hair cells; red: class III beta-tubulin (Tuj1), stains neuronal structures. OHC, outer hair cells; IHC, inner hair cells. Scale bar = 100 µm, applied to all panels. Please click here to view a larger version of this figure.
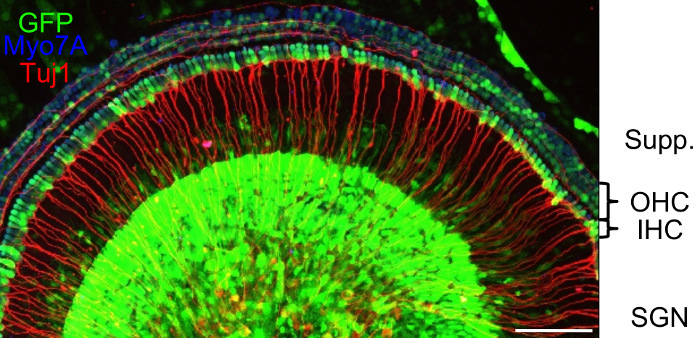 Figure 4:Confocal Microscopy Image of a Cochlear Explant after Exposure to the Synthetic Adeno-associated Virus Vector Anc80 for 48 h. Transduced cells express GFP (green). Myosin 7A (Myo7A, blue) stains Outer Hair Cells (OHC) and Inner Hair Cells (IHC); class III beta-tubulin (Tuj1, red) stains neuronal structures. Supp.: area of Sox2-positive supporting cells; SGN, spiral ganglion neurons. Scale bar: 100 µm. Please click here to view a larger version of this figure.
Figure 4:Confocal Microscopy Image of a Cochlear Explant after Exposure to the Synthetic Adeno-associated Virus Vector Anc80 for 48 h. Transduced cells express GFP (green). Myosin 7A (Myo7A, blue) stains Outer Hair Cells (OHC) and Inner Hair Cells (IHC); class III beta-tubulin (Tuj1, red) stains neuronal structures. Supp.: area of Sox2-positive supporting cells; SGN, spiral ganglion neurons. Scale bar: 100 µm. Please click here to view a larger version of this figure.
Discussion
Researchers must perfect the dissection technique before carrying out experiments involving cochlear explants. Hair cells are commonly damaged during dissections performed early on in the learning curve, and a particularly problematic moment for their integrity is the removal of the tectorial membrane, which requires steady hands, proper tools, and experience. To save time and resources, a visual control should be performed under the dissection microscope and potentially damaged areas should be noted. Instead of using expensive primary and secondary antibodies, cheaper reagents like phalloidin are more appropriate for preliminary experiments. Slightly damaged explants can potentially still be used as controls in certain experiments (analysis of sections that have not been affected) or for the testing of new combinations of antibodies.
Transfer of the specimens is also particularly important, because one must guarantee that the pieces are not oriented upside-down ("floating" hair cells without the guidance of the coated coverslip often degenerate). A central position on the coverslip facilitates all further pipetting steps.
For negative control experiments involving novel compounds (that obviously must be soluble in the culture medium), it is important to apply the appropriate vehicle to the explants and to not focus exclusively on untreated explants. For example, if a commercial drug includes sodium citrate in addition to the active ingredient, then sodium citrate should be added to the negative control cochlear explants because sodium citrate might itself have an effect.
Limitations to the use of cochlear explants include the fact that these organotypic cultures are typically derived from young pups that are still undergoing postnatal developmental changes and that there is a lack of an ionic gradient across the explant, which is essential for normal hearing in vivo. However, the utility of the protocol is compellingly shown in a recent publication regarding AAVs18. While this group of vectors is limited to about 4.7 kb of packaging capacity, it has become an important resource for the therapy of several human syndromes26. Using the cochlear explant model, the above-mentioned publication demonstrated that the Anc80 virus outperformed the traditional AAV serotypes in the transduction of a variety of cochlear cells, including hair cells and SGNs. These in vitro findings were subsequently confirmed by choosing the most promising candidates for injection through the round window in mouse pups in vivo. The ex vivo model provides a way to successfully recapitulate the anatomy of the cochlea, decreasing the time and resources needed for conducting in vivo work27.
Another advantage of the ex vivo model is its potential for application to the study of specific cellular mechanisms that contribute to SNHL. By applying compounds found in the tumor microenvironment (e.g., human tumor secretions) to an explant, the effect of these compounds on the cochlea can be directly visualized and the efficacy or toxicity of potential drug therapies assessed. This is important because the human cochlea cannot be biopsied, and the cells inside it cannot be visualized in vivo. For example, a recent publication examined the ototoxic potential of proteins secreted from vestibular schwannomas, which are intracranial tumors that arise from the vestibular nerves and cause hearing loss in 95% of patients. Applying human tumor secretions to cochlear explants, compared to secretions from control healthy human nerves, confirmed the toxic effects of these secretions on the cochlea19. The same experiment would be difficult to replicate in an in vivo model due to the immunogenic response against human proteins that would arise in an immunocompetent mouse.
In summary, this manuscript presents a technique that enables the simultaneous study of cochlear hair cells, synapses, neurites, and neurons in an ex vivo model. This model can help to provide insight into the mechanisms of action of molecules new to the cochlea28, including factors in human secretions19,20, while potentially accelerating the screening of candidate therapeutic compounds18 prior to their testing in vivo.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was supported by the National Institute of Deafness and Other Communication Disorders grants R01DC015824 (K.M.S.) and T32DC00038 (supporting S.D.), the Department of Defense grant W81XWH-15-1-0472 (K.M.S.), the Bertarelli Foundation (K.M.S.), the Nancy Sayles Day Foundation (K.M.S.), and the Lauer Tinnitus Research Center (K.M.S.). We thank Jessica E. Sagers, B.A. for insightful comments on the manuscript.
References
- Mathers C, Smith A, Concha M. Global burden of hearing loss in the year. World Health Organization (WHO) 2000. Available from: http://www.who.int/healthinfo/statistics/bod_hearingloss.pdf.
- Geleoc GS, Holt JR. Sound strategies for hearing restoration) Science. 2014;344(6184):1241062. doi: 10.1126/science.1241062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Acceleration of age-related hearing loss by early noise exposure: evidence of a misspent youth. J Neurosci. 2006;26(7):2115–2123. doi: 10.1523/JNEUROSCI.4985-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after "temporary" noise-induced hearing loss. J Neurosci. 2009;29(45):14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makary CA, Shin J, Kujawa SG, Liberman MC, Merchant SN. Age-related primary cochlear neuronal degeneration in human temporal bones. J Assoc Res Otolaryngol. 2011;12(6):711–717. doi: 10.1007/s10162-011-0283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JB, Lysaght AC, Liberman MC, Qvortrup K, Stankovic KM. Immediate and delayed cochlear neuropathy after noise exposure in pubescent mice. PLoS One. 2015;10(5) doi: 10.1371/journal.pone.0125160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landegger LD, Psaltis D, Stankovic KM. Human audiometric thresholds do not predict specific cellular damage in the inner ear. Hear Res. 2016. pp. 83–93. [DOI] [PMC free article] [PubMed]
- Wang HC, et al. Spontaneous Activity of Cochlear Hair Cells Triggered by Fluid Secretion Mechanism in Adjacent Support Cells. Cell. 2015;163(6):1348–1359. doi: 10.1016/j.cell.2015.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay M, Ryan AF, Housley GD. Type I vs type II spiral ganglion neurons exhibit differential survival and neuritogenesis during cochlear development. Neural Dev. 2011;6 doi: 10.1186/1749-8104-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh C, et al. Short interfering RNA against Bax attenuates TNFalpha-induced ototoxicity in rat organ of Corti explants. Otolaryngol Head Neck Surg. 2013;148(5):834–840. doi: 10.1177/0194599813477631. [DOI] [PubMed] [Google Scholar]
- Mazurek B, Yu Y, Haupt H, Szczepek AJ, Olze H. Salicylate modulates Hsp70 expression in the explanted organ of Corti. Neurosci Lett. 2011;501(2):67–71. doi: 10.1016/j.neulet.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Kao SY, et al. Loss of osteoprotegerin expression in the inner ear causes degeneration of the cochlear nerve and sensorineural hearing loss. Neurobiol Dis. 2013;56:25–33. doi: 10.1016/j.nbd.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan TA, Chai R, Sayyid ZN, Cheng AG. Isolating LacZ-expressing cells from mouse inner ear tissues using flow cytometry. J Vis Exp. 2011. p. e3432. [DOI] [PMC free article] [PubMed]
- Haque KD, Pandey AK, Kelley MW, Puligilla C. Culture of embryonic mouse cochlear explants and gene transfer by electroporation. J Vis Exp. 2015. p. e52260. [DOI] [PMC free article] [PubMed]
- Parker M, Brugeaud A, Edge AS. Primary culture and plasmid electroporation of the murine organ of Corti. J Vis Exp. 2010. [DOI] [PMC free article] [PubMed]
- Mulvaney JF, Dabdoub A. Long-term time lapse imaging of mouse cochlear explants. J Vis Exp. 2014. p. e52101. [DOI] [PMC free article] [PubMed]
- Wang Q, Green SH. Functional role of neurotrophin-3 in synapse regeneration by spiral ganglion neurons on inner hair cells after excitotoxic trauma in vitro. J Neurosci. 2011;31(21):7938–7949. doi: 10.1523/JNEUROSCI.1434-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landegger LD, et al. A synthetic AAV vector enables safe and efficient gene transfer to the mammalian inner ear. Nat Biotechnol. 2017;35(3):280–284. doi: 10.1038/nbt.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilwali S, Landegger LD, Soares VY, Deschler DG, Stankovic KM. Secreted Factors from Human Vestibular Schwannomas Can Cause Cochlear Damage. Sci Rep. 2015;5:18599. doi: 10.1038/srep18599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares VY, et al. Extracellular vesicles derived from human vestibular schwannomas associated with poor hearing damage cochlear cells. Neuro Oncol. 2016. [DOI] [PMC free article] [PubMed]
- Tong M, Brugeaud A, Edge AS. Regenerated synapses between postnatal hair cells and auditory neurons. J Assoc Res Otolaryngol. 2013;14(3):321–329. doi: 10.1007/s10162-013-0374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, et al. Ouabain-induced cochlear nerve degeneration: synaptic loss and plasticity in a mouse model of auditory neuropathy. J Assoc Res Otolaryngol. 2014;15(1):31–43. doi: 10.1007/s10162-013-0419-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez KA, Jeffers PW, Lall K, Liberman MC, Kujawa SG. Aging after noise exposure: acceleration of cochlear synaptopathy in "recovered" ears. J Neurosci. 2015;35(19):7509–7520. doi: 10.1523/JNEUROSCI.5138-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay M, Constable R, James NR, Thorne PR, Montgomery JM. Reduced sensory stimulation alters the molecular make-up of glutamatergic hair cell synapses in the developing cochlea. Neuroscience. 2016. pp. 50–62. [DOI] [PubMed]
- Zinn E, et al. In Silico Reconstruction of the Viral Evolutionary Lineage Yields a Potent Gene Therapy Vector. Cell Rep. 2015;12(6):1056–1068. doi: 10.1016/j.celrep.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Yang H, Colosi P. Effect of genome size on AAV vector packaging. Mol Ther. 2010;18(1):80–86. doi: 10.1038/mt.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, et al. Identification of Adeno-Associated Viral Vectors That Target Neonatal and Adult Mammalian Inner Ear Cell Subtypes. Hum Gene Ther. 2016. [DOI] [PMC free article] [PubMed]
- Kao SY, Soares VY, Kristiansen AG, Stankovic KM. Activation of TRAIL-DR5 pathway promotes sensorineural degeneration in the inner ear. Aging Cell. 2016;15(2):301–308. doi: 10.1111/acel.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]


