Abstract
Marfan syndrome is characterized by high expression of matrix metalloproteinases (MMPs) in aortic smooth muscle cells (AoSMCs) associated with medial elastolysis and aortic root aneurysm. We aimed to reduce aortic elastolysis through decrease of MMP expression with decoy oligodeoxynucleotides (dODNs) neutralizing the transcription factor activating factor-1 (AP-1). AP-1 abundance in nuclear extracts as well as MMP-2 and MMP-9 expression were significantly increased in isolated mAoSMC of mgR/mgR Marfan mice compared to wild-type cells. Exposure to AP-1 neutralizing dODNs resulted in a significant reduction of basal and interleukin-1β-stimulated MMP expression and activity in mAoSMCs. Moreover, increased migration and formation of superoxide radical anions was substantially decreased in mAoSMCs by AP-1 dODN treatment. Aortic grafts from donor Marfan mice were treated with AP-1- dODN ex vivo and implanted as infrarenal aortic interposition grafts in mgR/mgR mice. Pretreatment of aortic grafts with AP-1 dODN led to reduced elastolysis, macrophage infiltration, and MMP activity. Permeability of the endothelial monolayer was increased for dODN in mgR/mgR aortae with observed loss of tight junction proteins ZO-1 and occludin, enabling dODN to reach the tunica media. Targeting AP-1 activity offers a new potential strategy to treat the vascular phenotype associated with Marfan syndrome.
Keywords: Marfan syndrome, matrix metalloproteinases, AP-1, aneurysm, aorta
Introduction
In patients with Marfan syndrome (MFS), dilatation of the aortic root due to fibrillin fragmentation and deficiency of the aortic wall necessitates cardiovascular surgery in most patients.1 Aortic valve–sparing re-implantation technique represents a relatively safe method with favorable long-term results that avoids the need for lifelong anticoagulation.2, 3 Recently, using the selective AT1 receptor blocker losartan attenuating the canonical transforming growth factor-β (TGF-β) signaling in the aorta, an abrogated aneurysm progression in mice was demonstrated.4 However, recent clinical studies denied a protective effect of sartans in Marfan patients.5, 6 Therefore, an effective medical therapy that reliably prevents the development of the vascular phenotype with aortic root aneurysm in MFS is still not available.
It has been shown that high expression and activity of matrix metalloproteinases (MMPs) in aortic smooth muscle cells (AoSMC) lead to medial elastolysis in patients with infrarenal aneurysms (AAA) or MFS by destruction of structural matrix molecules.7, 8 Recent studies demonstrated upregulation of elastolytic MMP activity in these patients and in a murine model of MFS, especially MMP-2 and MMP-9.7, 9, 10, 11 Inhibition of MMP activity with doxycycline or losartan has been shown to reduce aortic dilatation.12, 13
Gene regulation of MMPs is influenced by the transcription factor activating factor-1 (AP-1).14 AP-1 complexes are heterodimers of proteins of the two proto-oncogene families (jun and fos) and have binding sites in the promoter region of MMPs,15 as well as in genes mediating inflammatory responses.16 The transcription factor decoy oligodeoxynucleotide (dODN) methodology allows neutralization of AP-1 and thereby inhibition of target gene expression, which has already been demonstrated by several preclinical animal studies.17, 18, 19, 20 dODNs are taken up efficiently by their target cells without any delivery aid through an energy-dependent carrier-mediated transport mechanism.21 These short double-stranded DNA sequences have successfully been used to bind specifically and neutralize transcription factors in cultured cells or in vivo, rendering them incapable of subsequent binding to the promoter region and induce the expression of target genes.22 We hypothesized that ex vivo aortic AP-1 neutralization by anti-AP-1 dODN leads to reduction of MMP expression and therefore reduces aortic elastolysis in an aortic transplantation murine Marfan model (mgR/mgR). Here, we report that in explanted aortic grafts of mgR/mgR mice pretreated with AP-1 dODN MMP activity and elastic fiber degradation were significantly decreased.
Results
Transcription Factor dODN Technology
The AP-1 consensus dODN (AP-1 cODN) was designed to neutralize the transcription factor AP-1, whereas the mutant control dODN (AP-1 mODN) as control does not affect the AP-1 transcription activity. The AP-1 dODN, which requires a hybridization step of the two complementary single strands, has already been successfully tested in various animal experimental models.17, 18, 19, 20 The so-called hairpin AP-1 dODN (hpODN) was designed as a single-stranded homoduplex molecule. This molecular beacon consists of a small loop and a long beacon stem that contains the transcription factor binding site. The initially single-stranded hpAP-1 dODN quickly hybridizes to itself, representing the active double-stranded AP-1 hpODN subsequently modulating gene expression. The hpODN constitutes a development of the complementary single-stranded dODNs designed for an adeno-associated virus-mediated gene therapy approach in Marfan mice and will provide for the first time a causal treatment perspective for Marfan patients.
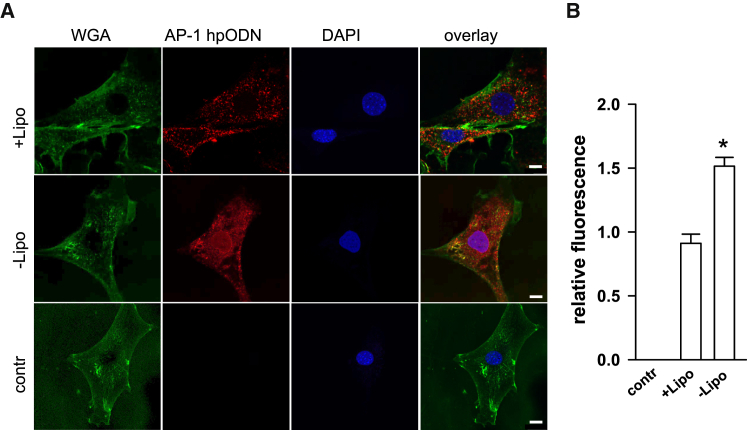
In this study, cultured cells and vessel grafts were incubated ex vivo with the dODN without any delivery aid like, e.g., cationic lipid transfection reagents. Cell culture experiments using mgR/mgR smooth muscle cells and the widely used cationic lipid transfection reagent Lipofectamine revealed no further improvement of hpODN uptake. The “naked” hpODN was already effectively taken up by the cells that demonstrated at least superior uptake efficacy in comparison to Lipofectamine (Figures 1A and 1B). It is of note that without Lipofectamine the hpODN was located in the cytosol and nucleus.
Figure 1.
AP-1 hpODN Uptake in mgR/mgR Smooth Muscle Cells
(A) mgR/mgR SMCs were seeded on gelatin-covered glass coverslips and incubated for 2 hr with an ATTO 590-labeled hpODN (red) without (“naked,” −Lipo) or with Lipofectamine (+Lipo). Wheat germ agglutinin (WGA)-Alexa 488 (green) was used for plasma membrane staining and DAPI to label nuclei. Exemplary pictures, scale bar corresponds to 10 μm. Images were taken using confocal microscope, and mean red fluorescence intensity was analyzed using ImageJ for statistical summary shown in (B); n = 8, *p = 0.0087, Mann-Whitney U-test. contr, control.
AP-1-Dependent Increase of MMP Expression in Cultured mgR/mgR Marfan SMCs
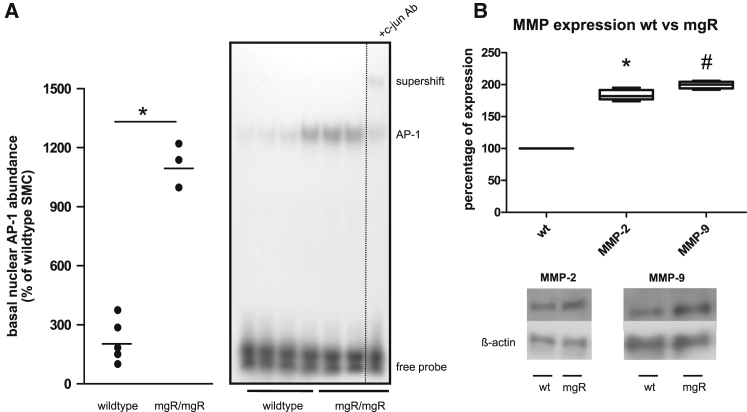
Supershift analysis showed a significantly increased abundance of c-Jun/AP-1 transcription factor in nuclear extracts from mgR/mgR Marfan-mAoSMCs as compared to wild-type cells (Figure 2A). The MMP-2 and MMP-9 promoters contain a number of potential AP-1 binding sites.14 Correspondingly, basal MMP-2 and MMP-9 expression was significantly increased in cultured Marfan-mAoSMCs as compared to wild-type cells (Figure 2B).
Figure 2.
Activation of AP-1 and MMP Expression Is Increased in Cultured mgR/mgR Marfan SMCs
(A) Increased AP-1 abundance in nuclear extracts from mgR/mgR mAoSMCs compared to wild-type (WT) mAoSMCs (*p = 0.0357, n = 3) probed by EMSA. Supershift analysis using a specific antibody showed the presence of the AP-1 subunit c-Jun. (B) Comparison of MMP expression in WT and mgR/mgR mice. Representative results and statistical summary (*p = 0.024, n = 4; #p = 0.016, n = 4).
AP-1 Consensus dODN-Dependent Inhibition of MMP Expression and Activity
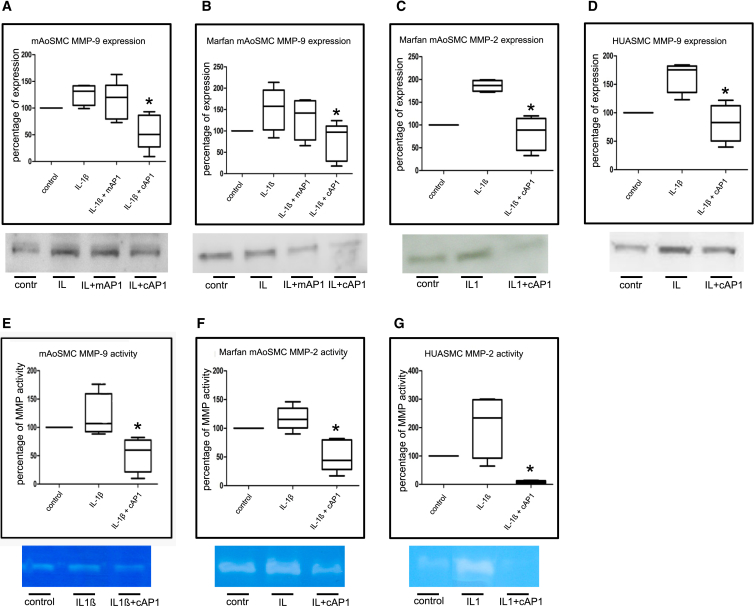
Pretreatment of wild-type mAoSMCs with AP-1 consensus dODN resulted in a reduction of MMP-9 expression by 87% and decreased MMP-9 activity by 50% as compared to untreated controls (Figures 3A and 3E). In mAoSMCs of Marfan mice, pretreatment resulted in reduction of MMP-2 expression and activity by 80% and 70%, respectively (Figures 3C and 3F). Moreover, MMP-9 expression was downregulated by 70% in these cells (Figure 3B). The mutant AP-1 dODN as appropriate control had no effect on MMP9 expression (Figures 2A and 2B). As these findings support our previously published findings using the mutant AP-1 dODN,17, 18, 20 we decided to forego the further experimental use of this dODN. Aimed to prove a potential translation into men, inhibition of MMPs by AP-1 consensus dODN was also evaluated in human umbilical artery smooth muscle cells (HUASMC), resulting in a reduction of MMP-9 expression by 80% and MMP-2 activity by 90% (Figures 3D and 3G). To induce AP-1 activation, cells were stimulated with IL-1β,23 except for controls cells. Our findings show that with exception of the human cells (HUASMC; Figure 3D) IL-1β only slightly but not significantly induce MMP expression and activity in murine cells. Nevertheless, these experiments clearly demonstrate that the AP-1 consensus dODN effectively neutralizes its target transcription factor because MMP expression and activity was reduced below the initial control level.
Figure 3.
AP-1-Dependent MMP Expression and Activity in Cultured Murine and Human SMCs
Effect of AP-1 cODN treatment on MMP expression by western blot (A–D) and activity by zymography (E–G) in IL-1β (IL1)-stimulated murine and human SMCs. Treatment with AP-1 cODN (cAP1), but not the mutated control ODN (mAP1), resulted in reduction of MMP-9 expression in WT mAoSMC (A, *p = 0.0159, n = 6) and mgR/mgR mAoSMC (B, *p = 0.0411, n = 6) and reduction of MMP-2 expression in mgR/mgR mAoSMC (C, *p = 0.025, n = 4), which was also shown for MMP-2 in HUASMCs (D, *p = 0.025, n = 4). Subsequently, MMP-9 (E, *p = 0.025, n = 5) and MMP-2 activity (F, *p = 0.022, n = 7; G, *p = 0.027, n = 4) was reduced as well. contr, control.
AP-1 hpODN Significantly Decreased IL-1β-Induced Migration and ROS Formation in mAoSMCs of Marfan Mice
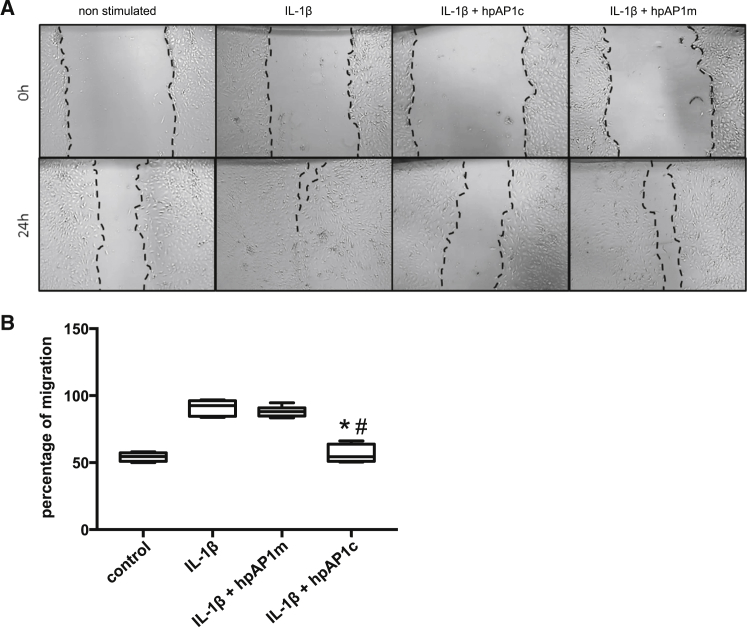
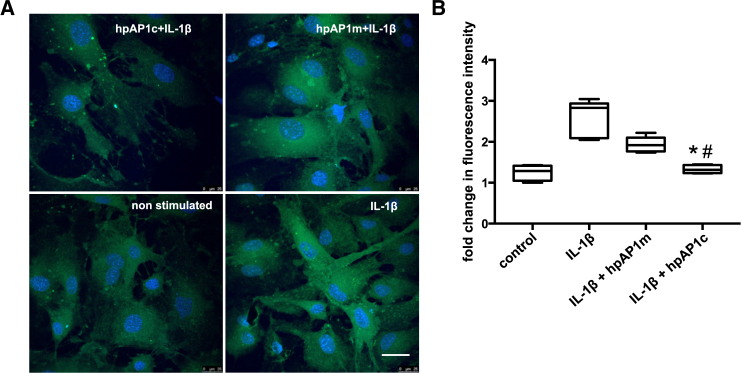
In normal mature blood vessels, the predominant smooth muscle phenotype is quiescent or contractile. This phenotype can switch into the proliferative, migratory, and synthetic type, which is known to be regulated by TGF-β and the transcription factor AP-1.24 IL-1β stimulation resulted in significant increase in the migration rate of SMCs (91% in IL-1-β-treated cells and 55.68% in non-stimulated control cells). Pretreatment with AP-1 consensus hpODNs significantly decreased IL-1β-induced migration (56% migration, p < 0.0001), while preincubation with the AP1-mutated hpODNs had no effect (88% migration; Figure 4). MAoSMC exposure to IL-1β enhanced formation of ROS (2.5-fold), as evaluated using the H2DCF-DA method. Incubation with AP1 hpODNs prior to IL-1β addition to cell culture medium significantly (p = 0.0152) reduced the ROS production (Figure 5).
Figure 4.
The Hairpin hpAP-1 cODN (hpAP1c), but Not the Mutated Control ODN (hpAP1m), Inhibits the Migration Ability of mgR/mgR SMCs
(A) Representative images showing the level of IL-1β-induced migration of SMCs in 24 hr. (B) Quantification of the percentage of migration of SMCs (*p < 0.0001, hpAP1c versus IL-1β-stimulated group; # p < 0.0001, hpAP1c versus hpAP1m; n = 5, one-way ANOVA).
Figure 5.
hpAP-1 cODN (hpAP1c), but Not the Mutated Control ODN (hpAP1m), Significantly Decreases the IL-1β-Induced Accumulation of ROS in mgR/mgR SMCs
(A) Analysis of cellular ROS production by DCF-DA stainings using confocal microscopy; scale bar corresponds to 25 μm. (B) Quantification of mean fluorescence intensity of DCF as a measure of oxidative stress. (*p = 0.0152 hpAP1c versus IL-1β-stimulated group; #p < 0.0001, hpAP1c versus hpAP1m, n = 4, one-way ANOVA).
Increased Endothelial Monolayer Permeability in mgR/mgR Mice
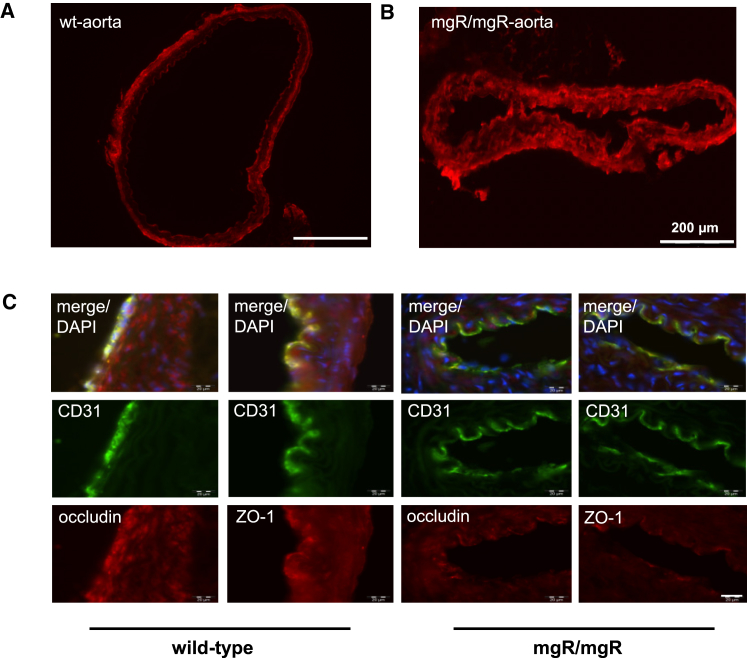
Vascular disease in MFS is characterized mainly by elastic matrix abnormalities in the medial layer of the aortic wall, including fragmentation and disorganization of elastic fibers but also by endothelial dysfunction.25, 26 Using fluorescent-labeled dODNs, we could show that dODNs are able to pass the endothelial barrier and reach the smooth muscle cells in the aortic media of Marfan mice but not of wild-type mice (Figures 6A and 6B). The assessment of tight junction coherence by ZO-1 and occludin stainings revealed a decrease of these proteins in Marfan mice (Figure 6C). This is shown by exhibition of a lower false orange fluorescence color intensity in mgR/mgR mouse aortic tissue staining than in wild-type mouse tissue after merging the endothelial cell marker CD31 (green) and tight junction protein stainings (red).
Figure 6.
The Endothelial Monolayer Permeability Is Increased in the Aorta of mgR/mgR Mice
Detection of ATTO 590-labeled fluorescent decoy penetration, apparent as bright red fluorescence, into aortic wall of WT (A, n = 1) and mgR/mgR mice (B, n = 1). ZO-1 and occludin stain (red) is reduced in mgR/mgR mice (n = 3) in comparison to WT animals (n = 2) (C). The decrease is visible because of lower false orange fluorescence color intensity in mgR/mgR mice aortic tissue staining after merging the endothelial cell marker CD31 (green) and tight junction protein stainings (red).
Reduction of MMP Activity, Medial Elastolysis, and Macrophage Infiltration in AP-1 Consensus ODN-Treated Aortic Grafts of Marfan Mice
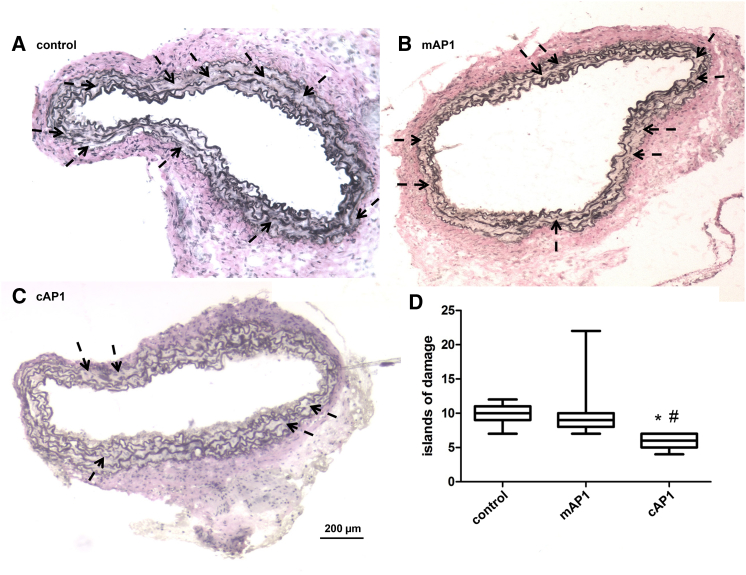
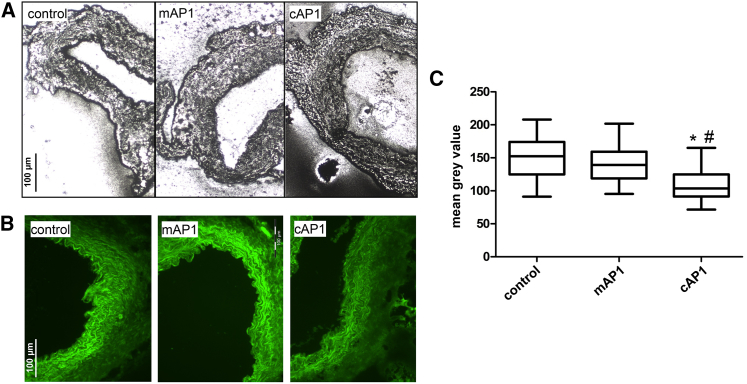
Aortic wall architecture disturbance was assessed by counting the number of islands of damage as described before by McLoughlin et al.30 Van Giesson staining demonstrated 30 days after transplantation that medial elastolysis was significantly reduced in aortic grafts of Marfan mice incubated ex vivo with AP-1 consensus dODN compared to the mutated control dODN and the untreated control groups (Figure 7). Thus, we demonstrated that short incubation with AP-1-neutralizing dODNs has a beneficial long-lasting effect on the aortic wall structure. In addition, we were able to demonstrate a decrease of gelatinase activity in AP-1 consensus dODN-treated aortic grafts compared to the AP-1-mutated dODN and untreated control groups, demonstrating in vivo reduction of MMP activity by AP-1 dODN even 30 days after treatment (Figure 8). In comparison to the AP-1-mutated ODN-treated animal, the macrophage infiltration in the aortic adventitia of AP-1 consensus dODN-treated animals was also significantly reduced (Figure 9).
Figure 7.
Verhoff van Giesson-Stained Axial Sections of Interposition Grafts of mgR/mgR Mice, Explanted after 30 Days, Show a Reduction of Fiber Breaks in the AP-1 cODN-Treated Group
Exemplary pictures of (A) untreated mice (control), (B) AP-1 mODN (mAP1)-treated mice, and (C) AP-1 cODN (cAP1)-treated mice. Arrows indicate “islands of damage,” defined as two strand breaks and widening of the aorta. (D) Statistical summary of the number of islands of damage survey, as counted by two observers blinded to groups (cAP1 versus control *p = 0.0002, n = 7; cAP1 versus mAP1 #p < 0.0001, n = 6).
Figure 8.
Representative In Situ Zymography with Aortic Sections Demonstrate a Decrease of MMP Activity in the Aortic Media of AP-1 cODN (cAP1)-Pretreated Grafts in Comparison to Non-Treated (Control) or AP-1 mODN (mAP1) Grafts
Either a photographic emulsion containing gelatin (A) or a fluorescin-labeled gelatin substrate (B) is brought into contact with the tissue sections. After incubation, MMP enzymatic activity is revealed as white spots on a dark background (A) or as bright green fluorescence (B), respectively. (C) Statistical summary of the mean brightness value analysis of each group (cAP1 versus control, *p < 0.0001, n = 5; cAP1 versus mAP1, #p < 0.0001, n = 4) of the fluorescin-gelatin zymography.
Figure 9.
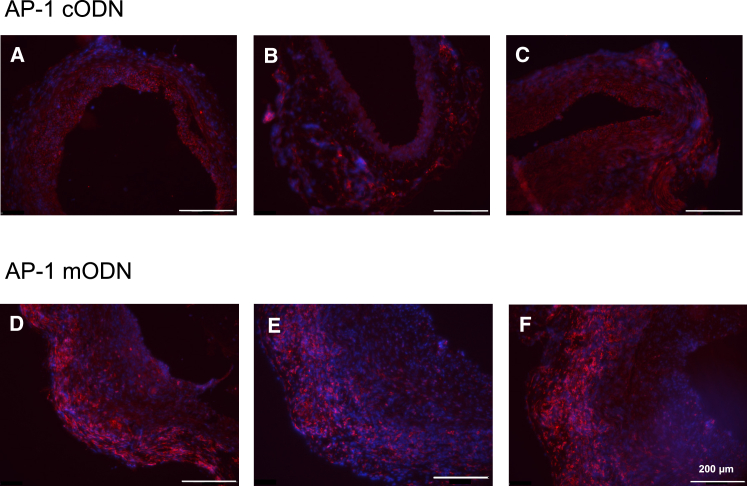
Macrophage Infiltration in the Aortic Adventitia of AP-1 cODN-Treated mgR/mgR Mice Is Reduced
Representative macrophage F4/80 staining (red, monocyte/macrophage marker) in mgR/mgR Marfan aortic sections, demonstrating a decrease of macrophage infiltration in the aortic adventitia of AP-1 cODN (animal, A–C), but not mutated control AP-1 mODN (animal, D–F)-pretreated grafts, explanted after 30 days. DAPI (blue) served as a nuclear marker; the scale bar represents 200 μm.
Discussion
Aortic root dilatation may lead to aortic rupture or dissection as the most life-limiting factor in Marfan syndrome.1 These patients need lifelong clinical and imaging surveillance, and operation for life-threatening aortic root dilatation is with few exceptions inevitable. New operative concepts such as aortic-valve-preserving implantation techniques for replacement of the aneurysmatic aortic root have shown favorable results; however, the underlying vascular pathology is not affected by surgery and the elastolysis may progress in the downstream aorta.28 Throughout the last years, medical treatment options changed from β-adrenergic blocker and calcium antagonists to doxycycline and losartan therapy with new insights in the pathomechanism of aortic wall degradation.12, 13, 29 Also statins, as HMG-CoA reductase inhibitors, accomplish similar effects to losartan in attenuating aortic root dilatation in a murine model of Marfan syndrome by reduction of MMPs.30 Interestingly, statins are also known to downregulate the activation of AP-1,31 which would explain the therapeutic effect that we have demonstrated here in Marfan mice. Deeper understanding of the pathology and molecular mechanisms of the vascular phenotype in MFS may help to develop new treatment strategies aiming to prevent root dilatation and consecutive need for open-heart surgery.
Disturbances in different molecular pathways caused by the underlying Fbn1 mutation may be responsible for the elastolysis of the aortic wall. To assess mechanisms and treatment options, various murine models of Marfan syndrome have been developed.9, 32 The hypomorphic mgR/mgR mouse provides a model with about only a quarter of Fbn1 production leading to phenotypic features in the skeleton and the aorta similar to those of patients with classic MFS. Recently, we were able to show that 49 out of 50 mgR/mgR mice die due to rupture and dissection of the ascending aorta.33 MMPs are presumed to play a key role in the pathogenesis of this genetic disorder through an imbalance between synthesis and proteolysis of extracellular matrix of the aortic media.7 MMP-2 and MMP-9 as elastolytic MMPs are secreted by AoSMC into the aortic media. Chung et al. demonstrated that elevated expression of MMP-2 and -9 lead to progression of thoracic aortic aneurysms associated with degeneration of elastic fibers, endothelial dysfunction, and reduced SMC contractility.34 MMP-2 and -9 have also been associated with TGF-β activation, which connects both MMP and TGF-β as the two major contributors of aortic elastolysis in Marfan patients.7 AP-1 has been described to be a downstream mediator of TGF-β signaling and profibrotic effects pointing out the potential importance of AP-1 in Marfan syndrome.35
AP-1 plays a pivotal role in transcriptional regulation of MMP expression.36 The decoy technique allows the neutralization of AP-1 without any transfection reagents for cellular uptake that cause potential side effects or immune reactions.17, 18 This energy-dependent transport mechanism of “naked” dODN is clearly distinct from the endocytotic pathway that has been described for antisense ODNs and involves both a saturable carrier-mediated component as well as a receptor-mediated podocytosis with subsequent intracellular release from caveloae vesicles.21 In addition, the length (i.e., uptake of decoy ODNs greater than 25-mer in size tends to be rather poor) but also certain sequence characteristics of the double-stranded DNA molecules seem to be important for the speed by which they are taken up by the target cell.21 Cationic lipids, such as the Lipofectamine that can greatly enhance plasmid DNA uptake, have only a modest impact on in vivo delivery of ODNs.22 ODN taken up via the Lipofectamine-mediated delivery could be exclusively detected in the cytosol and presumably ends in the lysosomal pathway for degradation,37, 38 whereas the “naked” dODN was detectable in the cytosol and nucleus. This result is in line with Bene et al.,39 demonstrating that lipid-complexed complexed AP-1 dODNs were readily transfectable into cells, but were consistently detectable only in the cytoplasm and not in the nucleus. In this context, it is particularly important that dODN become active immediately after absorption into the target cells. By contrast, the efficacy of antisense or RNA-interference ODNs is primarily dependent upon the conversion of the protein in the cell and therefore upon its re-synthesis. Nevertheless, we previously demonstrated the same effectiveness of an antisense ODN targeting the transcription factor STAT1 (signal transducers and activators of transcription) and a STAT1 transcription factor dODN40 to reduce CD40 expression on an mRNA and protein level. Because of the need of a delivery aid for antisense ODN, which may induce cellular stress response and change gene expression pattern in transfected cells,41 this method has to be considered as critical in regard of therapeutic use.
Treatment with decoys has been shown to limit restenosis after percutaneous transluminal coronary angioplasty in coronary arteries of hypercholesterolemic minipigs.17, 42 Moreover, the AP-1 dODN treatment suppresses graft endothelial adhesion molecule expression, reducees graft infiltration, and in turn significantly delays acute rejection in different rat transplantation models17 and prevents the occurrence of cardiac allograft vasculopathy in a fully allogenic rat heart transplantation model.18 Therefore, single ex vivo perfusion of cardiac allografts with AP-1 dODN targeting allograft immunogenicity during the cardiac transplantation procedure might be an attractive adjunct or even alternative concept to systemic immunosuppressive therapy of the recipient.17
In the present study, we demonstrated an increased AP-1 abundance and consecutively elevated MMP activity in aortic smooth muscle cells of Marfan mice. Subsequently, both MMP expression and activity were significantly reduced using the AP-1 dODN approach in these cells. AP-1 is a key player in regulating inflammatory processes but also required for basal expression of several genes, thus constantly activated.43 We therefore assume that in contrast to HUASMCs used in Figure 3D, IL-1β presumably failed in murine SMCs to further increase AP-1 activation and MMP expression because of an already increased high basal AP-1 activation level.
In line with this finding, we observed a reduced macrophage infiltration into the aortic adventitia of the AP-1 consensus dODN-treated grafts of mgR/mgR mice. Inflammatory infiltrates have already been described in the mgR/mgR mouse model and suggest that inflammation may represent a component of the complex pathogenesis of MFS.44 AP-1 dODN treatment also reduced ROS formation in smooth muscle cells, which is solely sufficient to create a pro-inflammatory environment and promote cell migration and MMP expression, subsequently leading to aneurysm formation.45 Indeed, a reduced IL-1β-induced migration of cultured smooth muscle cells could be demonstrated after AP-1 dODN treatment. It has been published previously that TGF-β can also increase vascular smooth muscle migration proliferation and also migration in patients with MFS.24
Another interesting aspect of neutralizing AP-1 is the inhibition of interactions between AP-1 and Smad proteins, which synergize to activate the TGF-β1-responsive genes involved in hypertrophic growth of the heart muscle and in the development of cardiac fibrosis.46 Through preventing AP-1 activation, we are not only able to reduce MMP expression and subsequently activity but may also achieve synergistic effects to treat MFS symptoms.
By using fluorescent-marked dODNs, we could show that these molecules are able to pass the endothelial barrier and reach the smooth muscle cells in the aortic media of Marfan, but not of wild-type mice. In wild-type mice, the endothelial cell layer represents a major barrier site to the passive movement of small molecules, as already shown previously in native hearts perfused with fluorescent-dye-labeled dODN18 and grafts incubated with fluorescent albumin.26 Moreover, the immunohistological ZO-1 and occludine staining suggest that mgR/mgR mice have a leakage in endothelial barrier function within the intercellular boundaries, as published recently.26 This is a prerequisite for understanding the effectiveness of dODN in Marfan aorta and may also explain why the migration of immunological cells in AP-1 dODN grafts is reduced. Guo et al. demonstrated that aortic extracts from mgR/mgR mice were able to stimulate macrophage chemotaxis by interaction with elastin-binding protein and showed that fibrillin-1 fragments are capable of chemotactic stimulatory activity.44 These findings are in line with the observation that patients with progressive aortic disease showed higher levels of macrophage colony stimulating factor (M-CSF) in the blood.47 It has been demonstrated previously that M-CSF gene expression is mediated by transcription factors like AP-1.48
The method of ex vivo incubation of the descending thoracic aorta and re-implantation into the infrarenal abdominal aorta of genetically identical mice has been previously described.27 Using this approach by incubation with AP-1 consensus dODN of the aortic grafts, we could demonstrate a significant reduction of elastolysis. Moreover, the neutralization resulted not only in a reduced elastolysis but also in a reduced MMP activity. The modification of our decoy structure into a hairpin molecule could enable possible catheter-based applications or a systemic application. Miyake et al. already described a systemic approach using chimeric ribbon-type decoy ODNs against nuclear factor-κB for successful prevention of abdominal aneurysm formation in a rat model.49
In conclusion, we demonstrated that AP-1 neutralization results in a significant inhibition of both MMP expression and activity in cultured aortic smooth muscle cells. Subsequently, medial MMP activity and elastolysis was reduced in AP-1 dODN-treated aortic grafts of Marfan mice. The observed loss of tight junction proteins may be related to increased endothelial cell permeability, which facilitates a deep dODN penetration into the aortic wall of Marfan mice. These findings may lead to a new nucleic-acid-based therapeutic concept to treat the vascular phenotype of Marfan syndrome.
Materials and Methods
Animal Experiments
All animal experiments were performed with permission of the Regional Council Karlsruhe (G187/11) and conformed to the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 2011). Inbred mgR/mgR mice, generously provided by P.N. Robinson, and gender-matched wild-type littermates were housed in the interfaculty biomedical research facility (IBF), University of Heidelberg, Germany. Age- and gender-matched mgR/mgR mice were used as isograft recipients. The mgR/mgR mouse is a gene-targeted hypomorphic murine model of MFS engineered by insertion of a neomycin cassette between exons 18 and 19 of the Fbn1 gene.9, 33
Aortic Isograft Model
Harvested from 21 mgR/mgR mice immediately after euthanasia using carbon dioxide (CO2) inhalation, the descending thoracic aorta was transplanted as end-to-end infrarenal interposition grafts into age- and gender-matched mgR/mgR mice at the age of 6–9 weeks. Grafts from donor animals (n = 7 per group) were perfused with 100 μL Ringer (B. Braun, Melsungen, Germany), AP-1-mutated dODN (10 μmol/L,) or AP-1 consensus dODN (10 μmol/L) and incubated ex vivo for 30 min at room temperature. During incubation, the recipient mice were anesthetized by inhalation of 5% isoflurane and intraperitoneal injection of Temgesic (0.3 mg/mL buprenorphine; 2 mg/100 g body weight). After laparotomy, the infrarenal abdominal vessels were carefully separated, vascular clamps were applied, and the aorta was dissected between the clamps. The heating was turned off, and 10 mL ice-cold 0.9% NaCl was applied to diminish the effects of warm ischemia. The ex vivo incubated isografts were anastomosed with 11-0 prolene in an end-to-end technique. After approximately 45 min of ischemia, blood flow was re-established and perfusion was assessed. Afterward, the abdomen was closed using absorbable 5-0 monofilament sutures. The mice were kept under standard conditions until sacrifice for graft explantation 30 days later, again using CO2 inhalation for euthanasia.27
Transcription Factor Decoy Technology
Double-stranded ODNs and hairpin dODNs (purity > 95%) were prepared from complementary single-stranded phosphorothioate-bonded ODNs obtained from Biomers (Ulm, Germany) as previously described.17 The uptake of an ATTO 590-labeled AP-1dODN by aortic tissue was monitored by fluorescence microscopy with an Olympus Spinning Disc Confocal microscope and Olympus Xcellence imaging software. A mutated decoy ODN was used as a control for the non-specific actions of DNA administration. The sequences of the single-stranded ODNs were as follows (bold letters denote phosphorothioate-bonded bases; mutations are set in italics).
AP-1 consensus dODN, 5′-GTGCTGACTCAGCAC-3′;
AP-1 mutated dODN, 5′-GTGCTTACTTAGCAC-3′;
Hairpin AP-1 consensus dODN, 5′-CTGCGGTGCTGACTCAGCACGAAACGTGCTCAGTGAGCACCGCAG-3′;
Hairpin AP-1 mutated dODN, 5′-CTGCGGTGCTTACTTAGCACGAAACGTGCTAAGTAAGCACCGCAG-3′
Lipofectamine-Mediated Delivery of dODN
mgR/mgR SMCs were seeded on gelatin-covered glass coverslips in 24-well plates. ATTO 590-labeled decoy ODNs were added in the medium to a final concentration of 10 μM. Lipofectamine-mediated delivery was performed by incubating the decoy ODNs with 1 μL/well Lipofectamine 3000 for 15 min in Opti-MEM medium prior to cell treatment. After 2 hr, cells were washed with PBS and incubated with 5 μg/mL WGA-Alexa 488 at 37°C for 10 min. Afterward, cells were fixed with 4% paraformaldehyde (PFA) and counterstained with DAPI. Images were taken using confocal microscope, and mean red fluorescence intensity was analyzed using ImageJ.
Histochemistry
Interposition aortic grafts were explanted after 30 days and embedded in Tissue-Tek (Weckert Labortechnik, Germany). Cryostat sections (Microm HM 500 O, Walldorf, Germany) were cut at 5 μm thickness. Thereafter, Van-Giesson differential staining was performed for assessing the elastic fibers in the aortic media. The degree of fragmentation of elastic fibers was examined by two independent observers blinded to the treatment groups. Each slide was scored using an arbitrary scoring system that counted the number of “islands of damage” within an aortic cross-section (n = 7 mice in each group). An island of damage was defined as an isolated area of aortic wall where two adjacent elastic fibers were fragmented with interposed excessive connective tissue matrix, as published elsewhere.27
Immunohistochemistry
Aortic cryosections of 9-week-old mgR/mgR and wild-type littermates were used for endothelial marker CD31, tight junction proteins ZO-1 and occludin stainings according to standard protocols published previously.50 In addition a F4/80 monocyte fluorescence immunohistochemistry was performed as described previously by Seppelt et al.26 Positive immunoreaction was detected using an Olympus Spinning Disc Confocal microscope and the Olympus Xcellence imaging software (Olympus Europa, Hamburg, Germany).
Cell Culture
HUASMC and murine aortic smooth muscle cells (mAoSMCs) from wild-type and mgR/mgR Marfan mice were obtained by explant culture from tissue stained positive for smooth muscle α-actin (ab5694, Abcam, UK). Tissue harvesting for cultivation of mAoSMCs from wild-type and mgR/mgR Marfan mice was performed after euthanasia through CO2 inhalation. Only cells cultured up to passage 4 were used. For the use of HUASMCs, informed consent was obtained from the patients (parents) after approval by the local ethics committee (Ethikkommission der Medizinischen Fakultät Heidelberg, Germany, S-182/2013), and experiments conformed to the principles outlined in the Declaration of Helsinki (1997). On the basis of previous electrophoretic mobility shift assay (EMSA) and PCR analyses, SMCs were preincubated for 2 hr with 10 μmol/L dODN in DMEM with 0% fetal calf serum (FCS; GIBCO Life Technologies, New York, USA) followed by stimulation with IL-1β (60 U/mL; Humanzyme, Chicago, USA) for the next 24 hr.18 Cellular dODN uptake was achieved without using any cationic lipid or liposomal complex. The supernatant was collected and frozen at −80°C until needed for zymography.
Western Blot Analysis and EMSA
Western immunoblotting analysis was performed by denaturating 8%–10% SDS-PAGE and transferred onto polyvinylidene fluoride (PVDF) membranes (Merck Millipore, Germany) as previously described.51 Protein quantification was done by using antibodies against MMP-2 (DLN-12481, Dianova, Germany), MMP-9 (ab38898, Abcam, UK), and β-actin (ab6276, Abcam, UK). Secondary antibodies were purchased from Dianova (Germany).
Gel or electrophoretic mobility shift analyses with the 32P-labeled AP-1 consensus dODN as a probe were performed as described previously.18 Nuclear extracts were prepared either from isolated and cultured SMCs of wild-type or Marfan mgR/mgR mice as also described before.18
Zymography
Gelatin zymography was performed to assess MMP activity in the cellular supernatant, according to standard protocols using gelatin-conjugated Sepharose beads (Gelatin Sepharose 4B; Amersham Pharmacia Biotech, Uppsala, Sweden) for affinity precipitation.51
For in situ zymography with aortic sections, either a photographic emulsion containing gelatin or a fluorescin-labeled gelatin substrate was brought into contact with tissue sections of explanted grafts 30 days after treatment according to Yan and Blomme.52
mAoSMC-Migration Assay
Murine aortal smooth muscle cells were cultured as mentioned above and put in 12-well plates (Greiner Bio One, Frickenhausen, Germany) in which silicone stoppers were positioned prior to seeding. After the cells reached confluency, they were serum-starved for 16 hr. For 2 hr, half of the wells were treated with the hpAP1 consensus decoy ODNs (Biomers, Ulm, Germany) and the other half with hpAP1 mutated decoy ODNs. IL-1β 20 ng/mL (Humanzyme, Chicago, USA) was added to the culture medium prior to stopper removal. Bright field microscopy images were captured at time points 0 and 24 hr after the silicone stoppers withdrawal. The area of migration was determined using ImageJ, and the migration rate was calculated according to the following formula: Percent migration = (A(0) − A(24)/A(0)) × 100. (A(0), initial area between the cells; A(24), area between the cells after 24 hr).
Reactive Oxygen Species Production
The intracellular amount of reactive oxygen species (ROS) was determined using dichlorodihydrofluorescein diacetate (H2DCFDA) (Thermo Fischer Scientific, Darmstadt, Germany) In brief, mAoSMCs were cultured on gelatin-coated glass coverslips and incubated with the hpAP1 decoy ODNs for 2 hr. Afterward, cells were stimulated with IL-1β 20 ng/mL (Humanzyme, Chicago, USA) for 5 hr and subsequently incubated with H2DCFDA for 30 min. The cells were then fixed with methanol and mounted on slides using a DAPI-containing medium. DCF fluorescence was measured using a confocal microscope.
Data Analysis
Results are presented as means ± SEM of n experiments. Comparisons of three or more groups were performed with one-way ANOVA followed by exact, two-sided Kruskal-Wallis tests. Mann-Whitney U tests were used to compare two samples (GraphPad Prism software package version 5.01, GraphPad Software, San Diego, USA) with p < 0.05 considered to be significantly different.
Author Contributions
R.A., M.Z., A.H.W., and K.K. conceived and designed the experiments. R.A., M.Z., P.S., A.R., R.K., H.S., S.M.E., A.H.W., and K.K. performed the experiments analyzed the data. R.A., M.Z., P.S., S.S., S.M.E., O.J.M., P.N.R., M.H., M.K., A.H.W., and K.K. contributed to the writing of the manuscript.
Conflicts of Interest
None of the authors have any disclosures to make.
Acknowledgments
This work was supported by the B. Braun Foundation, Melsungen, Germany, and in part by a grant of the German state of Baden-Württemberg (M.K.). We are indebted to Antje Weber and Franziska Mohr for expert technical assistance.
References
- 1.Murdoch J.L., Walker B.A., Halpern B.L., Kuzma J.W., McKusick V.A. Life expectancy and causes of death in the Marfan syndrome. N. Engl. J. Med. 1972;286:804–808. doi: 10.1056/NEJM197204132861502. [DOI] [PubMed] [Google Scholar]
- 2.David T.E., Feindel C.M. An aortic valve-sparing operation for patients with aortic incompetence and aneurysm of the ascending aorta. J. Thorac. Cardiovasc. Surg. 1992;103:617–621. discussion 622. [PubMed] [Google Scholar]
- 3.Kallenbach K., Baraki H., Khaladj N., Kamiya H., Hagl C., Haverich A., Karck M. Aortic valve-sparing operation in Marfan syndrome: what do we know after a decade? Ann. Thorac. Surg. 2007;83:S764–S768. doi: 10.1016/j.athoracsur.2006.10.097. [DOI] [PubMed] [Google Scholar]
- 4.Habashi J.P., Doyle J.J., Holm T.M., Aziz H., Schoenhoff F., Bedja D., Chen Y., Modiri A.N., Judge D.P., Dietz H.C. Angiotensin II type 2 receptor signaling attenuates aortic aneurysm in mice through ERK antagonism. Science. 2011;332:361–365. doi: 10.1126/science.1192152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milleron O., Arnoult F., Ropers J., Aegerter P., Detaint D., Delorme G., Attias D., Tubach F., Dupuis-Girod S., Plauchu H. Marfan Sartan: a randomized, double-blind, placebo-controlled trial. Eur. Heart J. 2015;36:2160–2166. doi: 10.1093/eurheartj/ehv151. [DOI] [PubMed] [Google Scholar]
- 6.Lacro R.V., Dietz H.C., Sleeper L.A., Yetman A.T., Bradley T.J., Colan S.D., Pearson G.D., Selamet Tierney E.S., Levine J.C., Atz A.M., Pediatric Heart Network Investigators Atenolol versus losartan in children and young adults with Marfan’s syndrome. N. Engl. J. Med. 2014;371:2061–2071. doi: 10.1056/NEJMoa1404731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ikonomidis J.S., Jones J.A., Barbour J.R., Stroud R.E., Clark L.L., Kaplan B.S., Zeeshan A., Bavaria J.E., Gorman J.H., 3rd, Spinale F.G., Gorman R.C. Expression of matrix metalloproteinases and endogenous inhibitors within ascending aortic aneurysms of patients with Marfan syndrome. Circulation. 2006;114(1, Suppl):I365–I370. doi: 10.1161/CIRCULATIONAHA.105.000810. [DOI] [PubMed] [Google Scholar]
- 8.Patel M.I., Melrose J., Ghosh P., Appleberg M. Increased synthesis of matrix metalloproteinases by aortic smooth muscle cells is implicated in the etiopathogenesis of abdominal aortic aneurysms. J. Vasc. Surg. 1996;24:82–92. doi: 10.1016/s0741-5214(96)70148-9. [DOI] [PubMed] [Google Scholar]
- 9.Pereira L., Lee S.Y., Gayraud B., Andrikopoulos K., Shapiro S.D., Bunton T., Biery N.J., Dietz H.C., Sakai L.Y., Ramirez F. Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proc. Natl. Acad. Sci. USA. 1999;96:3819–3823. doi: 10.1073/pnas.96.7.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiong W., Knispel R.A., Dietz H.C., Ramirez F., Baxter B.T. Doxycycline delays aneurysm rupture in a mouse model of Marfan syndrome. J. Vasc. Surg. 2008;47:166–172. doi: 10.1016/j.jvs.2007.09.016. discussion 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Segura A.M., Luna R.E., Horiba K., Stetler-Stevenson W.G., McAllister H.A., Jr., Willerson J.T., Ferrans V.J. Immunohistochemistry of matrix metalloproteinases and their inhibitors in thoracic aortic aneurysms and aortic valves of patients with Marfan’s syndrome. Circulation. 1998;98(Suppl 19):II331–II337. discussion II337–II338. [PubMed] [Google Scholar]
- 12.Habashi J.P., Judge D.P., Holm T.M., Cohn R.D., Loeys B.L., Cooper T.K., Myers L., Klein E.C., Liu G., Calvi C. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang H.H., Kim J.M., Chum E., van Breemen C., Chung A.W. Effectiveness of combination of losartan potassium and doxycycline versus single-drug treatments in the secondary prevention of thoracic aortic aneurysm in Marfan syndrome. J. Thorac.Cardiovasc. Surg. 2010;140:305–312. doi: 10.1016/j.jtcvs.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 14.Benbow U., Brinckerhoff C.E. The AP-1 site and MMP gene regulation: what is all the fuss about? Matrix Biol. 1997;15:519–526. doi: 10.1016/s0945-053x(97)90026-3. [DOI] [PubMed] [Google Scholar]
- 15.Chakraborti S., Mandal M., Das S., Mandal A., Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol. Cell. Biochem. 2003;253:269–285. doi: 10.1023/a:1026028303196. [DOI] [PubMed] [Google Scholar]
- 16.Schonthaler H.B., Guinea-Viniegra J., Wagner E.F. Targeting inflammation by modulating the Jun/AP-1 pathway. Ann. Rheum. Dis. 2011;70(Suppl 1):i109–i112. doi: 10.1136/ard.2010.140533. [DOI] [PubMed] [Google Scholar]
- 17.Stadlbauer T.H., Wagner A.H., Hölschermann H., Fiedel S., Fingerhuth H., Tillmanns H., Bohle R.M., Hecker M. AP-1 and STAT-1 decoy oligodeoxynucleotides attenuate transplant vasculopathy in rat cardiac allografts. Cardiovasc. Res. 2008;79:698–705. doi: 10.1093/cvr/cvn135. [DOI] [PubMed] [Google Scholar]
- 18.Hölschermann H., Stadlbauer T.H., Wagner A.H., Fingerhuth H., Muth H., Rong S., Güler F., Tillmanns H., Hecker M. STAT-1 and AP-1 decoy oligonucleotide therapy delays acute rejection and prolongs cardiac allograft survival. Cardiovasc. Res. 2006;71:527–536. doi: 10.1016/j.cardiores.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Feldner A., Otto H., Rewerk S., Hecker M., Korff T. Experimental hypertension triggers varicosis-like maladaptive venous remodeling through activator protein-1. FASEB J. 2011;25:3613–3621. doi: 10.1096/fj.11-185975. [DOI] [PubMed] [Google Scholar]
- 20.Buchwald A.B., Wagner A.H., Webel C., Hecker M. Decoy oligodeoxynucleotide against activator protein-1 reduces neointimal proliferation after coronary angioplasty in hypercholesterolemic minipigs. J. Am. Coll. Cardiol. 2002;39:732–738. doi: 10.1016/s0735-1097(01)01797-1. [DOI] [PubMed] [Google Scholar]
- 21.Hecker M., Wagner S., Henning S.W., Wagner A.H. Decoy oligodeoxynucleotides to treat inflammatory diseases. In: Kurreck J., editor. Therapeutic Oligonucleotides. Chapter 7. The Royal Society of Chemistry; 2008. pp. 163–188. [Google Scholar]
- 22.Mann M.J., Dzau V.J. Therapeutic applications of transcription factor decoy oligonucleotides. J. Clin. Invest. 2000;106:1071–1075. doi: 10.1172/JCI11459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung Y.D., Fan F., McConkey D.J., Jean M.E., Liu W., Reinmuth N., Stoeltzing O., Ahmad S.A., Parikh A.A., Mukaida N., Ellis L.M. Role of P38 MAPK, AP-1, and NF-kappaB in interleukin-1beta-induced IL-8 expression in human vascular smooth muscle cells. Cytokine. 2002;18:206–213. doi: 10.1006/cyto.2002.1034. [DOI] [PubMed] [Google Scholar]
- 24.Crosas-Molist E., Meirelles T., López-Luque J., Serra-Peinado C., Selva J., Caja L., Gorbenko Del Blanco D., Uriarte J.J., Bertran E., Mendizábal Y. Vascular smooth muscle cell phenotypic changes in patients with Marfan syndrome. Arterioscler. Thromb. Vasc. Biol. 2015;35:960–972. doi: 10.1161/ATVBAHA.114.304412. [DOI] [PubMed] [Google Scholar]
- 25.Syyong H.T., Chung A.W., Yang H.H., van Breemen C. Dysfunction of endothelial and smooth muscle cells in small arteries of a mouse model of Marfan syndrome. Br. J. Pharmacol. 2009;158:1597–1608. doi: 10.1111/j.1476-5381.2009.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seppelt P.C., Schwill S., Weymann A., Arif R., Weber A., Zaradzki M., Richter K., Ensminger S., Robinson P.N., Wagner A.H. Loss of endothelial barrier in Marfan mice (mgR/mgR) results in severe inflammation after adenoviral gene therapy. PLoS ONE. 2016;11:e0148012. doi: 10.1371/journal.pone.0148012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heim C., Abele-Ohl S., Eckl S., Ramsperger-Gleixner M., Mahmoudian S., Weyand M., Stamminger T., Ensminger S.M. Murine cytomegalovirus infection leads to increased levels of transplant arteriosclerosis in a murine aortic allograft model. Transplantation. 2010;90:373–379. doi: 10.1097/TP.0b013e3181e8a699. [DOI] [PubMed] [Google Scholar]
- 28.Kallenbach K., Karck M., Pak D., Salcher R., Khaladj N., Leyh R., Hagl C., Haverich A. Decade of aortic valve sparing reimplantation: are we pushing the limits too far? Circulation. 2005;112(Suppl 9):I253–I259. doi: 10.1161/01.CIRCULATIONAHA.104.525907. [DOI] [PubMed] [Google Scholar]
- 29.Browatzki M., Larsen D., Pfeiffer C.A., Gehrke S.G., Schmidt J., Kranzhofer A., Katus H.A., Kranzhofer R. Angiotensin II stimulates matrix metalloproteinase secretion in human vascular smooth muscle cells via nuclear factor-kappaB and activator protein 1 in a redox-sensitive manner. J. Vasc. Res. 2005;42:415–423. doi: 10.1159/000087451. [DOI] [PubMed] [Google Scholar]
- 30.McLoughlin D., McGuinness J., Byrne J., Terzo E., Huuskonen V., McAllister H., Black A., Kearney S., Kay E., Hill A.D. Pravastatin reduces Marfan aortic dilation. Circulation. 2011;124(Suppl 11):S168–S173. doi: 10.1161/CIRCULATIONAHA.110.012187. [DOI] [PubMed] [Google Scholar]
- 31.Dichtl W., Dulak J., Frick M., Alber H.F., Schwarzacher S.P., Ares M.P., Nilsson J., Pachinger O., Weidinger F. HMG-CoA reductase inhibitors regulate inflammatory transcription factors in human endothelial and vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2003;23:58–63. doi: 10.1161/01.atv.0000043456.48735.20. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein C., Liaw P., Jimenez S.A., Buchberg A.M., Siracusa L.D. Of mice and Marfan: genetic linkage analyses of the fibrillin genes, Fbn1 and Fbn2, in the mouse genome. Mamm. Genome. 1994;5:696–700. doi: 10.1007/BF00426075. [DOI] [PubMed] [Google Scholar]
- 33.Schwill S., Seppelt P., Grunhagen J., Ott C.E., Jugold M., Ruhparwar A., Robingson P.N., Karck M., Kallenbach K. The fibrillin-1 hypomorphic mgR/mgR murine model of Marfan syndrome shows severe elastolysis in all segments of the aorta. J. Vasc. Surg. 2013;57:1628–1636. doi: 10.1016/j.jvs.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Chung A.W., Au Yeung K., Sandor G.G., Judge D.P., Dietz H.C., van Breemen C. Loss of elastic fiber integrity and reduction of vascular smooth muscle contraction resulting from the upregulated activities of matrix metalloproteinase-2 and -9 in the thoracic aortic aneurysm in Marfan syndrome. Circ. Res. 2007;101:512–522. doi: 10.1161/CIRCRESAHA.107.157776. [DOI] [PubMed] [Google Scholar]
- 35.Palumbo K., Zerr P., Tomcik M., Vollath S., Dees C., Akhmetshina A., Avouac J., Yaniv M., Distler O., Schett G., Distler J.H. The transcription factor JunD mediates transforming growth factor beta-induced fibroblast activation and fibrosis in systemic sclerosis. Ann. Rheum. Dis. 2011;70:1320–1326. doi: 10.1136/ard.2010.148296. [DOI] [PubMed] [Google Scholar]
- 36.Bergman M.R., Cheng S., Honbo N., Piacentini L., Karliner J.S., Lovett D.H. A functional activating protein 1 (AP-1) site regulates matrix metalloproteinase 2 (MMP-2) transcription by cardiac cells through interactions with JunB-Fra1 and JunB-FosB heterodimers. Biochem. J. 2003;369:485–496. doi: 10.1042/BJ20020707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujiwara Y., Wada K., Kabuta T. Lysosomal degradation of intracellular nucleic acids-multiple autophagic pathways. J. Biochem. 2017;161:145–154. doi: 10.1093/jb/mvw085. [DOI] [PubMed] [Google Scholar]
- 38.Juliano R.L., Carver K. Cellular uptake and intracellular trafficking of oligonucleotides. Adv. Drug Deliv. Rev. 2015;87:35–45. doi: 10.1016/j.addr.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bene A., Kurten R.C., Chambers T.C. Subcellular localization as a limiting factor for utilization of decoy oligonucleotides. Nucleic Acids Res. 2004;32:e142. doi: 10.1093/nar/gnh139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagner, A.H., and Hecker, M. (2008). Modulation of the expression of genes dependent on STAT-1, US 10/491,644 patent US7320964 B2, filed October 2, 2002, and published January 22, 2008.
- 41.Fiszer-Kierzkowska A., Vydra N., Wysocka-Wycisk A., Kronekova Z., Jarząb M., Lisowska K.M., Krawczyk Z. Liposome-based DNA carriers may induce cellular stress response and change gene expression pattern in transfected cells. BMC Mol. Biol. 2011;12:27. doi: 10.1186/1471-2199-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stojanovic T., Wagner A.H., Wang S., Kiss E., Rockstroh N., Bedke J., Gröne H.J., Hecker M. STAT-1 decoy oligodeoxynucleotide inhibition of acute rejection in mouse heart transplants. Basic Res. Cardiol. 2009;104:719–729. doi: 10.1007/s00395-009-0028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buttice G., Quinones S., Kurkinen M. The AP-1 site is required for basal expression but is not necessary for TPA-response of the human stromelysin gene. Nucleic Acids Res. 1991;19:3723–3731. doi: 10.1093/nar/19.13.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo G., Booms P., Halushka M., Dietz H.C., Ney A., Stricker S., Hecht J., Mundlos S., Robinson P.N. Induction of macrophage chemotaxis by aortic extracts of the mgR Marfan mouse model and a GxxPG-containing fibrillin-1 fragment. Circulation. 2006;114:1855–1862. doi: 10.1161/CIRCULATIONAHA.105.601674. [DOI] [PubMed] [Google Scholar]
- 45.Fan L.M., Douglas G., Bendall J.K., McNeill E., Crabtree M.J., Hale A.B., Mai A., Li J.M., McAteer M.A., Schneider J.E. Endothelial cell-specific reactive oxygen species production increases susceptibility to aortic dissection. Circulation. 2014;129:2661–2672. doi: 10.1161/CIRCULATIONAHA.113.005062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schröder D., Heger J., Piper H.M., Euler G. Angiotensin II stimulates apoptosis via TGF-beta1 signaling in ventricular cardiomyocytes of rat. J. Mol. Med. (Berl.) 2006;84:975–983. doi: 10.1007/s00109-006-0090-0. [DOI] [PubMed] [Google Scholar]
- 47.Radonic T., de Witte P., Groenink M., de Waard V., Lutter R., van Eijk M., Jansen M., Timmermans J., Kempers M., Scholte A.J. Inflammation aggravates disease severity in Marfan syndrome patients. PLoS ONE. 2012;7:e32963. doi: 10.1371/journal.pone.0032963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Konicek B.W., Xia X., Rajavashisth T., Harrington M.A. Regulation of mouse colony-stimulating factor-1 gene promoter activity by AP1 and cellular nucleic acid-binding protein. DNA Cell Biol. 1998;17:799–809. doi: 10.1089/dna.1998.17.799. [DOI] [PubMed] [Google Scholar]
- 49.Miyake T., Aoki M., Osako M.K., Shimamura M., Nakagami H., Morishita R. Systemic administration of ribbon-type decoy oligodeoxynucleotide against nuclear factor κB and ets prevents abdominal aortic aneurysm in rat model. Mol. Ther. 2011;19:181–187. doi: 10.1038/mt.2010.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bauer A.T., Bürgers H.F., Rabie T., Marti H.H. Matrix metalloproteinase-9 mediates hypoxia-induced vascular leakage in the brain via tight junction rearrangement. J. Cereb. Blood Flow Metab. 2010;30:837–848. doi: 10.1038/jcbfm.2009.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kallenbach K., Fernandez H.A., Seghezzi G., Baumann F.G., Patel S., Grossi E.A., Galloway A.C., Mignatti P. A quantitative in vitro model of smooth muscle cell migration through the arterial wall using the human amniotic membrane. Arterioscler. Thromb. Vasc. Biol. 2003;23:1008–1013. doi: 10.1161/01.ATV.0000069880.81136.38. [DOI] [PubMed] [Google Scholar]
- 52.Yan S.J., Blomme E.A. In situ zymography: a molecular pathology technique to localize endogenous protease activity in tissue sections. Vet. Pathol. 2003;40:227–236. doi: 10.1354/vp.40-3-227. [DOI] [PubMed] [Google Scholar]