Abstract
Nox4-based NADPH oxidase is a major reactive oxygen species-generating enzyme in the vasculature, but its role in atherosclerosis remains controversial.
Objective
Our goal was to investigate the mechanisms of endothelial Nox4 in regulating atherosclerosis.
Approach and results
Atherosclerosis-prone conditions (disturbed blood flow, type I diabetes, and Western diet) downregulated endothelial Nox4 mRNA in arteries. To address whether the downregulated endothelial Nox4 was directly involved in the development of atherosclerosis, we generated mice carrying a human Nox4 P437H dominant negative mutation (Nox4DN), driven by the endothelial specific promoter Tie-2, on atherosclerosis-prone genetic background (ApoE deficient mice) to mimic the effect of decreased endothelial Nox4. Nox4DN significantly increased type I diabetes-induced aortic stiffness and atherosclerotic lesions. Gene analysis indicated that soluble epoxide hydrolase 2 (sEH) was significantly upregulated in Nox4DN endothelial cells (EC). Inhibition of sEH activity in Nox4DN EC suppressed inflammation and macrophage adhesion to EC. On the contrary, overexpression of endothelial wild type Nox4 suppressed sEH, ameliorated Western diet-induced atherosclerosis and decreased aortic stiffness.
Conclusions
Atherosclerosis-prone conditions downregulated endothelial Nox4 to accelerate the progress of atherosclerosis, at least in part, by upregulating sEH to enhance inflammation.
1. Introduction
Atherosclerosis occurs preferentially at lesion prone areas of disturbed flow characterized by low and oscillatory wall shear stress in branched or curved arteries. The link between oscillatory flow and atherosclerosis has been well characterized by using a partial left carotid artery (LCA) ligation model [1–3]. Western diet (WD) accelerates the development of atherosclerosis. Diabetes, by driving inflammation, dramatically accelerates atherosclerosis [4–6].
Nox4-based NADPH oxidase is a major source of reactive oxygen species (ROS) associated with cardiovascular health and diseases. Nox4 is abundantly expressed both in vascular smooth muscle cells (SMC) and endothelial cells (EC) but the relative contribution of Nox4 from each of these two cell types to atherosclerosis remains controversial. Transgenic mice overexpressing Nox4 in endothelium had decreased atherosclerotic lesions [7]. Similarly, two recent publications using whole body Nox4 deficient mice, indicate that Nox4 is protective in the progression of atherosclerotic lesions, although the molecular mechanisms remain unclear [8,9]. However, one study using Nox4 deficient mice, on ApoE deficient (ApoE −/−) genetic background, found no effect of Nox4 deficiency on atherosclerosis in a model of type I diabetes [10]. In cultured EC, oscillatory shear stress (OSS) decreases Nox4 mRNA level [11].
We recently reported that smooth muscle Nox4 is upregulated in atherosclerosis-prone conditions (disturbed blood flow and Western diet), and downregulation of smooth muscle Nox4 ameliorates WD-induced atherosclerosis by suppressing soluble epoxide hydrolase (sEH, gene EPHX2) [12]. In this study, we explored whether endothelial Nox4 contributes to the development of atherosclerosis and whether this effect is mediated by sEH, similar to SMC. We found that disturbed blood flow induced by partial LCA ligation downregulated endothelial Nox4, upregulated sEH and the inflammatory marker vascular cell adhesion molecule-1 (VCAM1) in the ligated artery. Streptozocin (STZ)-induced type I diabetes and WD downregulated endothelial Nox4, and increased atherosclerotic lesions. To mimic the effect of decreased endothelial Nox4 under atherosclerosis-prone conditions in vivo, we backcrossed transgenic mice overexpressing a human Nox4 dominant negative P437H mutant (Nox4DN), specifically in the endothelium under the control of a Tie-2 promoter [13], onto FVB/N ApoE −/− background to study atherosclerosis. We found that endothelial Nox4DN upregulated sEH and accelerated atherosclerosis, while overexpression of endothelial Nox4 wild type downregulated sEH and ameliorated atherosclerosis.
2. Material and methods
2.1. Transgenic mouse lines in FVB/N and FVB/N ApoE−/− background
Mouse lines overexpressing human Nox4 wild type (EWT) or its dominant negative form P437H (EDN) in the endothelium, driven by a Tie-2 promoter, were originally generated in Dr. Junichi Sadoshima's laboratory on a FVB/N genetic background [14]. The mutation of proline at position 437, crucial for Nox4 enzymatic activity, to histidine was confirmed by genomic DNA sequencing. For atherosclerosis studies, both EDN and EWT were backcrossed onto the FVB/N ApoE−/− background [15] to obtain EDN/ApoE−/− and EWT/ApoE−/−. Littermate FVB/N ApoE−/−, without Nox4DN or Nox4 transgene, served as controls. Backcrossing onto FVB/N ApoE −/− background did not affect Nox4DN or Nox4 mRNA expression levels, as confirmed by quantitative PCR. Male and female mice were used with equal number of mice in each gender group. All animal procedures were approved by the Boston University's Institutional Animal Care and Use Committee in accordance with the provisions of the Animal Welfare Act, Public Health Service Animal Welfare Policy, and the principles of the NIH Guide for the Care and Use of Laboratory Animals. Animals were maintained in an AALAC-approved Laboratory Animal Science Center staffed with licensed veterinarians.
2.2. Endothelial cell culture [14]
Cardiac ventricles pooled from two hearts from each transgenic mouse line from 10 to 20 weeks old mice were used for EC isolation and culture, as described previously [14]. At least 3 independent cell isolations were carried out for each transgenic mouse line along with age-matched littermate controls. Briefly, ventricles were digested with collagenase type II and DNAse I, and EC were selected with a rat anti-mouse CD31 antibody (BD Biosciences PharMingen). The resulting cardiac EC were resuspended in endothelial basal medium-2 (EBM-2) supplemented with 10% fetal bovine serum (FBS), EC mitogen and antibiotics. Cells from passages 2 to 8 were used. No difference on plating efficiency was noticed among EC from different transgenic mouse lines.
2.3. Real time quantitative PCR [16]
Total cellular RNA was isolated from EC cultured in 0.2% FBS EBM-2 or from common carotid arteries and aorta using standard procedures, and retro-transcribed to cDNA. Quantitative PCR was performed with the following gene specific primers: Nox4, Forward primer-5′TGGCCAACGAAGGGGTTAAA3′, Reverse primer-5′ACACAATCCTAGGCCCAACA3′; EPHX2: Forward primer-5′CAGGAGGACACAGACACCATA3′, Reverse primer-5′TCTCAGGTAGATTGGCTCCAC3′ using SYBR-Green-based detection, or Taqman gene specific primers (Applied Biosystems), using the following cycling conditions: denaturation, annealing, and extension at 95 °C, 57 °C and 72 °C for 10 s, 30 s, and 10 s, respectively, for 40 cycles. β-actin or 18S were used as endogenous control.
2.4. Western blot
EC were cultured in 0.2% FBS EBM-2 overnight before being lysed. Proteins were subjected to SDS-PAGE and immunoblotted with specific antibodies against Nox4 (provided by Dr. Ajah Shah), phospho-eNOS (p-eNOS, Ser1177, BD), phospho-p65 NFκB (Cell Signaling Technologies, CST), p65 NFκB (CST), VCAM1 (Santa Cruz), sEH (Santa Cruz); GAPDH (CST) was used as loading control. Proteins were visualized with an ECL system (Amersham Biosciences). Densitometry analysis was performed using NIH Image J software, and protein expression levels were normalized to GAPDH.
2.5. Treatment with sEH inhibitor 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl) urea (TPPU) [17]
TPPU (0.1 µM) was dissolved in DMSO and added to EDN/ApoE−/− EC in EBM-2 supplemented with 0.2% FBS for two days before proliferation assay, migration assay, NFκB promoter luciferase activity assay, macrophage adhesion assay or protein collection. DMSO served as vehicle control.
2.6. Cell proliferation assay [14]
EC were seeded in 96-well plates at a density of 5 × 103 cells per well in EBM-2 supplemented with 0.2% FBS with or without VEGF-A (50 ng/mL) and culture medium with or without VEGF-A was changed daily. Cell number in each well was determined 72 h after seeding using a tetrazolium-based non-radioactive proliferation assay kit (Quick Cell Proliferation Assay Kit II, BioVision), according to the manufacturer's protocol.
2.7. Wounded monolayer migration assay in EC
A detailed method is described [18]. Briefly, EC were seeded overnight in 0.2% FBS EBM-2 at a density of 8 × 105 cells per well (100% confluence) in 12-well plates coated with gelatin. Scratch wounds were applied to EC monolayer with a pipette tip. Immediately after scratching, the cells were treated with DMSO or TPPU in 0.2% FBS medium and photographs were taken at 0 h and 6 h at three fixed locations along the scratch with a light microscope and analyzed using NIH ImageJ software.
2.8. Adenoviral transfection of human aortic endothelial cells (HAEC) [18]
HAEC were transfected with empty adenoviral vector, human Nox4 adenovirus (provided by Dr. Sadoshima [19]), adenoviral control small interference RNA (siRNA) or adenoviral Nox4 siRNA (provided by Dr. Chen [20]) with 50 MOI/cell in EBM-2 without serum or antibiotics for 6 h before switching to EBM-2 containing 2% FBS and growth factors for 3 days.
2.9. NFκB promoter luciferase activity assay [21]
EC were cultured in 24-well plates with 0.2% FBS EBM-2 overnight, and then co-infected with firefly NFκB promoter luciferase adenovirus and a pre-packaged renilla luciferase adenovirus (Vector Biolabs) for 48 h. Briefly, cells were washed and lysed in lysis buffer as per manufacturer's recommendations. The ratio of firefly to renilla luciferase luminescence was calculated as indicative of NFκB promoter activity.
2.10. Bone marrow-derived macrophage (BMDM) adhesion assay [21]
Bone marrow-derived mononuclear cells were isolated [22] from ApoE−/− mice and cultured in high glucose medium supplemented with 10% FBS and 20% L929-conditioned medium for five days, when mononuclear cells became macrophages. For macrophage adhesion assay, EC were plated in 12-well plates, at a density of 106 cells/well, in 0.2% FBS EBM-2 overnight until confluent. BMDMs (5 × 105 cells/well) were added to EC and incubated for 1 h, then the media was removed and EC washed 3 times with PBS to remove unbound macrophages. Four images of macrophage adherent to EC in each well were taken and the number of bound macrophage was counted per image area.
2.11. Partial left common carotid artery (LCA) ligation [2]
Anesthesia was induced by intraperitoneal injection of xylazine (10 mg/kg) and ketamine (80 mg/kg) mixture. Mice underwent partial LCA ligation to rapidly induce disturbed blood flow. Briefly, three of the four caudal branches of the LCA (left external carotid, internal carotid, and occipital artery) were ligated with a 6-0 silk suture, while the superior thyroid artery was left intact. Mice which underwent similar manipulation but without ligation were used as sham control. Mice were sacrificed 2 days or 2 weeks after surgery, for RNA isolation or tissue collection, respectively.
2.12. Tissue preparation and immunohistochemistry analysis [16]
Tissues were collected as described previously [16]. For each animal, 6 identical 7 µm cross-sections at 250–300 µm intervals were prepared, starting at the bifurcation of the common carotid artery. The mean cross-sectional area of neotimal and medial areas were determined using NIH Image J analysis software. The medial area was calculated as the area encircled by the external elastic lamina minus the area encircled by the internal elastic lamina. The intima to media ratio was quantified by dividing the neointimal area by the medial area.
2.13. Streptozotocin (STZ)-induced atherosclerotic lesions
Ten weeks old EDN/ApoE−/− and ApoE−/− mice were administered 5 daily intraperitoneal injections of either vehicle (citrate acid buffer, pH 4.5) or STZ (50 mg/kg/d, dissolved in citrate acid buffer at pH 4.5). Diabetes was verified on the basis of a blood glucose level N 200 mg/dL one week later. Four weeks later, mice underwent partial LCA ligation to rapidly induce disturbed blood flow for 2 weeks before being sacrificed for tissue harvesting.
2.14. Western diet (WD)-induced atherosclerotic lesions [15]
Ten weeks old EWT/ApoE−/− and ApoE −/− mice were fed a WD (Teklad Adjusted Calories 88,137; 21% wt/wt fat, 0.15% wt/wt cholesterol, 19.5% wt/wt casein, and no sodium cholate) to accelerate the formation of atherosclerotic lesions. After 12 weeks on WD, mice underwent partial LCA ligation to rapidly induce disturbed blood flow. After surgery, mice continued on WD feeding for another 2 weeks before being sacrificed to collect tissues.
2.15. Intimal RNA isolation from common carotid arteries and aortas [2]
Two days after partial LCA ligation, one week after STZ-induced diabetes or 4 weeks of WD, mice were sacrificed to collect ligated LCA, LCA with sham surgery and aortas. The periadventitial fat was carefully cleaned. The carotid or aortic lumen was quickly flushed with 150 or 300 µL of QIAzol lysis reagent (QIAGEN) using a 29-gauge insulin syringe to collect endothelium, and the leftover was used to collect smooth muscle.
2.16. Measurement of plasma cholesterol and triglycerides [5]
Mice were sacrificed and blood was drawn from the right atrium into EDTA-containing tubes, for lipid measurements. Plasma was prepared via centrifugation at 850 × g for 15 min at 4 °C and stored at −20 °C. Measurements were carried out using an Infinity Cholesterol and Infinity Triglycerides measurement kit (Thermo Scientific), based on absorbance of samples normalized to the absorbance of a known concentration of a calibrator (200 mg/dL), provided with the kit. Data are expressed as mg/dL.
2.17. Oil Red O (ORO) staining for lipids
ORO staining was used to quantify lipid deposition. For aortic root analysis, the heart was isolated along with the aortic arch after perfusion with PBS. Aortic roots were embedded in OCT compound. Sequential sections, each 5 µm thick, were cut starting at the aortic sinus, which was identified by the appearance of the semilunar valve leaflets, and extending over the next 1.2 mm over the entire aortic sinus. Frozen whole neck sections were serially sectioned (7 µm), air-dried and mounted on superfrost glass slide. Briefly, slides were dipped in propylene glycol to dehydrate followed by ORO (American MasterTech) for 10 min at 60 °C. Sections were differentiated in propylene glycol for 1 min and counterstained with hematoxylin and mounted in an aqueous mounting media. For aortic roots, the areas of the ORO positive lesions in the proximal aortas were quantified. The mean cross-sectional area of ORO positive lesions and the total area were determined by using NIH Image J software.
2.18. In vivo measurement of aortic pulse wave velocity (PWV) [12]
PWV was measured using a high-resolution Doppler ultrasound instrument (Vevo770, VisualSonics), as we previously described [12]. Briefly, mice were anesthetized with 2% isoflurane and mounted on a heated (37 °C) platform to monitor electrocardiogram (ECG), heart rate (HR), respiratory rate, and to eliminate movement artifacts. Flow wave Doppler measurements were taken at two locations along the aorta, one proximal and one distal to the heart, at 30 MHz with a pulse-repetition frequency of 40 kHz at a depth of 6 mm. There was no difference in the HR between the proximal and distal point measurements. In offline analysis of the images, the time (ms) from the peak of the ECG R wave to the foot of the flow waves at both the proximal and distal locations were measured, in at least 5 replicates for each location per mouse. The difference between proximal and distal points arrival time yields the transit time (TT). PWV (mm/ms) was calculated from the distance between the measurement sites (Δd) and TT (Δt).
2.19. Statistical analysis
All data are presented as mean ± standard error. Student t-test was used to analyze data from experimental and control groups. Two-way ANOVA was used to compare data from 2 groups with two treatments, followed by repeated measures and post hoc analysis with Bonferroni multiple comparison test. A probability value of b 0.05 is considered statistically significant.
3. Results
3.1. Disturbed blood flow downregulates endothelial Nox4 and upregulates EPHX2 and VCAM1
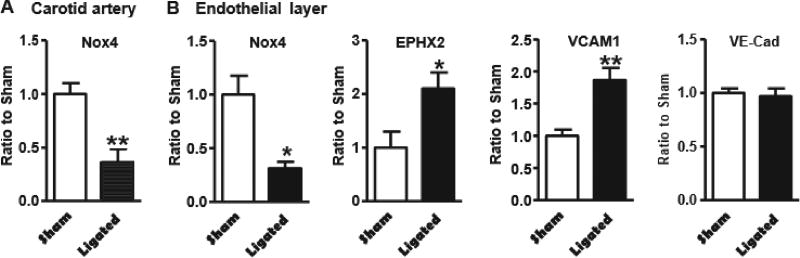
Using a partial LCA ligation to induce disturbed blood flow patterns in FVB/N ApoE−/− mice, we found that disturbed blood flow dramatically downregulated Nox4 mRNA level in whole carotid artery 2 days after ligation (Fig. 1A). As we previously demonstrated that smooth muscle Nox4 was upregulated by disturbed blood flow [12], we speculated that endothelial Nox4 was downregulated by disturbed blood flow. As shown in Fig. 1B, in pooled samples from endothelial layer isolated from the carotid arteries, Nox4 mRNA level was downregulated by disturbed blood flow. As we demonstrated that sEH is a downstream target of smooth muscle Nox4 in the regulation of atherosclerosis [12], we measured the expression of sEH and its downstream target, VCAM1. Similar to what we found in smooth muscle [12], disturbed blood flow upregulated EPHX2 and VCAM1 mRNA levels in endothelial layer, 2 days after partial LCA ligation (Fig. 1B). Endothelial phenotype was confirmed by measuring the expression of endothelial marker VE-cadherin (Fig. 1B). These results suggest that downregulation of Nox4 and upregulation of EPHX2 and VCAM1 in endothelium by disturbed blood flow may contribute to the development of atherosclerosis.
Fig. 1.
Disturbed blood flow downregulates Nox4 and upregulates both EPHX2 and VCAM1 in endothelium in FVB/N ApoE−/− mice. A. Genes in common carotid arteries 2 days after partial left common carotid artery (LCA) ligation. *p < 0.05, partial ligated LCA vs. sham surgery LCA, n = 5–6. B. *p < 0.05, ligated LCA vs. sham surgery control LCA, n = 5–6.
3.2. Type I diabetes downregulates endothelial Nox4 and upregulates sEH
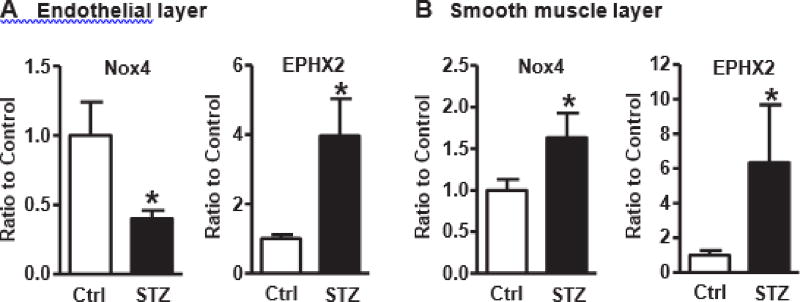
As type I diabetes accelerates the development of atherosclerosis, we tested the effect of type I diabetes on endothelial Nox4. As shown in Fig. 2A, ApoE−/− mice with STZ-induced type I diabetes for 1 week had significantly decreased Nox4 mRNA expression and increased sEH mRNA expression in aortic endothelium compared with citric acid control. In comparison, both Nox4 and sEH mRNA expression in aortic smooth muscle were upregulated by type I diabetes (Fig. 2B). These results indicate that endothelial Nox4 and smooth muscle Nox4 responded differently to type I diabetes, despite sEH being upregulated in both, and that the decreased Nox4 and increased sEH in endothelium may contribute to the accelerated progression of atherosclerosis under diabetic condition.
Fig. 2.
Type I diabetes downregulates endothelial Nox4 and upregulate sEH in ApoE−/− aortic endothelium. A. Genes in endothelial layer. Mice under type I diabetes for 1 week. *p < 0.05 streptozotocin (STZ) vs. citric acid vehicle control, n = 6–7. B. Genes in smooth muscle layer. Mice under type I diabetes for 1 week. *p < 0.05 STZ vs. citric acid vehicle control, n = 5–11.
3.3. Overexpression of human Nox4DN in EC accelerates type I diabetes-induced atherosclerotic lesions and arterial stiffness
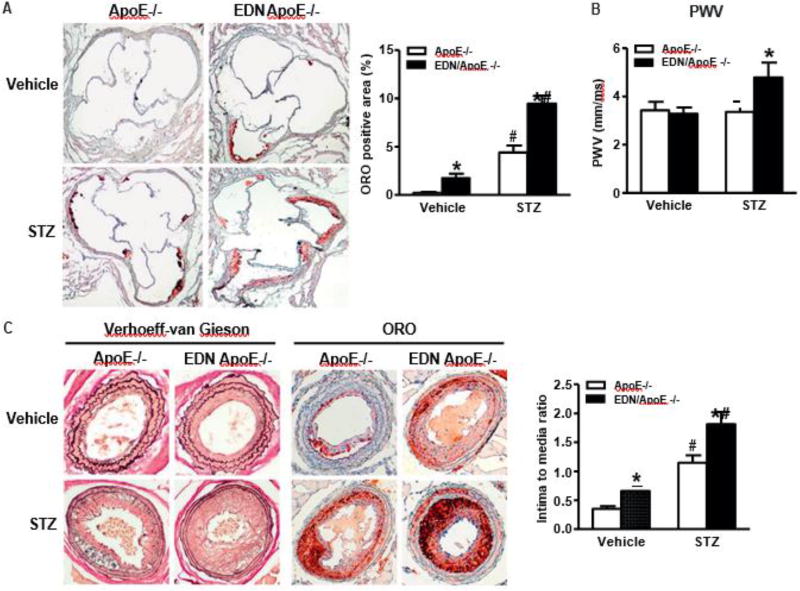
To address whether the downregulated EC Nox4 directly contributes to the development of atherosclerotic lesions, we used transgenic mice that overexpress a human Nox4 dominant negative P437H mutant (Nox4DN), specifically in EC (EDN) [14], to mimic the effect of decreased EC Nox4 under disturbed blood flow, and backcrossed them onto the FVB/N ApoE−/− genetic background for atherosclerosis analysis [15]. We previously reported that, in isolated cardiac ECs, Nox4DN decreased H2O2 production even though the total Nox4 protein levels were increased about 2.5-fold compared with EC from littermate controls, confirming the dominant negative functional effect of P437H on H2O2 production by endogenous Nox4 in vivo [14]. In comparison with ApoE −/− on C57BL/6 genetic background, obvious atherosclerotic lesions, in the aortic root, take much longer to develop in FVB/N ApoE−/− mice (up to 11 months). To accelerate the formation of atherosclerotic lesions in the aortic root, mice were injected STZ to induce type I diabetes for 6 weeks and, in the last 2 weeks, we performed a partial LCA ligation to induce advanced lesions [2]. STZ significantly increased the level of blood glucose and slightly increased the levels of total cholesterol and triglyceride, and equally in both groups, but had no effect on body weight (Table 1). Importantly, lipid deposition in the aortic root, detected by ORO staining, was increased in EDN/ApoE−/− compared with ApoE−/−, under both vehicle and STZ treated conditions (Fig. 3A). PWV is inversely proportional to aortic distensibility and directly correlates with aortic stiffness [23]. As shown in Fig. 3B, STZ increased PWV in EDN/ApoE−/− compared with ApoE −/−. To note, ApoE−/− and EDN/ApoE−/− had increased baseline PWV compared to what we usually observe for healthy, non-atherosclerotic control mice [12,24], suggesting that aortic wall stiffening and remodeling in ApoE−/− even before frank atherosclerotic lesions can be observed. Furthermore, the neointimal formation and atherosclerotic lesions were significantly increased in EDN/ApoE−/− compared with ApoE−/−, under both vehicle and STZ treated conditions after partial LCA ligation (Fig. 3C), further confirming that the decreased endothelial Nox4 under disturbed blood flow directly contributed to the development of atherosclerosis and type I diabetes accelerates the development of atherosclerosis.
Table 1.
Plasma total cholesterol and triglycerides levels.
| ApoE−/− vehicle | EDN/ApoE−/− vehicle | ApoE−/− | EDN/ApoE−/− | |
|---|---|---|---|---|
| Bodv weight (g) | 30.3 ± 1.1 | 30.5 ± 1.2 | 29.7 ± 1.0 | 29.0 ± 1.2 |
| Blood glucose (mg/dL) | 124.5 ± 8.6 | 128.6 ± 11.1 | 486.2 ± | 472.0 ± 28.7* |
| TG (mg/dL) | 327.9 ± 21.9 | 313.8 ± 15.7 | 454.1 ± | 441.2 ± 23.7* |
| Cholesterol (mg/dL) | 447.8 ± 18.2 | 455.7 ± 48.9 | 573.3 ± | 557.6 ± 61.3 |
Note:
p b 0.05 streptozotocin (STZ) vs. citric acid vehicle control (Vehicle) in the same mouse line 6 weeks after type I diabetes was induced, n = 13–19.
Fig. 3.
Overexpression of human Nox4DN in endothelium increases arterial stiffness and type I diabetes induced atherosclerotic lesions. A. Representative picture of Oil Red O (ORO) staining of aortic root lesions at 6 weeks after type I diabetes was induced (20×). Quantification in graph. *p < 0.05 vs. ApoE−/−, #p < 0.05 vs. vehicle control, n = 8–17. B. Pulse wave velocity (PWV) at 4 weeks after type I diabetes was induced. *p < 0.05 vs. ApoE−/−, n = 6. C. Representative picture of carotid artery lesions 2 weeks after partial LCA ligation with 6 weeks of type I diabetes induction (20×). Quantification in graph. *p < 0.05 vs. ApoE−/−, #p <| 0.05 vs. vehicle control, n = 9–12.
3.4. Overexpression of human Nox4DN upregulates expression of sEH and enhances inflammation in endothelial cells
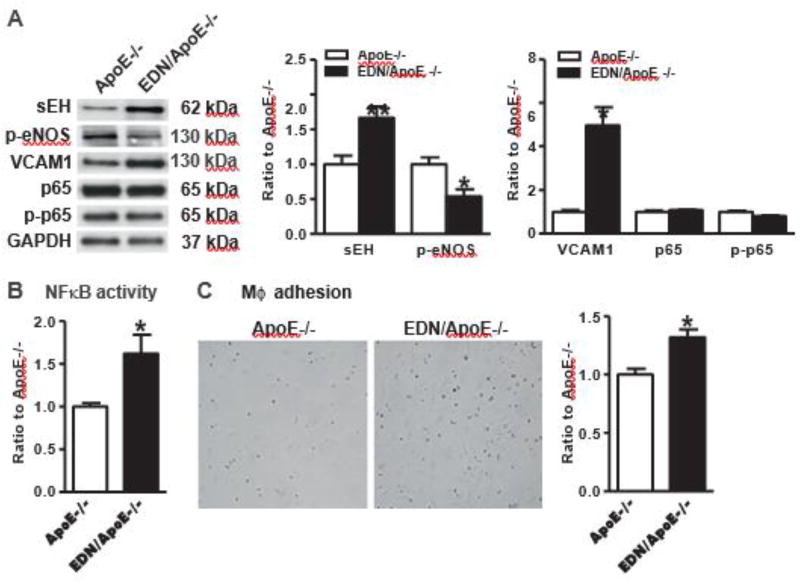
Our previous study in SMC indicates that Nox4 directly regulates sEH and inflammation [12]. To validate whether Nox4 in endothelium could similarly regulate sEH and inflammation, we isolated EC from hearts in both EDN/ApoE −/− and ApoE −/− mice. As shown in Fig. 4A, sEH was significantly upregulated in EC from EDN/ApoE −/− compared with ApoE −/−. Nitric oxide (NO) is the most important endothelium-derived hyperpolarizing factor released by EC to maintain endothelium homeostasis and vascular tone. Endothelium-derived NO production is mainly derived by the activity of endothelial nitric oxide synthase (eNOS). As sEH negatively regulates eNOS activation [25], we measured eNOS phosphorylation at serine 1177, a well established index of eNOS activation. As shown in Fig. 4A, eNOS phosphorylation was decreased in EC from EDN/ApoE −/− compared with ApoE −/−.
Fig.4.
Overexpression of human Nox4DN in endothelium upregulates the expression level of soluble epoxide hydrolase 2 and increases inflammation. A. Representative Western blots from cultured EC and quantification of band intensities in graph. *p < 0.05 vs. ApoE−/−, n = 4–11. B. NFκB activity in EC cultured in 0.2% FBS EBM-2. *p < 0.05 vs ApoE−/−, n = 6. C. Representative pictures if macrophage adhesion to EC and quantification in graph. *p <| 0.05 vs. ApoE−/−, n = 9.
Because sEH inhibition is known to downregulate proinflammatory genes and decrease inflammatory cell infiltration into the vascular wall [26], we assessed VCAM1 expression and NFκB promoter activity, as inflammatory markers, and the interaction between macrophage and EC in cultured EC. As shown in Fig. 4A, VCAM1 was significantly increased in EC isolated from EDN/ApoE −/− compared with ApoE −/−, but we did not detect any difference in protein level of phospho-p65 NFκB, a well-known component of NFκB activation. Despite phospho-p65 NFκB levels were similar, the NFκB promoter activity was increased in EDN/ApoE−/− compared with ApoE−/−, as measured by a luciferase reporter assay (Fig. 4B).
The endothelium is known to play a key role in regulating leukocyte adhesion to vessel wall via VCAM1 and NFκB, among other inflammatory mediators. Because human Nox4DN increased both VCAM1 expression and NFκB promoter activity in EC, we measured macrophage adhesion to EC isolated from EDN/ApoE −/− and ApoE −/−. As shown in Fig. 4C, macrophage adhesion to EDN/ApoE−/− EC was enhanced compared with ApoE−/− EC. Taken together, downregulation of eNOS activity, VCAM1 upregulation, increased NFkB promoter activity and increased macrophage adhesion in EDN/ApoE−/− EC are consistent with a role of decreased EC Nox4 in promoting vascular inflammation under atherosclerosis prone conditions, which may contribute to the increased atherosclerotic lesions observed in EDN/ApoE −/− mice (Fig. 3).
3.5. Inhibition of sEH activity suppresses inflammation and improves endothelial cell function in EDN/ApoE−/− EC
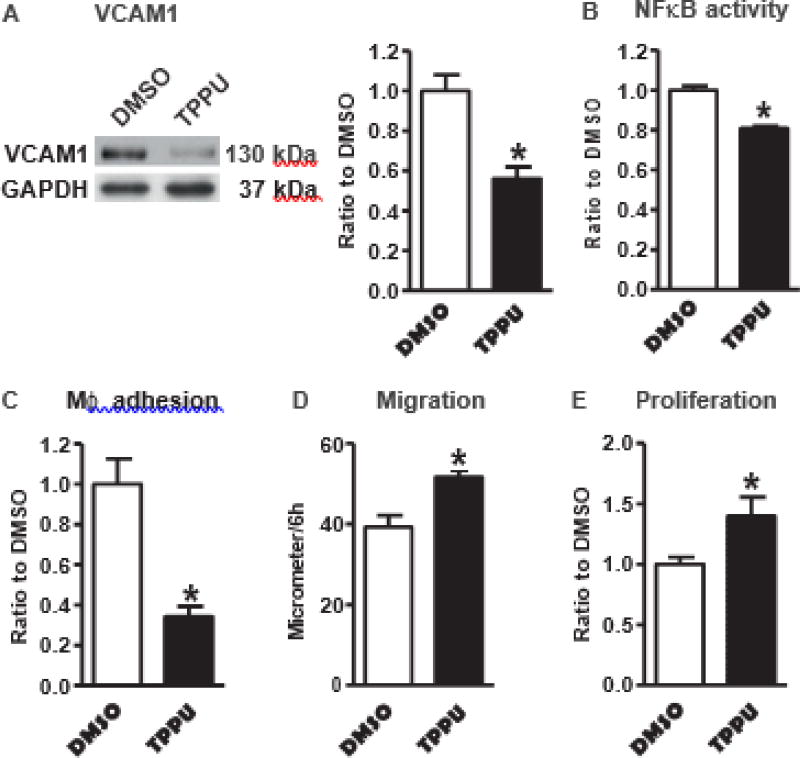
To test whether sEH upregulation in EDN/ApoE −/− EC accounts for the increased inflammation, we used a specific sEH inhibitor TPPU [17] in EDN/ApoE −/− EC. As shown in Fig. 5A–C, inhibition of sEH activity decreased VCAM1 expression, suppressed NFkB promoter activity, and inhibited macrophage adhesion to EDN/ApoE −/− EC, confirming the contribution of sEH in mediating the proinflammatory effect of decreased endothelial Nox4. Furthermore, inhibition of sEH activity increased EC migration and proliferation in EDN/ApoE −/− EC (Fig. 5D&E), consistent with our previous findings that EDN EC have impaired migration and proliferation [14]. No difference in plating efficiency was noticed among these cells.
Fig. 5.
Inhibition of soluble epoxide hydrolase 2 activity by TPPU suppresses inflammation and improves cell functions in EDN/ApoE−/− EC. A. Vascular cell adhesion molecule 1 (VCAM1). *p < 0.05 vs. DMSO, n = 4. B. NFκB activity. *p < 0.05 vs. DMSO, n = 6. C. Macrophage adhesion to EC. *p < 0.05 vs. DMSO, n = 6. D. Migration. *p < 0.05 vs. DMSO, n = 6. E. Proliferation. *p < 0.05 vs. DMSO, n = 10.
3.6. Overexpression of human Nox4 wild type in EC attenuates Western diet-induced atherosclerotic lesions and arterial stiffness
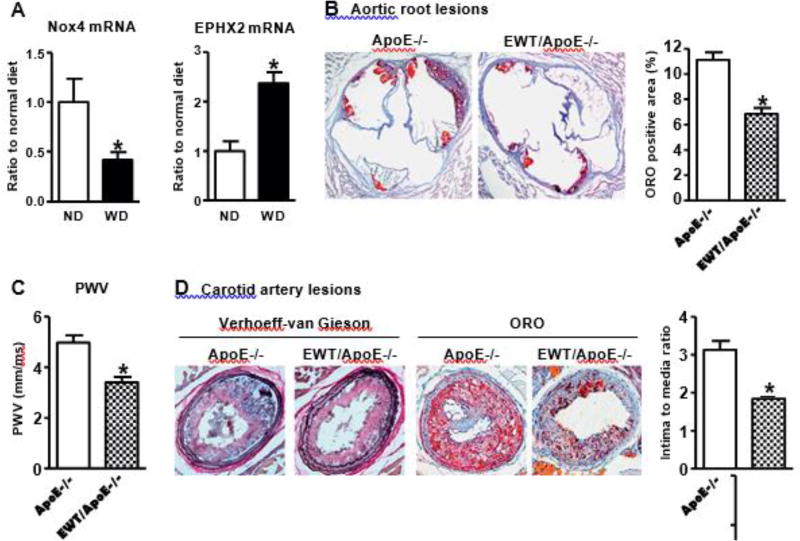
As WD accelerates the development of atherosclerosis, we tested its effect on endothelial Nox4. As shown in Fig. 6A, ApoE−/− mice fed WD for 4 weeks had significantly decreased Nox4 mRNA expression and increased sEH mRNA expression compared with normal diet (ND). These results imply that the decreased Nox4 and increased sEH in endothelium may contribute to the accelerated progress of atherosclerosis under WD-fed condition.
Fig. 6.
Overexpression of human Nox4 wild type in endothelium decreases arterial stiffness and Western diet induced atherosclerotic lesions. A. Western diet (WD) feeding 4 weeks downregulates endothelial Nox4 and upregulates sEH in ApoE−/− aortic endothelium. *p < 0.05 vs. normal diet (ND), n = 5–6. B. Representative picture of ORO staining of aortic root lesions in mice WD fed for 14 weeks (20×). Quantification in graph. *p < 0.05 vs. ApoE−/−, n = 12–16. C. PWV in mice WD fed for 12 weeks. *p < 0.05 vs. ApoE−/−, n = 12–18. D. Representative picture of carotid artery lesions after 2 weeks partial LCA ligation and 14 weeks WD feeding (20×). Quantification in graph. *p < 0.05 vs. ApoE−/−, n = 10–11.
Next, we used transgenic mice to overexpress human Nox4 wild type in endothelium (EWT) [14] and backcrossed them onto FVB/N ApoE−/− background to counter the effect of decreased endothelial Nox4 under disturbed blood flow (Fig. 1B). We previously showed that H2O2 production is increased in cardiac EC isolated from EWT mice, and the total Nox4 protein levels were increased about 2-fold compared with littermate controls [14]. Because atherosclerotic lesions form very slowly in FVB/N background, we used WD to accelerate lesion formation and compared EWT/ApoE −/− and ApoE −/− under this condition. As shown in Table 2, there was no difference in body weight, plasma total cholesterol and triglycerides levels between EWT/ApoE−/− and ApoE−/−. However, the lipid deposition in the aortic root and PWV were decreased in EWT/ApoE −/− compared with ApoE −/− (Fig. 6B&C). Furthermore, the neointimal formation and atherosclerotic lesions after partial LCA ligation were significantly decreased in EWT/ApoE −/− compared with ApoE −/− (Fig. 6D), further confirming that overexpression of endothelial Nox4 wild type could rescue the detrimental effect of decreased endothelial Nox4 under disturbed blood flow on atherosclerosis.
Table 2.
Plasma total cholesterol and triglycerides levels in mice fed Western diet.
| ApoE−/− | EWT/ApoE−/− | |
|---|---|---|
| Body weight (g) | 41.1 ± 2.2 | 41.4 ± 1.8 |
| TG (mg/dL) | 540.5 ± 23.3 | 519.9 ± 11.1 |
| Cholesterol (mg/dL) | 1101.6 ± 105.2 | 1181.9 ± 68.7 |
Note: Mice fed WD for 14 weeks, n = 16–18.
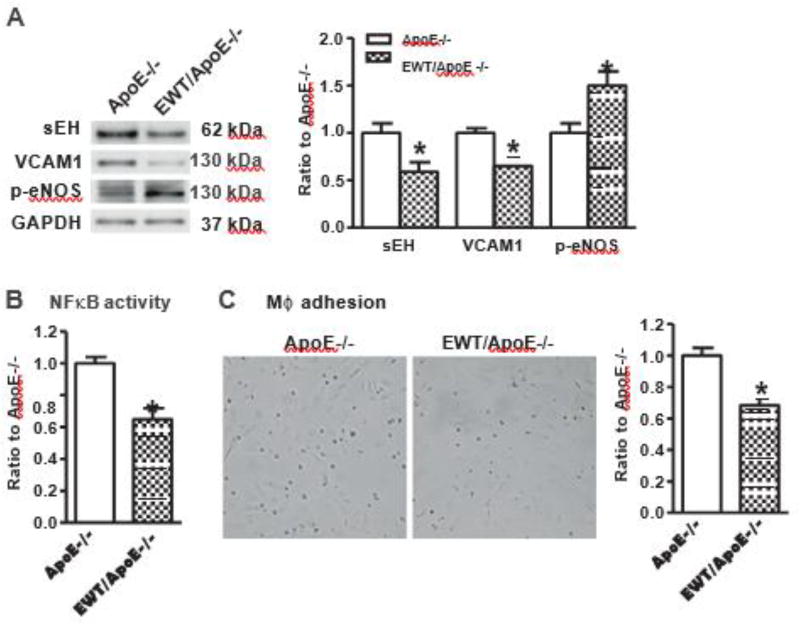
3.7. Overexpression of human Nox4 wild type downregulates expression of sEH and suppresses inflammation in endothelial cells
As shown in Fig. 7A, the expression levels of both sEH and VCAM1 were significantly downregulated while p-eNOS was upregulated in EC from EWT/ApoE −/− compared with ApoE−/−. NFκB promoter activity was decreased in EC from EWT/ApoE−/− compared with ApoE−/− (Fig. 7B). Macrophage adhesion to EC from EWT/ApoE−/− was decreased compared with ApoE−/− (Fig. 7C). These results confirmed that overexpression of human Nox4 wild type induced opposite effects on sEH expression and inflammation compared with its dominant negative form.
Fig. 7.
Overexpression of human Nox4 wild type in endothelium downregulates the expression levels of soluble epoxide hydrolase 2 and suppresses inflammation. A. Representative Western blots from cultured EC and quantification of band intensities in graph. *p < 0.05 vs. ApoE−/−, n = 6–10. B. NFκB activity in EC cultured in 0.2% FBS EBM-2. *p < 0.05 vs. ApoE−/−, n = 6. C. Representative pictures of macrophage adhesion to EC and quantification in graph. *p <| 0.05 vs. ApoE−/−, n = 10.
3.8. Nox4 inversely regulates expression of sEH in human aortic endothelial cells (HAEC)
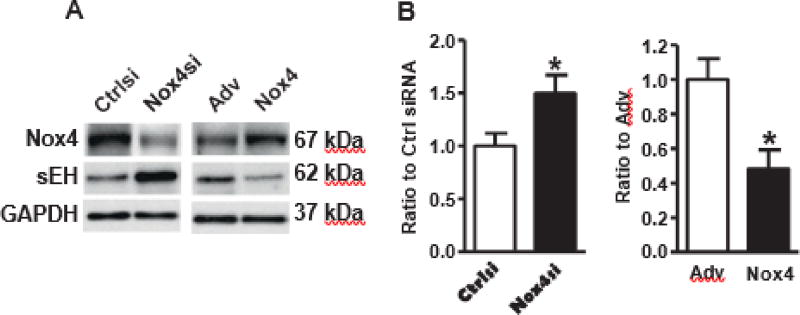
To validate the direct effects of Nox4 on sEH in EC, we carried out experiments with Nox4 downregulation or overexpression in cultured HAEC. Similar to Nox4DN mice, Nox4 siRNA upregulated sEH expression, while overexpression of human Nox4 downregulated sEH expression in HAEC (Fig. 8), further confirming a crucial role of EC Nox4 in regulating sEH
Fig. 8.
Nox4 inversely regulates expression of soluble epoxide hydrolase 2 in human aortic endothelial cells. A. Representative Western blots of sEH by knocking down of Nox4 or overexpression| of Nox4. B. Quantification of band intensities in graph. *p b 0.05 vs. control siRNA or empty adenovirus, n = 3–12.
4. Discussion
The role of vascular Nox4 in atherosclerosis remains controversial mainly because of overtly contrasting results using different animal models [7–10,12]. In part to address this limitation, we generated unique mouse models with Nox4 expression specifically in smooth muscle [10] and endothelium. In the current study, we engineered two endothelial specific Nox4 transgenic mouse lines (Nox4 dominant negative and Nox4 wild type transgenic) to respectively mimic or counteract the effect of disturbed blood flow on endothelial Nox4. Here we report that endothelial Nox4 1) plays a crucial role in the development of atherosclerosis, and 2) sEH is an endothelial Nox4 downstream target. Our study indicates that atherosclerosis-prone conditions, such as disturbed blood flow, STZ-induced type I diabetes and WD, all downregulate endothelial Nox4 to promote atherosclerosis, by stimulating inflammation through increased sEH. On the contrary, overexpression of endothelial Nox4 suppresses sEH thereby suppressing atherosclerosis. Overall, our findings strongly implicate that loss of endothelial Nox4 is a major determinant of atherosclerosis under atherosclerosis-prone conditions, at least in part, by increasing sEH revealing a protective role of endothelial Nox4 against atherosclerosis.
We previously reported that elevated glucose, in the setting of type I diabetes, was associated with upregulated Nox4 in SMC [27], results that were confirmed in the current study (Fig. 2B). In contrast, type I diabetes decreased Nox4 in the endothelium of diabetic ApoE−/− mice (Fig. 2A). These results are consistent with findings by others in which Nox4 mRNA levels were downregulated in advanced atherosclerotic plaques of diabetic patients [9], although the authors were not able to determine whether the decreased Nox4 mRNA was attributable to the endothelium or other cell type in the vascular wall. However, our studies with isolated EC and endothelial specific Nox4 transgenic mice support the conclusion that the endothelium is the major site of Nox4 downregulation in atherosclerotic plaque in the setting of diabetes. Similarly, WD, which accelerates atherosclerotic plaque formation, downregulated aortic Nox4 in ApoE−/− mice, in accordance with reports by others [7]. Interestingly, we found that the same WD upregulates Nox4 in aortic smooth muscle [12] while decreasing Nox4 in aortic endothelium. Overall, our studies indicate that disturbed blood flow, type I diabetes and WD regulates Nox4 expression in an opposite manner in endothelium and smooth muscle, underscoring the importance of using cell-specific, and not whole body Nox4 deficient mice, to study the role of Nox4 in atherosclerosis. Moreover, the net effect of disturbed blood flow is a decrease in artery Nox4 expression, despite Nox4 is increased in SMC [12] explaining the reportedly protective role of Nox4 in atherosclerosis in Nox4 deficient mice [8,9]. That is, in whole body Nox4 null mice, the detrimental effect of loss of endothelial Nox4 may overcome the protective effect of the loss of smooth muscle Nox4, resulting in increased atherosclerosis and implying endothelial Nox4 as a major contributing factor to the development of atherosclerosis and to a greater extent than SMC Nox4. To note, overexpressing Nox4DN and Nox4 WT in transgenic mice in the endothelium, did not affect plasma lipid levels, indicating that the effect of endothelial Nox4 on atherosclerosis is not related to an effect on lipid levels.
Atherosclerosis occurs preferentially at lesion prone areas of disturbed flow. EC in straight arteries experience unidirectional, high time-averaged wall shear stress, termed laminar shear stress (LSS). LSS induces acute and chronic changes in ECs leading to homeostatic cell alignment, vasodilation, and inhibition of inflammation and coagulation, which are atheroprotective responses. In contrast, OSS stimulates proatherogenic responses, including cell turnover, inflammation and thrombosis [28–30]. In cultured EC, LSS promotes Nox4-dependent H2O2 production [31], and decreases sEH mRNA, protein and activity [32] while OSS decreases Nox4 mRNA [11]. Consistent with these in vitro studies, here we report that disturbed blood flow, induced by a partial LCA ligation in mice, decreased Nox4 mRNA and increased sEH in arterial endothelium in vivo. Using EC isolated from Nox4 dominant negative and wild type transgenic mice and knocking-down or overexpressing Nox4 in human EC, we found that Nox4 inversely regulates sEH in EC. In addition, pharmacological inhibition of sEH suppressed inflammation in EDN/ApoE−/− EC, indicating that sEH upregulation mediates the effects of endothelial Nox4 downregulation on inflammation. Although inflammatory responses have been implicated in the protective effects of Nox4 against atherosclerosis, the molecular mechanisms remain controversial [8,9]. One potential molecular mechanism by which endothelial Nox4 overexpression may protect against atherosclerosis is through downregulation of gamma interferon-induced monokine, a cytokine known to recruit and activate T cells. Therefore, endothelial Nox4 may promote a T cell differentiation in the vascular wall that favors resolution rather than amplification of inflammatory cascades [7].
sEH is a proinflammatory gene by inversely regulating the concentration of epoxyeicosatrienoic acids (EETs) and endothelium-derived NO. sEH has both C-terminal epoxide hydrolase activity and N-terminal lipid phosphatase activity. N-terminal domain of sEH negatively regulates the activation of eNOS [25]. NO and EETs are the most important endothelium-derived hyperpolarizing factors released by EC to maintain endothelium homeostasis and vascular tone. Endothelium-derived NO is mainly controlled by the activity of eNOS. The acute loss of NO production in the blood vessel wall stimulates inflammatory processes including the increased recruitment of leukocytes to the vascular endothelium [33,34]. eNOS deficiency accelerates atherosclerosis in WD-fed ApoE−/− mice [35]. Targeting improvement of eNOS function can improve vascular function, reduce VCAM1 production and inhibit atherogenesis in diabetic rats and hypercholesterolemic rabbits [36, 37]. EETs are generated by the activity of cytochrome p450 enzymes on arachidonic acid and inactivated largely by sEH, which converts them to their corresponding dihydroxyeicosatrienoic acids. In EC, EETs inhibit inflammatory gene expression, by mechanisms which are largely unknown but may include direct binding to peroxisome proliferator-activated receptors, G-protein coupled receptors, or transient receptor potential channels, to initiate anti-inflammatory signaling cascades [38]. In cultured EC, EETs attenuate TNFα-induced VCAM1 expression and leukocyte adhesion, partially through inhibition of NFκB activation [39]. A role of the hydrolase activity of sEH in the metabolism of EETs and cardiovascular inflammation is well documented [40–42]. sEH inhibitors decrease proinflammatory factors, both circulating and in the aorta, as well as inflammatory cell infiltration into the vascular wall [26]. Administration of an sEH inhibitor to Ang II-treated ApoE−/− mice significantly attenuates atherosclerotic lesion area [26,43]. In addition, studies by us and others indicate that endothelial Nox4 is important for endothelial function and repair, especially after ischemia-injury [14,44,45]. We found that inhibition of sEH activity in EDN/ApoE−/− EC not only suppressed inflammation but also improved EC functions. One of innovative aspects of our study is that sEH is a downstream mediator of endothelial Nox4 in regulating inflammation, which is associated with an effect on atherosclerosis. However, currently we do not know the exact mechanism by which EC Nox4 regulates sEH expression. We did not observe consistent effects of H2O2, PEG-catalase or overexpression of catalase on sEH expression in cultured EC (data not shown). These findings can be explained by the fact that the conditions we tested could not fully mimic Nox4-dependent endogenous H2O2 production which is tightly regulated. Alternatively, considering that Nox4 is localized in the endoplasmic reticulum and nucleus, we cannot exclude it may transcriptionally regulate sEH gene expression in a direct manner, independently of H2O2. In HepG2 cells, DNA methylation of the sEH promoter silences its expression [46]. In lung fibroblasts, Nox4 is associated with DNA methyltransferase [47] therefore this may be one of the mechanisms by which Nox4 directly regulates sEH expression, warranting further investigation.
Compared with our previous findings [12], indicating that SMC Nox4 promotes atherosclerosis, the results of the current study underscore that SMC and EC Nox4 play an opposite role in the development of atherosclerosis. Similarly, OSS regulates endothelial Nox4 and smooth muscle Nox4 in an opposite way, indicating that SMC and EC respond differently under the same stimulation. Currently, the exact mechanisms of this differential cell-specific response remain to be elucidated.
Overall, our studies indicate that in atherosclerosis-prone vascular regions, both decreased EC Nox4 and the increased SMC Nox4 contribute to the development of atherosclerosis, at least in part through upregulation of sEH. Type I diabetes and WD accelerate the progress of atherosclerosis by further downregulating Nox4 and upregulating sEH in endothelium. In conclusion, decreased endothelial Nox4 plays an essential role in the development of atherosclerosis in disturbed blood flow region, and sEH is a crucial Nox4-dependent mediator of inflammation. Proatherosclerosis conditions, such as type I diabetes and WD, may accelerate atherosclerosis through downregulation of endothelial Nox4 and upregulation of sEH therefore targeting sEH activity could be beneficial in preventing atherosclerosis.
Acknowledgments
The authors thank Dr. Jan L. Breslow for FVB/N ApoE−/− mice, Dr. Junichi Sadoshima for endothelial Nox4 mice in FVB/N background, and Dr. Ajah Shah for Nox4 antibody. This work was supported by National Natural Science Foundation of China (31571172, Tong X), Fundamental Research Funds for the Central Universities (0236015202008, Tong X), Chongqing Research Program of Basic Research and Frontier Technology (cstc2016jcyjA0407, Tong X), American Diabetes Association award (7-09-JF-69, Tong X), NIH R01 HL031607 (Cohen RA and Tong X), R37 HL104017, Boston University Medical Center Department of Medicine Evans Center Arterial Stiffness ARC, China Postdoctoral Science Foundation (Xm2015033) (Hu P, 2016M592623), Postdoctoral Science Foundation (Hu P, Xm2015033). B.D.H and K.S.S.L. is partially supported by the NIEHS grant R01 ES002710, NIEHS Superfund Research Program grant P42 ES004699 and NIH CounterAct U54 NS079202 and K.S.S.L. has been partially supported by the NIH Pathway to Independence Award from NIH/NIEHS (R00 ES024806).
Footnotes
Disclosure
None.
Transparency document
The Transparency document associated with this article can be found, in online version.
References
- 1.Nam D, Ni C-W, Rezvan A, Suo J, Budzyn K, Llanos A, et al. A model of disturbed flow-induced atherosclerosis in mouse carotid artery by partial ligation and a simple method of RNA isolation from carotid endothelium. J. Vis. Exp. 2010 doi: 10.3791/1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nam D, Ni C-W, Rezvan A, Suo J, Budzyn K, Llanos A, et al. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H1535–H1543. doi: 10.1152/ajpheart.00510.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ni C-W, Qiu H, Rezvan A, Kwon K, Nam D, Son DJ, et al. Discovery of novel mechanosensitive genes in vivo using mouse carotid artery endothelium exposed to disturbed flow. Blood. 2010;116:e66–e73. doi: 10.1182/blood-2010-04-278192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bu DX, Rai V, Shen X, Rosario R, Lu Y, D'Agati V, et al. Activation of the ROCK1 branch of the transforming growth factor-beta pathway contributes to rage-dependent acceleration of atherosclerosis in diabetic ApoE-Null mice. Circ. Res. 2010;106:1040–1051. doi: 10.1161/CIRCRESAHA.109.201103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuccollo A, Shi C, Mastroianni R, Maitland-Toolan KA, Weisbrod RM, Zang M, et al. The thromboxane A2 receptor antagonist S18886 prevents enhanced atherogenesis caused by diabetes mellitus. Circulation. 2005;112:3001–3008. doi: 10.1161/CIRCULATIONAHA.105.581892. [DOI] [PubMed] [Google Scholar]
- 6.Woo C-H, Shishido T, McClain C, Lim JH, Li J-D, Yang J, et al. Extracellular signal-regulated kinase 5 SUMOylation antagonizes shear stress-induced antiinflammatory response and endothelial nitric oxide synthase expression in endothelial cells. Circ. Res. 2008;102:538–545. doi: 10.1161/CIRCRESAHA.107.156877. [DOI] [PubMed] [Google Scholar]
- 7.Craige SM, Kant S, Reif M, Chen K, Pei Y, Angoff R, et al. Endothelial NADPH oxidase 4 protects ApoE−/− mice from atherosclerotic lesions. Free Radic. Biol. Med. 2015;89:1–7. doi: 10.1016/j.freeradbiomed.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schürmann C, Rezende F, Kruse C, Yasar Y, Löwe O, Fork C, et al. The NADPH oxidase Nox4 has anti-atherosclerotic functions. Eur. Heart J. 2015:ehv460. doi: 10.1093/eurheartj/ehv460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray SP, Di Marco E, Kennedy K, Chew P, Okabe J, El-Osta A, et al. Reactive oxygen species can provide atheroprotection via NOX4-dependent inhibition of inflammation and vascular remodeling. Arterioscler. Thromb. Vasc. Biol. 2016;36:295–307. doi: 10.1161/ATVBAHA.115.307012. [DOI] [PubMed] [Google Scholar]
- 10.Gray SP, Di Marco E, Okabe J, Szyndralewiez C, Heitz F, Montezano AC, et al. Nox1 plays a key role in diabetes accelerated atherosclerosis. Circulation. 2013;127:1888–1902. doi: 10.1161/CIRCULATIONAHA.112.132159. [DOI] [PubMed] [Google Scholar]
- 11.Sorescu GP, Song H, Tressel SL, Hwang J, Dikalov S, Smith DA, et al. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a Nox1-based NADPH oxidase. Circ. Res. 2004;95:773–779. doi: 10.1161/01.RES.0000145728.22878.45. [DOI] [PubMed] [Google Scholar]
- 12.Tong X, Khandelwal AR, Wu X, Xu Z, Yu W, Chen C, et al. Proatherogenic role of smooth muscle Nox4-based NADPH oxidase. J. Mol. Cell. Cardiol. 2016;92:30–40. doi: 10.1016/j.yjmcc.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tong X, Hou X, Jourd'heuil D, Weisbrod RM, Cohen RA. Upregulation of Nox4 by TGF{beta}1 oxidizes SERCA and inhibits NO in arterial smooth muscle of the prediabetic Zucker rat. Circ. Res. 2010;107:975–983. doi: 10.1161/CIRCRESAHA.110.221242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L, Hou X, Xiao J, Kuroda J, Ago T, Sadoshima J, et al. Both hydrogen peroxide and transforming growth factor beta 1 contribute to endothelial Nox4 mediated angiogenesis in endothelial Nox4 transgenic mouse lines. Biochim. Biophys. Acta. 2014;1842:2489–2499. doi: 10.1016/j.bbadis.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Dansky HM, Charlton SA, Sikes JL, Heath SC, Simantov R, Levin LF, et al. Genetic background determines the extent of atherosclerosis in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 1999;19:1960–1968. doi: 10.1161/01.atv.19.8.1960. [DOI] [PubMed] [Google Scholar]
- 16.Tong X, Khandelwal AR, Qin Z, Wu X, Chen L, Ago T, et al. Role of smooth muscle Nox4-based NADPH oxidase in neointimal hyperplasia. J. Mol. Cell. Cardiol. 2015;89:185–194. doi: 10.1016/j.yjmcc.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Sanborn R, Hammock BD. NIH Public Access. 2012;53:7067–7075. [Google Scholar]
- 18.Tong X, Ying J, Pimentel DR, Trucillo M, Adachi T, Cohen RA. High glucose oxidizes SERCA cysteine-674 and prevents inhibition by nitric oxide of smooth muscle cell migration. J. Mol. Cell. Cardiol. 2008;44:361–369. doi: 10.1016/j.yjmcc.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc. Natl. Acad. Sci. U. S. A. 2010;107:15565–15570. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen K, Kirber MT, Xiao H, Yang Y, Keaney JF. Regulation of ROS signal transduction by NADPH oxidase 4 localization. J. Cell Biol. 2008;181:1129–1139. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bayat H, Xu S, Pimentel D, Cohen RA, Jiang B. Activation of thromboxane receptor upregulates interleukin (IL)-1beta-induced VCAM-1 expression through JNK signaling. Arterioscler. Thromb. Vasc. Biol. 2008;28:127–134. doi: 10.1161/ATVBAHA.107.150250. [DOI] [PubMed] [Google Scholar]
- 22.Wang H-W, Liu P-Y, Oyama N, Rikitake Y, Kitamoto S, Gitlin J, et al. Deficiency of ROCK1 in bone marrow-derived cells protects against atherosclerosis in LDLR−/− mice. FASEB J. 2008;22:3561–3570. doi: 10.1096/fj.08-108829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lane HA, Smith JC, Davies JS. Noninvasive assessment of preclinical atherosclerosis. Vasc. Health Risk Manag. 2006;2:19–30. doi: 10.2147/vhrm.2006.2.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weisbrod RM, Shiang T, Al Sayah L, Fry JL, Bajpai S, Reinhart-King CA, et al. Arterial stiffening precedes systolic hypertension in diet-induced obesity. Hypertension. 2013;62:1105–1110. doi: 10.1161/HYPERTENSIONAHA.113.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou H-H, Hammock BD, Su K-H, Morisseau C, Kou YR, Imaoka S, et al. N-terminal domain of soluble epoxide hydrolase negatively regulates the VEGF-mediated activation of endothelial nitric oxide synthase. Cardiovasc. Res. 2012;93:120–129. doi: 10.1093/cvr/cvr267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L-N, Vincelette J, Cheng Y, Mehra U, Chen D, Anandan S-K, et al. Inhibition of soluble epoxide hydrolase attenuated atherosclerosis, abdominal aortic aneurysm formation, and dyslipidemia. Arterioscler. Thromb. Vasc. Biol. 2009;29:1265–1270. doi: 10.1161/ATVBAHA.109.186064. [DOI] [PubMed] [Google Scholar]
- 27.Tong X, Schröder K. NADPH oxidases are responsible for the failure of nitric oxide to inhibit migration of smooth muscle cells exposed to high glucose. Free Radic. Biol. Med. 2009;47:1578–1583. doi: 10.1016/j.freeradbiomed.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li YSJ, Haga JH, Chien S. Molecular basis of the effects of shear stress on vascular endothelial cells. J. Biomech. 2005;38:1949–1971. doi: 10.1016/j.jbiomech.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 29.Chiu J-J, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol. Rev. 2011;91:327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berk BC, Min W, Yan C, Surapisitchat J, Liu YM, Hoefen R. Atheroprotective mechanisms activated by fluid shear stress in endothelial cells. Drug News Perspect. 2002;15:133–139. doi: 10.1358/dnp.2002.15.3.704684. [DOI] [PubMed] [Google Scholar]
- 31.Bretón-Romero R, González de Orduña C, Romero N, Sánchez-Gómez FJ, de Álvaro C, Porras A, et al. Critical role of hydrogen peroxide signaling in the sequential activation of p38 MAPK and eNOS in laminar shear stress. Free Radic. Biol. Med. 2012;52:1093–1100. doi: 10.1016/j.freeradbiomed.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Zhang Y, Schmelzer K, Lee T-S, Fang X, Zhu Y, et al. The antiinflammatory effect of laminar flow: the role of PPARgamma, epoxyeicosatrienoic acids, and soluble epoxide hydrolase. Proc. Natl. Acad. Sci. U. S. A. 2005;102:16747–16752. doi: 10.1073/pnas.0508081102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc. Natl. Acad. Sci. U. S. A. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma XL, Weyrich AS, Lefer DJ, Lefer AM. Diminished basal nitric oxide release after myocardial ischemia and reperfusion promotes neutrophil adherence to coronary endothelium. Circ. Res. 1993;72:403–412. doi: 10.1161/01.res.72.2.403. [DOI] [PubMed] [Google Scholar]
- 35.Kuhlencordt PJ, Gyurko R, Han F, Scherrer-Crosbie M, Aretz TH, Hajjar R, et al. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation. 2001;104:448–454. doi: 10.1161/hc2901.091399. [DOI] [PubMed] [Google Scholar]
- 36.Riad A, Westermann D, Van Linthout S, Mohr Z, Uyulmaz S, Becher PM, et al. Enhancement of endothelial nitric oxide synthase production reverses vascular dysfunction and inflammation in the hindlimbs of a rat model of diabetes. Diabetologia. 2008;51:2325–2332. doi: 10.1007/s00125-008-1159-9. [DOI] [PubMed] [Google Scholar]
- 37.Candipan RC, Wang B, Buitrago R, Tsao PS, Cooke JP. Regression or progression: dependency on vascular nitric oxide. Arterioscler. Thromb. Vasc. Biol. 1996;16:44–50. doi: 10.1161/01.atv.16.1.44. [DOI] [PubMed] [Google Scholar]
- 38.Thomson SJ, Askari A, Bishop-Bailey D. Anti-inflammatory effects of epoxyeicosatrienoic acids. Int. J. Vasc. Med. 2012;2012:11–12. doi: 10.1155/2012/605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog. Lipid Res. 2004;43:55–90. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 41.Inceoglu B, Jinks SL, Ulu A, Hegedus CM, Georgi K, Schmelzer KR, et al. Soluble epoxide hydrolase and epoxyeicosatrienoic acids modulate two distinct analgesic pathways. Proc. Natl. Acad. Sci. U. S. A. 2008;105:18901–18906. doi: 10.1073/pnas.0809765105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deng Y, Theken KN, Lee CR. Cytochrome P450 epoxygenases, soluble epoxide hydrolase, and the regulation of cardiovascular inflammation. J. Mol. Cell. Cardiol. 2010;48:331–341. doi: 10.1016/j.yjmcc.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ulu A, Davis BB, Tsai H-J, Kim I-H, Morisseau C, Inceoglu B, et al. Soluble epoxide hydrolase inhibitors reduce the development of atherosclerosis in apolipoprotein e-knockout mouse model. J. Cardiovasc. Pharmacol. 2008;52:314–323. doi: 10.1097/FJC.0b013e318185fa3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Craige SM, Chen K, Pei Y, Li C, Huang X, Chen C, et al. NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation. 2011;124:731–740. doi: 10.1161/CIRCULATIONAHA.111.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schroder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, et al. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ. Res. 2012;110:1217–1225. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- 46.Zhang D, Ai D, Tanaka H, Hammock BD, Zhu Y. DNA methylation of the promoter of soluble epoxide hydrolase silences its expression by an SP-1-dependent mechanism. Biochim. Biophys. Acta Gene Regul. Mech. 2010;1799:659–667. doi: 10.1016/j.bbagrm.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 47.Sanders YY, Liu H, Liu G, Thannickal VJ. Free radical biology and medicine epigenetic mechanisms regulate NADPH oxidase-4 expression in cellular senescence. Free Radic. Biol. Med. 2015;79:197–205. doi: 10.1016/j.freeradbiomed.2014.12.008. [DOI] [PubMed] [Google Scholar]