Abstract
This protocol describes 3D bioprinting of cardiac tissue without the use of biomaterials, using only cells. Cardiomyocytes, endothelial cells and fibroblasts are first isolated, counted and mixed at desired cell ratios. They are co-cultured in individual wells in ultra-low attachment 96-well plates. Within 3 days, beating spheroids form. These spheroids are then picked up by a nozzle using vacuum suction and assembled on a needle array using a 3D bioprinter. The spheroids are then allowed to fuse on the needle array. Three days after 3D bioprinting, the spheroids are removed as an intact patch, which is already spontaneously beating. 3D bioprinted cardiac patches exhibit mechanical integration of component spheroids and are highly promising in cardiac tissue regeneration and as 3D models of heart disease.
Keywords: Bioengineering, Issue 125, Cardiac Tissue Engineering, 3D Printing, Bioprinting, Biofabrication, Additive Manufacturing, Heart Failure, Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes
Introduction
There are many different methods of 3D bioprinting1,2,3. 3D bioprinting is frequently classified by printing technology1, with examples such as inkjet bioprinting, microextrusion bioprinting, laser assisted bioprinting, a combination of methods, or newer approaches. 3D bioprinting can also be classified into scaffold-free or scaffold-dependent methods4. Most methods of 3D bioprinting are scaffold-dependent, where there is a need for biomaterials, e.g. bioinks5 or scaffolds6. However, scaffold-dependent 3D bioprinting face many issues and limitations4,7, such as immunogenicity of scaffolding material, cost of proprietary bioinks, slow speed and toxicity of degradation products.
Scaffold-free cardiac tissue engineering using spheroids has been attempted8, with the potential to overcome these disadvantages of scaffold-dependent tissue engineering. However, as acknowledged by the authors in that paper, it had been difficult to robustly handle and position spheroids in fixed locations, in the process of biofabrication. The concomitant use of 3D bioprinting and spheroid-based tissue engineering has the potential to overcome these difficulties. In this protocol, we describe 3D bioprinting of cardiac tissue without other biomaterials, using only cells in the form of spheroids.
Scaffold-free spheroid-based 3D bioprinters9 have the ability to pick up individual spheroids using vacuum suction and position them on a needle array. The concept of positioning spheroids on a needle array in 3D bioprinting, is inspired from the use of needle arrays (known as "kenzan") in the ancient Japanese art of flower arrangement, ikebana. This system allows spheroids to be precisely positioned in any configuration and results in the individual spheroids fusing together over a short period to create a 3D bioprinted tissue. This method thus allows spheroids to be manipulated with ease, with potential implications for the future of scaffold-free organ biofabrication.
Protocol
1. Preparation of Cardiomyocytes
Generate and culture human induced pluripotent stem cells (hiPSCs) on 6-well plates coated with basement membrane matrix as described10.
Differentiate hiPSCs into hiPSC-derived cardiomyocytes (hiPSC-CMs) using previously described methods11,12.
On Day 19 after differentiation, isolate the cardiomyocytes using 2 mL of Trypsin/EDTA 0.05% in each well for 5 min at room temperature.
Monitor the cardiomyocytes under an optical microscope to watch for cell dissociation.
Neutralize Trypsin using 2 mL of Trypsin inhibitor 0.0125%.
Pool the isolated cardiomyocytes and transfer them into one 50 mL conical tube using a motorized pipette filler.
Centrifuge the cell suspension for 5 min at 250 x g at room temperature to obtain a pellet.
Resuspend the pellet in 5 mL of Roswell Park Memorial Institute (RPMI) cell media supplemented with B-27 (RPMI/B-27 cell media).
Pipette 20 µL of cells in cell media and stain with an equal amount of 0.4% Trypan Blue solution.
Use an automated cell counter or a manual hemocytometer to count and obtain the concentration and cell viability of the cell suspension.
2. Preparation of Fibroblasts
Initiate the human cardiac fibroblast (HCF) (adult ventricular type) cell line13.
Passage and isolate them in accordance to HCF culturing protocols13.
Pipette 20 µL of cells in cell media and stain with an equal amount of Trypan Blue solution 0.4%.
Use an automated cell counter or a manual hemocytometer to count and obtain the concentration and cell viability of the new cell suspension.
3. Preparation of Endothelial Cells
Initiate the human umbilical vein endothelial cell (HUVEC) line as described14. Passage and isolate them in accordance to HUVEC culturing protocols as described14.
Pipette 20 µL of cells in cell media and stain with an equal amount of 0.4% Trypan Blue solution.
Use an automated cell counter or a manual hemocytometer to count and obtain the concentration and cell viability of the cell suspension.
4. Co-culture:
Mix the three cell types (hiPSC-CM, HCFs, and HUVECs) in RPMI/B-27 cell media to generate a stock of mixed cell solution in a 50 mL conical tube, at a hiPSC-CM:HCF:HUVEC cell ratio of 70:15:15 and concentration of 165,000 cells per mL.
Distribute the mixed cell solution into ultra-low attachment 96-well U-bottom plates at 200 µL per well, or 33,000 cells per well, using a multi-channel pipette.
Incubate the 96-well plates for 3 days (37°C, 5% carbon dioxide, 95% humidity).
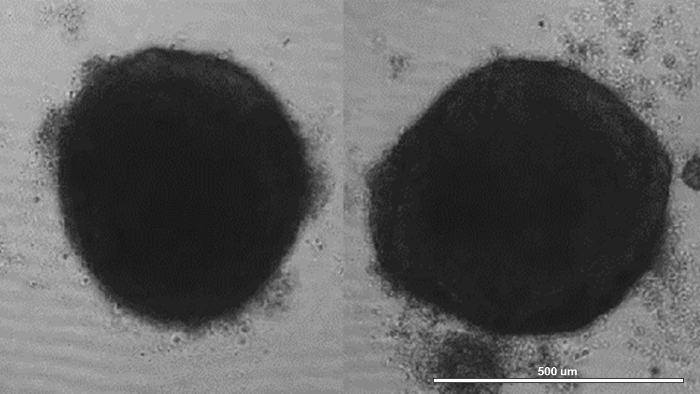
Inspect for the presence of mixed cell multicellular spheroids at the center and bottom of individual wells, under light microscopy. NOTE: In a two-dimensional microscopic projection, these spheroids will appear circular in shape (Figure 1). Spheroids should form within 24 h, after cell seeding into ultra-low attachment 96-well plates. Spheroids should start beating within 48 h, after cell seeding into 96-well plates. Spheroids that are not beating should not be used for 3D bioprinting to ensure that the final 3D bioprinted cardiac patch is functional. Immunofluorescence of spheroids for connexin 43 (Cx43) and troponin may be used to assess spheroid cellular composition and quality15.
5. 3D Bioprinting of Scaffold-free Cardiac Tissues
Load the plates containing the spheroids into the magazines of the scaffold-free spheroid-based 3D bioprinter.
- Turn on the 3D bioprinter and execute the 3D bioprinting software.
- Click "Auxillary", "Operation Preparation", "Inspect Mode", and then "Home Return".
- Click the green icon under "Start" to begin spheroid inspection.
- Watch for the spheroid diameters to appear under the column "(2) Dia (µm)" on the screen.
To estimate the average spheroid diameter to the nearest 100 µm, click "Initial settings" and enter the average spheroid diameter into Stack Z pitch (µm).
Click "Total", the number under "Layers" should readjust automatically. Click "Apply" to confirm the settings.
Click "Needle Array Map", go to "Layer No.", select the desired layer for 3D bioprinting, and draw the desired 3D bioprinting design on the map on the left of the screen by selecting individual spheroid positions. Click "Apply" to confirm the 3D bioprinting design.
Click "Needle Array Check" to begin needle array inspection. If the needle array is out of focus, input values into "OffsetZ (µm)", ranging from 0 to 500 µm to adjust camera focus, starting with 500 µm and decreasing by 100 µm to 0 µm.
Click "Inspection Parameters", "Type 1", and set "Diameter (µm)" to the desired diameter range. Set "Roundness (%)" and "Smoothness (%)" to desired percentages. Click "Apply" to confirm the settings. NOTE: Here the spheroid diameters were set from 450 µm to 550 µm. "Roundness (%)" and "Smoothness (%)" are computer calculated assessment of each individual spheroid. Here, the settings used for "Roundness (%)" is 60% and "Smoothness (%)" is 70%.
Click "Presence" and update the "Diameter (µm)" to the same settings in the previous step. Click "Apply" to confirm the settings.
Click "Stack Mode", then the green icon under "Start" to begin spheroid pick up using the nozzle of the 3D bioprinter by vacuum suction, and placement of spheroids in specific locations in a needle array. NOTE: For a cardiac patch consisting of 81 spheroids (9 spheroids x 9 spheroids), 3D bioprinting should take about 20 to 30 min.
After bioprinting, using sterile forceps, pick up the needle array containing the 3D bioprinted patch and transfer it into a 250 mL sterile beaker containing 150 mL of RPMI/B-27 cell media.
Incubate the 3D bioprinted patch with the needle array for 3 days (37°C, 5% carbon dioxide, 95% humidity).
6. Removal of 3D Bioprinted Patch from the Needle Array and Patch Maturation
After 3 days of incubation, with one hand at the base of the needle array and the other on the plastic cover under the patch, gently slide the plastic cover upwards to remove the 3D bioprinted patch from the needle array. NOTE: Lubrication with phosphate-buffered saline (PBS) may be applied to the needles just before this step, as needed, for smoother and easier removal.
Using a pair of sterile forceps, pick up the plastic cover with the 3D bioprinted patch on top of it and transfer the patch and plastic cover into a 35 mm dish containing RPMI/B-27 cell media.
Gently shake the plastic cover with the patch in the RPMI/B-27 cell media, with the forceps still holding on to the plastic cover, to release the patch into the media.
Inspect the 3D bioprinted patch under light microscopy to visualize whether it is beating to ensure that the 3D bioprinted patch is functional.
Incubate the 3D bioprinted patch in the 35 mm dish for 3 days or longer (37°C, 5% carbon dioxide, 95% humidity). NOTE: After 3 days, the spheroids fuse together and the holes in the patch, where the needles were, should no longer be visible.
Representative Results
At the end of step 4.4 (co-culture), the cells in each well should aggregate at the bottom of the ultra-low attachment 96-well U-bottom plates to form spheroids by gravity. These spheroids contain hiPSC-CM, HCFs, and HUVECs, and can be visually inspected under light microscopy, where they should appear circular by two-dimensional projection (Figure 1). At the end of step 6.3, the 3D bioprinted cardiac patch should contain tissue voids, due to needle holes created by the needle array (Figure 2, left). At this point, the boundaries between the spheroids should have become indistinct and the patch should have begun to beat spontaneously, exhibiting mechanical integration of spheroids. At the end of step 6.5, the tissue voids should be filled in by surrounding tissue (Figure 2, right), while the 3D bioprinted cardiac patch should continue to beat spontaneously.
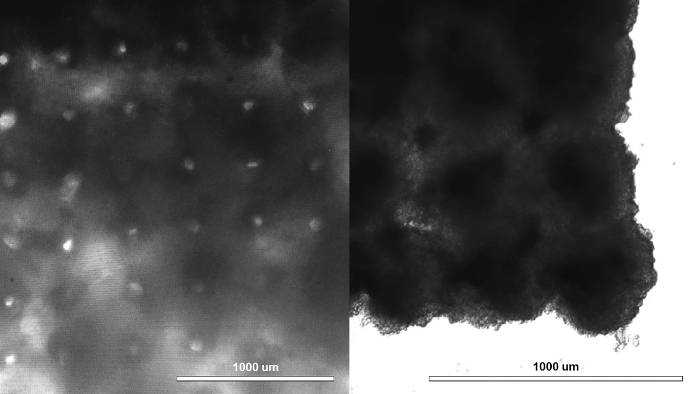
Figure 1: Creation of mixed cell multicellular spheroids. hiPSC-CM, HCFs, and HUVECs were mixed at a hiPSC-CM:HCF:HUVEC cell ratio of 70:15:15, and the cell mixture was distributed into ultra-low attachment 96-well U-bottom plates. Within 24 h, mixed cell multicellular spheroids (left, right) formed, one in each well. Scale bar = 500 µm. Please click here to view a larger version of this figure.
Figure 2: Creation of cardiac tissue exhibiting mechanical integration of spheroids. A 3D bioprinted cardiac patch immediately after removal from the needle array with visible needle holes (left). At this time, the boundaries between the spheroids had already become indistinct and the patch had already begun beating spontaneously, thus the spheroids had become mechanically integrated. Three days after removal from the needle array, the tissue voids caused by the needle array were filled in by surrounding tissue (right), and the patch continued beating spontaneously. Scale bar = 1 mm. Please click here to view a larger version of this figure.
Discussion
It is important to use beating, functional spheroids for 3D bioprinting. If spheroids are not beating, continuing to use them will invariably result in a non-functional 3D bioprinted patch.
One benefit of this approach is the ability to manipulate the cell content of the patch by varying the total number of cells and the percentage of cardiomyocytes, endothelial cells, and fibroblasts in the spheroids. This allows for many different types of cardiac patches to be printed, with varying histological and mechanical properties.
This approach also allows different types of spheroids to be used to make a patch, as well as the 3D bioprinting of cardiac patches with complex 3D designs. This complexity of the final 3D construct is achievable by 3D bioprinting with precise spheroid positioning, but may be difficult to achieve by gentle rotation of floating spheroids7 or simple molding of spheroids into a cylindrical 3D construct16.
The limitations of this approach are the relatively fixed size of the spheroids between 450 to 550 µm due to the fixed distance of 400 µm between the needles in the needle array, as well as possibly reduced cell viability if the spheroids are too large in diameter. The patches are mechanically fragile immediately after removal from the need array; however, the strength and durability are improved with culture for longer durations of time.
Finally, 3D bioprinting of cardiac patches has promising clinical applications17, in cardiac regeneration18,19 and as 3D models of heart disease. These 3D models can be used for applications in predictive pharmacology/toxicology and development of cell and genome directed therapies.
In conclusion, it is feasible to 3D bioprint cardiac tissue without the use of biomaterials. 3D bioprinted cardiac patches exhibit mechanical integration of component spheroids. 3D bioprinting of cardiac tissue has promising clinical applications in cardiac tissue regeneration and as 3D models of heart disease.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors acknowledge the following funding sources: Magic That Matters Fund for Cardiovascular Research and the Maryland Stem Cell Research Fund (2016-MSCRFI-2735).
References
- Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotech. 2014;32:773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- Bajaj P, Schweller RM, Khademhosseini A, West JL, Bashir R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Ann Rev Biomed Eng. 2014;16:247–276. doi: 10.1146/annurev-bioeng-071813-105155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra S, Young V. A Review of 3D Printing Techniques and the Future in Biofabrication of Bioprinted Tissue. Cell Biochem Biophy. 2016;74:93–98. doi: 10.1007/s12013-016-0730-0. [DOI] [PubMed] [Google Scholar]
- Moldovan NI, Hibino N, Nakayama K. Principles of the Kenzan Method for Robotic Cell Spheroid-Based Three-Dimensional Bioprinting. Tissue Eng Part B Rev. 2017. [DOI] [PubMed]
- Cui X, Boland T, D'Lima DD, Lotz MK. Thermal inkjet printing in tissue engineering and regenerative medicine. Recent Pat Drug Deliv Formul. 2012;6:149–155. doi: 10.2174/187221112800672949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CM, Haugh MG, O'Brien FJ. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomater. 2010;31:461–466. doi: 10.1016/j.biomaterials.2009.09.063. [DOI] [PubMed] [Google Scholar]
- Noguchi R, et al. Development of a three-dimensional pre-vascularized scaffold-free contractile cardiac patch for treating heart disease. J Heart Lung Transpl. 2016;35:137–145. doi: 10.1016/j.healun.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Mironov V, et al. Organ printing: tissue spheroids as building blocks. Biomater. 2009;30:2164–2174. doi: 10.1016/j.biomaterials.2008.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, et al. Scaffold-Free Tubular Tissues Created by a Bio-3D Printer Undergo Remodeling and Endothelialization when Implanted in Rat Aortae. PloS One. 2015;10:e0136681. doi: 10.1371/journal.pone.0136681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu PT, Fontes A, Vemuri MC, Macarthur CC. Generation of induced pluripotent stem cells with CytoTune, a non-integrating Sendai virus. Methods Mol Biol. 2013;997:45–56. doi: 10.1007/978-1-62703-348-0_5. [DOI] [PubMed] [Google Scholar]
- Li S, Cheng H, Tomaselli GF, Li RA. Mechanistic basis of excitation-contraction coupling in human pluripotent stem cell-derived ventricular cardiomyocytes revealed by Ca2+ spark characteristics: direct evidence of functional Ca2+-induced Ca2+ release. Heart Rhythm. 2014;11:133–140. doi: 10.1016/j.hrthm.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Boheler KR, et al. A Human Pluripotent Stem Cell Surface N-Glycoproteome Resource Reveals Markers, Extracellular Epitopes, and Drug Targets. Stem Cell Rep. 2014;3:185–203. doi: 10.1016/j.stemcr.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ScienCell Research Laboratories. Human Cardiac Fibroblasts (adult ventricular) Product Sheet. 2017. https://www.sciencellonline.com/PS/6310.pdf.
- Lonza Walkersville, Inc. Endothelial Cell Systems - Technical Information & Instructions., 2015. http://bio.lonza.com/uploads/tx_mwaxmarketingmaterial/Lonza_ManualsProductInstructions_Instructions__Technical_Info_-_Endothelial_Cell_Systems.pdf.
- Thermo Fisher Scientific, Inc. Immunofluorescence Method for IHC Detection. 2017. https://www.thermofisher.com/us/en/home/life-science/protein-biology/protein-biology-learning-center/protein-biology-resource-library/pierce-protein-methods/immunofluorescence-method-ihc-detection.html.
- Murata D, et al. A preliminary study of osteochondral regeneration using a scaffold-free three-dimensional construct of porcine adipose tissue-derived mesenchymal stem cells. J Orthop Surg Res. 2015;10 doi: 10.1186/s13018-015-0173-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosadegh B, Xiong G, Dunham S, Min JK. Current progress in 3D printing for cardiovascular tissue engineering. Biomed Mater. 2015;10:034002. doi: 10.1088/1748-6041/10/3/034002. [DOI] [PubMed] [Google Scholar]
- Chang CC, Boland ED, Williams SK, Hoying JB. Direct-write Bioprinting Three-Dimensional Biohybrid Systems for Future Regenerative Therapies. J Biomed Mater Res. Part B, Appl Biomater. 2011;98:160–170. doi: 10.1002/jbm.b.31831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Sing SL, Tan EYS, Yeong WY. Bioprinting in cardiovascular tissue engineering: a review. International J Bioprinting. 2016;2(2016) [Google Scholar]


