Abstract
Chronic Traumatic Encephalopathy (CTE) is an established neurodegenerative disease that is closely associated with exposure to repetitive mild Traumatic Brain Injury (mTBI). The mechanisms responsible for its complex pathological changes remain largely elusive, despite a recent consensus to define the neuropathological criteria. Here, we describe a novel method to develop a model of CTE in Drosophila melanogaster (Drosophila ) in an attempt to identify the key genes and pathways that lead to the characteristic hyperphosphorylated tau accumulation and neuronal death in the brain. Adjustable-strength impacts to inflict mild closed injury are delivered directly to the fly head, subjecting the head to rapid acceleration and deceleration. Our method eliminates the potential problems inherent with other Drosophila mTBI models (e.g.,animal death might be induced by damage to other parts of the body or to internal organs). The less labor- and cost-intensive animal care, short life span, and extensive genetic tools make the fruit fly ideal to study CTE pathogenesis and make it possible to perform large-scale, genome-wide forward genetic and pharmacological screens. We anticipate that the ongoing characterization of the model will generate important mechanistic insights on disease prevention and therapeutic approaches.
Keywords: Neuroscience, Issue 125, Mild traumatic brain injury, chronic traumatic encephalopathy, Drosophila, concussion, neurodegeneration, animal model
Introduction
Chronic Traumatic Encephalopathy (CTE) has recently been recognized as a distinct neurodegenerative disorder, separate from other tauopathies such as Alzheimer's disease1. Unlike Alzheimer's disease and other common tauopathies-whose most important risk factors are advancing age and a family history of dementia, CTE, as indicated by its name, implies a close association with a history of brain trauma, most likely seen in contact sports athletes, such as boxers and football players, as well as in military veterans2,3,4,5. It is thought to be initiated by repeated concussive and subconcussive blows to the head. Patients may present symptoms and signs such as cognitive deficits, mood and behavior changes, and movement dysfunction, which overlap significantly with Alzheimer's disease, frontotemporal dementia, Lewy body dementia, and Parkinson's disease6. In contrast, post-mortem examinations of brain tissue reveal a distinct pattern of hyperphosphorylated tau accumulation surrounding small blood vessels at the depths of the cortical sulci, a pathognomonic feature not observed in the other degenerative conditions7. However, so far, very little is known about the pathogenesis leading to disease manifestation. This is in large part due to the lack of a faithful animal model - only recently have rodent models been generated5,8. These model organisms have the disadvantages of cost-intensive care and a relatively long life span, which are not well-suited for neurodegenerative disease studies.
Compared to mammalian counterparts, invertebrate animals such as Drosophila are an excellent alternative, with their cost-effective maintenance, extensive tools for dissecting genetic determinants, and relatively short lifespan9. Remarkably, fly and human brains share evolutionarily conserved molecular and cellular pathways, as well as anatomical similarities10,11,12. Two ingenious Drosophila models to study traumatic brain injury have been reported previously13,14. The first "High Impact Trauma" (HIT) device designed by Katzenberger and colleagues contained free-moving flies in a plastic vial that was tied to the free end of a metal spring13,15. When the plastic vial was tilted upright and released, it hit a polyurethane pad and imparted trauma to the flies as they bounced to the vial wall and rebounded. In contrast, Barekat and colleagues designed a different delivery method using the Omni Bead Ruptor-24 homogenizer platform14. Flies were incapacitated with CO2 and placed in a 2 mL screwcap tube that was secured to the homogenizer and subjected to preprogrammed shaking conditions. One benefit of using the tissue homogenizer system is that the experimenter could modulate the intensity of injury, duration of injury, and number of injury bouts. However, both regimes suffer the same drawback: primary injuries to the head are randomly inflicted in terms of impact location and strength. In addition, both methods resulted in considerable mortality, caused by inevitable collateral damage to other parts of the body and internal organs. Here, we describe a novel method to induce mTBI in fruit flies. Our apparatus consists of a gas-propelled ballistic impactor. Compared to the existing Drosophila models14,15, our method has the unique advantage of delivering measurable impact, directed only at the free-moving fly head, thus allowing for the accurate control of various factors, such as impact severity, the time interval between impacts, and the total number of impacts sustained.
Protocol
1. Assembly of the Strike Device (Figure 1)
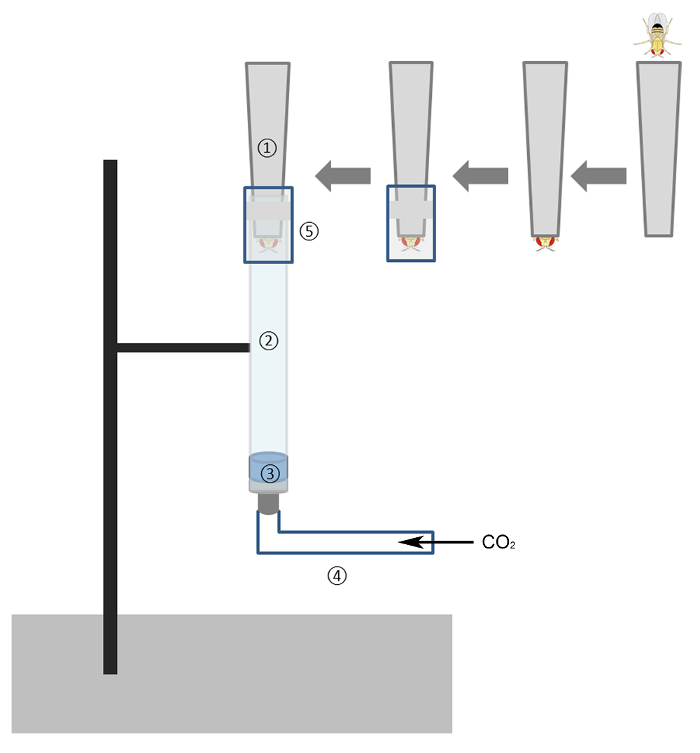
Remove the plunger from a 1 mL tuberculin syringe. Cut the barrel at the 1 mL mark.
Remove an aerosol barrier (3 mm height x 4 mm diameter) from a 200 µL pipette tip and use it as an impactor. Place the impactor inside the syringe barrel. Gently tap the barrel to move the impactor to the tip end, with the flat side covering the nozzle opening.
Attach the barrel tip end to plastic tubing that is connected to the carbon dioxide (CO2) flow regulator of a Drosophila anesthesia station.
Hold the barrel vertically and clamp it to a standard clamp holder stand so that the impactor stays at the bottom of the barrel.
- Modify a 200 µL pipette tip to make the fly holder.
- Cut 4 mm from the tip to make a 0.8 mm-diameter opening, allowing only the fly head to be exposed. NOTE: The thorax and all the other parts of the fly body will stay inside the pipette tip.
- Modify a 1,000 µL pipette tip and a 1 mL syringe needle cap to make the connector.
- Cut off 44 mm from the opening of the tip. Take a 6 mm length of a 1 mL syringe needle cap and push it tightly into the remaining segment of the 1,000 µL pipette tip.
2. Operation of the Strike Device
Anesthetize a single 2-day-old adult female fly using CO2 on a fly pad.
Gently transfer it to the fly holder using a fine brush. Tap the holder gently so that the fly head is seen outside of the tip end. If the fly proboscis is exposed outside the tip, gently tuck it back inside the tip with a blunted 1 mL syringe needle. NOTE: Make sure to keep the fly proboscis inside the holder. Otherwise, the fly may die from a sucking proboscis injury.
Tighten the fly holder to the syringe barrel with the connector so that the fly head is facing downwards.
Set the gas pressure at 100 kPa. Adjust the flow rate according to the experiment design.
Quickly turn the flow regulator toggle switch on and off so that the impactor strikes the fly head once.
Lift the fly holder and move it over a fly pad. Reverse the fly holder and gently tap the side to let the fly out. Leave the fly in an empty vial to recover.
3. Video-assisted Movement Tracking
Fill a 6-cm-diameter Petri dish with transparent silicon elastomer to make the tracking arena. Leave a 3 mm space between the silicon and the dish lid to allow the flies to walk freely but not fly.
Anesthetize four flies from either the sham or treated group each time and place them in the arena. Leave the flies at 22 °C for 1 h.
Position a Charge-Coupled Device (CCD) camera above the arenas and record for 5 min.
Analyze the recorded movement trajectories using Ctrax software (freely available from Caltech)16. Export the tracked data in a programming language (e.g., Matlab)-compatible format and analyze the data based on distance traveled per frame17. Calculate the mean walking distance for each fly and combine it with all other recorded flies/group to obtain a mean cumulative distance travelled by the population of files in the same group.
Representative Results
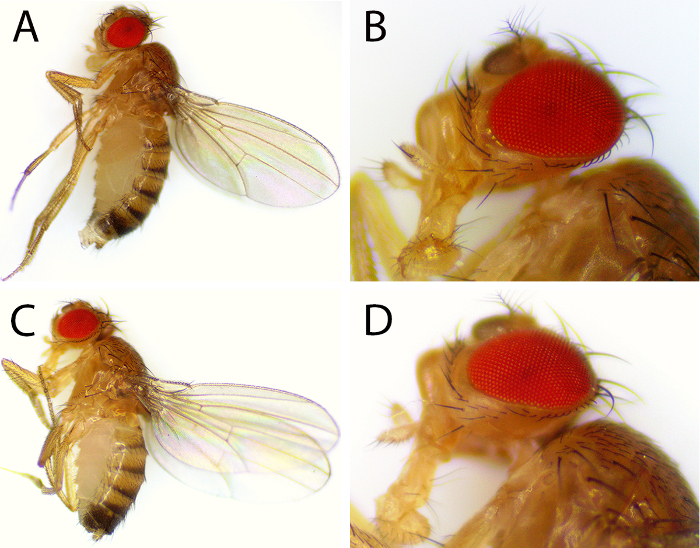
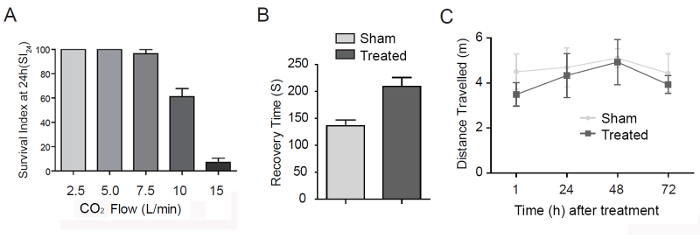
To establish a CTE model using adult Drosophila, we determined the effectiveness of our device at inflicting a single closed-head injury. To eliminate the variations relating to genotype, sex, or age, we used 2-day-old Canton-S WT female flies in the experiment. We could easily control the strength of the impactor by regulating the flow rate of CO2 at a constant gas pressure of 100 kPa. Flies exposed to a single strike at the highest flow rate (15 L/min) exhibited minimal external defects (Figure 2). Both the external eyes and mouthpart appeared intact. Nevertheless, we found that a flow rate of 15 L/min was acutely lethal, resulting in less than a 10% survival rate within 24 h (Figure 3A). In contrast, exposure to a 10 or 7.5 L/min strike resulted in 61.1% and 96.3% survival, respectively, while at 5.0 and 2.5 L/min flow rates, all flies survived after 24 h (Figure 3A). Therefore, we standardized our future studies to the 5.0 L/min CO2 flow rate. After one strike at 5.0 L/min, flies gradually recovered their mobility within 4 min (Figure 3B). Over the next 2 d, their locomotive function, measured by walking capability, slowly restored to normal (Figure 3C). As a preliminary assessment, these phenotypes are reminiscent of those presented by people who have experienced concussion or mild levels of trauma18. Patients suffering from traumatic brain injury often present with a progressive deterioration of muscular movements and premature death.
To evaluate the long-term effects of multiple brain injuries, we applied an mTBI protocol of five consecutive strikes at the 5.0 L/min flow rate, with 24 h recovery periods between each strike. To investigate the impact of mTBI on movement function, we used a video-assisted motion tracking system to analyze the fly motor behavior. After aging the flies 20 d post-treatment, the mTBI-treated group showed reduced walking activity and distance traveled compared to the sham group (Figure 4 A & B; sham group: 6.49 ±0.72, n = 28 versus treated group: 4.3 ±0.47, n = 32; p <0.05). To investigate whether repeat brain injuries have any chronic effects on survival, we determined the lifespan of flies that endured the mTBI protocol. Shown in Figure 4C, compared to the sham group (n = 129), treated flies (n = 100) had a substantially reduced median lifespan (36.9 ±2.7 Vs. 26.3 ±0.9 d) and significantly reduced maximum life span (49.3 ±2.4 Vs. 34.7 ±1.4 d).
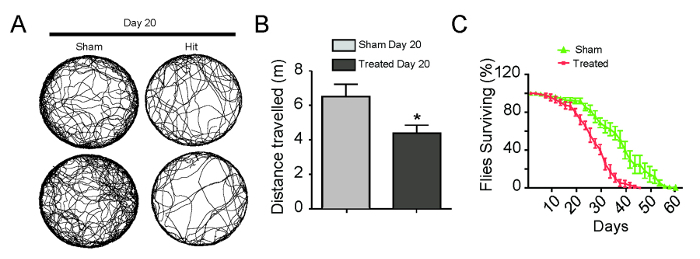
Figure 1. Diagram of the Strike Device.The device used materials that are readily available in a Drosophila lab: 1) 200 µL pipette tip, 2) 1 mL tuberculin syringe, 3) impactor, 4) connecting tube hooked to a Drosophila anesthesia station, and 5) connector to tighten the tip onto the syringe. Please click here to view a larger version of this figure.
Figure 2. Mild Closed-head Traumatic Injury. One single strike at the highest gas flow did not incur obvious external damage to the head, body, or appendages. (A & B) Strike-inflicted fly. (C & D) Sham fly. The sham flies underwent exactly the same procedures as the treated group, including transfers on and off the fly holder, except no impactor was placed in the syringe barrel when the CO2 flow was applied. Please click here to view a larger version of this figure.
Figure 3. The Effect of a Single Strike. (A) The survival index at 24 h (SI24), defined by the percentage of flies that survived within 24 h after a strike, is graphed versus the gas flow rate. Flies that have been struck once with the 5.0 L/min gas flow recovered locomotor activity within 4 min (B), and their mobility slowly restored to normal over a 2 d period (C). Error bar, Standard Error of the Mean (SEM). Please click here to view a larger version of this figure.
Figure 4. Development of the mTBI Model in Drosophila. Repeated strikes over five days resulted in significantly impaired locomotor activity (A & B) and reduced life span (C). Representative walking tracks recorded over 5 min for 4 sham and treated flies are shown in A. Error bars, SEM. * p <0.05, Mann-Whitney U-test with a Bonferroni correction. Total flies: treated group n = 100, sham group n = 129. Please click here to view a larger version of this figure.
Discussion
Animal models that faithfully model CTE features, including neurophysiological alterations, neuropathological hallmarks, and neurobehavioral deficits, are essential for uncovering disease mechanisms and for developing diagnostic and therapeutic targets. It is understandable that no animal model of a human disease is perfect at mimicking all clinically relevant endpoints. However, we believe that a robust CTE model should satisfy the following three requirements: (1) the impact must be directly applied to a head that has an intact scalp and skull protection; (2) the head should not be immobilized during impact exposure so that rapid acceleration-deceleration and rotational and linear head movements are allowed; and (3) the experimental design should include both single and repetitive regimes, and the impact consequences should be mild in nature, without inflicting visible damage, such as tissue edema, contusion, or frank hemorrhage.
Our fruit fly model is distinguished from other methods that inflict traumatic brain injury in flies13,14,15. Both the Bead Ruptor model and the HIT device inflict injury with random impact location and strength. In both methods, variable numbers of flies contained in either a standard plastic vial or a 2 mL screwcap tube contact the container wall and rebound multiple times during each strike or injury bout. Since the flies are freely movable inside the container, the primary injuries inflicted are considerably different for individual flies, as different parts of their bodies and/or heads are injured with different forces. Thus, it is not surprising that, using those models, the flies suffered from intestinal barrier dysfunction. This was directly correlated with the mortality rate in both regimens13,14, strongly indicating that non-neuronal alterations were responsible for trauma-induced death in both systems. In contrast, our method is capable of repeatedly delivering direct head impacts to the same location. The body is protected from any direct exposure, eliminating the potential caveats that death might be caused by damage to other parts of the body or to internal organs. In addition, the flow rate of the CO2 gas used to propel the weight can be regulated to achieve controlled, adjustable strength. Most importantly, the fly head is not constrained at the time of strike, allowing for a very rapid acceleration-deceleration process that mimics the most common form of mTBI that occurs in the human population19.
Although it would be ideal to install an automatic on/off switch to control the CO2 flow regulator, the current manual switch does not appear to affect the reproducibility of the impactor force because the design of our device allows the force to be quickly exerted once the impactor strikes the head.
In summary, our system provides a novel regime to better mimic mTBI, introducing a new platform to model CTE. Like other Drosophila models that have proven valuable at deciphering human neurodegenerative disorders9,20, it is anticipated that the ongoing detailed characterization of the model in terms of neuronal loss, tau hyperphosphorylation, TDP-43 proteinopathy, and neuroinflammatory response to the inflicted mTBI will generate important mechanistic insights into CTE disease processes and will help to answer some of the critical questions in the field.
Disclosures
This work was supported by the Johns Hopkins University School of Medicine faculty startup fund to L.C.
Acknowledgments
The authors have nothing to disclose.
References
- McKee AC, et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol. 2016;131:75–86. doi: 10.1007/s00401-015-1515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martland HS. Punch drunk. JAMA. 1928;91(15):1103–1107. [Google Scholar]
- Millspaugh JA. Dementia pugilistica. US Naval Med Bull. 1937;35:297–303. [Google Scholar]
- Omalu BI, et al. Chronic traumatic encephalopathy in a national football league player: part II. Neurosurgery. 2006;59(5):1086–1092. doi: 10.1227/01.NEU.0000245601.69451.27. [DOI] [PubMed] [Google Scholar]
- Goldstein LE, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med. 2012;4(134) doi: 10.1126/scitranslmed.3003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mez J, Stern RA, McKee AC. Chronic traumatic encephalopathy: where are we and where are we going? Curr Neurol Neurosci Rep. 2013;13(12):407. doi: 10.1007/s11910-013-0407-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136(Pt 1):43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petraglia AL, et al. The spectrum of neurobehavioral sequelae after repetitive mild traumatic brain injury: a novel mouse model of chronic traumatic encephalopathy. J Neurotrauma. 2014;31(13):1211–1224. doi: 10.1089/neu.2013.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirth F. Drosophila melanogaster in the study of human neurodegeneration. CNS Neurol Disord Drug Targets. 2010;9(4):504–523. doi: 10.2174/187152710791556104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littleton JT, Ganetzky B. Ion channels and synaptic organization: analysis of the Drosophila genome. Neuron. 2000;26(1):35–43. doi: 10.1016/s0896-6273(00)81135-6. [DOI] [PubMed] [Google Scholar]
- Appel LF, et al. The Drosophila Stubble-stubbloid gene encodes an apparent transmembrane serine protease required for epithelial morphogenesis. Proc Natl Acad Sci USA. 1993;90(11):4937–4941. doi: 10.1073/pnas.90.11.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piyankarage SC, Featherstone DE, Shippy SA. Nanoliter hemolymph sampling and analysis of individual adult Drosophila melanogaster. Anal Chem. 2012;84(10):4460–4466. doi: 10.1021/ac3002319. [DOI] [PubMed] [Google Scholar]
- Katzenberger RJ, et al. A Drosophila model of closed head traumatic brain injury. Proc Natl Acad Sci USA. 2013;110(44):E4152–E4159. doi: 10.1073/pnas.1316895110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barekat A, et al. Using Drosophila as an integrated model to study mild repetitive traumatic brain injury. Sci Rep. 2016;6:25252. doi: 10.1038/srep25252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenberger RJ, et al. A Method to Inflict Closed Head Traumatic Brain Injury in Drosophila. J Vis Exp. 2015. [DOI] [PMC free article] [PubMed]
- Branson K, Robie AA, Bender J, Perona P, Dickinson MH. High-throughput ethomics in large groups of Drosophila. Nat Methods. 2009;6(6):451–457. doi: 10.1038/nmeth.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straw AD, Dickinson MH, et al. Motmot, an open-source toolkit for realtime video acquisition and analysis. Source Code Biol Med. 2009;4(5):1–10. doi: 10.1186/1751-0473-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavage TM, et al. Functionally-detected cognitive impairment in high school football players without clinically-diagnosed concussion. J Neurotrauma. 2014;31(4):327–338. doi: 10.1089/neu.2010.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theadom A, et al. Frequency and impact of recurrent traumatic brain injury in a population-based sample. J Neurotrauma. 2015;32(10):674–681. doi: 10.1089/neu.2014.3579. [DOI] [PubMed] [Google Scholar]
- Drobysheva D, et al. An optimized method for histological detection of dopaminergic neurons in Drosophila melanogaster. J Histochem Cytochem. 2008;56(12):1049–1063. doi: 10.1369/jhc.2008.951137. [DOI] [PMC free article] [PubMed] [Google Scholar]


