Abstract
Epidemiological studies indicate that increased flavonoid intake correlates with decreased mortality due to cardiovascular diseases (CVD) in the United States (US) and Europe. Berries are widely consumed in the US and have a high polyphenolic content. Polyphenols have been shown to interact with many molecular targets and to exert numerous positive biological functions, including antioxidant, anti-inflammatory, and cardioprotective effects. Polyphenols isolated from blackberry (BL), raspberry (RB), and black raspberry (BRB) reduce oxidative stress and cellular senescence in response to angiotensin II (Ang II). This work provides a detailed description of the protocol used to prepare the polyphenol extracts from freeze-dried berries. Polyphenol extractions from freeze-dried berry powder were performed using 80% aqueous ethanol and an ultrasonic-assisted extraction method. The crude extract was further purified and fractionated using chloroform and ethyl acetate, respectively. The effects of both crude and purified extracts were tested on Vascular Smooth Muscle Cells (VSMCs) in culture.
Keywords: Chemistry, Issue 125, Polyphenols, blackberry, vascular smooth muscle cells, angiotensin II, cell signaling, senescence
Introduction
Polyphenols are compounds containing at least one phenolic ring in their structure and are abundantly present in the plant kingdom1. Humans have been consuming plants for millennia for medicinal purposes without being aware of the existence of such compounds2. Many fruits and vegetables have some shared polyphenolic compounds, albeit with different quantities, including flavonoids, stilbenes, and phenolic acids3. Although polyphenols are often associated with colorful fruits and vegetables, this is not strictly true. For example, zeaxanthin and xanthine are present in vegetables that are not highly colorful, such as onions and garlic, which are from the family of scallions and are associated with numerous health benefits4. Aside from being associated with several health benefits5, polyphenols also serve plants by protecting them from insects and ultraviolet radiation2. Polyphenols are commonly found in the human diet and are considered powerful antioxidants, as they can scavenge Reactive Oxygen Species (ROS)6,7,8. They also have anti-inflammatory9, antimicrobial10, anti-hypertensive11, and anti-carcinogenic12,13 properties.
Epidemiological studies demonstrate an inverse association between the consumption of flavonoids and cardiovascular disease (CVD) incidence16,17 and mortality14,15. Berries are widely consumed in the US and have high amounts of polyphenols, including flavonoids. For instance, consumption of blackberry (BL) juice (300 mL/d) for eight weeks significantly decreased systolic blood pressure in dyslipidemic patients18. Jeong et al.19 reported that pre-hypertensive men and women consuming 2.5 g of black raspberry (BRB) extract per day had lower 24-h and nighttime blood pressure compared to those consuming a placebo. Raspberries (RB) decreased blood pressure while increasing the expression of superoxide dismutase (SOD) in spontaneously hypertensive rats20. It has recently been shown that BL, RB, and BRB reduce the levels of ROS and senescence induced by angiotensin II (Ang II) in Vascular Smooth Muscle Cells (VSMCs)21. In addition, the anthocyanin fraction from BL extract reduced the expression of inducible nitric oxide synthase (iNOS) and inhibited the activity of Nuclear Factor kappa B (NF-κB) and extracellular signal-regulated kinase (ERK) in lipopolysaccharide (LPS)-stimulated J774 cells22. BRB extracts decreased the NF-κB activation and cyclooxygenase 2 (COX-2) expression in vitro 23, improved the lipid profile, and prevented atherosclerosis lesion formation in mice fed a high-fat diet24. Anthocyanins, which are considered the most abundant flavonoids in berries, modulate the inflammatory response in LPS-stimulated RAW 264.7 macrophages by decreasing Tumor Necrosis Factor alpha (TNF-α) production25 and decrease the proliferation and migration of VSMCs26.
Since there has been growing interest in understanding the role of polyphenols in human health and disease, it is important to optimize the extraction method. Solvent extraction is widely used for that purpose, as it is cost-effective and easily reproducible. In this study, a solvent extraction was used with ethanol, along with an ultrasonic-assisted extraction method, which was adapted from Kim and Lee27. The purification and fractionation of crude extracts (CE) using chloroform and ethyl acetate were performed to obtain the purified extract (PE) fraction that was adapted from Queires et al28. Furthermore, the efficacy of crude versus purified polyphenol extracts from BL at reducing the basal phosphorylation of ERK1/2 were compared, and representative examples of the inhibitory effect of purified BL polyphenol extract on Ang II-induced signaling reductions in VSMCs were provided.
Protocol
1. Preparation of Reagents
Prepare 80% ethanol (100 mL) by mixing 80 mL of absolute ethanol (molecular biology-grade) and 20 mL of cell culture-grade sterile water.
To prepare polyphenol extract (10 mg/mL), weigh 10 mg of CE or PE. Add 1 mL of plain Dulbecco's Modified Eagle Medium (DMEM) under a cell culture hood. Vortex the solution. Aliquot in 200-µL portions and store at -20 °C.
- Prepare lysis buffer.
- Add 5 mL of 1 M HEPES stock solution (pH 7.4; 50 mM final), 1 mL of 5 M NaCl stock solution (50 mM final), 1 mL of 0.5 M EDTA stock solution (5 mM final), 2 mL of 0.5 M NaF stock solution (10 mM final), 286 µL of 700 mM Na3VO4 stock solution (2 mM final), 5 mL of 200 mM Na4P2O7 stock solution (10 mM final), and 5 mL of 20% Triton-X-100 stock solution prepared in water (1% final). Add water to reach a volume of 100 mL. Keep at 4 °C.
- Add 10 µL/mL of protease inhibitor cocktail. Add fresh when the cells are ready for lysis.
- Prepare Laemmli sample buffer (4x).
- Add 31.25 mL of 2 M Tris stock solution (pH 6.8), 100 mL of glycerol, 50 mL of a 20% Sodium Dodecyl Sulfate (SDS) solution prepared in water, and 50 mL of β-mercaptoethanol. Add water up to 250 mL. Keep at 4 °C.
To prepare Tris-Buffered Saline (TBS) buffer, weigh 3 g of Tris (25 mM final), 0.18 g of KCl (2.5 mM final), and 8.76 g of NaCl (150 mM final). Adjust the pH to 7.4 and add water up to 1 L. Keep at RT.
To prepare TBS-T, add 997.5 mL of TBS and 2.5 mL of a 20% Triton-X-100 solution prepared in water. Keep at RT.
2. Preparation of Blackerry Extracts
- Preparation of crude extract from freeze-dried berry powder.
- Weigh 10 g of freeze-dried blackberry powder (or 5 g of fresh powder). If frozen fruit is used, cut it into very small pieces before starting the extraction.
- Mix the fruit powder with 100 mL of 80% aqueous ethanol in a 1 L round-bottom Erlenmeyer flask.
- Sonicate the mixture for 20 min at 42 kHz, 135 W using an ultrasonic bath at 25 °C, without any time interval in between. Perform the sonication with continuous nitrogen purging in subdued light. For frozen fruit, increase the incubation time to 4 h.
- Filter the mixture through a #2 filter paper using a chilled Buchner funnel with vacuum suction. NOTE: At the end of this step, residuals of the sample appear on the filter paper; this is called filter cake.
- Rinse the filter cake with 50 mL of 100% ethanol. Save the filtrate and add the filter cake to a 1 L round-bottom flask containing 100 mL of 80% aqueous ethanol.
- Repeat the extraction process for the residue (steps 2.1.2 - 2.1.4).
- Combine the two filtrates into a round-bottom flask with an additional 50 mL of 80% aqueous ethanol. NOTE: The final volume should be approximately 300 mL.
- Use a rotary evaporator at 62 °C and 50 rpm to evaporate the solvent. Continue this process until the ethanol does not evaporate anymore. NOTE: This process takes approximately 45 min.
- Transfer the sample to a 50 mL conical tube. Evaporate the ethanol by injecting nitrogen gas at the top of the tube for 10 min. NOTE: This step is needed to ensure the complete evaporation of the ethanol. The volume of the sample at this step is approximately 20 mL.
- Freeze the sample at -80 °C for at least 24 h. NOTE: This step is needed to allow for a more efficient freeze-drying process.
- Freeze-dry the sample at -50 °C for approximately 8 h. Store the samples at -20 °C. NOTE: The freeze dryer creates a powerful vacuum at -50 °C inside the chamber to dry the samples but preserves most of the compounds, such as polyphenols.
- Preparation of purified polyphenol extracts from crude extracts.
- Weigh the freeze-dried extracted samples. NOTE: The volume of the extract at this step is approximately 10 mL.
- Add two volumes (~20 mL) of chloroform to the crude extract and shake the solution for 5 min in a stir-plate.
- Pour the mixture into a separating funnel. Let the crude extract decant for 1 h at RT to obtain the purified fraction. NOTE: At this step, a two-phase mixture will form.
- Discard the bottom layer (chloroform phase) from the separating funnel and collect the aqueous layer into a clean beaker.
- Add two volumes of ethyl acetate to the aqueous fraction and use a magnetic stir bar to stir the mixture for 5 min at RT.
- Filter the mixture through #2 filter paper using a chilled Buchner funnel with vacuum suction.
- Collect the filtered sample and transfer it to a round-bottom flask to be used with the rotary evaporator.
- Set the rotary evaporator to 62 °C and 50 rpm and evaporate the ethyl acetate for about 30 min.
- Freeze-dry the sample at -50 °C for approximately 8 h. Transfer the sample to a 50 mL conical tube and store it at -20 °C.
3. Treatment of VSMCs with Berry Extracts
- VSMCs culture.
- Prepare complete medium for the culture of VSMCs using DMEM containing 1 g/L glucose and supplemented with 10% Fetal Bovine Serum (FBS), 100 U/mL penicillin, 100 mg/mL streptomycin, and 2 mM L-glutamine. Store the complete medium at 4 °C.
- Incubation of VSMCs with polyphenol extracts.
- To split the VSMCs, discard the culture medium and wash the cells twice with phosphate-buffered saline (PBS). Add 4 mL of PBS and 2 mL of trypsin EDTA 0.25%. Incubate the cells for 5 min at 37 °C in a CO2 incubator.
- Collect the cells, mix them with 2 mL of complete medium in a 15 mL centrifuge tube, and centrifuge at 1,100 × g for 5 min. Discard the supernatant and resuspend the cell pellet with 1 mL of PBS. Count the cells using a hemocytometer.
- Seed 50,000 VSMCs per well in 6-well culture plates and grow them in complete medium containing 10% FBS until they reach 90% confluency (about 3 d). Maintain the VSMCs at 37 °C in a humidified 5% CO2 incubator.
- Prepare the medium for the treatment using DMEM medium containing 1 g/L glucose, 100 U/mL penicillin, 100 mg/mL streptomycin, 2 mM L-glutamine, and 0.5% FBS. Store the treatment medium at 4 °C. NOTE: Polyphenol extracts and Ang II are added directly to cells using this medium.
- Prepare a stock solution of 10 mg/mL Crude Extract (CE) or Purified Extract (PE) of polyphenols by dissolving 10 mg of extract in 1 mL of plain DMEM medium. Keep the stock in 200 µL aliquots at -20 °C.
- Incubate the VSMCs with 2 mL of treatment medium containing 0.5% FBS and add either CE or PE extracts to achieve a concentration of 50 - 500 µg/mL. Change the medium every 24 h by adding fresh polyphenol extracts. Treat the cells for 3 d.
- For treatment with Ang II or other hormones and growth factors, starve the cells for at least 24 h by incubating the cells with treatment medium containing 0.5% FBS before the addition of Ang II (100 nM). NOTE: Incubation with and without CE and PE in 0.5% FBS treatment medium before the addition of Ang II is recommended.
- After 24 h of starvation, add 100 nM Ang II. Replace the treatment medium with fresh CE, PE, or Ang II every 24 h for 3 d.
- Preparation of cell samples.
- Wash the treated cells twice in cold PBS and lyse them with 200 µL of lysis buffer on ice.
- Collect cell lysates using cell scrapers and transfer the lysates to 1.5 mL microcentrifuge tubes. Incubate the total cell lysates for 20 min on ice and vortex every 5 min.
- Sonicate the cell extracts with three bursts at 125 W for 10 s each, with a 2 s pause in between. Keep the samples on ice throughout the sonication.
- Measure the protein concentration using a protein assay reagent at 595 nm.
- Store the samples at -20 °C for analysis by Western blot.
- Western blot.
- Mix equal amounts of protein (about 50 µg) from control non-treated and polyphenol-treated or Ang II-treated samples with lysis buffer to obtain a maximum total volume of 50 µL. Add 16 µL of a 4x Laemmli sample buffer. Heat the samples for 5 min at 75 °C.
- Separate the samples in 10% SDS-PAGE gels and transfer them to PDVF membranes at 10 V for 75 min in a semi-dry transfer system for Western blot analysis with specific antibodies.
- Block the membrane with 2% milk in TBS 0.05% Triton-X100 buffer (TBS-T) for 20 min.
- Remove the milk by washing the membrane at least three times with TBS (5 min each) and incubate with primary antibodies for 1 h at RT or O/N at 4 °C.
- Wash the membrane three times, 10 min each, with TBS-T and incubate with an HRP-linked secondary antibody for 45 min.
- Wash the membrane three times, 10 min each, with TBS-T and develop by enhanced chemiluminescence (ECL).
Representative Results
It has been previously demonstrated that polyphenol extracts isolated from BL, RB, and BRB reduced the senescence of VSMCs in response to Ang II21. It has been shown that these purified polyphenol extracts modulate Ang II signaling by reducing the phosphorylation of Akt, p38 Mitogen-Activated Protein Kinase (MAPK), and ERK1/2. BL prevents senescence by reducing the expression of the NADPH oxidase (Nox) 1, an enzyme that produces superoxide anions and is strongly upregulated by Ang II. In contrast, RB and BRB prevent senescence by a Nox1-independent mechanism, as they increase the expression of the antioxidant enzymes SOD1, SOD2, and glutathione peroxidase 1 (GPx-1). BL fails to increase SOD2 expression, while none of the extracts attenuate the downregulation of catalase by Ang II21. BL was focused on to determine whether the lack of effect of BL on SOD2 and catalase expression could be explained by a loss of polyphenol compounds during the purification process or by an inadequate concentration of a certain polyphenolic compound in the extract. VSMCs were incubated with 50-500 µg/mL CE (Figure 1A) or PE (Figure 1B) in medium containing 0.5% FBS for three days. Neither CE nor PE increased SOD2 or catalase levels at any of the concentrations tested. As a positive control, ERK1/2 phosphorylation was measured, and it was found that CE reduced the phosphorylation of this kinase at concentrations higher than 300 µg/mL. In contrast, PE was effective at about 100 µg/mL (Figure 1B). Low phosphorylation levels were observed at concentrations of 200-500 µg/mL. These data support the previous observation showing that 200 µg/mL BL PE is sufficient to reduce Ang II signaling21. These results suggest that higher concentrations of polyphenol compounds in PE, compared with CE, could explain the higher efficiency of this extract at reducing ERK1/2 phosphorylation. To test this idea, the polyphenol compositions of both extracts were compared. The identification and quantification of polyphenol compounds in CE was performed by HPLC, as previously described21 (Table 1). 3-O-caffeoylquinic acid and quercetin were present at higher levels in PE compared to CE, while ferulic acid and rutin were found only in CE. Next, VSMCs were treated with 200 µg/mL BL PE for 24 h before the addition of 100 nM Ang II (Figure 1C). As previously reported21, the BL polyphenol extract reduced Ang II-induced ERK1/2 phosphorylation but demonstrated no effect on catalase and SOD2 expression.
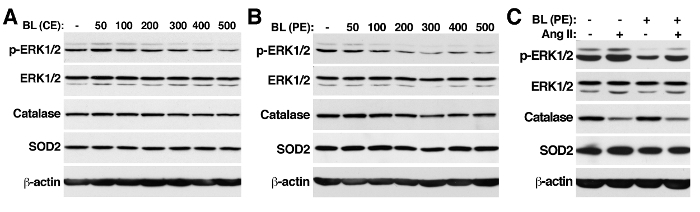
Figure 1: Blackberry Polyphenols Reduce Basal and Ang II-induced ERK1/2 Phosphorylation in VSMCs. VSMCs cultured in 10% FBS at about 90% confluency were incubated with 50 - 500 µg/mL BL Crude Extract (CE) (A), Purified Extract (PE) (B), or 200 µg/mL PE (C) in 0.5% FBS DMEM medium for 3 d. C) After 24 h of incubation with BL PE, Ang II (100 nM) was added and the cells were incubated for 3 d. The medium, with fresh extracts and Ang II, was changed every day. The cells were then washed and lysed, and the total cell extracts were separated in 10% PAGE-SDS gels. Western blots were tested with rabbit antibodies against phosphorylated ERK1/2 (Thr 202/Tyr 204), ERK1/2, catalase, and SOD2 and mouse antibody against β-actin. Please click here to view a larger version of this figure.
| Analytes (ppm) | PE | CE |
| Phenolic acids | ||
| gallic acid | 243.5 | 321.9 |
| p-coumaric acid | 32.9 | 46.5 |
| Ferulic acid | - | 236.5 |
| Chlorogenic acids | ||
| 3-O-caffeoylquinic acid | 235.3 | 170.5 |
| 4-O-caffeoylquinic acid | 13 | 76.9 |
| 5-O-caffeoylquinic acid | 14.1 | 49.9 |
| FLAVONOIDS | ||
| Flavonols | ||
| Quercetin | 95 | 24.5 |
| Flavanones | ||
| Rutin | - | 37.8 |
Table 1: Analysis of the Polyphenol Composition of Blackberry in Crude and Polyphenol-purified Extracts. Phenolic acids and flavonoids in Crude Extract (CE) and Purified Extract (PE) were analyzed using High Performance Liquid Chromatography (HPLC). The concentration of analytes is expressed as ppm. The composition of polyphenols in BL PE was recently published21 and added to the table to be compared to CE.
Discussion
Polyphenols isolated from berries contain distinct compositions. The ethanol-based extraction protocol described here allowed for the identification of different levels of phenolic acids and flavonoids present in crude and purified polyphenol extracts of BL (Table 1). CE was enriched in gallic acid, ferulic acid, 4-O-caffeoylquinic acid, and 5-O-caffeoylquinic acid. The purification process did not significantly alter the levels of gallic acid and p-coumaric acid. However, it increased the levels of 3-O-caffeoylquinic acid from 170.5 - 235.3 ppm and of quercetin from 24.5 - 95 ppm. In contrast, ferulic acid and rutin were lost during the purification of CE.
The treatment of VSMCs with 50 - 500 µg/mL CE and PE showed that both extracts were effective in reducing the basal phosphorylation of ERK1/2. However, PE showed a stronger downregulation in the activity of this kinase at a lower concentration: 100 µg/mL for PE compared with 400 - 500 µg/mL for CE. These results may reflect the higher concentration of 3-O-caffeoylquinic acid or quercetin found in PE. The use of individual phenolic compounds in cells in culture is needed to identify the specific compound(s) responsible for the downreguation of ERK1/2 phosphorylation.
The decreased activity of signaling kinases, including Akt, ERK1/2, and p38MAPK, caused by purified polyphenols isolated from BL, RB, and BRB21, as well as of ERK1/2 caused by BL CE, shown here, is in agreement with previous reports. For instance, polyphenol extracts isolated from blueberry decreased the tumor growth of mammary cancer cells, in part by reducing the activity of Akt and ERK1/230. As mentioned before, these kinases are also involved in the induction of cellular senescence by Ang II 21, suggesting that polyphenols from BL, RB, and BRB should reduce vascular aging and dysfunction associated with CVD.
As we reported previously21, catalase and SOD2 expression were not upregulated by PE, even at concentrations as high as 500 µg/mL. Since CE was also ineffective at increasing catalase and SOD2 expression, these data suggest that the phenolic compounds lost during the purification protocol are not involved in the regulation of these antioxidant enzymes. These data also suggest that the purification protocol shown here effectively concentrated phenolic compounds relevant to the regulation of Ang II signaling, oxidative stress, and cellular senescence. For example, the Representative Results show that PE strongly downregulated Ang II-induced ERK1/2 phosphorylation. The assessment of the phosphorylation of this kinase is relevant because the inhibition of ERK1/2 activity prevented Ang II-induced cellular senescence21.
In terms of modifications to previous protocols, a sonication was added here to increase the extraction yield for CE. Additionally, for the purification of polyphenols, instead of using a C18 Cartridge, as described by Kim and Lee27, a more traditional method from Queires et al.28 was adopted here. A filtration step, using filter paper to remove impurities, was added to the purification of polyphenol section. In terms of limitations, the sonication and evaporation steps should be carefully monitored according to the type of instrument used, since the duration and temperature of the sonication and evaporation, are the critical steps in this protocol. The modifications added to this method resulted in the highest yield of polyphenols when compared to previous methods27,28 used in our laboratory (data not shown), most likely because of the addition of a sonication step. As mentioned in the protocol section, this protocol can be used for freeze-dried powder from various berries, as well as for frozen fruits. This method has been successfully used to extract and purify polyphenols from raspberries and black raspberries21, as well as from blueberries and strawberries (data not shown). Thus, this method could be also used to extract polyphenols from other types of foods, including vegetables.
In conclusion, this work details a fast, cost-effective, and easily reproducible method to isolate polyphenols from berries, which allows for the retention and concentration of compounds that are protective against oxidative stress in VSMCs.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was funded by the American Heart Association (14GRNT20180028) and the Florida State University Council on Research and Creativity (COFRS).
References
- Morton LW, Abu-Amsha Caccetta R, Puddey IB, Croft KD. Chemistry and biological effects of dietary phenolic compounds: relevance to cardiovascular disease. Clin Exp Pharmacol Physiol. 2000;27(3):152–159. doi: 10.1046/j.1440-1681.2000.03214.x. [DOI] [PubMed] [Google Scholar]
- Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90(3):859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79(5):727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- Griffiths G, Trueman L, Crowther T, Thomas B, Smith B. Onions--a global benefit to health. Phytother Res. 2002;16(7):603–615. doi: 10.1002/ptr.1222. [DOI] [PubMed] [Google Scholar]
- Mazzoni L, et al. The genetic aspects of berries: from field to health. J Sci Food Agric. 2016;96(2):365–371. doi: 10.1002/jsfa.7216. [DOI] [PubMed] [Google Scholar]
- Wang SY, Jiao H. Scavenging capacity of berry crops on superoxide radicals, hydrogen peroxide, hydroxyl radicals, and singlet oxygen. J Agric Food Chem. 2000;48(11):5677–5684. doi: 10.1021/jf000766i. [DOI] [PubMed] [Google Scholar]
- Choi MH, Shim SM, Kim GH. Protective effect of black raspberry seed containing anthocyanins against oxidative damage to DNA, protein, and lipid. J Food Sci Technol. 2016;53(2):1214–1221. doi: 10.1007/s13197-015-2094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes-Hernandez TY, et al. The Healthy Effects of Strawberry Polyphenols: Which Strategy behind Antioxidant Capacity? Crit Rev Food Sci Nutr. 2016;56(Suppl 1):S46–S59. doi: 10.1080/10408398.2015.1051919. [DOI] [PubMed] [Google Scholar]
- Figueira ME, et al. Protective effects of a blueberry extract in acute inflammation and collagen-induced arthritis in the rat. Biomed Pharmacother. 2016;83:1191–1202. doi: 10.1016/j.biopha.2016.08.040. [DOI] [PubMed] [Google Scholar]
- Daglia M. Polyphenols as antimicrobial agents. Curr Opin Biotechnol. 2012;23(2):174–181. doi: 10.1016/j.copbio.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Hügel HM, Jackson N, May B, Zhang AL, Xue CC. Polyphenol protection and treatment of hypertension. Phytomedicine. 2016;23(2):220–231. doi: 10.1016/j.phymed.2015.12.012. [DOI] [PubMed] [Google Scholar]
- Niedzwiecki A, Roomi MW, Kalinovsky T, Rath M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients. 2016;8(9):E552. doi: 10.3390/nu8090552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kresty LA, Mallery SR, Stoner GD. Black raspberries in cancer clinical trials: Past, present and future. J Berry Res. 2016;6(2):251–261. doi: 10.3233/JBR-160125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertog MG, et al. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch Intern Med. 1995;155(4):381–386. [PubMed] [Google Scholar]
- Peterson JJ, Dwyer JT, Jacques PF, McCullough ML. Associations between flavonoids and cardiovascular disease incidence or mortality in European and US populations. Nutr Rev. 2012;70(9):491–508. doi: 10.1111/j.1753-4887.2012.00508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy A, et al. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation. 2013;127(2):188–196. doi: 10.1161/CIRCULATIONAHA.112.122408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques PF, Cassidy A, Rogers G, Peterson JJ, Dwyer JT. Dietary flavonoid intakes and CVD incidence in the Framingham Offspring Cohort. Br J Nutr. 2015;114(9):1496–1503. doi: 10.1017/S0007114515003141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghababaee SK, et al. Effects of blackberry (Morus nigra L.) consumption on serum concentration of lipoproteins, apo A-I, apo B, and high-sensitivity-C-reactive protein and blood pressure in dyslipidemic patients. J Res Med Sci. 2015;20(7):684–691. doi: 10.4103/1735-1995.166227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong HS, et al. Effects of Rubus occidentalis extract on blood pressure in patients with prehypertension: Randomized, double-blinded, placebo-controlled clinical trial. Nutrition. 2016;32(4):461–467. doi: 10.1016/j.nut.2015.10.014. [DOI] [PubMed] [Google Scholar]
- Jia H, et al. The antihypertensive effect of ethyl acetate extract from red raspberry fruit in hypertensive rats. Pharmacogn Mag. 2011;7(25):19–24. doi: 10.4103/0973-1296.75885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feresin RG, et al. Blackberry, raspberry and black raspberry polyphenol extracts attenuate angiotensin II-induced senescence in vascular smooth muscle cells. Food Funct. 2016;7(10):4175–4187. doi: 10.1039/c6fo00743k. [DOI] [PubMed] [Google Scholar]
- Pergola C, Rossi A, Dugo P, Cuzzocrea S, Sautebin L. Inhibition of nitric oxide biosynthesis by anthocyanin fraction of blackberry extract. Nitric Oxide. 2006;15(1):30–39. doi: 10.1016/j.niox.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Lu H, Li J, Zhang D, Stoner GD, Huang C. Molecular mechanisms involved in chemoprevention of black raspberry extracts: from transcription factors to their target genes. Nutr Cancer. 2006;54(1):69–78. doi: 10.1207/s15327914nc5401_8. [DOI] [PubMed] [Google Scholar]
- Kim S, et al. Aqueous extract of unripe Rubus coreanus fruit attenuates atherosclerosis by improving blood lipid profile and inhibiting NF-κB activation via phase II gene expression. J Ethnopharmacol. 2013;146(2):515–524. doi: 10.1016/j.jep.2013.01.016. [DOI] [PubMed] [Google Scholar]
- Wang J, Mazza G. Effects of anthocyanins and other phenolic compounds on the production of tumor necrosis factor alpha in LPS/IFN-gamma-activated RAW 264.7 macrophages. J Agric Food Chem. 2002;50(15):4183–4189. doi: 10.1021/jf011613d. [DOI] [PubMed] [Google Scholar]
- Pascual-Teresa S, Moreno DA, Garcia-Viguera C. Flavanols and anthocyanins in cardiovascular health: a review of current evidence. Int J Mol Sci. 2010;11(4):1679–1703. doi: 10.3390/ijms11041679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DO, Lee CY. Curr Protoc Food Analyt Chem. Vol. 1. New York: John Wiley & Sons; 2002. Extraction and Isolation of Polyphenolics; pp. 2.1–2.12. [Google Scholar]
- Queires LC, et al. Polyphenols purified from the Brazilian aroeira plant (Schinus terebinthifolius, Raddi) induce apoptotic and autophagic cell death of DU145 cells. Anticancer Res. 2006;26(1A):379–387. [PubMed] [Google Scholar]
- Griendling KK, Taubman MB, Akers M, Mendlowitz M, Alexander RW. Characterization of phosphatidylinositol-specific phospholipase C from cultured vascular smooth muscle cells. J Biol Chem. 1991;266(23):15498–15504. [PubMed] [Google Scholar]
- Vuong T, et al. Role of a polyphenol-enriched preparation on chemoprevention of mammary carcinoma through cancer stem cells and inflammatory pathways modulation. J Transl Med. 2016;14 doi: 10.1186/s12967-016-0770-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


