Abstract
Objective:
Increasing evidence has shown that skeletal muscle damage plays a role in neuromyelitis optica spectrum disorder (NMOSD). The objective of this study was to compare the serum creatine kinase (sCK) levels in NMOSD patients with different clinical statuses.
Methods:
In the observational study, levels of sCK were measured during the acute and stable phases for patients with NMOSD and healthy controls (HCs).
Results:
We enrolled 168 patients with NMOSD (female:male ratio, 153:15; age: 43.9 ± 13.1 years) in the acute phase, and blood samples were collected from 85 of the patients with NMOSD during both acute and stable phases to determine the sCK levels. The mean log sCK levels of the patients with NMOSD in the acute phase were higher (4.51 ± 1.17, n = 85) than those of the patients with NMOSD in the stable phase (3.85 ± 0.81, n = 85, p = 0.000). Furthermore, the log sCK levels of the patients with NMOSD in the stable phase were lower than those of the HCs (4.31 ± 0.39, n = 200, p = 0.000). In patients with sCK levels within the normal limits, these differences were also observed (p < 0.05). In the multivariable linear regression model performed for the patients with NMOSD in the acute phase, it suggested that a higher estimated glomerular filtration rate (p = 0.026), patients with the core clinical characteristics of optic neuritis (p = 0.005), and serum anti-SSA positivity (p = 0.019) predicted lower log sCK levels.
Conclusions:
Muscle damage occurs in patients with NMOSD and is aggravated during the acute phase.
Neuromyelitis optica spectrum disorder (NMOSD) is an inflammatory disease of the CNS that is characterized by severe optic neuritis and longitudinally extensive transverse myelitis. One of the major advances in recent years is the discovery that circulating autoantibodies (NMO-immunoglobulin G [NMO-IgG]) against water channel aquaporin 4 (AQP4) are associated with NMOSD.1
Outside the CNS, AQP4 is also expressed in the plasma membranes of other cells, including the cells of the stomach, airway, kidneys, and fast-twitch skeletal muscle fibers.2 During muscle contractions, AQP4 is believed to be involved in the rapid osmotic transfer of water from blood to muscle.3 In recent years, increasing evidence has shown that peripheral organs are involved in NMOSD, particularly skeletal muscle.4 Several patients have been reported with prominent hyperCKemia as an antecedent or accompaniment of acute NMO attacks, and these patients experienced general fatigue, myalgia, or no symptoms.5–11 In addition, 3 patients with NMOSD showed muscle pathology compatible with complement-activating IgG targeting sarcolemmal AQP4, confirming the involvement of muscle as a component of NMOSD.5,6 However, in medical practice, milder hyperCKemia might be asymptomatic and overlooked in patients with NMOSD.
Therefore, the aim of this study was to compare the serum creatine kinase (sCK) levels in NMOSD patients with different clinical statuses.
METHODS
Samples and participants.
This study was approved by the Medical Ethics Committee of the West China Hospital of Sichuan University, and written informed consent was obtained from all included patients.
From January 2009 to February 2017, we prospectively and consecutively enrolled 168 Chinese Han patients with NMOSD. These patients with NMOSD received outpatient and inpatient treatments at the Department of Neurology, West China Hospital of Sichuan University, which is the largest general hospital in Western China. None of the patients with NMOSD had preexisting myopathy or NMO-IgG–related myopathy. The diagnosis of NMOSD encompassed NMO based on the 2006 revised diagnostic criteria for NMO12 and a set of limited forms of NMO with seropositivity for NMO-IgG (based on a cell-based flow cytometry serum assay).
Venous blood was collected from the patients with NMOSD during the acute phase and stable phase. Patients who had experienced a relapse within the past month were considered to be in the acute phase, whereas those who were in a stable condition and had not experienced a clinical relapse for more than 1 month were considered to be in the stable phase. A relapse was defined as new or worsening CNS symptoms or signs that persisted for more than 24 hours. Pseudo-relapses were defined as cases in which the patients experienced only subjective changes, such as numbness, with no changes in objective symptoms, as confirmed by physical examination, Expanded Disability Status Scale assessment, or MRI. The concentration levels of sCK and creatinine (calculated for estimated glomerular filtration rate, eGFR) were measured in the morning after an overnight fast. The measurements were taken using a dry chemistry method with a Vitros 5600 clinical analyzer, located in the Clinical Laboratory of West China Hospital of Sichuan University. In addition, the patient's serum antinuclear antibody (ANA), anti-Sjogren syndrome A (anti-SSA) antibody, anti-Sjogren syndrome B(anti-SSB) antibody, and NMO-IgG statuses were detected. We also tested rheumatoid factors, anti–double-stranded DNA, and anti-SM antibodies; however, because of their low positive frequencies (≤4), they were excluded from our analysis. In addition, total sCK levels were measured for 200 age- and sex-matched healthy controls (HCs).
The other clinical data collected were as follows: current age, age at the time of blood collection, sex, race, and disease duration. During the acute phase, the following information was collected: core clinical characteristics (optic neuritis, acute myelitis, etc.), date when the acute phase began, date of blood collection, and method and time point of therapy for acute attacks.
Statistical analyses.
Because a test of normality showed that the sCK levels were not normally distributed, a log transformation was applied to the variant to improve the normality of the data. The results were expressed as mean ± SD log sCK levels. One-way analysis of variance (ANOVA) and the Scheffe post hoc test were used for the statistical comparisons among the HC, acute phase, and stable phase groups. To eliminate the effects of age and sex, a multivariable linear regression model was used to determine the differences in the log sCK levels among the groups.
To determine the associations between the demographic and clinical characteristics and log sCK levels, unadjusted univariate and adjusted multivariable linear regression models were performed for the patients with NMOSD in the acute phase. First, unadjusted univariate analyses of the clinical characteristics and log sCK levels were conducted to determine the potential predictors. Second, independent predictors for which p <0.05 in the univariate analyses were included in the adjusted multivariate analyses. Coefficients (coef.) with their 95% confidence intervals (CIs) were calculated to determine the risk of increased log sCK levels. All statistical analyses were performed using STATA 12.1. A 2-sided p <0.05 was considered statistically significant.
RESULTS
Baseline characteristics.
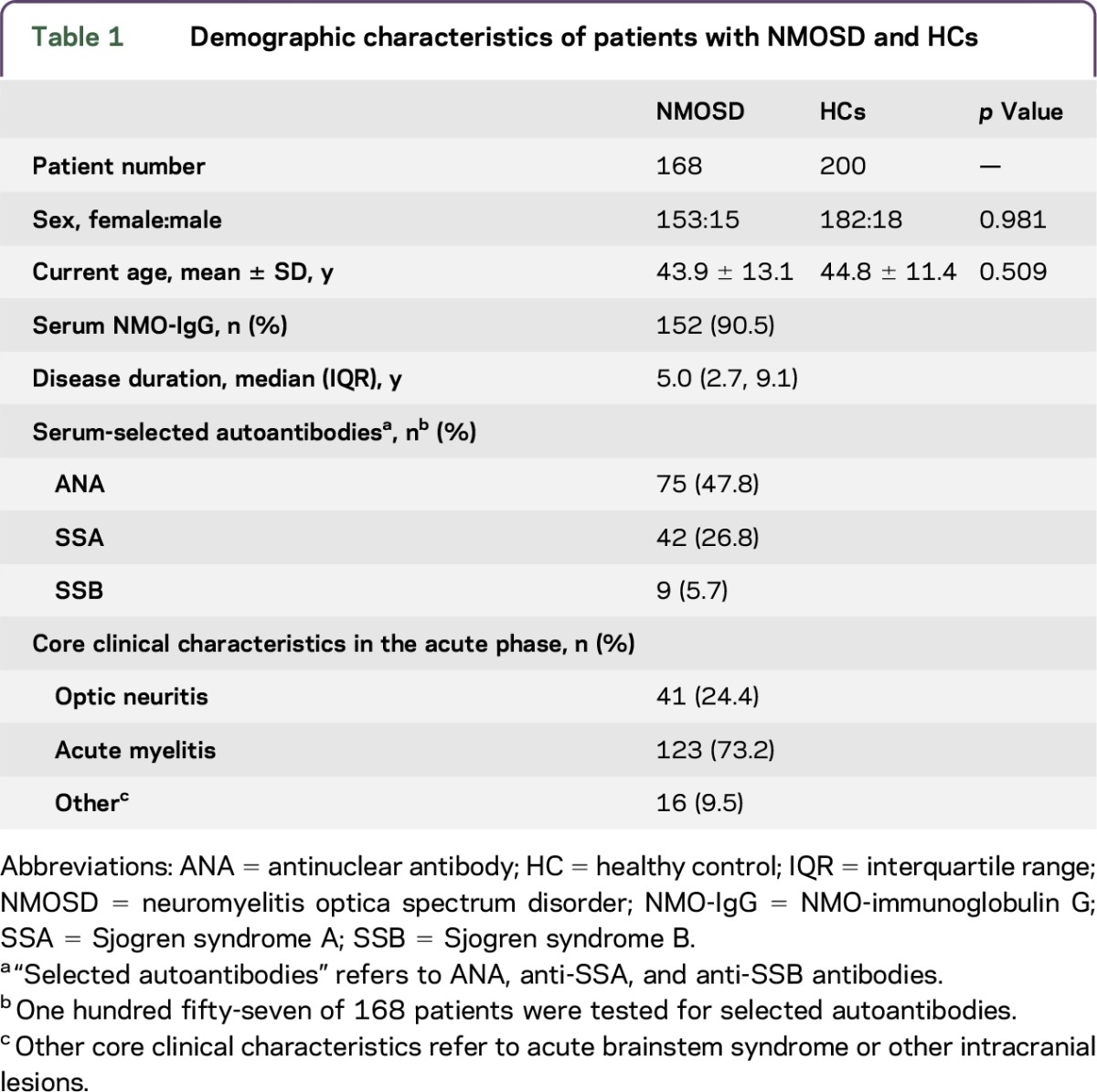
In this study, we enrolled 168 patients with NMOSD (female:male ratio, 153:15). The current age of the patients was 43.9 ± 13.1 years. The average disease duration was 5.0 (2.7, 9.1) (median [interquartile range (IQR)]) years. A total of 152 (90.5%) of the patients were NMO-IgG seropositive, and 157 of 168 patients were tested to detect selected autoantibodies. The selected autoantibodies (ANA, anti-SSA, and anti-SSB) were positive in 75 (47.8%), 42 (26.8%), and 9 (5.7%) of these patients, respectively (table 1).
Table 1.
Demographic characteristics of patients with NMOSD and HCs
During the acute phase, 133 samples were collected before treatment (methylprednisolone pulse therapy [MPT, n = 132] or IV immunoglobulin [IVIG, n = 1]). For the blood samples obtained during the acute phase in patients with NMOSD, the time from the onset of relapse to blood collection was 9 (5, 16) (median [IQR]) days. According to the core clinical characteristics during the acute phase, 41 patients had optic neuritis, 123 patients had acute myelitis, and 16 patients had other core clinical characteristics (acute brainstem syndrome or other intracranial lesions).
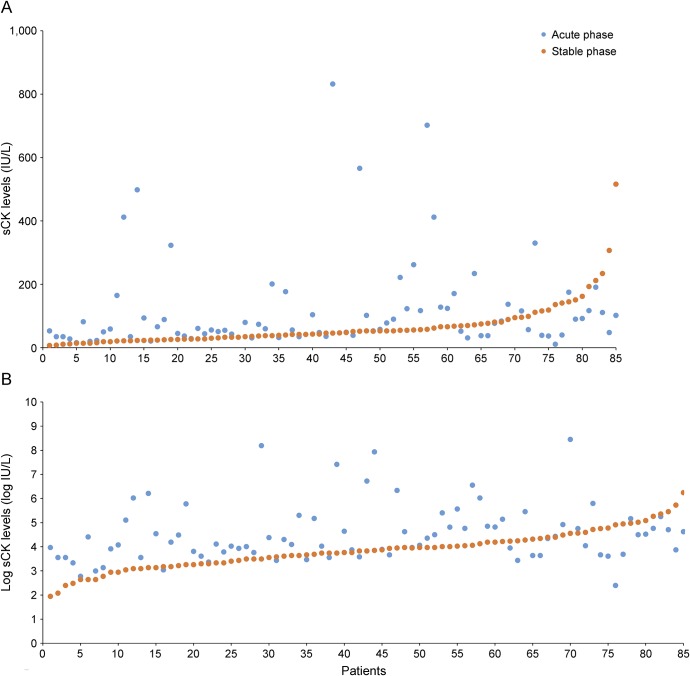
In addition, blood samples were collected from 85 of the patients with NMOSD (female:male ratio, 77:8; 46.1 ± 12.7 years) during both acute and stable phases to test the sCK levels (figure). However, blood samples were collected from the remaining 83 patients only during the acute phase, with no blood samples collected during the stable phase. Furthermore, 200 age- and sex-matched HCs were enrolled (female:male ratio, 182:18; 44.8 ± 11.4 years).
Figure. sCK levels and log sCK levels of the same patients in acute and stable phases.
(A) sCK levels of the same patients in acute and stable phases; sCK levels of 4 patients at the acute phase are not shown (2,787, 3,610, 4,681, and 1,666 IU/L). (B) Log sCK levels of the same patients at the acute and stable phases. sCK = serum creatine kinase.
Analysis of the log sCK levels.
Comparisons among HCs, acute phase, and stable phase patients.
In the unadjusted model (ANOVA and the Scheffe post hoc test), the mean log sCK levels of the patients with NMOSD in the acute phase were significantly higher (4.51 ± 1.17, n = 85) than those of the patients with NMOSD in the stable phase (3.85 ± 0.81, n = 85, p = 0.000). In addition, the log sCK levels of the patients with NMOSD in the stable phase were significantly lower than those of the HCs (4.31 ± 0.39, n = 200, p = 0.000). However, the log sCK levels of the HCs and patients with NMOSD in the acute phase were not significantly different (p = 0.137). After adjusting for age and sex in the multivariable linear regression model, we found that the adjusted mean log sCK levels were significantly higher in the samples from the patients with NMOSD in the acute phase than those from HCs (p = 0.032). In addition, the adjusted mean log sCK levels of the samples from the patients with NMOSD in the acute phase (p = 0.000) and HCs (p = 0.000) were both significantly higher than those from patients with NMOSD in the stable phase (table 2).
Table 2.
Log sCK levels of samples from HCs or patients with NMOSD in the acute phase or stable phase
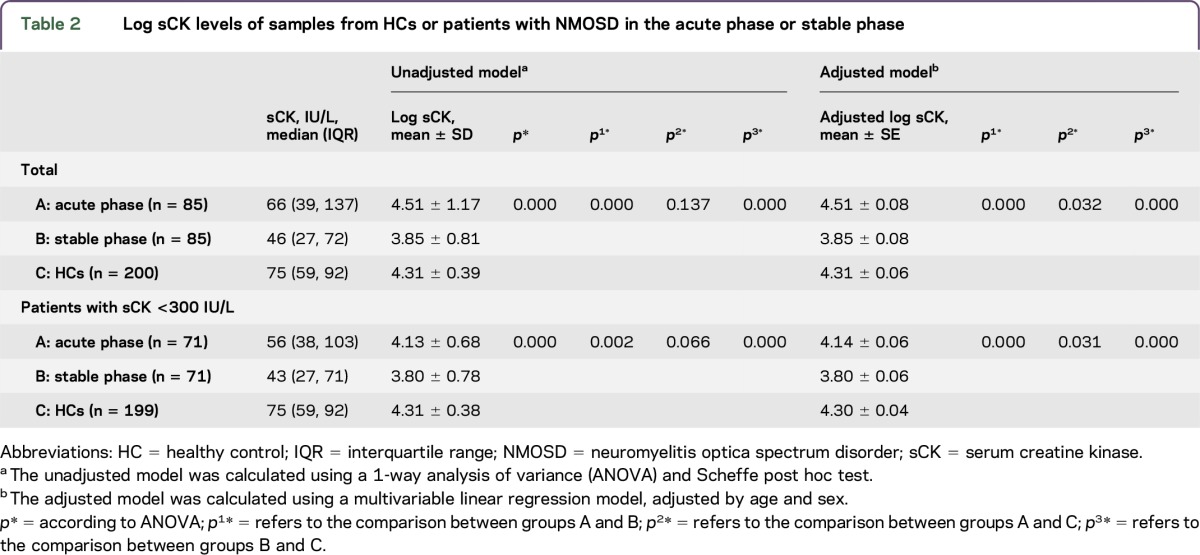
To eliminate the influence of huge increases in sCK, we excluded patients with NMOSD and HCs whose sCK levels were higher than 300 IU/L and compared the patients with log sCK levels within the normal limits at different clinical statuses (table 2). We found that the log sCK levels of patients with NMOSD in the acute phase were significantly higher than those of patients in the stable phase (unadjusted model, p = 0.002; adjusted models, p = 0.000). The log sCK levels of the patients with NMOSD in the stable phase were significantly lower than those from the HCs (p = 0.000 for both unadjusted and adjusted models). Moreover, the log sCK levels of the patients with NMOSD in the acute phase were significantly lower than those from the HCs (unadjusted model, p = 0.066; adjusted models, p = 0.031) (table 2).
The log sCK levels of patients with acute-phase NMOSD.
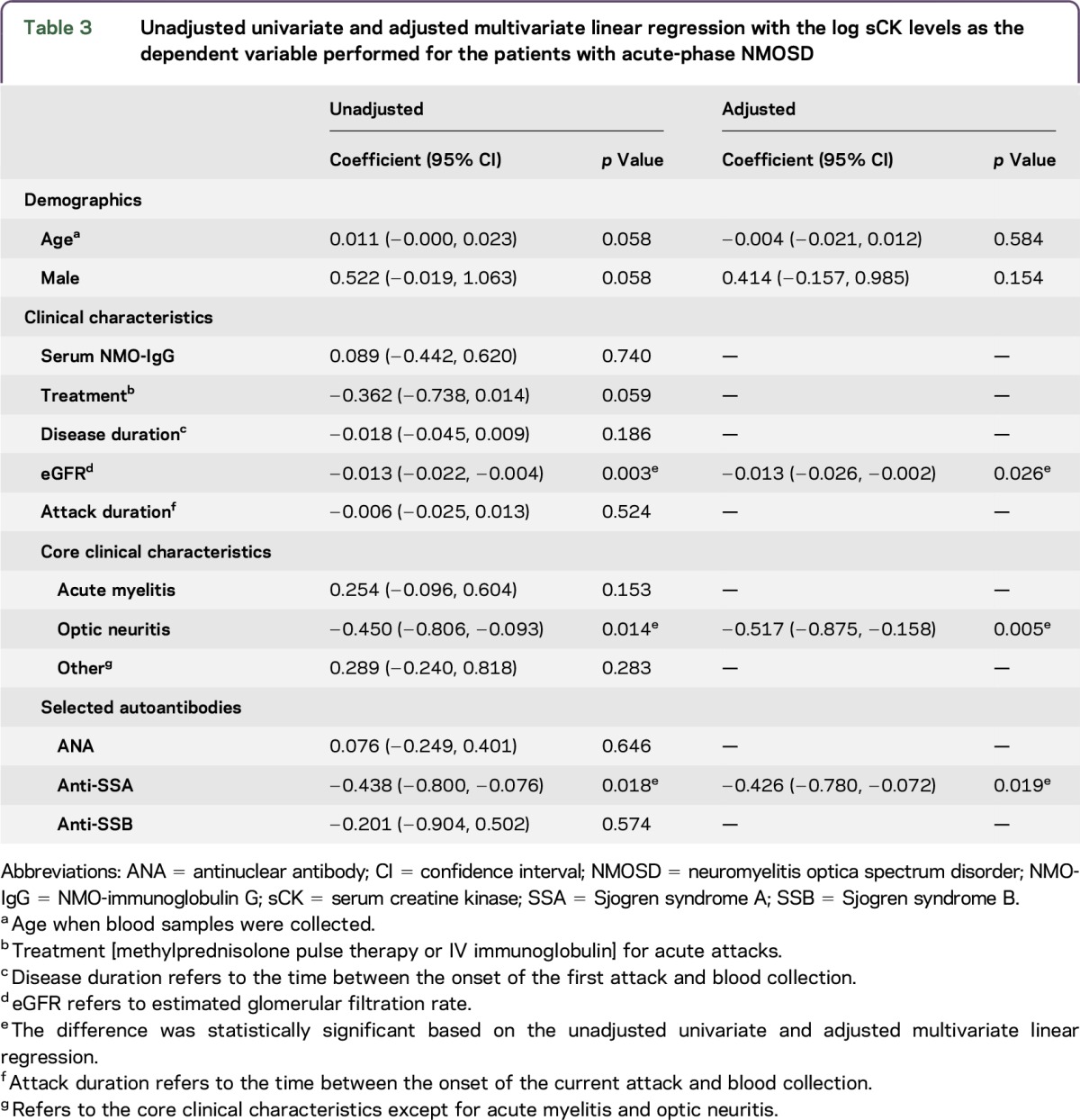
To determine the associations between the demographic and clinical characteristics and the log sCK levels, unadjusted univariate and adjusted multivariable linear regression models were performed for the patients with acute-phase NMOSD. The independent predictors of higher log sCK levels that remained significant in the adjusted analysis were eGFR (p = 0.026), optic neuritis (p = 0.005), and anti-SSA (p = 0.019). A higher eGFR predicted lower log sCK levels (coef. = −0.013; 95% CI: −0.026, −0.002). Patients with the core clinical characteristics of optic neuritis (coef. = −0.517; 95% CI: −0.875, −0.158) had lower log sCK levels. Serum anti-SSA positivity predicted lower log sCK levels (coef. = −0.426; 95% CI: −0.780, −0.072). However, sex, age, serum NMO-IgG status, treatment for acute attacks, disease duration, duration from relapse onset to blood collection, acute myelitis, other core clinical characteristics except acute myelitis and optic neuritis, and serum ANA and anti-SSB status failed to predict changes in log sCK levels (p > 0.05) (table 3).
Table 3.
Unadjusted univariate and adjusted multivariate linear regression with the log sCK levels as the dependent variable performed for the patients with acute-phase NMOSD
DISCUSSION
Previous studies4 have confirmed that muscle damage should be considered a component of NMOSD. Muscle biopsies from patients with NMOSD have revealed mild inflammatory exudates and scattered myofibers with internal nuclei, atrophy, and regeneration, but no necrosis. In addition, the sarcolemma exhibited the loss of AQP4 and the deposition of IgG and complement activation products.5,10 However, other than several case reports of hyperCKemia,5–11 no studies have investigated serum CK levels in NMOSD. In the present study, we first found that the sCK levels of patients in the acute phase of NMOSD were significantly higher than those of NMOSD patients in the stable phase. In addition, during the acute phase of NMOSD, eGFR, anti-SSA, and the core clinical characteristics of optic neuritis were associated with the sCK levels.
As previously mentioned,5,10 the deposition of AQP4-specific IgG and the loss of AQP4 are alternative primary or secondary causes of elevated CK levels. AQP4 is anchored in the sarcolemma, and the major CK isoform of muscle is located in the cytoplasm and is largely not bound to the cytoskeleton. Thus, the loss of AQP4 could result in structural disorganization of the sarcolemma and leakage of CK into the serum. On the other hand, in bioenergetic pathways, calcium homeostasis is particularly perturbed in fast-twitch muscle fibers, which highly express AQP4. IgG-induced loss of AQP4 could lead to metabolic damage and CK leakage.5
As Jarius et al.13 reported, the AQP4-Ab serum levels of patients with NMOSD correlate with the clinical disease activity, with relapses being preceded by an up to 3-fold increase in AQP4-Ab titers. It is plausible that the increase of AQP4-Ab with relapse aggravates structural and metabolic myofibre damage in fast-twitch muscle fibers, causing leakage of CK into the serum. In the stable phase, the decrease of AQP4-Ab relieves muscle damage.
However, according to the multivariable linear regression model performed for patients with NMOSD in the acute phase, seropositive NMO-IgG was not statistically associated with increased sCK levels. This may be due to the small sample size of patients with seronegative NMO-IgG (n = 16). In addition, we failed to test the serum NMO-IgG titers each time we measured the sCK levels; instead, serum NMO-IgG was only tested once for each patient. Further studies regarding the relationship between serum NMO-IgG and sCK levels are needed.
Furthermore, there may have been other mechanisms involved in the observed results. First, in our report, higher eGFR predicted lower sCK levels. To date, little is known about the relationship between sCK levels and GFR in humans, although sCK has a molecular mass of approximately 86 KDa, whereas the physiologic threshold for glomerular filtration is approximately 65 KDa. However, previous studies14,15 and the present study show that sCK levels might be related to GFR. Second, treatment may also affect sCK levels. After MPT or IVIG during the acute phase, patients with NMOSD had lower serum CK levels (coef. = −0.362; p = 0.059), which is consistent with previous studies.16,17 However, it remains unclear whether this led to lower sCK activity or only reflects a need for higher doses of steroids because the disease was more active.
In addition, we found that patients with NMOSD in the stable phase had lower sCK levels than HCs. Even in patients with NMOSD in the acute phase, whose sCK levels were within the normal limits, had lower sCK levels than the HCs. This phenomenon may be attributed to systemic lupus erythematosus (SLE), Sjögren syndrome (SS), and other inflammatory rheumatic diseases that may coexist with NMOSD. Several studies have observed low sCK activity in some inflammatory rheumatic diseases, such as SS, SLE,15,16,18 etc., which was consistent with our report. The present study found that serum anti-SSA positivity predicted lower log sCK levels.
In conclusion, the present study indicates that muscle damage occurs in patients with NMOSD and is aggravated during the acute phase, even when sCK levels are within the normal limits. However, further studies are needed to determine the exact mechanisms.
GLOSSARY
- ANOVA
analysis of variance
- AQP4
aquaporin 4
- CI
confidence interval
- coef.
coefficient
- eGFR
estimated glomerular filtration rate
- HC
healthy control
- IQR
interquartile range
- IVIG
IV immunoglobulin
- MPT
methylprednisolone pulse therapy
- NMO-IgG
NMO-immunoglobulin G
- NMOSD
neuromyelitis optica spectrum disorder
- sCK
serum creatine kinase
- SLE
systemic lupus erythematosus
- SS
Sjögren syndrome
AUTHOR CONTRIBUTIONS
Hong-xi Chen: study design, data collection, and manuscript writing. Qin Zhang: study design, data collection, and statistic analysis. Zhi-yun Lian, Ju Liu, Zi-yan Shi, Xiao-hui Miao, Hui-ru Feng, Qin Du, Jing-lu Xie, and Shao-li Yao: data collection. Hong-yu Zhou: manuscript writing.
STUDY FUNDING
This work was supported by the National Natural Science Foundation of China (Grant No. 81271321) and the Department of Science and Technology of Sichuan Province of China (Grant No. 2013FZ0015).
DISCLOSURE
The authors report no disclosures. Go to Neurology.org/nn for full disclosure forms.
REFERENCES
- 1.Wingerchuk DM, Banwell B, Bennett JL, et al. . International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015;85:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verkman AS. Aquaporins in clinical medicine. Annu Rev Med 2012;63:303–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wakayama Y. Aquaporin expression in normal and pathological skeletal muscles: a brief review with focus on AQP4. J Biomed Biotechnol 2010;2010:731569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He D, Li Y, Dai Q, et al. . Myopathy associated with neuromyelitis optica spectrum disorders. Int J Neurosci 2016;126:863–866. [DOI] [PubMed] [Google Scholar]
- 5.Guo Y, Lennon VA, Popescu BF, et al. . Autoimmune aquaporin-4 myopathy in neuromyelitis optica spectrum. JAMA Neurol 2014;71:1025–1029. [DOI] [PubMed] [Google Scholar]
- 6.Malik R, Lewis A, Cree BA, et al. . Transient hyperckemia in the setting of neuromyelitis optica (NMO). Muscle Nerve 2014;50:859–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iyer A, Rathnasabapathi D, Elsone L, et al. . Transverse myelitis associated with an itchy rash and hyperckemia: neuromyelitis optica associated with dermatitis herpetiformis. JAMA Neurol 2014;71:630–633. [DOI] [PubMed] [Google Scholar]
- 8.Yokoyama N, Niino M, Takahashi T, Matsushima M, Maruo Y. Seroconversion of neuromyelitis optica spectrum disorder with hyperCKemia: a case report. Eur J Neurol 2012;19:e143. [DOI] [PubMed] [Google Scholar]
- 9.Deguchi S, Deguchi K, Sato K, et al. . HyperCKemia related to the initial and recurrent attacks of neuromyelitis optica. Intern Med 2012;51:2617–2620. [DOI] [PubMed] [Google Scholar]
- 10.Di Filippo M, Franciotta D, Massa R, et al. . Recurrent hyperCKemia with normal muscle biopsy in a pediatric patient with neuromyelitis optica. Neurology 2012;79:1182–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki N, Takahashi T, Aoki M, et al. . Neuromyelitis optica preceded by hyperCKemia episode. Neurology 2010;74:1543–1545. [DOI] [PubMed] [Google Scholar]
- 12.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006;66:1485–1489. [DOI] [PubMed] [Google Scholar]
- 13.Jarius S, Aboul-Enein F, Waters P, et al. . Antibody to aquaporin-4 in the long-term course of neuromyelitis optica. Brain 2008;131:3072–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van der Meulen JH, Kuipers H, Drukker J. Relationship between exercise-induced muscle damage and enzyme release in rats. J Appl Physiol (1985) 1991;71:999–1004. [DOI] [PubMed] [Google Scholar]
- 15.Font J, Ramos-Casals M, Vilas AP, et al. . Low values of creatine kinase in systemic lupus erythematosus. Clinical significance in 300 patients. Clin Exp Rheumatol 2002;20:837–840. [PubMed] [Google Scholar]
- 16.Sanmarti R, Collado A, Gratacos J, et al. . Reduced serum creatine kinase activity in inflammatory rheumatic diseases. J Rheumatol 1996;23:310–312. [PubMed] [Google Scholar]
- 17.Lee YH, Choi SJ, Ji JD, Song GG. Serum creatine kinase in patients with rheumatic diseases. Clin Rheumatol 2000;19:296–300. [DOI] [PubMed] [Google Scholar]
- 18.Wei N, Pavlidis N, Tsokos G, Elin RJ, Plotz PH. Clinical significance of low creatine phosphokinase values in patients with connective tissue diseases. JAMA 1981;246:1921–1923. [PubMed] [Google Scholar]