Abstract
Background
The Inter-professional Spine Assessment and Education Clinics (ISAEC) were developed to improve primary care assessment, education and management of patients with persistent or recurrent low back pain–related symptoms. This study aims to determine the effect of ISAEC on access for surgical assessment, referral appropriateness and efficiency for patients meeting a priori referral criteria in rural, urban and metropolitan settings.
Methods
We conducted a retrospective review of prospective data from networked ISAEC clinics in Thunder Bay, Hamilton and Toronto, Ontario. For patients meeting surgical referral criteria, wait times for surgical assessment, surgical referral–related magnetic resonance imaging (MRI) scans and appropriateness of referral were recorded.
Results
Overall 422 patients, representing 10% of all ISAEC patients in the study period, were referred for surgical assessment. The average wait times for surgical assessment were 5.4, 4.3 and 2.2 weeks at the metropolitan, urban and rural centres, respectively. Referral MRI usage for the group decreased by 31%. Of the patients referred for formal surgical assessment, 80% had leg-dominant pain and 96% were deemed appropriate surgical referrals.
Conclusion
Contrary to geographic concentration of health care resources in metropolitan settings, the greatest decrease in wait times was achieved in the rural setting. A networked, shared-cared model of care for patients with low back pain–related symptoms significantly improved access for surgical assessment despite varying geographic practice settings and barriers. The greatest reductions were noted in the rural setting. In addition, significant improvements in referral appropriateness and efficiency were achieved compared with historical reports across all sites.
Abstract
Contexte
Les cliniques interprofessionnelles d’évaluation de la colonne vertébrale et d’éducation (Inter-professional Spine Assessment and Education Clinics [ISAEC]) ont été mises sur pied pour améliorer les soins primaires d’évaluation, d’éducation et de prise en charge des patients atteints de symptômes persistants ou récurrents de lombalgie. Cette étude a pour but d’évaluer l’effet des ISAEC sur l’accès à une évaluation chirurgicale et sur la pertinence et l’efficacité de la référence des patients en milieux ruraux, urbains et métropolitains répondant a priori aux critères de référence.
Méthodes
Nous avons mené une étude rétrospective de données prospectives issues de cliniques du réseau des ISAEC situées à Thunder Bay, à Hamilton et à Toronto, en Ontario. Nous avons retenu pour l’étude les patients répondant aux critères de référence en chirurgie; pour chacun de ces patients, nous avons consigné le temps d’attente pour obtenir une évaluation chirurgicale, les images obtenues par résonance magnétique (IRM) aux fins de référence et la pertinence de la référence.
Résultats
Au total, 422 patients, soit 10 % des patients des ISAEC au cours de la période étudiée, ont été dirigés en évaluation chirurgicale. Les temps d’attente moyens pour obtenir une évaluation chirurgicale étaient de 5,4 semaines, de 4,3 semaines et de 2,2 semaines dans les centres métropolitains, urbains et ruraux, respectivement. Le recours à l’IRM aux fins de référence a diminué de 31 % par rapport à la situation initiale. Parmi les patients référés en évaluation chirurgicale formelle, 80 % présentaient une douleur principalement localisée dans les jambes. La référence de 96 % des patients a été jugée adéquate.
Conclusion
Même si les ressources en soins de santé sont concentrées en milieu métropolitain, c’est le milieu rural qui a connu la plus grande baisse du temps d’attente. La mise sur pied d’un modèle de soins partagés en réseau pour les patients aux prises avec des symptômes de lombalgie a amélioré l’accès aux évaluations chirurgicales de façon significative, malgré la variété géographique des milieux de pratique et les divers obstacles rencontrés. Les baisses les plus importantes ont été observées en milieu rural. De plus, des améliorations significatives de la pertinence et de l’efficacité des références ont été observées lors de la comparaison avec les rapports antérieurs, pour tous les sites de l’étude.
Low back pain (LBP) has a global 1-month prevalence of 23%, with estimates of a North American lifetime incidence of 80%.1,2 In light of a high potential for chronicity,3,4 negative impact on productivity,5 inappropriate health care utilization6,7 and inefficiencies in care delivery, LBP is increasingly the target of studies to reduce its burden on the health care system. 8,9
In Canada, the traditional referral process from a primary care provider to a spine surgeon has many regional barriers and inefficiencies.10,11 The majority of surgeons report wait times from primary care referral to consultation for nonurgent spinal conditions in excess of 6 months despite a substantial proportion closing their practices to new referrals or screening them.10 Approximately 75%–85% of patients referred to a spine surgeon in Canada are not surgical candidates.12–14 As a result, the majority of Canadian surgeons require advanced imaging (77% require magnetic resonance imaging [MRI] of the spine) prior to referral in an attempt to screen out nonsurgical patients).11
To improve access, referral and imaging appropriateness and delivery of care for both surgical and nonsurgical patients, several spine models of care have recently been established in Ontario, Saskatchewan, Quebec and Manitoba. 5,13,15 These models have used nurse practitioners, chiropractors and physical therapists as adjunctive members of the medical team to assess and educate patients referred by their primary care providers and in some scenarios as a screening tool for surgical referral (i.e., serving a triage function). Initial reports have shown a reduction in wait times and MRI utilization, and an increase in the surgical conversion rate.16,17
The purpose of this study was to examine the effect of the Inter-professional Spine Assessment and Education Clinics (ISAEC), Ontario’s low back shared-care model, on surgical referral MRI utilization rates, surgical assessment wait times, and referral appropriateness and efficiency as compared with historical practices in rural (northern), urban, and metropolitan settings.
Methods
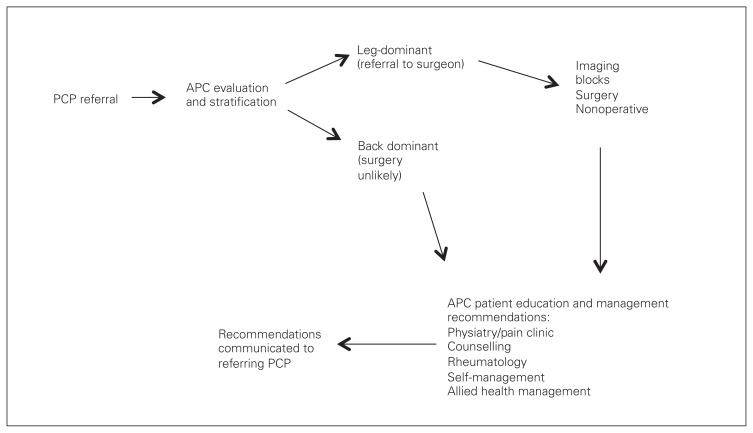
The ISAEC was established in Ontario in November 2012 with funding from the Ontario Ministry of Health and Long-Term Care to enable shared-care management of LBP among primary care providers (doctors and nurse practitioners), allied health providers (physiotherapists and chiropractors) and specialists (surgeons, pain specialists and rheumatologists). The model utilizes a network structure among primary care providers, specially trained physiotherapists and chiropractors (advanced-practice clinicians [APCs]) and specialists to deliver evidence-based LBP assessment, education and care recommendations; timely access; and support to enable patients to self-manage LBP. Purposeful operational variation regarding method and location of service delivery was instituted to meet regional and geographic needs (e.g., community v. centrally located APCs, telemedicine). However, to provide a consistent and reproducible assessment, stratification and patient/provider education process, all providers in the ISAEC network undergo a standardized and relevant education process (continuing medical education [CME] and non-CME training). Further details on ISAEC processes and education are available at www.ISAEC.org. For the present study, 386 participating primary care providers referred patients with LBP for assessment when they had unmanageable, persistent LBP for more than 6 weeks but less than 52 weeks, or recurrent LBP (regardless of duration) that had become unmanageable. Exclusion criteria were diagnosed pain disorder, narcotic dependency (i.e., actively treated by a specialist), pregnancy or postpartum less than a year, and presence of red flags in the patient presentation (e.g., fever/chills, symptoms of cauda equine syndrome, tumour, history of intravenous drug use). Figure 1 shows the pattern of surgical referral in the ISAEC model.
Fig. 1.
Inter-professional Spine Assessment and Education Clinics (ISAEC) referral pattern. APC = advanced-practice clinician; PCP = primary care provider.
Patients were seen at ISAEC by APCs, who are physical therapists and chiropractors with additional clinical training specific to the multidisciplinary aspects (e.g., surgical referral criteria and clinical assessment) of LBP assessment and management. Comprehensive baseline clinical information was obtained, and the patients were categorized into 1 of 4 clinical mechanical LBP presentations based on the classification system proposed by Hall and colleagues.18,19 Pattern 1 was back-dominant pain exacerbated by flexion. Pattern 2 was back-dominant pain aggravated by extension but not increased with flexion. Pattern 3 was leg-dominant pain that is constant with or without neurologic manifestations (i.e., radiculopathy, typically caused by a symptomatic disc herniation). Pattern 4 was leg-dominant pain that is intermittent, aggravated by extension and relieved by rest and/or flexion (i.e., neurogenic claudication, typically due to significant spinal stenosis). Patients were also stratified by presence or absence of risk factors for surgical or nonsurgical red flags (e.g., inflammatory LBP, narcotic dependency and chronicity risk).4,20,21
If patients were deemed by the APCs to be a surgical candidate (i.e., meeting a priori criteria for surgical referral: patients with leg-dominant pain, or neurologic symptoms or signs), they were sent on for surgical assessment at the centre of the ISAEC network spine surgeon. Each ISAEC site had only 1 network spine surgeon. Prior to APC or surgical assessment, primary care providers had the discretion of ordering an MRI (based on ISAEC education recommendations); however, this was not a requirement for surgical referral as it has been in the pre-ISAEC practices of the 3 network ISAEC surgeons. At surgical assessment, if a patient was deemed to be a surgical candidate and was interested in surgical treatment, required imaging (if not already completed) was organized and the standard perioperative surgical process of the surgeon was initiated. The number and type of imaging, the presenting pain pattern, the referral appropriateness for surgery, and the wait time from primary care provider referral to assessment at ISAEC were recorded at the initial assessment and after the surgeons’ assessment. Referral appropriateness was defined as current or past presenting symptoms and signs amenable to surgical intervention. For example, a patient with resolved or partially resolved radiculopathy from a disc herniation treated nonsurgically or a surgically appropriate patient not interested in pursing surgery following a discussion about the risks and benefits of surgery at the time of surgical assessment was still categorized as an appropriate surgical referral.
Statistical analysis
We used descriptive statistics and analysis of variance (ANOVA) to describe and compare differences between the subpopulation of primary care LBP patients seen at the various ISAEC centres who were deemed to be surgical candidates and seen by an ISAEC network spine surgeon. We considered results to be significant at p < 0.05.
Results
The study group consisted of 422 consecutive potential surgical candidates (out of 4059 patients seen by ISAEC) with nonemergent low back and/or leg symptoms assessed by APCs in Toronto, Hamilton and Thunder Bay and were referred for surgical consultation between January 2013 and August 2015. The number of patients referred was 166 at the Toronto centre, 83 in Hamilton and 173 in Thunder Bay. Wait times for assessment at each centre were 5.4, 4.3 and 2.4 weeks, respectively. Differences in wait times among the centres were statistically significant (p < 0.001).
Of the 422 patients assessed in the various ISAEC locations, 227 (54%) did not have an MRI upon referral to the surgeon by the APC. Of that group, 140 subsequently had MRIs (62%), for a total of 335 MRIs from 422 patients (79%). Table 1 summarizes the MRI utilization data per ISAEC centre.
Table 1.
Summary of MRI utilization
| Centre | No. of patients without MRI before surgical assessment | No. (%) of MRIs ordered | No. of patients with MRI before surgical assessment | Overall MRI utilization, % |
|---|---|---|---|---|
| Toronto | 91 | 40 (44) | 75 | 69 |
| Hamilton | 50 | 34 (68) | 33 | 81 |
| Thunder Bay | 86 | 66 (77) | 87 | 88 |
| Total | 227 | 140 (62) | 195 | 79 |
MRI = magnetic resonance imaging.
The clinical patterns seen at each respective centre are summarized in Table 2. At both the metropolitan and urban centres, the type 3 pattern (i.e., radicular leg pain) predominated the other diagnoses. The type 4 pattern (i.e., claudicant leg symptoms) was the most common in the rural setting.
Table 2.
Summary of diagnostic patterns
| Pain pattern | Centre; no. (%) of patients | |||
|---|---|---|---|---|
| Total | Toronto | Hamilton | Thunder Bay | |
| Pattern 1 | 30 (7) | 7 (4) | 3 (4) | 5 (3) |
| Pattern 2 | 14 (3) | 8 (5) | 4 (5) | 2 (1) |
| Pattern 3 | 230 (55) | 96 (59) | 61 (73) | 73 (45) |
| Pattern 4 | 151 (36) | 53 (32) | 15 (18) | 83 (51) |
Clinical agreement between the surgeon and the APC was 93% overall. Agreement was present for 151 patients in Toronto (96%), 77 patients in Hamilton (94%) and 163 patients (95%) in Thunder Bay. After assessment by the surgeon the number of patients deemed to be appropriate surgical candidates was 153 (92%) in Toronto, 75 (90%) in Hamilton and 168 (97%) in Thunder Bay.
Discussion
The present study shows the ability of a networked shared-care LBP model to improve access and referral appropriateness for surgical spine assessments while eliminating the participating surgeon’s requirement for MRI before acceptance of a referral. Reduction in wait time was the most striking finding in our study. Prior to ISAEC implementation the senior author’s elective wait time was a mean of 6 (range 1–19) months, depending on the referring diagnosis compared with 38 days at the Toronto ISAEC site. Wait times for surgical assessment before implementation of ISAEC at the Hamilton site ranged from 6 to 18 months, with urgent referrals seen in 6 months. At the Thunder Bay site, wait times ranged from 12 to 24 months for elective referrals and varied by case for urgent referrals. Although lengthy, the pre-ISAEC wait times were achieved with periodic closure of practices to new consults and/or refusal to see a percentage of referrals (deemed nonsurgical cases based on clinical referral information and imaging reports) in order to reduce wait times. If all surgical referrals were seen, pre-ISAEC surgical wait times would be longer.22 The reductions in wait times in our study are similar to those found in Saskatchewan; Wilgenbusch and colleagues23 found that after implementation of a multidisciplinary care pathway wait times for surgical assessment dropped from an average of 130 days to 69 days. Recent implementation of a similar pathway in Manitoba has allowed wait times to decrease from an average of 24 months to 30 days (Dr. Michael Johnson, University of Manitoba, Winnipeg, Man., personal communication; 2015).
To our knowledge, our study is the first to show that this shared-care model functions across different regions with distinct system and geographic barriers. With a higher concentration of health care resources in metropolitan and urban areas, we expected wait times in these locations would be the lowest. However, wait times had an unexpected inverse association with the population density of the city in which the ISAEC was located, with the population densities of Toronto, Hamilton and Thunder Bay being 4149/km2, 465/km2 and 47.6/km2, respectively.24 Given the similar number of surgical referrals between the Toronto and Thunder Bay sites, these differences are likely due to 2 effects. The decreased population density in rural settings requires patients to travel longer distances to receive health care; subsequently, a greater effort is made to coordinate all the required tests and consultations. In addition, in the smaller ISAEC clinics in Hamilton and Thunder Bay, there was closer interaction between the surgeons and the APCs. In Thunder Bay, where the APC and surgeon are located in the same clinic, it was not uncommon for the surgeon to see the surgical referrals on the same day that they had been assessed by the APC. As geographical maldistribution of health care providers, especially physicians, toward higher population centres is a ubiquitous problem,25 this finding is particularly relevant to the delivery of health care in less populated regions. In the ISAEC model the majority of patients do not require surgical or specialist assessment, thus wherever possible, APCs are located in proximity to primary care providers, not the specialists (i.e., decentralization of services). In a region like Thunder Bay, where there is a limited number of physiotherapists or chiropractors to train as APCs and the geographical distance between primary care providers and possible APCs was prohibitive, a more centralized service delivery process with the support of telemedicine as required was instituted.
In the Canadian health care system a number of factors increase the wait time to surgical assessment in the traditional pathway. Low back pain is one of the most common presenting symptoms of patients seen by primary care physicians. 26 At present, there is a large number of referrals to spine surgeons with a high proportion of nonsurgical patients; 75%–85% of patients referred to a spine surgeon for assessment are not surgical candidates. With the exception of spine surgical red flags (e.g., cauda equina syndrome), there is substantial variation in surgical decisionmaking for degenerative spinal disorders among spine surgeons.27 The more recent reliance on MRI to differentiate between the different sources of LBP has compounded the problem, as MRI has been shown to be unreliable in detecting pathology that should be managed surgically.7 Consequently, it would be unrealistic to expect primary care providers to achieve a high level of referral appropriateness without an active process like ISAEC. Unfortunately, the impact of a high degree of referrals is the delay in assessment of the 15%–25% of referrals who are surgical candidates and would likely benefit from surgical treatment. This is of concern, as Braybrooke and colleagues3 showed that longer wait time to surgery had a negative impact on patient-reported outcomes following elective spine surgery. The parameters most affected by a longer wait to surgery included physical function and subjective pain severity measures. 3 Basic science research has also solidified the link between effective treatment of LBP and changes in neural structure and function in patients with chronic LBP.28 In other words, the development of what can be irreversible pain-related changes in the brain is time-dependent.29
Our study focused only on the portion of primary care patients with LBP seen by ISAEC APCs who met surgical referral criteria and were sent for formal surgical assessment by the network ISAEC spine surgeon. However, our shared-care model also had benefits for patients who did not require assessment by a surgeon. Of the 4059 patients assessed in the various ISAEC clinics, approximately 90% were deemed to be nonsurgical by the screening APCs. Patients deemed nonsurgical, many of whom would have had to wait to see a specialist (before ISAEC), were assessed faster and received evidenced-based nonoperative treatment recommendations more quickly. Many studies show that a variety of nonoperative treatments are effective for chronic LBP. Psychological interventions, exercise, interdisciplinary rehabilitation, functional restoration, and spinal manipulation have shown statistically significant moderate effects on pain in patients with chronic LBP.30–34 Other patients may simply need reassurance that they do not have serious spine pathology, which would also have been expedited in our care pathway. Although not the focus of this paper, earlier clinical assessment of this group has benefits beyond expediting the pathway for surgical candidates.
Of the 422 patients referred from the ISAEC clinics for surgical assessment, clinical patterns 3 and 4 were the most common, representing 56% and 37%, respectively. This pattern was seen across all centres, as the dominant a priori criteria for surgical referral within the ISAEC network is that of leg-dominant symptoms. This mirrors patterns of diagnosis after triage by APCs in the Saskatchewan Spine Pathway clinics, wherein pattern 3 and pattern 4 represented 25% and 40%, respectively, of the patients assessed and referred for surgical assessment.17 Although the combined rate of 65% represented a significant increase from 15% before implementation of the Saskatchewan Spine Clinics, it still falls below our combined rate of 93% across all ISAEC centres. However, our study consisted of 422 patients referred for surgical assessment compared with 25 patients in the Saskatchewan study, therefore this may represent selection bias. In addition, appropriateness of referrals was improved as a result of the change in the clinical presentation of surgical referrals. Of the total 4059 patients referred to ISAEC only 422 (10%) were deemed to be surgically appropriate after assessment by APCs. Once assessed by the accepting surgeons 93% (Toronto), 85% (Hamilton) and 94% (Thunder Bay) were deemed appropriate surgical candidates. There was also close agreement (90%–93%) between the APCs and surgeons in regards to diagnostic categorization of the patients referred for surgical assessment. The rate of agreement between APCs and surgeons is very high despite the surgeons having no standardized criterion when assessing surgical candidacy. This shows flexibility in our shared-care model to accommodate differences in surgical practices. Our study also shows that a shared-care model can positively influence the type and appropriateness of surgical spine referrals.
The cost savings associated with our multidisciplinary clinic in terms of MRI utilization are potentially substantial. It is common practice for spine surgeons in Canada to require an MRI upon referral.11,12 Faced with a large number of nonsurgical referrals, this requirement may be an attempt to exclude patients with nonsurgical pathology.7 It is also quite common for primary care providers to order MRIs for patients with chronic back pain, possibly owing to worsening, persistent or recurrent symptoms.35 This has contributed to a significant increase in the use of MRI. Between 1993–94 and 2003–04 there was a 600% increase in the number of MRI scans.36 This utilization rate is particularly concerning, as MRIs have failed to show any effect on patient treatment in the majority of patients referred for surgical assessment.7,18,37,38 In the present study 227 patients were assessed at the 3 centres without pre-existing MRIs. Of those, 87 (39%) MRIs were not ordered after assessment. In the simplest manner, this represents a cost savings of $87 000 based on the cost of an MRI being $1000. Of the 422 patients referred for surgical assessment, 195 patients had pre-existing MRIs ordered by their primary care physicians. Estimating conservatively that 25% of the MRIs the patients obtained before assessment at the ISAEC would not have been ordered if they had first been seen at the ISAEC, another 49 MRIs may have been avoided, for a total potential savings of $136 000. It must be noted that the modest reduction in MRI utilization occurred in a population of referrals among whom more than 90% were deemed appropriate surgical candidates. Actual cost savings would likely be higher if the care model was applied to all initial referrals to ISAEC. Indeed, in current practice, where a large number of referrals are not considered surgical candidates,10 the senior author has previously shown that effective surgical spine triage could result in annual spine imaging–related cost avoidance of $24 million in Ontario.22
Limitations
This study has several limitations, 1 of which is its retrospective analysis of prospective data. Costs were estimated only in respect to MRI utilization rather than a systemic analysis of total costs of our shared-care model per spine patient seen in both the surgical and nonsurgical treatment arms. Unfortunately gathering health utilization data is difficult, even when considering the small subset of patients who go on to surgical management. In regards to nonoperative management, there is currently no practical method to estimate health care costs. In regards to patient outcome, our study focused on surgical candidacy rather than the actual number of patients who went on to receive surgery or to be placed on a surgical wait list. Our analysis assessed surgical candidacy upon initial referral. It is likely that nonoperative management failed in some of the patients who were deemed nonsurgical and that these patients were reassessed and offered surgical management at a subsequent date. Although we know our model produces a high level of agreement between the APC and surgeon in regards to surgical candidacy and diagnostic categorization for patients referred to the surgeon, these factors are not known in the 90% of patients who were deemed nonsurgical by the APC. Our shared-care model is specific, but we don’t know its sensitivity as the study assesses only the cohort of patients considered to be surgical candidates at each centre.
Despite the limitations, our study mirrors the findings of other groups, both in Canada and abroad, that a shared-care model for patients with spine pathology can expedite assessment and diagnosis, reduce costs, and streamline the delivery of care. The fact that these results occurred in metropolitan, urban and rural settings expands their applicability.
Conclusion
The ISAEC spine model of care, which is a shared-care interprofessional model with a stratified approach to LBP assessment, self-management and care recommendations, is adaptable and functions well in metropolitan, urban and rural settings. In all regions, ISAEC has resulted in a dramatic decrease in wait times for surgical assessment, improved referral appropriateness and efficiency of MRI utilization. A high level of APC and surgeon agreement was achieved both in terms of clinical categorization and surgical candidacy across settings and makes this interprofessional model particularly relevant to areas suffering from shortages of specialist surgical care.
Footnotes
Presented at the 2016 Canadian Spine Society Scientific Meeting, Feb. 24–27, Whistler, BC.
Competing interests: None declared.
Contributors: R. Rampersaud designed the study. A. Bidos, C. Fanti, B. Young, B. Drew and D. Puskas acquired the data, which M. Zarrabian, A. Bidos and R. Rampersaud analyzed. M. Zarrabian and R. Rampersaud wrote the article, which all authors reviewed and approved for publication.
References
- 1.World Health Organization. The burden of musculoskeletal conditions at the start of the new millennium. Switzerland: WHO; 2003. [PubMed] [Google Scholar]
- 2.Hoy D, Bain C, Williams G, et al. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012;64:2028–37. doi: 10.1002/art.34347. [DOI] [PubMed] [Google Scholar]
- 3.Braybrooke J, Ahn H, Gallant A, et al. The impact of surgical wait time on patient-based outcomes in posterior lumbar spinal surgery. Eur Spine J. 2007;16:1832–9. doi: 10.1007/s00586-007-0452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill JC, Whitehurst DG, Lewis M, et al. Comparison of stratified primary care management for low back pain with current best practice (STarT Back): a randomised controlled trial. Lancet. 2011;378:1560–71. doi: 10.1016/S0140-6736(11)60937-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris SA, Rampersaud Y. The importance of identifying and modifying unemployment predictor variables in the evolution of a novel model of care for low back pain in the general population. Spine J. 2016;16:16–22. doi: 10.1016/j.spinee.2015.09.034. [DOI] [PubMed] [Google Scholar]
- 6.Srinivas SV, Deyo RA, Berger ZD. Application of “less is more” to low back pain. Arch Intern Med. 2012;172:1016–20. doi: 10.1001/archinternmed.2012.1838. [DOI] [PubMed] [Google Scholar]
- 7.You JJ, Bederman SS, Symons S, et al. Patterns of care after magnetic resonance imaging of the spine in primary care. Spine. 2013;38:51–9. doi: 10.1097/BRS.0b013e3182611182. [DOI] [PubMed] [Google Scholar]
- 8.Paskowski I, Schneider M, Stevans J, et al. A hospital-based standardized spine care pathway: report of a multidisciplinary, evidence-based process. J Manipulative Physiol Ther. 2011;34:98–106. doi: 10.1016/j.jmpt.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Klein BJ, Radecki RT, Foris MP, et al. Bridging the gap between science and practice in managing low back pain: a comprehensive spine care system in a health maintenance organization setting. Spine. 2000;25:738–40. doi: 10.1097/00007632-200003150-00015. [DOI] [PubMed] [Google Scholar]
- 10.Busse JW, Riva JJ, Nash JV, et al. Surgeon attitudes toward non-physician screening of low back or low back–related leg pain patients referred for surgical assessment: a survey of Canadian spine surgeons. Spine. 2013;38:E402–8. doi: 10.1097/BRS.0b013e318286c96b. [DOI] [PubMed] [Google Scholar]
- 11.Busse JW, Riva J, Rampersaud R, et al. Spine surgeons’ requirements for imaging at the time of referral: a survey of Canadian spine surgeons. Can J Surg. 2014;57:E25–30. doi: 10.1503/cjs.003713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deis N, Findlay JM. Appropriateness of lumbar spine referrals to a neurosurgical service. Can J Neurol Sci. 2010;37:843–8. doi: 10.1017/s0317167100051544. [DOI] [PubMed] [Google Scholar]
- 13.Fourney DR, Dettori JR, Hall H, et al. A systematic review of clinical pathways for lower back pain and introduction of the Saskatchewan spine pathway. Spine. 2011;36:S164–71. doi: 10.1097/BRS.0b013e31822ef58f. [DOI] [PubMed] [Google Scholar]
- 14.Mayman D, Yen D. Maximizing use of a surgical clinic for referrals of patients having back problems. Can J Surg. 1999;42:117. [PMC free article] [PubMed] [Google Scholar]
- 15.McGill University. McGill Spine Care Algorithms. 2010. [accessed 2016 Jun 11]. Available: www.mcgill.ca/spineprogram/algorithms.
- 16.Rampersaud RY, Bidos A, Fanti CL, et al. Preliminary report from the Ontario Interprofessional Spine Assessment and Education Clinics (ISAEC) Spine J. 2014;14:S40. [Google Scholar]
- 17.Kindrachuk DR, Fourney DR. Spine surgery referrals redirected through a multidisciplinary care pathway: effects of nonsurgeon triage including MRI utilization: clinical article. J Neurosurg Spine. 2014;20:87–92. doi: 10.3171/2013.10.SPINE13434. [DOI] [PubMed] [Google Scholar]
- 18.Hall H, McIntosh G, Boyle C. Effectiveness of a low back pain classification system. Spine J. 2009;9:648–57. doi: 10.1016/j.spinee.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 19.Wilson L, Hall H, McIntosh G, et al. Intertester reliability of a low back pain classification system. Spine. 1999;24:248–54. doi: 10.1097/00007632-199902010-00009. [DOI] [PubMed] [Google Scholar]
- 20.Sieper J, van der Heijde D, Landewe R, et al. New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS) Ann Rheum Dis. 2009;68:784–8. doi: 10.1136/ard.2008.101501. [DOI] [PubMed] [Google Scholar]
- 21.Furlan AD, Reardon R, Weppler C National Opioid Use Guideline Group (NOUGG) Opioids for chronic noncancer pain: a new Canadian practice guideline. CMAJ. 2010;182:923–30. doi: 10.1503/cmaj.100187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JSM, Dong JZ, Brener S, et al. Cost-effectiveness analysis of a reduction in diagnostic imaging in degenerative spinal disorders. Healthc Policy. 2011;7:e105–21. [PMC free article] [PubMed] [Google Scholar]
- 23.Wilgenbusch CS, Wu AS, Fourney DR. Triage of spine surgery referrals through a multidisciplinary care pathway: a value-based comparison with conventional referral processes. Spine. 2014;39:S129–35. doi: 10.1097/BRS.0000000000000574. [DOI] [PubMed] [Google Scholar]
- 24.Statistics Canada. Statistics Canada: 2011 Census Profile. 2012. [accessed 2016 Apr 24]. Available: www12.statcan.ca/census-recensement/2011/dp-pd/prof/details/page.cfm?Lang=E&Geo1=CSD&Code1=3558004&Geo2=CD&Code2=3558&Data=Count&SearchText=Thunder&SearchType=Begins&SearchPR=01&B1=All&Custom=&TABID=1.
- 25.Pong RW. Strategies to overcome physician shortages in northern Ontario: a study of policy implementation over 35 years. Hum Resour Health. 2008;6:24. doi: 10.1186/1478-4491-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simon D, Coyle M, Dagenais S, et al. Potential triaging of referrals for lumbar spinal surgery consultation: a comparison of referral accuracy from pain specialists, findings from advanced imaging and a 3-item questionnaire. Can J Surg. 2009;52:473. [PMC free article] [PubMed] [Google Scholar]
- 27.Irwin ZN, Hilibrand A, Gustavel M, et al. Variation in surgical decision making for degenerative spinal disorders. Part I: lumbar spine. Spine. 2005;30:2208–13. doi: 10.1097/01.brs.0000181057.60012.08. [DOI] [PubMed] [Google Scholar]
- 28.Seminowicz DA, Wideman TH, Naso L, et al. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J Neurosci. 2011;31:7540–50. doi: 10.1523/JNEUROSCI.5280-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lynch ME, Campbell F, Clark AJ, et al. A systematic review of the effect of waiting for treatment for chronic pain. Pain. 2008;136:97–116. doi: 10.1016/j.pain.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 30.Schonstein E, Kenny D, Keating J, et al. Physical conditioning programs for workers with back and neck pain: a Cochrane systematic review. Spine. 2003;28:E391–5. doi: 10.1097/01.BRS.0000092482.76386.97. [DOI] [PubMed] [Google Scholar]
- 31.McNeely ML, Torrance G, Magee D. A systematic review of physiotherapy for spondylolysis and spondylolisthesis. Man Ther. 2003;8:80–91. doi: 10.1016/s1356-689x(02)00066-8. [DOI] [PubMed] [Google Scholar]
- 32.Hayden JA, Van Tulder MW, Malmivaara A, et al. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst Rev. 2005:CD000335. doi: 10.1002/14651858.CD000335.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chou R, Huffman LH. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:492–504. doi: 10.7326/0003-4819-147-7-200710020-00007. [DOI] [PubMed] [Google Scholar]
- 34.Assendelft WJ, Morton SC, Yu EI, et al. Spinal manipulative therapy for low-back pain. Cochrane Database Syst Rev. 2004 doi: 10.1002/14651858.CD000447.pub2. [DOI] [PubMed] [Google Scholar]
- 35.Matzkin E, Smith MEL, Freccero CD, et al. Adequacy of education in musculoskeletal medicine. J Bone Joint Surg Am. 2005;87:310–4. doi: 10.2106/JBJS.D.01779. [DOI] [PubMed] [Google Scholar]
- 36.Busse J, Alexander PE, Abdul-Razzak A, et al. Appropriateness of spinal imaging use in Canada. Ottawa (ON): Canadian Institutes of Health Research; 2013. [Google Scholar]
- 37.Chou R, Deyo RA, Jarvik JG. Appropriate use of lumbar imaging for evaluation of low back pain. Radiol Clin North Am. 2012;50:569–85. doi: 10.1016/j.rcl.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Chou R, Qaseem A, Owens DK, et al. Diagnostic imaging for low back pain: advice for high-value health care from the American College of Physicians. Ann Intern Med. 2011;154:181–9. doi: 10.7326/0003-4819-154-3-201102010-00008. [DOI] [PubMed] [Google Scholar]