Abstract
Background
Patients with Alzheimer’s disease (AD) are more prone to seizures and myoclonus, but relative risk of these symptoms among other dementia types is unknown.
Objective
To determine incidence of seizures and myoclonus in the three most common neurodegenerative dementias: AD, dementia with Lewy bodies (DLB), and frontotemporal dementia (FTD).
Methods
Our institution’s medical records were reviewed for new-onset unprovoked seizures and myoclonus in patients meeting criteria for AD (n=1,320), DLB (n=178), and FTD (n=348). Cumulative probabilities of developing seizures and myoclonus were compared between diagnostic groups, whereas age-stratified incidence rates were determined relative to control populations.
Results
The cumulative probability of developing seizures after disease onset was 11.5% overall, highest in AD (13.4%) and DLB (14.7%) and lowest in FTD (3.0%). The cumulative probability of developing myoclonus was 42.1% overall, highest in DLB (58.1%). The seizure incidence rates, relative to control populations, were nearly 10 fold in AD and DLB, and 6 fold in FTD. Relative seizure rates increased with earlier age-at-onset in AD (age <50, 127 fold; 50–69, 21 fold; 70+, 2 fold) and FTD (age <50, 53 fold; 50–69, 9 fold), and relative myoclonus rates increased with earlier age-at-onset in all groups. Seizures began an average of 3.9 years after the onset of cognitive or motor decline, and myoclonus began 5.4 years after onset.
Conclusions
Seizures and myoclonus occur with greater incidence in patients with AD, DLB, and FTD than in the general population, but rates vary with diagnosis, suggesting varied pathomechanisms of network hyperexcitability. Patients often experience these symptoms early in disease, suggesting hyperexcitability could be an important target for interventions.
Keywords: Alzheimer’s disease, dementia with Lewy bodies, epilepsy, frontotemporal dementia
INTRODUCTION
Neurodegenerative disease represents a major cause of seizures in older adults. Seizures are known to occur more frequently in patients with Alzheimer’s disease (AD) than in the general population, despite widely varied estimates [1–3]. Emerging evidence suggests that seizures can accompany AD in earlier stages than previously recognized [4, 5]. Seizures in AD can escape detection because they are often non-motor in nature [6, 7]. Additionally, subclinical epileptiform activity, the occurrence of epileptiform activity in the absence of seizures, can be detected in over 40% of AD patients when they undergo extended neurophysiological monitoring [8]. Both seizures and subclinical epileptiform activity in AD are associated with accelerated cognitive decline [8–10], a phenomenon that could relate to increased amyloid-beta (Aβ) and tau production due to periodic increases in synaptic activity [11–13] as well as chronic compensatory remodeling of neuronal circuits [14, 15]. Previous studies suggest that seizures and myoclonus, which are both signs of network hyperexcitability, could predict shortened survival in AD [16, 17].
Network hyperexcitability in AD has been attributed to aberrant network activity within disease-specific circuits induced by amyloid precursor protein (APP) and its metabolites, most notably Aβ [18]. Recent investigation implicates disease proteins that are not unique to AD in the propagation of aberrant network excitability, such as microtubule-associated protein tau, present in both AD and frontotemporal dementia (FTD) pathology, and α-synuclein, which is associated with Parkinson’s disease (PD) and dementia with Lewy bodies (DLB) [18, 19]. Selective expression of tau containing four repeats in the repeat domain, which is the predominant tau isoform that aggregates in progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD), worsens functional outcome in mice compared to three-repeat tau, possibly through increases in network excitability [20]. Overexpression of tau with mutations linked to FTD or the A152T variant, which is linked to several tauopathies, also increases seizure susceptibility in transgenic mice [21, 22]. Additionally, apolipoprotein E4, the most common risk factor for AD, contributes to network hyperexcitability in animal models by causing tau-dependent decrease in GABAergic interneurons in the hippocampus [23]. Finally, overexpression of α-synuclein is sufficient to cause aberrant network excitability in experimental models of DLB, AD, and comorbidity [24].
The occurrence of network hyperexcitability in dementia has also been explored from the perspective of calbindin-D28K levels, which are reduced in brain regions where chronic over-excitation occurs. Overexpression of APP/Aβ and α-synuclein have each been associated with a reduction in calbindin in the hippocampi of transgenic animal models, and similar changes are observed in postmortem brain samples from patients with AD and DLB [18, 24].
These animal studies strongly implicate numerous disease proteins in the generation of network hyperexcitability in dementia. The relative contribution of each protein and their potential interrelationships remain unknown. Furthermore, the incidence of epileptic activity in patients with non-AD forms of dementia has not yet been analyzed in depth. The rate at which patients with non-AD dementia develop seizures has been suggested to be lower than the rate in AD [25], but the comparison of seizure occurrence has not gone beyond this level of distinction. Our knowledge of the relative rates and timing of onset of myoclonus between dementias is also limited.
Here, we examined the incidence of network hyperexcitability in the forms of seizures and myoclonus in the three most common forms of degenerative dementia syndromes, AD, FTD, and DLB. Comparisons were also conducted between clinical subtypes of each dementia group, including DLB and concurrent AD (DLB-AD) and FTD-spectrum disorders including behavioral variant frontotemporal dementia (bvFTD), semantic variant primary progressive aphasia (svPPA), PSP, corticobasal syndrome due to CBD, and non-fluent variant primary progressive aphasia (nfvPPA). Since network hyperexcitability is associated with faster cognitive decline in patients with AD [8–10] and possibly non-AD dementias, recognizing the risk and typical presentations of new-onset seizures across multiple forms of dementia is important. Correct diagnosis and early treatment of epilepsy in patients with dementia could improve quality of life, and trials will determine if such strategies could help stabilize or delay disease progression [26, 27].
MATERIALS AND METHODS
Patient Selection and Chart Review
Electronic medical records at the UCSF Memory and Aging Center were reviewed for patients evaluated between January 1, 2007 to December 31, 2013 (n=7,925) and who met diagnostic criteria for AD, FTD, or DLB (n=2,408) at their most recent clinical evaluation. Patients lacking sufficient records for review were excluded (n=183), as were those with diagnostic uncertainty who met probable criteria for multiple neurodegenerative diseases (n=333). Patients meeting both probable DLB and probable AD were not excluded due to the high coincidence of these forms of dementia, and were included in the DLB cohort [28, 29]. Patients meeting probable AD and probable corticobasal syndrome criteria were included in the AD cohort [30].
Patients with seizure risk factors were excluded, including those with remote history of seizures (defined as seizure onset at least 10 years prior to symptoms of neurodegenerative disease; n=16) or those with previous seizures provoked by cortical lesions, acute metabolic disorders, or subdural hematomas (n=25). This approach corresponds with exclusion criteria in similar studies investigating seizures in dementia patients [31, 32] Records of patients with seizures were additionally reviewed to ascertain whether patients were taking bupropion or typical antipsychotics at the time of their first unprovoked seizure, given the ability of these medications to lower seizure threshold [33]. One of 78 patients with new-onset seizures included in this study was taking bupropion concurrent with their first seizure, although it was only taken once a week, and none were taking typical antipsychotics. Patients with a history of provoked myoclonus, such as drug-induced (n=5), were excluded. The remaining 1,846 patients were categorized into diagnostic cohorts based on their syndrome: AD (n=1,320), DLB (n=178; consisting of 149 DLB [28] and 29 DLB-AD [28, 29]) cases, and FTD (n=348; consisting of 115 bvFTD [34], 82 svPPA [35], 37 nfvPPA [35], 53 PSP [36], and 61 corticobasal syndrome due to CBD [30, 37]) cases. All subjects classified as AD in the present study met the “probable AD based on clinical criteria” guidelines outlined in McKhann et al. 2011 [29], including subjects diagnosed prior to 2011 who met the McKhann et al. 1984 criteria for probable AD [38]. Patient records were manually reviewed to determine age-at-onset of first neurodegenerative symptoms and date of dementia diagnosis. The neurologists’ evaluations included documentation of standardized forms querying for seizures and myoclonus.
Records were then compiled and searched using an automated text processing script that monitored the occurrence of word stems related to seizures (“seiz”, “epil”, “EEG”, “spell”, “shak”), myoclonus (“myoclon”, “jerk”), cognitive fluctuations (“fluc”), and head trauma (“head”). Records containing indications of possible epileptiform abnormalities were individually reviewed to determine if patients had experienced (a) one or more unprovoked seizures diagnosed by the clinician based on characteristic clinical features and/or epileptiform activity on EEG, (b) suspected but unconfirmed seizures, or (c) little to no clinical suspicion for seizures. Records with indications of myoclonus were reviewed to establish the date of myoclonus onset, defined as the date myoclonus was first stated in records if a specific date was not explicitly reported, and to screen for benign physiological myoclonus such as hypnagogic jerks. Seizures were classified according to established guidelines based on clinical presentation [39]. Inclusion within the seizure-positive group did not necessitate meeting diagnostic criteria for epilepsy, although a majority in this group (70 of 78) were subsequently diagnosed with epilepsy. Suspected but unconfirmed seizures referred to episodic symptoms that were suspicious for seizures to the clinician, but unconfirmed by witness accounts or epileptiform activity on concurrent or subsequent EEG, and did not meet criteria for inclusion as a seizure in the study. Clinical fluctuations in cognition, awareness, or attention, occurring within a single day, were based on either caregiver reports or observations by clinicians.
The electronic database was also searched for biomarker evidence of underlying AD pathology using positron emission tomography (PET) with amyloid-β binding radiotracers, including carbon 11–labeled Pittsburgh Compound B (PIB-PET) and 18F–AV-45 (florbetapir). PET scans were reviewed by a trained neurologist and classified as being either supportive or unsupportive of underlying AD pathology. Amyloid PET positivity in AD and FTD diagnosed participants was 93.5% (86/92) and 8.8% (8/91), respectively. Amyloid PET was performed on three patients with suspected DLB, of which 2 were positive and who were categorized as DLB-AD by clinical criteria. Baseline Mini-Mental State Examination (MMSE) and Clinical Dementia Rating Sum-of-Boxes (CDR-B) scores obtained within 90 days of diagnosis of dementia and MMSE within 90 days of the first unprovoked seizure or myoclonus were extracted from the database when available. Records of patients with seizures were also reviewed for EEG results, including descriptions of epileptiform abnormalities and slowing indicative of cerebral dysfunction.
DNA extracted from whole blood samples was analyzed when available to assess for autosomal-dominant disease causing mutations.[8, 40] The genes included AD-causing mutations (amyloid precursor protein (APP), presenilin 1 (PSEN1), and presenilin 2 (PSEN2)) and FTD-causing mutations (chromosome 9 open reading frame 72 (C9ORF72), FUS RNA binding protein (FUS), granulin precursor (GRN), microtubule-associated protein tau (MAPT), and TAR DNA binding protein (TDP43)). Genetic variants associated with dementia risk and potential genetic disease modifiers were also assessed including apolipoprotein E (APOE) alleles,[8], MAPT Ala152Thr (A152T) substitution,[41] granulin precursor rs5848 allele,[42] MAPT H1/H2 haplotype,[8] transmembrane protein 106B (TMEM106B) rs1990622 allele,[43] and triggering receptor expressed on myeloid cells 2 (TREM2) rs75932628 allele.[44] Genotype data was available in 25–30% of all 1,846 subjects.
Epidemiological Analyses
Cumulative probability curves were calculated for the development of unprovoked seizures and myoclonus according to established Kaplan-Meier failure methods. Person-time contributing to Kaplan-Meier curves was calculated from date of onset of cognitive or motor symptoms until one of two criteria was met: (a) a new-onset seizure or new-onset myoclonus occurred, or (b) the date of the last available physician’s note. Detailed notes from the time of death were unavailable; therefore, patients were censored at last available follow-up.
Incidence rates were calculated for new-onset seizures and myoclonus according to established formulas [45]. Patients contributed a maximum of eight years observed person-time (January 1st 2006 to December 31st 2013) and were censored as described above for cumulative incidence. Rates were then compared to corresponding incidence rates in general populations established by large-scale epidemiological studies of seizures [46] and myoclonus [47] to generate incidence rate ratios (IRR), the ratios of incidence rates in disease groups to incidence rates in age-matched control populations. The onset of seizures and myoclonus in all patient groups tended to cluster near the time of diagnosis. Therefore, patients without seizures or myoclonus were stratified according to age at dementia diagnosis.
Ethics
This study underwent review and was approved by the University of California, San Francisco Committee on Human Research.
Statistical analyses
All statistical analyses were conducted using Stata 14 software, and tests were computed at two-tailed alpha = 0.05, with Bonferroni corrections for multiple comparisons when necessary. Non-parametric tests were applied to compare non-normally distributed demographic and clinical data. Prevalence data was compared using Χ2 test, and post-hoc pairwise comparisons were subsequently carried out applying the generalized linear model. Cumulative incidence curves were compared by age-at-diagnosis-stratified log-rank test to account for the influence of age on the presentation of seizures and myoclonus. Genetic data were tested for associations of autosomal-dominant disease causing mutations with seizures and myoclonus in AD (genes APP, PSEN1, and PSEN2) and FTD (genes C9ORF72, FUS, GRN, MAPT, and TDP43) by Fisher’s exact test. Genetic variants were also tested for associations with seizures and myoclonus across all subjects, as well as within each diagnostic cohort (AD, DLB, and FTD) by Fisher’s exact test correcting for multiple comparisons. A logistic regression model was developed to estimate the independent effects of age-at-onset, diagnosis, and MMSE on the likelihood of developing seizures or myoclonus (see Supplementary Methods).
RESULTS
Demographics
Demographic information of participants is shown in Table 1. Patients with AD were predominately female whereas those with DLB were predominately male. FTD participants were younger at symptom onset and had more years of education compared to AD and DLB participants. Supplementary Table 1 presents demographics of the sample population by secondary diagnosis, including AD, DLB, DLB-AD, svPPA, bvFTD, PSP, CBD, and nfvPPA.
Table 1.
Patient demographics and clinical information
| AD (n = 1,320) | DLB (n = 178) | FTD (n = 348) | p value | |
|---|---|---|---|---|
| Female (%) | 60.5 | 39.3 | 51.2 | < 0.001b |
| Right-handed (%) | 90.9 | 88.8 | 91.6 | 0.557c |
| Education (median (IQR)) | 16 (12 – 18) | 16 (13 – 18) | 16 (14 – 18) | 0.030d |
| Age-at-onset (median (IQR))a | 71 (62 – 78) | 71 (64 – 76) | 61 (56 – 67) | 0.0001e |
| Years from onset to diagnosis (median (IQR)) | 4 (2 – 6) | 4 (2 – 6) | 4 (3 – 6) | 0.378f |
| Age-at-diagnosis (median (IQR)) | 75 (67 – 82) | 74 (68 – 79) | 65 (60– 71) | 0.0001g |
| MMSE nearest diagnosis (median (IQR)) | 22 (18 – 25) | 25 (22 – 27) | 26 (23 – 28) | 0.0001h |
| CDR-B nearest diagnosis (median (IQR)) | 4.5 (3.5 – 6) | 4.5 (3 – 9) | 4 (2 – 7.3) | 0.592f |
Age-at-onset refers to onset of cognitive or motor symptoms.
Χ2 test. All comparisons significantly differ with Χ2 corrected for multiple corrections. AD vs. DLB, p <0.001; DLB vs. FTD, p=0.010; and AD vs. FTD, p=0.002 (Mann-Whitney).
Χ2 test.
Kruskal-Wallis. FTD had more years of education than AD, p=0.011 (Mann-Whitney).
Kruskal-Wallis. Age-at-onset significantly lower in FTD vs. AD, p<0.0001 and DLB, p<0.0001 (Mann-Whitney).
Kruskal-Wallis.
Kruskal-Wallis. Age-at-diagnosis significantly lower in FTD vs. AD, p<0.0001 and DLB, p<0.0001 (Mann-Whitney).
Kruskal-Wallis. MMSE significantly lower in AD vs. DLB, p < 0.0001 and FTD, p < 0.0001. MMSE significantly lower in DLB vs. FTD, p = 0.028 (Mann-Whitney).
AD, Alzheimer’s disease; CDR-B, Clinical Dementia Rating Box Score; DLB, dementia with Lewy bodies; FTD, frontotemporal dementia; IQR, interquartile range; MMSE, Mini-Mental Status Examination.
Incidence of Seizures and Myoclonus Differs by Dementia Type
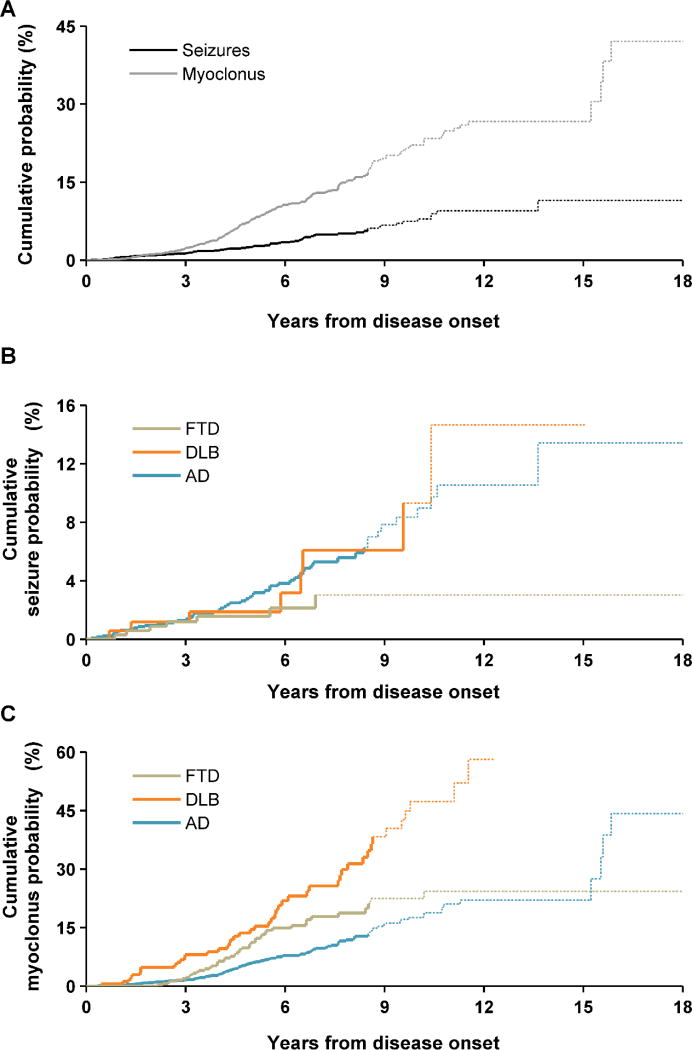
The cumulative probability of developing seizures across diagnosis groups was 11.5%, and cumulative probability of developing myoclonus was 42.1%, as shown in Figure 1A. Figure 1B displays cumulative seizure probability curves of the three primary dementia cohorts. While the prevalence of new-onset seizures and myoclonus did not differ between the three groups (Table 2), their cumulative probabilities were distinct. The lengths of the observation periods in which seizures could occur for cumulative probability analysis did not significantly differ (Kruskal-Wallis p = 0.20; AD median 5.2 years, IQR 3.3 – 7.6; DLB median 5.5 years, IQR 3.7 – 8.3; FTD median 5.5 years, IQR 3.6 – 7.8). The seizure probability reached 13.4% in AD, 14.7% in DLB, and 3.0% in FTD, and the three curves were significantly different. For myoclonus, the cumulative probability was highest in DLB, reaching 58.1%, and the probability curves for the three cohorts were significantly different as well (Figure 1C). Incidence rates of seizures compared to age-matched controls were nearly 10 times higher in patients with AD and DLB, and 6 times higher in patients with FTD (Table 3). The incidence rate of myoclonus was 689 to 1,481 times higher in dementia patients than in age-matched controls.
Figure 1.
Cumulative probability curves of new-onset seizures and myoclonus. (A) Cumulative probability curves for all patients. (B and C) Cumulative probability curves of new-onset seizures (B) and myoclonus (C), by diagnostic cohort (panel B, p=0.005 and panel C, p<0.0001, age-at-diagnosis-stratified log-rank test comparing the three groups). Curves become dotted at the point where 80% of patients are censored.
Table 2.
Prevalence of new-onset seizures and other potential indicators of network hyperexcitability
| New-onset seizures (%) |
Suspected but unconfirmed seizures (%) |
Myoclonus (%) |
Clinical fluctuations (%) |
|
|---|---|---|---|---|
| AD (n = 1,320) | 4.5 | 3.8 | 8.5 | 10.2 |
| DLB (n = 178) | 5.1 | 14.6 | 27.0 | 69.1 |
| FTD (n = 348) | 2.6 | 2.3 | 12.9 | 6.9 |
|
| ||||
| p valuea | 0.229 | < 0.001b | < 0.001b,c | < 0.001b† |
Χ2 test.
DLB significantly higher than FTD, p<0.001 and AD, p<0.001.
FTD significantly higher than AD, p=0.017.
AD, Alzheimer’s disease; DLB, dementia with Lewy bodies; FTD, frontotemporal dementia.
Table 3.
Incidence rates of new-onset seizures and myoclonus in dementia groups, expressed as ratios relative to age-matched control populations [46, 47].
| New-onset seizures | Myoclonus | |
|---|---|---|
| AD | 9.7 (6.8 – 13.7) | 689.2 (383.9 – 1,343) |
| DLB | 10.0 (4.2 – 20.4) | 1,481 (752.5 – 3,179) |
| FTD | 6.4 (2.7 – 13.0) | 1,097 (573.1 – 2,239) |
Values in parentheses are 95% confidence intervals.
AD, Alzheimer’s disease; DLB, dementia with Lewy bodies; FTD, frontotemporal dementia.
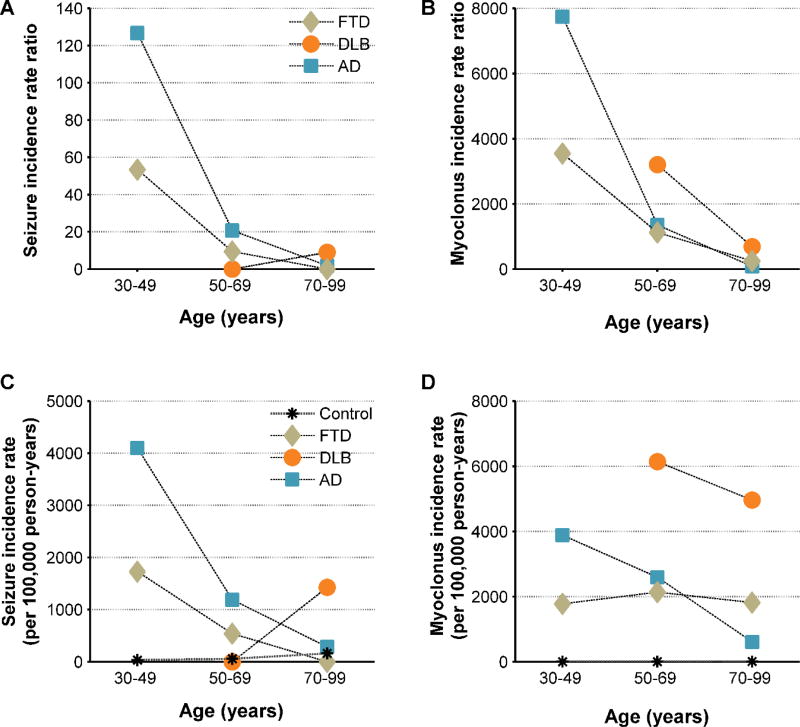
Figure 2 displays age-stratified incidence rates of seizures and myoclonus for the three dementia cohorts, as well as the incidence rate ratios (IRR), the ratios of incidence rates in dementia groups to incidence rates in age-matched control populations. The seizure IRR increased with earlier age in AD (<50 IRR 126.7, 50–69 IRR 20.7, 70+ IRR 1.8, p < 0.0001) (Figure 2A). The corresponding incidence rates of seizures in AD showed a similar age-related pattern (Figure 2C). The seizure IRR also increased with earlier age in FTD (<50 IRR 53.4, 50–69 IRR 9.4, 70+ IRR 0, p = 0.012), although the FTD cohort included only 11 patients in the youngest stratum. Additionally, no DLB patients were diagnosed before age 50. Myoclonus IRR increased with earlier age for patients in all three groups (Figure 2B). This relationship was also evident in the absolute incidence rates for AD and DLB but not FTD (Figure 2D).
Figure 2.
Age-stratified incidence rate ratios (IRR), the ratios of incidence rates in dementia groups to incidence rates in age-matched control populations, and raw incidence rates for new-onset seizures and myoclonus, by diagnostic cohort. (A and B) IRR for new-onset seizures (A) and myoclonus (B) (panel A, seizure IRR increased with earlier age in AD, p<0.0001, and FTD, p=0.012, Χ2 test, and panel B, myoclonus IRR increased with earlier age in AD, p<0.0001, DLB, p=0.022, and FTD, p=0.033, Χ2 test). The IRR values and 95% confidence intervals are provided in Supplementary Table 2. (C and D) Component incidence rates from patients and control data in epidemiological studies[46, 47] for seizures (C) and myoclonus (D). No patients with DLB were seen in the stratum of 30 – 49 years of age.
Suspected but unconfirmed seizures, as well as apparent non-epileptic fluctuations in cognition and awareness, were most prevalent in patients with DLB (Table 2). Myoclonus was also most prevalent in DLB, followed by FTD. Supplementary Table 4 contains the prevalence of new-onset seizures and myoclonus at the level of dementia subtypes. Of the dementia subtypes, the group meeting probable criteria for both DLB and AD (DLB-AD) had the highest prevalence of seizures (20.7%), whereas none of the patients with svPPA had seizures. Myoclonus was most prevalent in the CBD group (41.0%) and least prevalent in the svPPA group (4.9%). Of 78 patients with seizures, one bvFTD patient had an autosomal-dominant GRN mutation and one AD patient had an autosomal-dominant PSEN1 mutation. Of 205 patients with myoclonus, one had an autosomal-dominant form of AD (same PSEN1 case with seizures). All remaining patients with evidence of hyperexcitability had sporadic forms of dementia. In analyses limited to patients with syndromes that can be caused by autosomal-dominant mutations, there were no statistically significant differences in the frequencies of mutations in patients with or without new-onset seizures or myoclonus (Fisher’s exact test). There also were no significant associations between genetic variants that modify dementia risk or clinical presentation and development of new-onset seizures or myoclonus when assessed within each diagnostic group or pooled across all patients (Fisher’s exact test, Supplementary Table 3).
Temporal Associations between Seizures and Myoclonus and Dementia
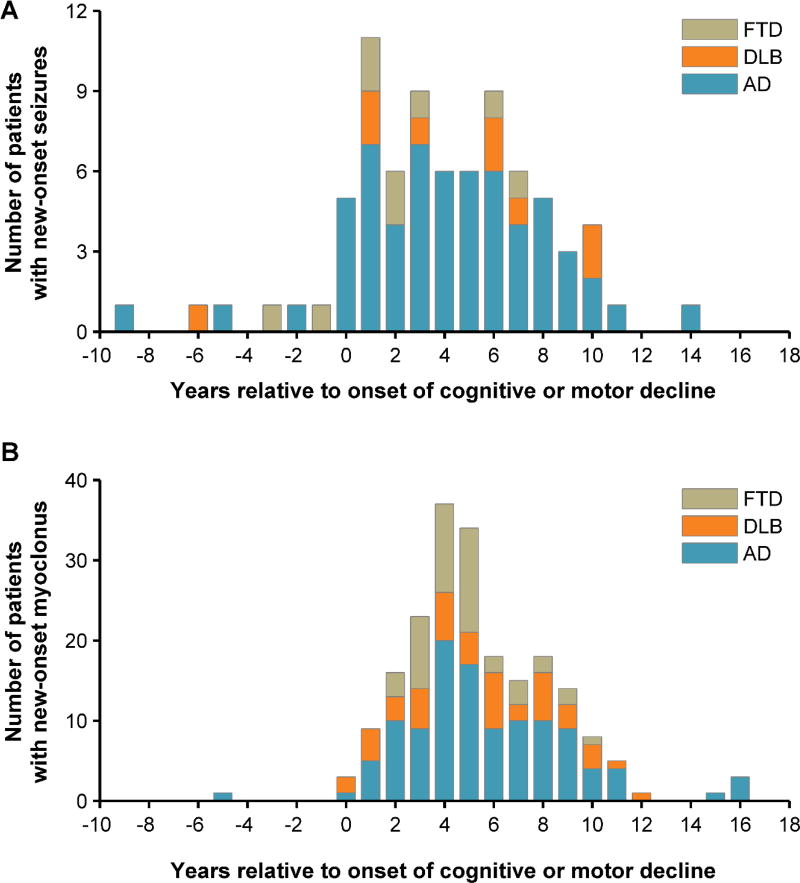
Seizures began an average of 3.9 ± 3.9 standard deviation years after the onset of cognitive or motor decline, whereas myoclonus began 5.4 ± 3.0 years after symptom onset (Figure 3). The time difference between onset of seizures or myoclonus and onset of dementia did not differ between dementia syndromes (Kruskal-Wallis). Seizures and myoclonus were significantly associated; 33.3% of patients with seizures had myoclonus, whereas 10.1% of patients without seizures had myoclonus (p<0.001, Χ2). Of the 26 patients with both seizures and myoclonus, seizures preceded myoclonus in 13 and they occurred simultaneously in 2. The median difference between onset of seizure and myoclonus in patients with both symptoms was 1.2 years (IQR 0.6 – 3.6).
Figure 3.
Onset of seizures and myoclonus in relation to onset of cognitive or motor decline, by diagnostic cohort. (A) Occurrence of new-onset seizures in relation to age-at-onset of cognitive or motor decline. (B) Occurrence of myoclonus in relation to age-at-onset of cognitive or motor decline.
In logistic regression analyses that included diagnosis, age-at-onset, and disease severity measured by MMSE as independent predictors of seizures and myoclonus, MMSE was not associated with myoclonus (p = 0.40) or seizures (p = 0.65) (Supplementary Tables 5 and 6). As suggested by the absolute rates of seizures and myoclonus within each age stratum (Figure 2C and D), seizures were associated with younger age in AD (p < 0.001) and older age in DLB (p = 0.04), and myoclonus was associated with younger age in AD (p < 0.001). See Supplementary Results for a complete description of these models.
Seizure Semiology
Of 78 patients identified with new-onset seizures, sufficient information to classify seizures was available for 71. Seizures were exclusively non-motor in 54.9% of cases (39 of 71), and were classified as generalized, without clear focal or lateralizing symptoms, in 52.1% (37 of 71). There were no significant relationships between dementia syndrome and the frequency of motor versus non-motor or generalized versus focal onset seizures (Fisher exact test). Clinical characteristics of these seizures are summarized by dementia syndrome in Table 4, and electroencephalography (EEG) results for patients with seizures are summarized in Table 5.
Table 4.
Clinical characteristic of seizures in patients with neurodegenerative diseases
| AD (n = 56) | DLB (n = 8) | FTD (n = 7) | p valuea | |
|---|---|---|---|---|
| Generalized/Focal | 29/27 | 6/2 | 2/5 | 0.246 |
| Non-motor/Motor | 32/24 | 4/4 | 3/4 | 0.763 |
Fisher exact test.
AD, Alzheimer’s disease; DLB, dementia with Lewy bodies; FTD, frontotemporal dementia.
Table 5.
Electroencephalographic characteristics of 78 patients with seizures
| Cohort Characteristic | Value (n / total n (%)) |
|---|---|
| Patients with EEG | 48/78 (61.5) |
| At least one >12 hour | 12/48 (25.0) |
| EEG acquired | |
| EEG Resulta | |
| Normal | 11/48 (22.9) |
| Slowing | 31/48 (64.6) |
| Diffuse | 19/31 (61.3) |
| Focal | 15/31 (48.4) |
| Epileptiform | 22/48 (45.8) |
| Asymmetric | 16/22 (72.7) |
| Focal temporal or frontotemporal | 13/22 (59.1) |
| Generalized | 3/22 (13.6) |
Results pooled across all available EEG acquisitions for each patient. Patients may contribute to more than one result
DISCUSSION
The results of this study indicate that development of seizures and myoclonus differs amongst patients with the three most common forms of neurodegenerative disease: AD, DLB, and FTD. Overall, allowing for co-pathology, the results suggest that seizure propensity is highest when Aβ or α-synuclein pathology is present (e.g., AD, DLB, and DLB-AD), whereas myoclonus propensity, at least in early stages of disease, is higher in primary tau disorders (e.g., CBD) or when α-synuclein is present. Notably, pathological tau is common to the above disorders. In this regard, it is informative that patients with svPPA, which is commonly a TDP-43 disorder that lacks tau co-pathology [48], had the lowest rates of seizures and myoclonus. These findings support complex mechanisms of network hyperexcitability in the different dementia syndromes, reflecting their heterogeneous neuropathology.
Cumulative Incidence, Prevalence, and Clinical Presentations
Patients with AD and DLB had a higher cumulative incidence of seizures than FTD, although they did not differ significantly in terms of point prevalence of seizures. The overall cumulative probability of developing seizures for patients in this study is in close agreement with other studies of seizures in neurodegenerative diseases [2, 32, 49].
Seizures were more frequently suspected and unconfirmed in DLB compared to both AD and FTD. This finding may be attributable to the fluctuations in cognition and awareness that are hallmarks of DLB diagnosis and were present in a majority of patients with the disease. Previous literature has indicated that seizures can precede or follow cognitive symptoms in both AD and non-AD dementias [4, 5, 31, 50, 51]. The characteristics of seizure presentation in this study agree with those findings and provide more detail about seizure onset within the DLB and FTD cohorts. The seizures in patients with AD, DLB, and FTD lacked obvious distinguishing clinical features, suggesting that seizure semiology is not specific to the neurodegenerative syndrome.
In terms of myoclonus, DLB patients had a consistently higher cumulative incidence of myoclonus than patients with AD or FTD. Myoclonus probability in AD increased gradually over time, whereas in FTD the probability rose steeply in the years immediately following disease onset. These different patterns could partially reflect inclusion of myoclonus in some FTD diagnostic criteria such as CBD, since the upslope was near the time interval when diagnosis was most likely. Patients with DLB also had a high point prevalence of myoclonus, significantly higher than the prevalence in both AD and FTD and similar to values obtained in pathologically confirmed cases [52].
Temporal Relationship between Symptoms of Hyperexcitability and Onset of Disease
Seizure rates in were markedly increased in younger patients with AD and, to a lesser extent, FTD. The strong relationship between younger age and seizure incidence in AD is strikingly similar to previous reports [2], and our findings extend on previous data to show an even higher seizure risk when disease starts before age 50. In addition to the age-stratified analysis that compared incidence rates with control populations, a predictive model that included age-at-onset as a continuous variable demonstrated a similar relationship between younger age and occurrence of seizures in AD. This model also revealed an association between older age and seizures in DLB, as suggested by Figure 2C, which could reflect development of AD pathology or other comorbidities in the DLB group with aging.
Myoclonus rates were increased at younger ages in all three dementia syndromes, in comparison with age-matched control populations. The relationship between young age and occurrence of myoclonus was strongest in AD, as indicated by the supplementary logistic regression model. Taken together, these findings indicate that clinical signs of hyperexcitability develop more commonly with younger age in AD and variably in relation to age in FTD and DLB.
Both seizures and myoclonus developed early in disease course in all three dementias, and in many cases the two features coexisted. Associations between seizures and myoclonus have also been reported in patients with early-onset AD, and are suggested to have a common underlying cortical pathology [16]. From a clinical perspective, recognizing the co-occurrence of seizures and myoclonus may guide clinicians to their earlier detection and treatment. The development of seizures and/or myoclonus might hasten disease onset or represent a more aggressive neurodegenerative phenotype. The cohort contained only two patients with seizures or myoclonus in the context of an early-onset, familial form of disease, raising the possibility that there are other genetic or epigenetic predispositions to seizures and myoclonus in early-onset dementia cases.
Pathological Implications
The observation that the DLB-AD group had the highest prevalence of seizures (20.7%) amongst the dementia subtypes (Supplementary Table 2) warrants further discussion. Increased seizure risk in such cases could be simply attributed to increased neuropathological burden associated with dual disease, but there may also be an epileptogenic synergy between the two disease processes. Such synergistic interactions have been previously demonstrated in doubly transgenic mice expressing human α-synuclein and APP [53]. In contrast, no seizures were observed in the svPPA cohort, and this group had the lowest incidence of myoclonus among the FTD subtypes. This result is surprising given the high correlation of svPPA with ubiquitin- and TDP-43-positive pathology in the temporal lobes, and may reflect relatively confined involvement of anterior temporal lobe structures or lesser influence of TDP-43 on synaptic activity [35, 54]. Possibly related to the lower incidence of network hyperexcitability in svPPA, this group also has a longer survival rate than other variants of FTD [55].
Strengths and Limitations
A major strength of this study is its novel exploration of differences in susceptibility to network hyperexcitability in specific types of dementia. Some limitations should also be discussed. The retrospective nature of the analysis limited our ability to precisely distinguish the differences in seizure and myoclonus rates, and we were not able to employ validated tools common to prospective cohort studies. EEG confirmation of seizures was not obtained in all cases; however, it should be noted that routine EEG has low sensitivity in capturing epileptiform activity, even in patients with established seizure disorders [56]. Since clinical seizures were not highly prevalent, the DLB and FTD groups provide a relatively small sample size for epidemiological analysis of seizure development. Additionally, selection bias towards patients with rare forms of dementia who are suitable for research may reduce the generalizability of the findings. Demographic data in this study, however, do suggest that patients are somewhat representative of broader populations. For example, sex distributions are in line with previous reports in patients with neurodegenerative diseases [57, 58], and rates of amyloid PET positivity in patients with AD and FTD are consistent with results from broader dementia populations [59]. Finally, lack of autopsy information constrains our ability to accurately gauge the precision of clinical diagnosis and degree of co-pathology. The current results provide groundwork for future studies to prospectively elucidate precise mechanisms by which different disease processes might contribute to neural network hyperexcitability.
CONCLUSION
Seizures and myoclonus occur in AD, DLB, and FTD at higher rates than in the general population, and should therefore raise suspicion of a dementia diagnosis when other etiologies have been ruled out. This study’s findings illustrate that network hyperexcitability may develop from pathomechanisms that vary between neurodegenerative diseases and that clinical signs of hyperexcitability often appear near the time of dementia diagnosis.
Antiseizure drug therapy improves cognitive and behavioral function in transgenic mouse models of AD, and clinical trials using antiseizure drugs for AD-associated network hyperexcitability are ongoing. The findings of this study raise the possibility that similar strategies might benefit patients with FTD and DLB. Earlier detection of network hyperexcitability in patients with dementia, and more precise understanding of the mechanisms involved, could lead to better treatment of the disease.
Supplementary Material
Acknowledgments
The authors would like to first and foremost acknowledge the patients and family members who were seen at the Memory and Aging Center. We would also like to thank the clinical and research staff of the Memory and Aging Center; Andreas Lazaris, William Jagust and Gil Rabinovici for providing the amyloid imaging; and Anna Karydas for genetics support. This study was supported by the National Institutes of Health (NIH) grants K23 AG038357 (KAV) and F32 AG050434 (KGR), a grant from the Alzheimer’s Association, PCTRB-13-288476, made possible by Part the Cloud™ (KAV), the John Douglas French Alzheimer’s Foundation (KAV and EK), the Larry L. Hillblom Foundation, 2015-A-034-FEL (KGR), and the Robert Katzman, MD, Clinical Research Training Fellowship in Alzheimer’s Research sponsored by the American Brain Foundation and Alzheimer’s Association (EK).
Footnotes
AUTHOR CONTRIBUTIONS
AJB and SMD contributed equally to this study and were involved with study design, data collection, analysis, interpretation, and manuscript preparation. KGR contributed to study design, analysis, interpretation, and manuscript preparation. AL contributed to data collection and manuscript preparation. EK contributed to analysis, interpretation, and manuscript preparation. KAV conceptualized the study and contributed to design, data collection, analysis, interpretation, and manuscript preparation. All authors have reviewed and approve of the final draft of this manuscript.
COMPETING INTERESTS
The authors have no conflict of interest to report.
References
- 1.Scarmeas N, Honig LS, Choi H, Cantero J, Brandt J, Blacker D, Albert M, Amatniek JC, Marder K, Bell K, Hauser WA, Stern Y. Seizures in Alzheimer disease: who, when, and how common. Arch Neurol. 2009;66:992–997. doi: 10.1001/archneurol.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amatniek JC, Hauser WA, DelCastillo-Castaneda C, Jacobs DM, Marder K, Bell K, Albert M, Brandt J, Stern Y. Incidence and predictors of seizures in patients with Alzheimer’s disease. Epilepsia. 2006;47:867–872. doi: 10.1111/j.1528-1167.2006.00554.x. [DOI] [PubMed] [Google Scholar]
- 3.Romanelli MF, Morris JC, Ashkin K, Coben LA. Advanced Alzheimer’s disease is a risk factor for late-onset seizures. Arch Neurol. 1990;47:847–850. doi: 10.1001/archneur.1990.00530080029006. [DOI] [PubMed] [Google Scholar]
- 4.Vossel KA, Beagle AJ, Rabinovici GD, Shu H, Lee SE, Naasan G, Hegde M, Cornes SB, Henry ML, Nelson AB, Seeley WW, Geschwind MD, Gorno-Tempini ML, Shih T, Kirsch HE, Garcia PA, Miller BL, Mucke L. Seizures and epileptiform activity in the early stages of Alzheimer disease. JAMA Neurol. 2013;70:1158–1166. doi: 10.1001/jamaneurol.2013.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cretin B, Sellal F, Philippi N, Bousiges O, Di Bitonto L, Martin-Hunyadi C, Blanc F. Epileptic Prodromal Alzheimer’s Disease, a Retrospective Study of 13 New Cases: Expanding the Spectrum of Alzheimer’s Disease to an Epileptic Variant. J Alzheimers Dis. 2016 doi: 10.3233/JAD-150096. [DOI] [PubMed] [Google Scholar]
- 6.Vossel KA, Tartaglia MC, Nygaard HB, Zeman AZ, Miller BL. Epileptic activity in Alzheimer’s disease: causes and clinical relevance. Lancet Neurol. 2017;16:311–322. doi: 10.1016/S1474-4422(17)30044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lam AD, Deck G, Goldman A, Eskandar EN, Noebels J, Cole AJ. Silent hippocampal seizures and spikes identified by foramen ovale electrodes in Alzheimer’s disease. Nat Med. 2017 doi: 10.1038/nm.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vossel KA, Ranasinghe KG, Beagle AJ, Mizuiri D, Honma SM, Dowling AF, Darwish SM, Van Berlo V, Barnes DE, Mantle M, Karydas AM, Coppola G, Roberson ED, Miller BL, Garcia PA, Kirsch HE, Mucke L, Nagarajan SS. Incidence and impact of subclinical epileptiform activity in Alzheimer’s disease. Ann Neurol. 2016;80:858–870. doi: 10.1002/ana.24794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volicer L, Smith S, Volicer BJ. Effect of seizures on progression of dementia of the Alzheimer type. Dementia. 1995;6:258–263. doi: 10.1159/000106956. [DOI] [PubMed] [Google Scholar]
- 10.Lott IT, Doran E, Nguyen VQ, Tournay A, Movsesyan N, Gillen DL. Down syndrome and dementia: seizures and cognitive decline. J Alzheimers Dis. 2012;29:177–185. doi: 10.3233/JAD-2012-111613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lesne S, Ali C, Gabriel C, Croci N, MacKenzie ET, Glabe CG, Plotkine M, Marchand-Verrecchia C, Vivien D, Buisson A. NMDA receptor activation inhibits alpha-secretase and promotes neuronal amyloid-beta production. J Neurosci. 2005;25:9367–9377. doi: 10.1523/JNEUROSCI.0849-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 13.Wu JW, Hussaini SA, Bastille IM, Rodriguez GA, Mrejeru A, Rilett K, Sanders DW, Cook C, Fu H, Boonen RA, Herman M, Nahmani E, Emrani S, Figueroa YH, Diamond MI, Clelland CL, Wray S, Duff KE. Neuronal activity enhances tau propagation and tau pathology in vivo. Nat Neurosci. 2016;19:1085–1092. doi: 10.1038/nn.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palop JJ, Mucke L. Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat Neurosci. 2010;13:812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noebels J. A perfect storm: Converging paths of epilepsy and Alzheimer’s dementia intersect in the hippocampal formation. Epilepsia. 2011;52(Suppl 1):39–46. doi: 10.1111/j.1528-1167.2010.02909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samson WN, van Duijn CM, Hop WC, Hofman A. Clinical features and mortality in patients with early-onset Alzheimer’s disease. Eur Neurol. 1996;36:103–106. doi: 10.1159/000117218. [DOI] [PubMed] [Google Scholar]
- 17.Prasher VP, Corbett JA. Onset of seizures as a poor indicator of longevity in people with down syndrome and dementia. Int J Geriatr Psychiatry. 1993;8:923–927. [Google Scholar]
- 18.Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, Finkbeiner S, Noebels JL, Mucke L. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberson ED, Halabisky B, Yoo JW, Yao J, Chin J, Yan F, Wu T, Hamto P, Devidze N, Yu GQ, Palop JJ, Noebels JL, Mucke L. Amyloid-β/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer’s disease. J Neurosci. 2011;31:700–711. doi: 10.1523/JNEUROSCI.4152-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schoch KM, DeVos SL, Miller RL, Chun SJ, Norrbom M, Wozniak DF, Dawson HN, Bennett CF, Rigo F, Miller TM. Increased 4R–Tau Induces Pathological Changes in a Human-Tau Mouse Model. Neuron. 2016;90:941–947. doi: 10.1016/j.neuron.2016.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Cabrero AM, Guerrero-Lopez R, Giraldez BG, Llorens-Martin M, Avila J, Serratosa JM, Sanchez MP. Hyperexcitability and epileptic seizures in a model of frontotemporal dementia. Neurobiol Dis. 2013;58:200–208. doi: 10.1016/j.nbd.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Maeda S, Djukic B, Taneja P, Yu GQ, Lo I, Davis A, Craft R, Guo W, Wang X, Kim D, Ponnusamy R, Gill TM, Masliah E, Mucke L. Expression of A152T human tau causes age-dependent neuronal dysfunction and loss in transgenic mice. EMBO Rep. 2016;17:530–551. doi: 10.15252/embr.201541438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andrews-Zwilling Y, Bien-Ly N, Xu Q, Li G, Bernardo A, Yoon SY, Zwilling D, Yan TX, Chen L, Huang Y. Apolipoprotein E4 causes age- and Tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J Neurosci. 2010;30:13707–13717. doi: 10.1523/JNEUROSCI.4040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris M, Sanchez PE, Verret L, Beagle AJ, Guo W, Dubal D, Ranasinghe KG, Koyama A, Ho K, Yu GQ, Vossel KA, Mucke L. Network dysfunction in alpha-synuclein transgenic mice and human Lewy body dementia. Ann Clin Transl Neurol. 2015;2:1012–1028. doi: 10.1002/acn3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sherzai D, Losey T, Vega S, Sherzai A. Seizures and dementia in the elderly: Nationwide Inpatient Sample 1999–2008. Epilepsy Behav. 2014;36:53–56. doi: 10.1016/j.yebeh.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 26.Bakker A, Albert MS, Krauss G, Speck CL, Gallagher M. Response of the medial temporal lobe network in amnestic mild cognitive impairment to therapeutic intervention assessed by fMRI and memory task performance. Neuroimage Clin. 2015;7:688–698. doi: 10.1016/j.nicl.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cumbo E, Ligori LD. Levetiracetam, lamotrigine, and phenobarbital in patients with epileptic seizures and Alzheimer’s disease. Epilepsy Behav. 2010;17:461–466. doi: 10.1016/j.yebeh.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 28.McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 29.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, Boxer AL, Dickson DW, Grossman M, Hallett M, Josephs KA, Kertesz A, Lee SE, Miller BL, Reich SG, Riley DE, Tolosa E, Troster AI, Vidailhet M, Weiner WJ. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80:496–503. doi: 10.1212/WNL.0b013e31827f0fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarkis RA, Dickerson BC, Cole AJ, Chemali ZN. Clinical and Neurophysiologic Characteristics of Unprovoked Seizures in Patients Diagnosed With Dementia. J Neuropsychiatry Clin Neurosci. 2016;28:56–61. doi: 10.1176/appi.neuropsych.15060143. [DOI] [PubMed] [Google Scholar]
- 32.Hesdorffer DC, Hauser WA, Annegers JF, Kokmen E, Rocca WA. Dementia and adult-onset unprovoked seizures. Neurology. 1996;46:727–730. doi: 10.1212/wnl.46.3.727. [DOI] [PubMed] [Google Scholar]
- 33.Pisani F, Oteri G, Costa C, Di Raimondo G, Di Perri R. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25:91–110. doi: 10.2165/00002018-200225020-00004. [DOI] [PubMed] [Google Scholar]
- 34.Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini ML, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam MM, Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, Goetz CG, Golbe LI, Grafman J, Growdon JH, Hallett M, Jankovic J, Quinn NP, Tolosa E, Zee DS. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- 37.Lee SE, Rabinovici GD, Mayo MC, Wilson SM, Seeley WW, DeArmond SJ, Huang EJ, Trojanowski JQ, Growdon ME, Jang JY, Sidhu M, See TM, Karydas AM, Gorno-Tempini ML, Boxer AL, Weiner MW, Geschwind MD, Rankin KP, Miller BL. Clinicopathological correlations in corticobasal degeneration. Ann Neurol. 2011;70:327–340. doi: 10.1002/ana.22424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 39.Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, Engel J, French J, Glauser TA, Mathern GW, Moshe SL, Nordli D, Plouin P, Scheffer IE. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 40.Ranasinghe KG, Rankin KP, Pressman PS, Perry DC, Lobach IV, Seeley WW, Coppola G, Karydas AM, Grinberg LT, Shany-Ur T, Lee SE, Rabinovici GD, Rosen HJ, Gorno-Tempini ML, Boxer AL, Miller ZA, Chiong W, DeMay M, Kramer JH, Possin KL, Sturm VE, Bettcher BM, Neylan M, Zackey DD, Nguyen LA, Ketelle R, Block N, Wu TQ, Dallich A, Russek N, Caplan A, Geschwind DH, Vossel KA, Miller BL. Distinct Subtypes of Behavioral Variant Frontotemporal Dementia Based on Patterns of Network Degeneration. JAMA Neurol. 2016;73:1078–1088. doi: 10.1001/jamaneurol.2016.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coppola G, Chinnathambi S, Lee JJ, Dombroski BA, Baker MC, Soto-Ortolaza AI, Lee SE, Klein E, Huang AY, Sears R, Lane JR, Karydas AM, Kenet RO, Biernat J, Wang LS, Cotman CW, Decarli CS, Levey AI, Ringman JM, Mendez MF, Chui HC, Le Ber I, Brice A, Lupton MK, Preza E, Lovestone S, Powell J, Graff-Radford N, Petersen RC, Boeve BF, Lippa CF, Bigio EH, Mackenzie I, Finger E, Kertesz A, Caselli RJ, Gearing M, Juncos JL, Ghetti B, Spina S, Bordelon YM, Tourtellotte WW, Frosch MP, Vonsattel JP, Zarow C, Beach TG, Albin RL, Lieberman AP, Lee VM, Trojanowski JQ, Van Deerlin VM, Bird TD, Galasko DR, Masliah E, White CL, Troncoso JC, Hannequin D, Boxer AL, Geschwind MD, Kumar S, Mandelkow EM, Wszolek ZK, Uitti RJ, Dickson DW, Haines JL, Mayeux R, Pericak-Vance MA, Farrer LA, Alzheimer’s Disease Genetics C, Ross OA, Rademakers R, Schellenberg GD, Miller BL, Mandelkow E, Geschwind DH. Evidence for a role of the rare p.A152T variant in MAPT in increasing the risk for FTD-spectrum and Alzheimer’s diseases. Hum Mol Genet. 2012;21:3500–3512. doi: 10.1093/hmg/dds161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsiung GY, Fok A, Feldman HH, Rademakers R, Mackenzie IR. rs5848 polymorphism and serum progranulin level. J Neurol Sci. 2011;300:28–32. doi: 10.1016/j.jns.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cruchaga C, Graff C, Chiang HH, Wang J, Hinrichs AL, Spiegel N, Bertelsen S, Mayo K, Norton JB, Morris JC, Goate A. Association of TMEM106B gene polymorphism with age at onset in granulin mutation carriers and plasma granulin protein levels. Arch Neurol. 2011;68:581–586. doi: 10.1001/archneurol.2010.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JS, Younkin S, Hazrati L, Collinge J, Pocock J, Lashley T, Williams J, Lambert JC, Amouyel P, Goate A, Rademakers R, Morgan K, Powell J, St George-Hyslop P, Singleton A, Hardy J, Alzheimer Genetic Analysis G. TREM2 variants in Alzheimer’s disease. N Engl J Med. 2013;368:117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. Lippincott Williams & Wilkins; Philadelphia, PA: 2008. [Google Scholar]
- 46.Forsgren L, Bucht G, Eriksson S, Bergmark L. Incidence and clinical characterization of unprovoked seizures in adults: a prospective population-based study. Epilepsia. 1996;37:224–229. doi: 10.1111/j.1528-1157.1996.tb00017.x. [DOI] [PubMed] [Google Scholar]
- 47.Caviness JN, Alving LI, Maraganore DM, Black RA, McDonnell SK, Rocca WA. The incidence and prevalence of myoclonus in Olmsted County, Minnesota. Mayo Clin Proc. 1999;74:565–569. doi: 10.4065/74.6.565. [DOI] [PubMed] [Google Scholar]
- 48.Robinson AC, Thompson JC, Weedon L, Rollinson S, Pickering-Brown S, Snowden JS, Davidson YS, Mann DM. No interaction between tau and TDP-43 pathologies in either frontotemporal lobar degeneration or motor neurone disease. Neuropathol Appl Neurobiol. 2014;40:844–854. doi: 10.1111/nan.12155. [DOI] [PubMed] [Google Scholar]
- 49.Hauser WA, Morris ML, Heston LL, Anderson VE. Seizures and myoclonus in patients with Alzheimer’s disease. Neurology. 1986;36:1226–1230. doi: 10.1212/wnl.36.9.1226. [DOI] [PubMed] [Google Scholar]
- 50.Breteler MM, de Groot RR, van Romunde LK, Hofman A. Risk of dementia in patients with Parkinson’s disease, epilepsy, and severe head trauma: a register-based follow-up study. Am J Epidemiol. 1995;142:1300–1305. doi: 10.1093/oxfordjournals.aje.a117597. [DOI] [PubMed] [Google Scholar]
- 51.Lozsadi DA, Larner AJ. Prevalence and causes of seizures at the time of diagnosis of probable Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;22:121–124. doi: 10.1159/000093664. [DOI] [PubMed] [Google Scholar]
- 52.Louis ED, Klatka LA, Liu Y, Fahn S. Comparison of extrapyramidal features in 31 pathologically confirmed cases of diffuse Lewy body disease and 34 pathologically confirmed cases of Parkinson’s disease. Neurology. 1997;48:376–380. doi: 10.1212/wnl.48.2.376. [DOI] [PubMed] [Google Scholar]
- 53.Masliah E, Rockenstein E, Veinbergs I, Sagara Y, Mallory M, Hashimoto M, Mucke L. beta-amyloid peptides enhance alpha-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer’s disease and Parkinson’s disease. Proc Natl Acad Sci U S A. 2001;98:12245–12250. doi: 10.1073/pnas.211412398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davies RR, Hodges JR, Kril JJ, Patterson K, Halliday GM, Xuereb JH. The pathological basis of semantic dementia. Brain. 2005;128:1984–1995. doi: 10.1093/brain/awh582. [DOI] [PubMed] [Google Scholar]
- 55.Roberson ED, Hesse JH, Rose KD, Slama H, Johnson JK, Yaffe K, Forman MS, Miller CA, Trojanowski JQ, Kramer JH, Miller BL. Frontotemporal dementia progresses to death faster than Alzheimer disease. Neurology. 2005;65:719–725. doi: 10.1212/01.wnl.0000173837.82820.9f. [DOI] [PubMed] [Google Scholar]
- 56.Baldin E, Hauser WA, Buchhalter JR, Hesdorffer DC, Ottman R. Yield of epileptiform electroencephalogram abnormalities in incident unprovoked seizures: a population-based study. Epilepsia. 2014;55:1389–1398. doi: 10.1111/epi.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hebert LE, Scherr PA, McCann JJ, Beckett LA, Evans DA. Is the risk of developing Alzheimer’s disease greater for women than for men. Am J Epidemiol. 2001;153:132–136. doi: 10.1093/aje/153.2.132. [DOI] [PubMed] [Google Scholar]
- 58.Barker WW, Luis CA, Kashuba A, Luis M, Harwood DG, Loewenstein D, Waters C, Jimison P, Shepherd E, Sevush S, Graff-Radford N, Newland D, Todd M, Miller B, Gold M, Heilman K, Doty L, Goodman I, Robinson B, Pearl G, Dickson D, Duara R. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord. 2002;16:203–212. doi: 10.1097/00002093-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 59.Ossenkoppele R, Jansen WJ, Rabinovici GD, Knol DL, van der Flier WM, van Berckel BN, Scheltens P, Visser PJ, Amyloid PETSG, Verfaillie SC, Zwan MD, Adriaanse SM, Lammertsma AA, Barkhof F, Jagust WJ, Miller BL, Rosen HJ, Landau SM, Villemagne VL, Rowe CC, Lee DY, Na DL, Seo SW, Sarazin M, Roe CM, Sabri O, Barthel H, Koglin N, Hodges J, Leyton CE, Vandenberghe R, van Laere K, Drzezga A, Forster S, Grimmer T, Sanchez-Juan P, Carril JM, Mok V, Camus V, Klunk WE, Cohen AD, Meyer PT, Hellwig S, Newberg A, Frederiksen KS, Fleisher AS, Mintun MA, Wolk DA, Nordberg A, Rinne JO, Chetelat G, Lleo A, Blesa R, Fortea J, Madsen K, Rodrigue KM, Brooks DJ. Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. JAMA. 2015;313:1939–1949. doi: 10.1001/jama.2015.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.