Abstract
Objective
In this study, we aimed to determine whether plasma NGAL levels could be used as a biomarker for distinguishing between AKI and CKD in emergency medicine.
Materials and methods
This prospective study was conducted at the ED of a training and research hospital over a six-month period in 2015. Three groups were defined: an AKI group – defined as a new onset of at least a 1.5-fold or ≥0.3 mg increment increase of SCr values from the normal baseline, a stable CKD group – only included presence of stages 2 through 4 of CKD according to the National Kidney Foundation's KDIGO 2012, and a control group. After the initial evaluation of patients, venous blood samples were taken for routine biochemical, counter blood cell, and plasma NGAL measurement at admission.
Results
A total of 25 patients with AKI, 22 patients with stable CKD, and 22 control subjects were enrolled. Level of plasma NGAL in AKI group was higher than those of the stable CKD group (median: 794 ng/ml IQR: 317–1300 & 390 ng/ml IQR: 219–664, p < 0.001). AUC was measured as 0.68 (p = 0.02, 95% CIs: 0.54–0.84) to assess the utility of plasma NGAL levels at varying cut-off values for distinguishing between AKI and CKD. For plasma NGAL, the best cut-off level was found to be 457 ng/ml (sensitivity: 72.0%, specificity: 64%).
Conclusion
This study has clearly demonstrated that plasma NGAL levels were higher in AKI patients than in CKD patients. However, in clinical practice, the use of plasma NGAL levels to distinguish between AKI and CKD is limited.
Keywords: Acute kidney injury, Chronic kidney disease, Neutrophil gelatinase-associated lipocalin (NGAL), Emergency department
1. Introduction
Kidney disease represents a significant health problem with high rates of morbidity and mortality, and it accounts for a significant proportion of emergency department admissions. Although several guidelines have been published to standardize the definitions and classifications of acute kidney injury (AKI) and chronic kidney disease (CKD), there are no reliable, applicable, and simple clinical decision rules or markers for distinguishing between AKI and CKD, especially in the emergency department (ED). Classically, AKI is defined as increasing serum creatinine (SCr) levels based on previous levels within hours to days, and CKD is defined as abnormalities of kidney structure or function present for more than three months with significant health implications.[1], [2] However, when patients are admitted with any complaints which are not associated uremic and present with elevated SCr in the ED, it is often difficult to decide whether these patients are suffering from AKI or CKD if they do not have identifying symptoms (such as hypervolemia, hyperkalemia, severe metabolic acidosis, or uremia symptoms) and if previous SCr levels are unavailable.
Neutrophil gelatinase-associated lipocalin (NGAL), which is a member of the lipocalin family of proteins, is expressed and secreted from renal tubular cells at low concentrations. Several studies have shown that NGAL is produced in the kidney after ischemic or nephrotoxic injury.[3], [4], [5], [6] After kidney injury, NGAL can be detected in the plasma of patients with AKI within 2 h, and plasma levels peak approximately 6 h after injury. These peak levels may be sustained for as long as five days, after which they begin to decrease.[7], [8] Therefore, we hypothesized that plasma NGAL levels during the acute period of kidney injury are higher than those present in chronic kidney injury. If so, we believe that plasma NGAL levels can be useful in distinguishing between AKI and CKD.
In this study, we aimed to determine whether plasma NGAL levels could be used as a biomarker for distinguishing between AKI and CKD in emergency medicine.
2. Materials and methods
2.1. Study design
This prospective cross-sectional study was conducted at the ED of a training and research hospital over a six-month period in 2015. The local ethics committee approved the study, and written informed consent was obtained from all patients or from legally authorized relatives.
2.2. Selection of patients, groups, and definitions
In this study, three groups were defined: an AKI group, a stable CKD group, and a control group. AKI was defined as a new onset of at least a 1.5-fold or ≥ 0.3 mg increment increase of SCr values from the normal baseline. For AKI groups, patients over 18 years and under 80 years of age who were admitted to our ED and diagnosed at the “Risk” (R), “Injury” (I), or “Failure” (F) stages of AKI were included. According to the RIFLE classification, patients diagnosed at the “Loss” (L) or “End” (E) stage of AKI were excluded from this study. In addition, to minimize potential confounding factors, patients with AKI who had identifying symptoms—such as hypervolemia, hyperkalemia (potassium>6.8 mEq/L), severe metabolic acidosis (pH < 7.1), SCr levels above 8 mg/dl, or uremia symptoms with dialysis indication—were excluded from the AKI group. Similarly, patients who had malignancy, critical illness, infectious diseases, alterations in leukocyte count or formula, and those undergoing treatment with steroids or immunosuppressants were excluded from the study. Finally, patients who were diagnosed with post-renal AKI were excluded.
Patients over 18 years and under 80 years of age who were admitted to our ED with minor symptoms (such as minor trauma, primary headache, or lumbago) and those who had known CKD were included this study in the stable CKD group. Inclusion criteria were as follows: presence of stages 2 through 4 of CKD according to the National Kidney Foundation's KDIGO 2012 clinical practice guideline classification and stable renal function. Stable CKD was defined as the absence of any transitory or permanent 1.5-fold increase in SCr levels compared to SCr levels six months prior. Patients who had SCr levels that had increased 1.5-fold from previous levels and patients whose previous SCr levels were not available were excluded. Finally, patients with a calculated glomerular filtration rate (GFR) of <15 ml per minute (stage 5 CKD) were excluded. GFR was calculated using the Modification of Diet in Renal Disease Equation. For AKI and CKD groups, patients selected upon consecutively during the day in a 7/24 basis.
The control group consisted of healthy age and sex matched subjects who were admitted to our ED with minor symptoms (such as minor trauma, primary headache, or lumbago) and those who did not have any diseases such as malignancy, active infection, chronic metabolic disease, or diabetes.
2.3. NGAL and other parameter measurements
After the initial evaluation of patients, venous blood samples were taken for routine biochemical, counter blood cell, and plasma NGAL measurement at admission. At the same time, arterial blood samples were obtained by puncture of the radial or brachial artery for measurements of pH, HCO3, and PCO2 levels. To measure NGAL levels, plasma samples were collected in EDTA tubes and examined using a triage NGAL device with bedside kit (the Alere Triage CardioRenal Panel, Alere, USA).
2.4. Statistical analyses
Statistical analyses were performed using SPSS 15.0 (Chicago, IL, USA). Demographic data related to patients and control subjects were expressed as number, percentage, median values, and inter-quartile-range (IQR) 25%–75%. The Kolmogorov-Smirnov test was used to assess the normal distribution of the variables. Non-parametric parameters were analyzed using the Mann-Whitney U and Kruskal-Wallis tests. To assess the utility of plasma NGAL levels at varying cut-off values for the distinction between AKI and CKD, a receiver-operating characteristic (ROC) curve was generated, and the area under curve (AUC) was calculated. The 95% confidence intervals (95% CIs) were also calculated whenever appropriate, and a p-value less than 0.05 was considered statistically significant. The sample size was estimated with G-Power for Mac OS X (version 3.1.9.2; Universitat Düsseldorf, Germany). Our goal was power to detect a 300 ng/ml difference between AKI and CKD groups. And also, we considered that standard deviation of value as 290 ng/ml according to the study of Özkan et al..9 Thus, assuming a two-sided α = 0.05, we anticipated a sample size of 18 patients for each groups to achieve 80% power.
3. Results
During the study period, 45 patients with AKI and 49 patients with CKD were admitted to our ED. In the AKI group, 11 patients who had dialysis indications, severe metabolic acidosis, or hyperkalemia, five patients who were diagnosed with post-renal AKI, and four patients who had several critical illnesses were excluded. In the CKD group, 12 patients whose previous SCr levels were not available, eight patients who were diagnosed with progressive CKD (presence of a 1.5-fold increase in SCr levels compared to SCr levels six months prior), and seven patients diagnosed with stage 5 CKD were excluded. Finally, a total of 25 patients with AKI, 22 patients with stable CKD, and 22 control subjects were enrolled for statistical analyses. Median age and gender ratios (male/female) of the AKI, stable CKD, and control groups were 68 (IQR: 57–76)-14/11, 68 (IQR: 56–76)-11/11, and 66 (IQR: 61–74)-11/11, respectively. There was no significant difference among the three groups in terms of age or gender (p = 0.97 and p = 0.89, respectively). The main baseline characteristics of the patients are shown in Table 1.
Table 1.
Baseline characteristics of the study population in AKI and stable CKD groups median (IQR 25–75%).
| AKI | Stable CKD | P values | |
|---|---|---|---|
| Gender M/F | 14/11 | 11/11 | 0.89 |
| Age-years | 68 (57–76) | 68 (56–76) | 0.97 |
| GFR, ml/min/1.73 m2 | 38 (23–56) | 42 (32–57) | 0.1 |
| Creatinine, mg/dl | 2.15 (1.80–2.98) | 2.07 (1.64–2.33) | 0.08 |
| BUN mg/dl | 50 (40–71) | 39 (31–48) | 0.01 |
| Hemoglobin, g/dl | 11.5 (10.5–12.8) | 11.2 (10.4–12.1) | 0.4 |
| White blood cell (103/mm3) | 12.7 (10.5–15.8) | 10 (8–11) | <0.001 |
| pH | 7.38 (7.30–7.40) | 7.38 (7.34–7.40) | 0.8 |
| HCO3, mEq/L | 24 (21–27) | 24 (21–26) | 0.8 |
| PCO2 mmHg | 42 (39–44) | 40 (38–44) | 0.4 |
| Sodium, mmol/L | 137 (133–140) | 140 (135–141) | 0.1 |
| Potassium, mmol/L | 4.6 (4.1–5.0) | 4.5 (4.0–5.1) | 0.8 |
| Plasma NGAL, ng/ml | 794 (317–1300) | 390 (219–664) | 0.02 |
AKI: Acute kidney injury, CKD: Chronic kidney disease, GFR: Glomerular filtration rate.
The highest level of plasma NGAL was detected in the AKI group (median: 794 ng/ml IQR: 317–1300) and the median level of plasma NGAL was detected as 390 ng/ml (IQR: 219–664) in the stable CKD group. The median level of plasma NGAL was 112 ng/ml (IQR: 90–188) in the control group. Significant differences were determined in terms of plasma NGAL levels among all groups (p < 0.001).
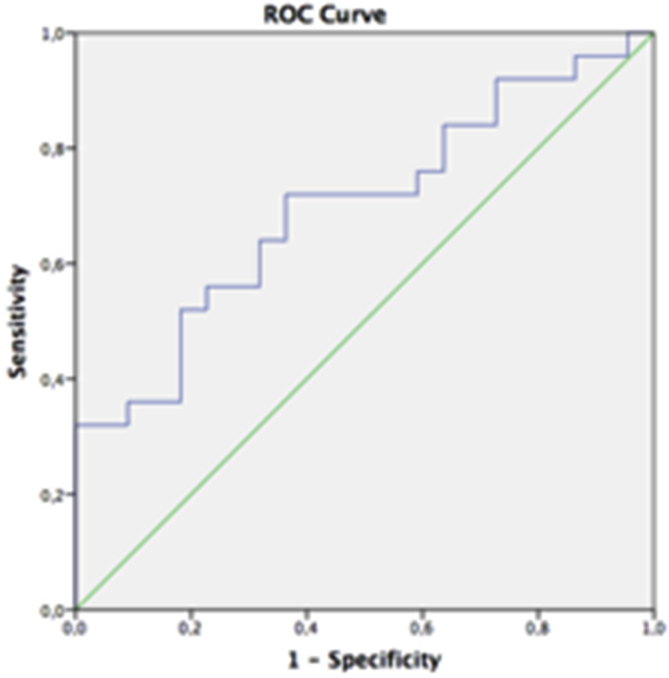
When analyzed to assess the utility of plasma NGAL levels at varying cut-off values to diagnose AKI, AUC was measured as 0.68 (p = 0.02, 95% CIs: 0.54–0.84) (Fig. 1). When was considered plasma NGAL as 457 ng/ml, sensitivity and specificity were found as 72.0% and 64%, respectively. In order to more reliably distinguish AKI from CKD, which means higher sensitivity, the 221 ng/ml levels can be used with 92% sensitivity and 28% specificity. The sensitivity and specificity values for different cut-off levels of plasma NGAL are shown in Table 2.
Fig. 1.
The area under curve (AUC) of as 0.68 (95% CIs: 0.54–0.84) The best cut-off level for NGAL for distinguishing between AKI and CKD was found as a 457 ng/ml with a sensitivity of 72.0% and a specificity of 64%.
Table 2.
The sensitivity and specificity values for different cut-off levels of plasma NGAL.
| pNGAL values (ng/mL) | Sensitivity (%) | Specificity (%) |
|---|---|---|
| 181.5 | 96 | 14 |
| 221.0 | 92 | 28 |
| 262.0 | 84 | 37 |
| 320.5 | 76 | 41 |
| 457.0 | 72 | 64 |
| 527.0 | 60 | 69 |
4. Discussion
In EDs, physicians often encounter patients who have elevated SCr levels, but it is often difficult to determine whether this elevation of SCr levels is related to AKI or CKD, especially when previous SCr levels are unavailable. Several clinical approaches have been used to distinguish between AKI and CKD in clinical practice. Renal ultrasonography is often used to evaluate kidney size and parenchyma echogenicity. However, although small kidney size and increased renal parenchyma echogenicity suggest CKD, normal kidney size or parenchyma echogenicity do not exclude the possibility of CKD.[10], [11] Similarly, although anemia, increased serum phosphate levels, and decreased vitamin D levels can be encountered in CKD, the correlation is not absolute, and the evaluation of these findings in ED is not practical or quick. Although SCr levels progressively increasing on a daily basis often suggests AKI, this cannot be used at the initial evaluation of patients in the ED. Initial SCr levels are not helpful in distinguishing between AKI and CKD12. Similarly, when our results were reviewed, there was no significant difference between the SCr levels of the AKI and CKD groups. Therefore, we believe that there is a need to discover a novel marker for distinguishing between AKI and CKD. We hypothesized that if plasma NGAL increases and peaks within hours after kidney injury, and if these levels start to decrease after the first five days, we can use this to distinguish between AKI and CKD upon the initial evaluation of patients with elevated SCr in the ED. When we analyzed our results, we identified two important points: first, as previous studies have found, the plasma NGAL levels of the AKI and the stable CKD groups were higher than those of the control group. Second, the plasma NGAL levels of the AKI group were higher than those of the stable CKD group, and if this difference is to be used to distinguish between AKI and CKD, 457 ng/ml for plasma NGAL is the best cut-off level; it offers a sensitivity of 72.0% and a specificity of 64%. Of course, we believe that these values of sensitivity and specificity are not acceptable for excluding AKI in ED practice. However, if the cut-off value is 221 ng/ml, sensitivity increases to 92.0%, but the specificity value decreases to 28%. We believe that using plasma NGAL to distinguish between AKI and CKD should not be recommend routinely in clinical practice. However, it varies whether the cut-off value can be used in clinical practice from country to country, depending on cost-benefit concerns.
Previous studies have shown that plasma NGAL levels increase under several clinical conditions—such as after cardiac surgery, poisoning, contrast-nephropathies, and after renal transplantation—which can cause AKI.[3], [4], [5], [6] In addition, a recent study conducted by Avci Çicek et al. showed that plasma NGAL levels of patients with CKD (4.01 ng/ml) were higher than those of control subjects (1.66 ng/mL) (p < 0.001).13 Similarly, another study conducted by Bolignano et al. showed that the plasma NGAL levels of the progressive CKD group (906.8 ng/ml) were higher than those of the stable CKD group (420 ng/ml), and the levels of both groups were higher than those of the control subjects (35.4 ng/ml) (p = 0.002).14 In another study, Somma et al. have reported that blood NGAL measurements are useful in the early diagnosis of AKI.15 Although several studies have been published on the association between plasma NGAL and AKI/CKD, studies directly comparing patients with AKI and CKD are limited. In a study conducted by Ozkan et al. on the plasma NGAL levels of patients with acute and chronic renal failure (not only CKD), plasma NGAL levels were found to be higher in the chronic renal failure group than in the acute group (428.50 pg/ml and 261.50 pg/ml, respectively) (p = 0.012).9 In contrast with this result, our results have shown that plasma NGAL levels were higher in AKI patients than in CKD patients. We believe that this discrepancy was caused by differences in the selection of the study population. In contrast with the study by Ozkan et al., patients with chronic renal failure, stage 5 CKD, and those with dialysis indications were not included in our study.
In another study conducted Nickolas et al., patients with AKI, prerenal azotemia, and non-progressive CKD were enrolled, and urinary NGAL levels of these patients were analyzed. They reported that urinary NGAL levels were higher than CKD groups. And also, to diagnose AKI, at a cutoff value of 130 μ g/g, sensitivity and specificity values of urinary NGAL were found as 90% and 99%, respectively.16 Although these results are similar to our results, we did not found high sensitivity and specificity values. We believe that this discrepancy was caused by our strict exclusion criteria such as patients who had clearly dialysis indications or stage 5 CKD.
In another prospective study conducted by Soto et al., patients with AKI (n = 130), transient azotemia (n = 159), stable CKD (n = 15), and normal kidney function (n = 312) were enrolled, and the plasma NGAL levels of these patients were analyzed. They reported that the median levels of plasma NGAL (which were measured at admission and after 6 h) in stable CKD patients were not significantly different from those of all patients with AKI. However, patients with higher grades of AKI (AKIN>2) had significantly higher plasma NGAL levels than stable CKD patients.17 The authors have stated that their study involved a small number of patients with stable CKD as a limitation. Probably because of this limitation, they did not perform ROC analysis. In contrast to the results of previous studies, in our study, the median levels of plasma NGAL in AKI patients were higher than those of stable CKD patients. Finally, we believe that the strengths of our study include the following: the AKI and CKD patients in our study were well classified and standardized, and our study also involved a larger cohort population than previous studies directly comparing patients with AKI and CKD.
5. Conclusion
We believe that this study has clearly demonstrated that plasma NGAL levels were higher in AKI patients than in CKD patients. However, in clinical practice, the use of plasma NGAL levels to distinguish between AKI and CKD is limited because of low sensitivity and specificity values of NGAL.
6. Limitations
This study had some limitations. First, in this study, we did not include all AKI and CKD patients to minimize potential confounding factors. For AKI groups, patients who had clearly dialysis indications did not included. Similarly, for CKD group, patients who had known CKD with stage 1 and 5 did not included. If we included all AKI and CKD patients, we might find higher sensitivity and specificity values of NGAL. Second, this study include the fact that it involved a relatively small cohort of patients and that it is a single-center study. Therefore, we believe that there is need for further studies involving a larger cohort population.
Conflict of interest
The authors declared that there is no conflict of interest.
Footnotes
Peer review under responsibility of The Emergency Medicine Association of Turkey.
References
- 1.Kidney disease: improving global outcomes CKD work group: KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 2.Bellomo R., Ronco C., Kellum J.A., Mehta R.L., Palevsky P. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mishra J., Dent C., Tarabishi R., Mitsnefes M.M., Ma Q., Kelly C. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365(9466):1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 4.Ahn J.Y., Lee M.J., Seo J.S., Choi D., Park J.B. Plasma neutrophil gelatinase-associated lipocalin as a predictive biomarker for the detection of acute kidney injury in adult poisoning. Clin Toxicol (Phila) 2015 Dec 18:1–7. doi: 10.3109/15563650.2015.1118487. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Khatami M.R., Sabbagh M.R., Nikravan N., Khazaeipour Z., Boroumand M.A., Sadeghian S. The role of neutrophil-gelatinase-associated lipocalin in early diagnosis of contrast nephropathy. Indian J Nephrol. 2015;25:292–296. doi: 10.4103/0971-4065.147370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mishra J., Ma Q., Kelly C., Mitsnefes M., Mori K., Barasch J. Kidney NGAL is a novel early marker of acute injury following transplantation. Pediatr Nephrol. 2006;21(6):856–863. doi: 10.1007/s00467-006-0055-0. [DOI] [PubMed] [Google Scholar]
- 7.Alge J.L., Arthur J.M. Biomarkers of AKI: a review of mechanistic relevance and potential therapeutic implications. Clin J Am Soc Nephrol. 2015;10:147–155. doi: 10.2215/CJN.12191213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wasung M.E., Chawla L.S., Madero M. Biomarkers of renal function, which and when? Clin Chim Acta. 2015;438:350–357. doi: 10.1016/j.cca.2014.08.039. [DOI] [PubMed] [Google Scholar]
- 9.Ozkan S., Durukan P., Kavalci C., Duman A., Sayhan M.B., Salt O. A. Importance of neutrophil gelatinase-associated lipocalin in differential diagnosis of acute and chronic renal failure. Iran Red Crescent Med J. 2014;16(8):e14133. doi: 10.5812/ircmj.14133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moghazi S., Jones E., Schroepple J., Arya K., McClellan W., Hennigar R.A. Correlation of renal histopathology with sonographic findings. Kidney Int. 2005;67:1515. doi: 10.1111/j.1523-1755.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- 11.Manley J.A., O'Neill W.C. How echogenic is echogenic? Quantitative acoustics of the renal cortex. Am J Kidney Dis. 2001;37:706. doi: 10.1016/s0272-6386(01)80118-9. [DOI] [PubMed] [Google Scholar]
- 12.Devarajan P. Emerging biomarkers of acute kidney injury. Contrib Nephrol. 2007;156:203–212. doi: 10.1159/000102085. [DOI] [PubMed] [Google Scholar]
- 13.Avci Çiçek E., Rota S., Dursun B., Kavalci E. Evaluation of serum NGAL and hepcidin levels in chronic kidney disease patients. Ren Fail. 2016;38:35–39. doi: 10.3109/0886022X.2015.1107823. [DOI] [PubMed] [Google Scholar]
- 14.Bolignano D., Lacquaniti A., Coppolino G., Donato V., Campo S., Fazio M.R. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:337–344. doi: 10.2215/CJN.03530708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Somma S., Magrini L., De Berardinis B., Marino R., Ferri E., Moscatelli P. Additive value of blood neutrophil gelatinase-associated lipocalin to clinical judgement in acute kidney injury diagnosis and mortality prediction in patients hospitalized from the emergency department. Crit Care. 2013 Feb 12;17:R29. doi: 10.1186/cc12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nickolas T.L., O'Rourke M.J., Yang J., Sise M.E., Canetta P.A., Barasch N. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148:810–819. doi: 10.7326/0003-4819-148-11-200806030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soto K., Papoila A.L., Coelho S., Bennett M., Ma Q., Rodrigues B. Plasma NGAL for the diagnosis of AKI in patients admitted from the emergency department setting. Clin J Am Soc Nephrol. 2013;8:2053–2063. doi: 10.2215/CJN.12181212. [DOI] [PMC free article] [PubMed] [Google Scholar]