Abstract
Objectives
The primary aim of this study was to report the vital signs, hemodynamic parameters and pain scores of the patients who have received procedural sedation and analgesia (PSA) with either ketofol (combination of ketamine and propofol) or etofen (combination of etomidate and fentanyl) and compare the proportion of patients with airway or respiratory adverse events (AEs) requiring an intervention and calculate the relative risk of AEs with each combination.
Methods
This study is a prospective observational study with survey analysis. All patients received procedural sedation and analgesia (PSA) with either ketofol (combination of ketamine and propofol) or etofen (combination of etomidate and fentanyl) were prospectively observed. Vital and hemodynamic parameters and pain scores of the patients were recorded by automated equipment and visual analog scale (VAS) charts.
Results
112 patients were enrolled, 55 received ketofol and 57 received etofen. All patients with a respiratory AE (n = 27) observed to receive a respiratory intervention. Respiratory AE rate and proportion of patient who required a respiratory intervention were significantly higher with ketofol (p = 0.0029). Overall AE rate, and rates of desaturation, emergence reaction were also significantly higher in ketofol group.
Conclusion
Etofen is a promising combination for the PSA of adult patients with lower respiratory AE and intervention rates and with better hemodynamic profile.
Keywords: Procedural sedation, Emergency department, Ketamine, Etomidate, Fentanyl, Propofol
1. Introduction
Procedural sedation and analgesia (PSA) is defined as the technique of administering sedatives or dissociative agents with or without analgesics to induce an altered state of consciousness that allows the patient to tolerate painful or unpleasant procedures while preserving cardiorespiratory function.1 American College of Emergency Medicine (ACEP) recommends propofol (A), etomidate (B), ketofol (B), ketamine (C) and alfentanil (C) for the PSA of adults with given levels of recommendations.1
The use of short-acting sedative agents such as propofol and etomidate for emergency department (ED) PSA has been widely accepted since shorter periods of impaired levels of consciousness created by those agents and less risk for respiratory adverse events (AE).1 Also, the combination of ketamine and propofol (ketofol) has been one of the most studied of combinations because of the theoretical synergy of those agents.1 Fewer respiratory AEs with and higher provider satisfaction was reported by ketofol compared to propofol.2, 3, 4
Etomidate is a non-barbiturate, non-benzodiazepine, imidazole derivate providing rapid onset of action, short duration of sedation, clinically insignificant hemodynamic alterations with minimal respiratory and hemodynamic effects.5, 6, 7, 8 The biggest disadvantage of etomidate is the associated myoclonus which is extensively described in 20–40% of patients.5, 8, 9, 10 Since, etomidate does not have any analgesic property, it has to be combined with an analgesic agent like fentanyl. However, to our knowledge, etofen (combination of etomidate and fentanyl) and ketofol has not been compared in PSA to date.
The primary aim of this study was to observe and compare the number and proportion of patients with airway or respiratory AEs requiring an intervention, and calculate the relative risk of adverse events with ketofol compared to etofen. Secondary aims were to compare the incidence of overall and non-respiratory AE rates, maximum changes in hemodynamic parameters, number and proportion of patients who received additional drug dose, duration of sedation, level of sedation, patient pain and physician satisfaction scores.
2. Methods
2.1. Study design and setting
This study is a prospective observational study with survey analysis which has been performed at the EM Department of a Level 3 Adult Trauma and Burn Center with an annual patient load of 500.000 in Turkey. Study protocol was examined and approved in by the Hospital Ethics Board (12/06/2012; B104ISM4340029/1009/49).
2.2. Selection of participants
All the patients who have been decided to receive PSA for the treatment of their isolated upper extremity orthopedic injuries in the procedure room by emergency physicians (EPs) and orthopedic surgeons (OS) have been enrolled for observation if they had an American Society of Anesthesiologists (ASA) physical status of two or less. Patients were excluded if they were below 16 years of age, had a known hypersensitivity to the study agents, were pregnant, already treated with analgesics or oxygen, or indicated to have surgery.
2.3. Data collection
All demographic information (age, sex, weight, comorbid diseases) were gathered and recorded before the procedure. At the beginning of the PSA, baseline values of the following hemodynamic parameters, and pain and sedation scores of the patients were recorded by automated equipment, visual analog scale (VAS) charts and EPs: end-tidal CO2 capnography (ETCO2), systolic [SBP] and diastolic blood pressure [DBP], mean arterial pressure [MAP], heart rate [HR], respiratory rate [RR], peripheral oxygen saturation [pSO2], Ramsay Sedation Score and VAS. All monitors have been set to automatically measure and record all hemodynamic parameters at every 5 min until the patients were recovered to the baseline alertness and sedation score, which were also recorded at the same time intervals. According to the departmental PSA protocol, patients with Ramsay score of 1–3 were re-evaluated for the need for an additional dose of treatment. Since the design was observational, EPs decided this need by themselves without a strict supervision.
All PSA procedures have been attended by an EP, an EM resident, and an EM nurse. Orthopedic procedures have been performed by OSs. The sole responsibility of the OS was the reduction and splinting. All other patient related medical interventions and evaluations have been performed and monitored by EPs. All the data and adverse events were recorded by the EM nurses and researchers.
Maximum recorded increase and decrease of each hemodynamic parameter during PSA have been obtained from the electronic records of the automated equipment. Pain intensity of each patient was evaluated by using a 10-cm long VAS chart before and after the PSA that had marks of 0 for no pain and 10 for worst possible pain. After the PSA was finished and patients were recovered to the baseline alertness and sedation score, OSs were asked to evaluate their satisfaction using a 10-cm long VAS chart which was marked by 0 for very unsatisfied and 10 for completely satisfied.
According to previous research, cardiopulmonary arrest, desaturation (defined as pSO2 below 94%), myoclonus (rhythmic, shock-like muscle contractions), emergence reaction (unpleasant dreams or hallucinations when emerging from the dissociative state), vertigo (dizziness), nausea, cough and dysrhythmia (any rhythm other than patient's primary rhythm) were selected as AEs and the presence of those AEs were selectively sought and documented during the PSA.
2.4. Interventions
According to the written departmental protocol for PSAs, ketofol was given as 0.75 mg/kg IV bolus of ketamine and propofol, etofen was given as 0.15 mg/kg IV bolus of etomidate and 0.15 μg/kg fentanyl as the initial dose. Repeated doses of 0.375 mg/kg of ketamine and propofol were given in every 3 min and 0.1 mg/kg of etomidate was administered in every 2 min as needed to achieve and maintain adequate sedation. Patients in whom the above protocols were violated were excluded.
2.5. Outcome measures
Our primary outcome measure was the number and proportion of patients with airway or respiratory AEs requiring an intervention. Respiratory AEs were predefined as desaturation (pSPO2<94%), apnea (cessation of respiration for more than 6 s on waveform capnography), respiratory depression (clinical evaluation), airway obstruction (complete absence of waveform) and aspiration (clinical evaluation). Respiratory interventions have been predefined and grouped as airway maneuvers (repositioning and airway opening maneuvers), maneuvers plus nasal oxygen administration (addition of supplemental oxygen), bag-mask-valve administration and any other airway intervention (intubation). Indications for each of those interventions have been clinically evaluated according to ACLS guidelines.
Secondary outcome measures were also predefined as any observed non-respiratory AE, the maximum recorded change in each hemodynamic parameter during the PSA, additional drug need, sedation duration (from the beginning of the first dose to the time full alertness has been gained), sedation levels, and patient pain and physician satisfaction scores.
2.6. Sample size estimation and power analysis
The sample size was estimated using the respiratory AE rates reported at previously published articles (22%–38%) for ketofol.2, 3, 11 We estimated a sample size of 49 to find at least 20% difference between groups with an alpha value of 0.05 and power of 0.80. Therefore we enrolled 55 patients (49 + 10%) for each group. The post-hoc power of this study for the comparison of the respiratory AE, need for a respiratory intervention, and any AE rates were 0.85, 0.85, and 0.86, respectively.
2.7. Statistical analysis
Categorical data were presented as number and proportions, with the difference of proportions between groups and their 95% confidence intervals (CI). Continuous data were presented as means and standard deviations with CIs or as medians and interquartile ranges (IQR) with 95% CI as defined by Campbell according to their distribution.12 Fisher's exact, Mann-Whitney U, Wilcoxon and t tests were used accordingly. Relative Risks (RR) were reported CI and NNHs (Number Needed to Harm). G*Power v3.1.7 and MedCalc v15.11.4 (MedCalc Software bvba, Belgium) were used in analysis.13
3. Results
3.1. Characteristics of study subjects
This study was conducted between July 2012, 1st and January 2013, 31th. Study population included 55 patients in ketofol and 57 patients in etofen groups. All eligible patients were successfully enrolled to the study and no patients met the exclusion criteria. Both treatment arms were similar with regard to baseline demographic characteristics (Table 1). All procedures were completed successfully, and no procedure was stopped due to AEs.
Table 1.
Demographic characteristics of patients.
| Scale variables | Ketofol, n = 55 Median (IQR) [Range] |
Etofen, n = 57 Median (IQR) [Range] |
P |
|---|---|---|---|
| Age, y | 37 (22, 60) [16, 92] | 35 (25, 53) [16, 88] | NS |
| Weight, kg | 66 (52, 80) [46,130] | 66 (45, 75) [35, 130] | 0.025 |
| SBP, mmHg | 130 (120, 130) [80, 170] | 130 (120, 130) [90, 210] | NS |
| DBP, mmHg | 80 (70, 80) [60, 100] | 70 (70, 70) [60, 110] | NS |
| MAP, mmHg | 93.33 (86.67, 93.33) [66.67, 120.00] |
91.67 (86.67, 91,67) [70.00, 136,67] |
NS |
| HR, bpm | 82 (76, 82) [60, 130) | 87 (79, 87) [60, 125] | NS |
| RR,/min | 18 (16, 18) [14, 23] | 18 (16, 28) [15, 24] | NS |
| SO2, % | 98 (98, 98) [94, 100] | 97 (96, 97) [40, 100] | <0.001 |
| ETCO2, % | 32 (29, 32) [27, 41] | 38 (36, 38) [27, 43] | <0.001 |
| Pain (VAS, cm) | 9 (8, 9) [7, 10] | 8 (8, 9) [7, 10] | <0.001 |
| Categorical variables | Ketofol, n = 55 n (%) |
Etofen, n = 57 n (%) |
Difference % [95% CI] |
P |
|---|---|---|---|---|
| Male | 32 (58.2) | 38 (66.7) | −8.5 (−26.37, 9.39) | NS |
| Any comorbidity | 19 (34.5) | 28 (49.1) | −14.6 (−32.64, 3.50) | NS |
| CAD | 2 (3.6) | 5 (8.8) | −5.1 (−13.99, 3.73) | NS |
| DM | 5 (9.1) | 5 (8.8) | 0.3 (−10.25, 10.89) | NS |
| HT | 9 (16.4) | 15 (26.3) | −10.0 (−25.00, 5.08) | NS |
| CHF | 1 (1.8) | 2 (3.5) | −1.7 (−7.63, 4.25) | NS |
| Other comorbidities | 9 (16.4) | 14 (24.6) | −8.2 (−23.05, 6.65) | NS |
| Location | ||||
| Hand | 6 (10.9) | 14 (24.6) | −13.7 (−27.53, 0.23) | NS |
| Wrist | 15 (27.3) | 8 (14.0) | 13.2 (−1.60, 28.06) | NS |
| Forearm | 15 (27.3) | 19 (33.3) | −6.1 (−23.04, 10.92) | NS |
| Elbow | 7 (12.7) | 2 (3.5) | 9.2 (−0.80, 19.24) | NS |
| Arm | 4 (7.3) | 4 (7.0) | 0.3 (−9.29, 9.79) | NS |
| Shoulder | 15 (27.3) | 17 (29.8) | −2.6 (−19.27, 14.17) | NS |
CAD: Coronary Artery Disease; DM: Diabetes Mellitus; HT: Hypertension; CHF: Congestive Heart Failure; p values by Mann-Whitney U and Fischer's Exact test.
3.2. Main results
All patients with a respiratory AE (n = 27) received a respiratory intervention. Respiratory AE rate and proportion of patient who required a respiratory intervention were significantly higher with ketofol (p = 0.0029). Overall AE rate, and rates of desaturation, emergence reactions were also significantly higher in ketofol group. Relative Risk of having any adverse event with ketofol compared to etofen was 1.85 (95% CI: 1.22, 2.83; p = 0.0041) with an NNH of 3.51. Patients in etofen group required significantly more additional doses (p < 0.001) and myoclonus was significantly more common (p = 0.0052). All results were given in Table 2, Table 3.
Table 2.
Comparison of adverse event rates.
| Harm | Ketofol, n = 55 n (%) |
Etofen, n = 57 n (%) |
Difference, % [95% CI] |
P |
|---|---|---|---|---|
| Any adverse event | 34 (61.8) | 19 (33.3) | 28.5 (10.75, 46.23) | 0.0025 |
| Desaturation | 20 (36.4) | 7 (12.3) | 24.1 (8.78, 39.38) | 0.0029 |
| Myoclonus | 1 (1.8) | 10 (17.5) | −15.7 (−26.21, −5.23) | 0.0052 |
| Emergence reaction | 6 (10.9) | 0 (0.0) | 10.9 (2.67, 19.15) | 0.0104 |
| Vertigo | 3 (5.5) | 0 (0.0) | 5.4 (−0.55, 11.45) | NS |
| Nausea | 2 (3.6) | 1 (1.8) | 1.9 (−4.12, 7.90) | NS |
| Cough | 2 (3.6) | 0 (0.0) | 3.6 (−1.31, 8.59) | NS |
| Dysrhythmia | 0 (0.0) | 1 (1.8) | −1.8 (−5.15, 1.65) | NS |
| Respiratory Interventions | 20 (36.4) | 7 (12.3) | 24.1 (8.78, 39.38) | 0.0029 |
| Airway maneuver | 2 (3.6) | 3 (5.3) | −1.6 (−9.24, 6.00) | NS |
| + nasal oxygen | 16 (29.1) | 3 (5.3) | 23.8 (10.50, 37.16) | <0.001 |
| + bag-valve-mask | 2 (3.6) | 1 (1.8) | 1.9 (−4.12, 7.90) | NS |
| Need for an additional dose | 1 (1.8) | 24 (42.1) | −40.3 (−53.59, −26.99) | <0.001 |
Table 3.
Significant Relative Risk of adverse events of Ketofol compared to Etofen.
| Harm | RR | 95% CI | P | NNH |
|---|---|---|---|---|
| Any adverse event | 1.85 | 1.22, 2.83 | 0.0041 | 3.51 (Harm) |
| Desaturation | 2.96 | 1.36, 6.44 | 0.0062 | 4.15 (Harm) |
| Myoclonus | 0.10 | 0.01, 0.78 | 0.0280 | 6.34 (Benefit) |
| Respiratory Interventions | 2.96 | 1.36, 6.44 | 0.0062 | 4.15 (Harm) |
| Need for an additional dose | 0.04 | 0.01, 0.31 | 0.0017 | 2.48 (Benefit) |
RR: Relative Risk, NNH: Number Needed to Harm.
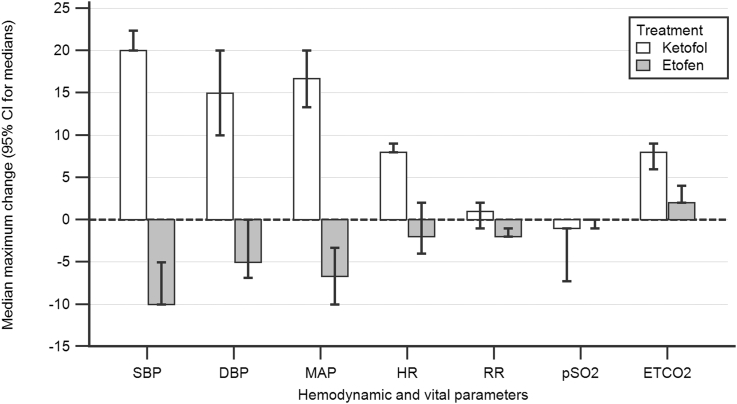
The median maximum changes observed in vital and hemodynamic parameters is shown in Table 3 and compared in Fig. 1. In ketofol group, maximum changes in SBP, DBP, MAP, HR and RR were consistently positive (increased), and significantly greater compared to etofen group. In etofen group, same parameters were consistently negative (decreased) but with smaller amplitudes. pSO2 decrease and ETCO2 increase were significantly greater in ketofol group (see Table 4).
Fig. 1.
Comparison of the maximum changes observed in vital and hemodynamic parameters during PSA.
Table 4.
Comparison of the secondary outcomes.
| Hemodynamic parameters, maximum change |
Ketofol, n = 55 Median (IQR) [95% CI] |
Etofen, n = 57 Median (IQR) [95% CI] |
Significance |
|---|---|---|---|
| SBP, mmHg | 20 (20, 30) [20.00, 22.36] |
−10 (−10, −3) [−10.00, −5.00] |
<0.001 |
| DBP, mmHg | 15 (10, 20) [10.00, 20.00] |
−5 (−10, 0) [−6.84, 0.00] |
<0.001 |
| MAP, mmHg | 16.67 (13.33, 20.00) [13.33, 20.00] |
−6.67 (−10.00, 0.42) [−10.00, 3.33] |
<0.001 |
| HR, bpm | 8 (7.25, 10.00) [8.00, 9.00] |
−2 (−6, 4) [−4.00, 2.00] |
<0.001 |
| RR, rpm | 1 (−2, 2) [−1.00, 2.00) |
−2 (−2.00, 0.25) [−2.00, −1.00] |
0.0013 |
| pSO2, % | −1 (−9, 0) [−7.24, −1.00] |
0 (−1, 1) [−1, 0] |
0.0019 |
| ETCO2, % | 8 (5, 10) [6.00, 9.00] |
2 (−1, 4) [2.00, 4.00] |
<0.001 |
| Sedation duration, min | 18 (16.25, 19.75) [18, 19] |
8 (4, 12) [6, 10] |
<0.001 |
| Ramsey Sedation Score | 5 (5, 6) [5, 6] |
4 (4, 4) [4, 4] |
<0.001 |
| Pain, pre (VAS, cm) | 9 (8, 9) [9, 9] |
8 (8, 9) [8, 9] |
<0.001 |
| Pain, post (VAS, cm) | 3 (2.25, 4) [3, 3] |
2 (2, 3) [2, 2] |
<0.001 |
| VAS decrease | −6 (−6, −5) [−6, −5.76] |
−6 (−7, −5) [−7, −6] |
0.0926 |
| Physician Satisfaction Score | 8 (7, 10) [8, 9] |
8 (7, 8) [7.63, 8.00] |
0.0269 |
P values by Mann-Whitney U test.
In ketofol group, median sedation duration was 10 min longer and Ramsey Sedation Score (RSS) was 1 point higher (Table 3) despite lower need for additional doses. In both treatment arms, post-PSA pain scores were significantly lower than pre-PSA values; however, magnitude of this change was similar. Physician satisfaction scores were significantly higher in ketofol group; however, the difference was within the clinical significance limit of 13 mm (Table 3).
4. Discussion
In this study, we compared the observed safety and efficacy of ketofol with etofen combination for PSA in adult ED patients for the first time, to our knowledge. In our study, significantly higher rate of overall AEs was observed with ketofol. The only observed respiratory AE was desaturation and it was significantly more common with ketofol (Table 2). Others studies also reported similar overall and respiratory AE rates. In previous studies, the overall AE rate was reported as 41.2%–58.1%.14, 15 Respiratory AE rate and respiratory intervention rates were reported as 6.2% and 3.2% for ketofol by Nejati et al.15 Recently, Miner et al. compared the safety and efficacy of propofol, with 1:1 and 4:1 mixtures of ketofol in a randomized, controlled study.16 They reported the respiratory AE rate as 35% (30/85) in 1:1 ketofol arm, which was also similar to our findings.16 The low incidence of respiratory AEs in Nejati et al.’s study might be because of their lower threshold for the definition of desaturation (pSO2 < 90%) in their study.15
Addition of fentanyl to etomidate was proposed to decrease the required dose of etomidate and rate of myoclonus, and this combination was studied in several different clinical situations for almost 40 years.17, 18, 19, 20, 21, 22 However, in several studies no desaturation, respiratory AE or need for an airway intervention were reported.21, 22 The routine use nasal continuous oxygen at the rate of 4–5 L/min during PSA may have prevented patients to desaturate in those studies. On the contrary, Kalogridaki et al. reported that 12 of 21 (57%) patients in etofen and 7 of 25 (28%) patients in propofol group suffered respiratory AE requiring at least an airway maneuver in patients undergoing electrical cardioversion.23 This rate was significantly higher (57% vs 12.3%) than our findings. We think that this difference is the result of the significant difference between study cohorts. In our study, patients in etofen group are significantly younger (median age of 35) than in the Kalogridaki's study (mean age of 61). In addition, we can speculate a higher cardiovascular risk status in Kalogridaki's study since it is a cohort of patients who had electrical cardioversion. Therefore, since our findings for respiratory AE rate lies between those two ends, we think it is compatible with our low risk patient population.
There are also some recent studies that have evaluated the incidence of respiratory AEs and respiratory intervention rates with etomidate alone for PSA in ED, and in all of those studies similar overall AE (47.3%), respiratory AE (8.3%–15.7%) and respiratory intervention rates (15.7%) were reported compared to our findings.6, 7, 24
In our study, the most common non-respiratory AEs were myoclonus (10/55, 17.5%), and emergence reaction (10.9%) in etofen and ketofol groups, respectively (Table 2). In previous research with etofen or etomidate alone, myoclonus was also the most commonly reported AE with a proportion of 6.6%–52%.6, 7, 8, 20, 23, 24 In our study, no treatment has been needed for emergence reactions, and the rate of this AE was similar to previous reports (1.8%–41.9%).11, 14, 15, 16, 25
In our study, the median sedation duration (8 min) was significantly shorter and median sedation depth (RSS = 4) was significantly lighter in etofen compared to ketofol group (18 min, RSS = 5) despite higher rate of additional doses with etofen. Previous studies reported a sedation duration of 7.3–15 min with etomidate or etofen, and the sedation duration of our etofen cohort is among this range.6, 7, 8, 20 Kalogridaki et al. reported that 40% of the patient in etofen group needed additional doses, which is also similar to our findings (42.1%).23
Propofol is known to have a tendency for hypotension and bradycardia, however, in a recent meta-analysis, ketofol was shown to lower those incidences.26 In almost all previous research, the mean BP and HR during PSA have been used for those evaluations. However, we used the maximum changes in all vital parameters since we think it is more important to see the maximum stress patients have been exposed with different agents. We observed that maximum changes with etofen and ketofol to be in opposite direction for almost all vital and hemodynamic parameters, as we have anticipated. With ketofol, median maximum changes in BP (SBP: +20 mmHg, DBP: +15 mmHg, MAP: +16.67 mmHg), RR (+1 breaths/min) and HR (+8 bpm) were all on the positive side (hypertension, tachypnea, and tachycardia), which were significantly different from etofen group. However, Jalili et al. reported the pooled relative risk (RR) for hypotension and bradycardia with ketofol as 0.41 (95% CI 0.17 to 0.97, p = 0.04) and 0.47 (95% CI 0.27 to 0.82, p = 0.008), respectively, where both were significant.26 We think that this difference has emerged from the difference in the reporting of values. In almost all of the previous studies, proportion of patients with an abnormality of a vital or hemodynamic sign (hypotension, bradycardia etc) at any given time have been reported. However, we calculated the absolute values and direction of the maximum changes of each vital parameter compared to the baseline values at each time point. Therefore, for example, the maximum change we observed with ketofol was 16.67 mmHg increase in MAP, however, 6.67 mmHg decrease with etofen (Table 3). As noted in Table 3, maximum changes in all vital and hemodynamic parameters were significantly different between groups. Median of the maximum changes with etofen were consistently on the side of slight decrease in BP (SBP: −10 mmHg, DBP: −5 mmHg, MAP: −6.67 mmHg), RR (−2 breaths/min) and HR (−2 bpm). The amplitude of those maximum changes was much smaller compared to ketofol. In previous studies, minimal hypotension was consistently reported with etofen and etomidate and this decrease was reported as clinically insignificant by authors, which is consistent with our findings.8, 20, 24 Last, in ketofol group, we observed a significantly higher median maximum change in ETCO2 (+8% vs +2%), which is consistent with the higher rate of observed respiratory AEs with ketofol. We concluded from our findings that ketofol creates hypertension, and tachycardia at a significant level. However, etofen seems not to create a clinically significant hemodynamic change, and may be a better agent for patients vulnerable to such differences.
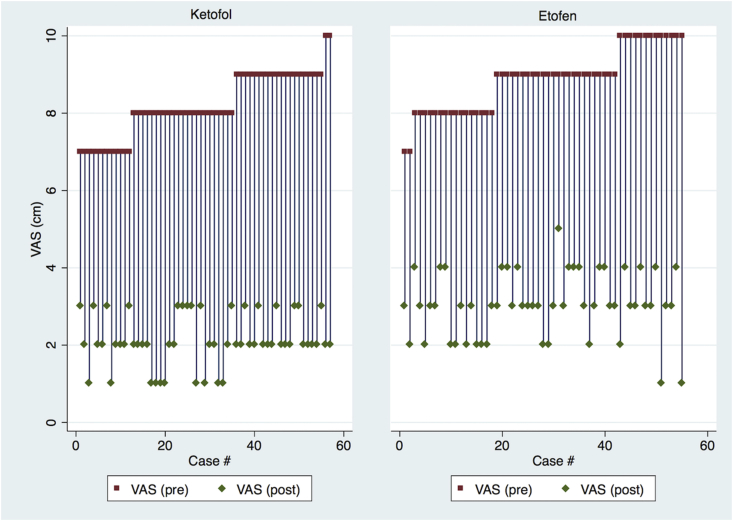
We also found that post-PSA pain scores of patients were significantly decreased in both groups, as expected. However, magnitude of this change was similar as summarized in Fig. 2. Median of the physician satisfaction scores were also clinically similar (8/10).
Fig. 2.
Comparison of pre- and post-procedural pain scores of patients among intervention groups.
In conclusion, a significantly lower rate of overall AEs, respiratory AEs and respiratory interventions with shorter sedation duration with lighter sedation depth were observed with etofen without hemodynamic compromise which results with a higher need for additional doses. Therefore, Etofen is a promising combination for the PSA of adult patients.
4.1. Limitations
First, to decrease the bias in outcomes related with the subjective evaluations of EPs, observed outcomes were approved by the researchers. Second, EPs may have decided to use more additional doses with etofen since they were aware of its shorter duration. Also, even the use of additional doses were limited by departmental protocols, since it was an observational study, EPs may have decided to use additional doses in higher or lower Ramsay scores. Therefore, reported median Ramsay Scores may be biased. Third, none of the reported AEs may have clinical significance since all PSAs were completed without any complications. Fourth, since this is an observational study, it has the methodological disadvantages regarding to this design.
Funding
No funding
Declaration of interest
The authors declare that they have no conflict of interest.
Ethical approval
Local hospital ethics committee approved this protocol as an observational study since there were no previous comparative trials of ketofol and etofen.
Author contributions
Initials of the contributing authors were listed in brackets after the relevant parts of the research: Literature search (ES, SK, HA), study design (ES, HA, ÖG), legislative applications (ES, SK, BK), data collection (ES, SK, BK), supervision and quality control (HA, ÖG), statistical data analysis (ES, HA), data interpretation (ES, HA, ÖG), drafting the manuscript (ES, HA, SK, BK, ÖG). All authors were involved in the writing and critical revision of the manuscript and approved the final version and take responsibility for the paper as a whole.
Footnotes
Peer review under responsibility of The Emergency Medicine Association of Turkey.
Contributor Information
Erkman Sanri, Email: erkmansanri@gmail.com.
Sinan Karacabey, Email: karacabeysinan@gmail.com.
Haldun Akoglu, Email: haldun.akoglu@marmara.edu.tr.
Bora Kaya, Email: drborakaya@hotmail.com.
Ozlem Guneysel, Email: guneysel@gmail.com.
References
- 1.Godwin S.A., Burton J.H., Gerardo C.J. Clinical policy: procedural sedation and analgesia in the emergency department. Ann Emerg Med. 2014;63(2) doi: 10.1016/j.annemergmed.2013.10.015. 247–58.e18. [DOI] [PubMed] [Google Scholar]
- 2.Yan J.W., McLeod S.L., Iansavitchene A. Ketamine-propofol versus propofol alone for procedural sedation in the emergency department: a systematic review and meta-analysis. Acad Emerg Med. 2015;22(9):1003–1013. doi: 10.1111/acem.12737. [DOI] [PubMed] [Google Scholar]
- 3.David H., Shipp J. A randomized controlled trial of ketamine/propofol versus propofol alone for emergency department procedural sedation. Ann Emerg Med. 2011;57(5):435–441. doi: 10.1016/j.annemergmed.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 4.Shah A., Mosdossy G., McLeod S., Lehnhardt K., Peddle M., Rieder M. A blinded, randomized controlled trial to evaluate ketamine/propofol versus ketamine alone for procedural sedation in children. Ann Emerg Med. 2011;57(5) doi: 10.1016/j.annemergmed.2010.08.032. 425–33.e2. [DOI] [PubMed] [Google Scholar]
- 5.Falk J., Zed P.J. Etomidate for procedural sedation in the emergency department. Ann Pharmacother. 2004;38(7-8):1272–1277. doi: 10.1345/aph.1E008. [DOI] [PubMed] [Google Scholar]
- 6.Hunt G.S., Spencer M.T., Hays D.P. Etomidate and midazolam for procedural sedation: prospective, randomized trial. Am J Emerg Med. 2005;23(3):299–303. doi: 10.1016/j.ajem.2005.02.042. [DOI] [PubMed] [Google Scholar]
- 7.Burton J.H., Bock A.J., Strout T.D., Marcolini E.G. Etomidate and midazolam for reduction of anterior shoulder dislocation: a randomized, controlled trial. Ann Emerg Med. 2002;40(5):496–504. doi: 10.1067/mem.2002.126607. [DOI] [PubMed] [Google Scholar]
- 8.Miner J.R., Danahy M., Moch A., Biros M. Randomized clinical trial of etomidate versus propofol for procedural sedation in the emergency department. Ann Emerg Med. 2007;49(1):15–22. doi: 10.1016/j.annemergmed.2006.06.042. [DOI] [PubMed] [Google Scholar]
- 9.Van Keulen S.G., Burton J.H. Myoclonus associated with etomidate for ED procedural sedation and analgesia. Am J Emerg Med. 2003;21(7):556–558. doi: 10.1016/j.ajem.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Dişel N.R., Yilmaz H.L., Sertdemir Y., Yeşilağaç H., Avci A. Etomidate versus ketamine: effective use in emergency procedural sedation for pediatric orthopedic injuries. Pediatr Emerg Care. April 2015:1. doi: 10.1097/PEC.0000000000000373. [DOI] [PubMed] [Google Scholar]
- 11.Willman E.V., Andolfatto G. A prospective evaluation of “ketofol” (ketamine/propofol combination) for procedural sedation and analgesia in the emergency department. Ann Emerg Med. 2007;49(1):23–30. doi: 10.1016/j.annemergmed.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Campbell M.J., Gardner M.J. Calculating confidence intervals for some non-parametric analyses. Br Med J Clin Res Ed. 1988;296(6634):1454–1456. doi: 10.1136/bmj.296.6634.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faul F., Erdfelder E., Lang A.-G., Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 14.Andolfatto G., Abu-Laban R.B., Zed P.J. Ketamine-propofol combination (ketofol) versus propofol alone for emergency department procedural sedation and analgesia: a randomized double-blind trial. Ann Emerg Med. 2012;59(6) doi: 10.1016/j.annemergmed.2012.01.017. 504–12.e1–2. [DOI] [PubMed] [Google Scholar]
- 15.Nejati A., Moharari R.S., Ashraf H., Labaf A., Golshani K. Ketamine/propofol versus midazolam/fentanyl for procedural sedation and analgesia in the emergency department: a randomized, prospective, double-blind trial. Acad Emerg Med. 2011;18(8):800–806. doi: 10.1111/j.1553-2712.2011.01133.x. [DOI] [PubMed] [Google Scholar]
- 16.Miner J.R., Moore J.C., Austad E.J., Plummer D., Hubbard L., Gray R.O. Randomized, double-blinded, clinical trial of propofol, 1:1 propofol/ketamine, and 4:1 propofol/ketamine for deep procedural sedation in the emergency department. Ann Emerg Med. 2015;65(5) doi: 10.1016/j.annemergmed.2014.08.046. 479–488.e2. [DOI] [PubMed] [Google Scholar]
- 17.Fassoulaki A., Pateras C., Kaniaris P. Fentanyl in the prevention of etomidate-induced myoclonus. Cah Anesthesiol. 1987;35(3):201–202. [PubMed] [Google Scholar]
- 18.Ko B.J., Oh J.N., Lee J.H., Choi S.R., Lee S.C., Chung C.J. Comparison of effects of fentanyl and remifentanil on hemodynamic response to endotracheal intubation and myoclonus in elderly patients with etomidate induction. Korean J Anesthesiol. 2013;64(1):12–18. doi: 10.4097/kjae.2013.64.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Souvatzis X., Kalogridaki M., Mavrakis H.E. Adding fentanyl to etomidate fails to reduce painful recall of external direct current cardioversion in adults: a randomised trial. Hell J Cardiol. 2015;56(2):142–148. [PubMed] [Google Scholar]
- 20.Desai P.M., Kane D., Sarkar M.S. Cardioversion: what to choose? Etomidate or propofol. Ann Card Anaesth. 2015;18(3):306–311. doi: 10.4103/0971-9784.159798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang C.-H., Tian X., Yin H.-B., Gao X.-H., Li N. Sedation and analgesia with fentanyl and etomidate for intrathecal injection in childhood leukemia patients. Med Baltim. 2015;94(1):e361. doi: 10.1097/MD.0000000000000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banihashem N., Alijanpour E., Basirat M. Sedation with etomidate-fentanyl versus propofol-fentanyl in colonoscopies: a prospective randomized study. Casp J Intern Med. 2015;6(1):15–19. [PMC free article] [PubMed] [Google Scholar]
- 23.Kalogridaki M., Souvatzis X., Mavrakis H.E. Anaesthesia for cardioversion: a prospective randomised comparison of propofol and etomidate combined with fentanyl. Hell J Cardiol. 2011;52(6):483–488. [PubMed] [Google Scholar]
- 24.Ruth W.J., Burton J.H., Bock A.J. Intravenous etomidate for procedural sedation in emergency department patients. Acad Emerg Med. 2001;8(1):13–18. doi: 10.1111/j.1553-2712.2001.tb00539.x. [DOI] [PubMed] [Google Scholar]
- 25.Andolfatto G., Willman E. A prospective case series of single-syringe ketamine-propofol (Ketofol) for emergency department procedural sedation and analgesia in adults. Acad Emerg Med. 2011;18(3):237–245. doi: 10.1111/j.1553-2712.2011.01010.x. [DOI] [PubMed] [Google Scholar]
- 26.Jalili M., Bahreini M., Irani A.D., Masoomi R., Arbab M., Mirfazaelian H. Ketamine-propofol combination (ketofol) versus propofol for procedural sedation and analgesia: systematic review and meta-analysis. Am J Emerg Med. December 2015 doi: 10.1016/j.ajem.2015.12.074. [DOI] [PubMed] [Google Scholar]