Abstract
Objectives
Ketamine is commonly used in anesthetic and sedation before surgical procedures and acts as an analgesic in smaller doses. The aim of this study was to assess the effects of intranasal (IN) ketamine in patients with moderate to severe limb trauma (visual analog scale (VAS) > 60 mm).
Methods
In a triple-blind randomized controlled clinical trial; 154 patients with isolated orthopedic trauma and visual analog scale (VAS) ≥60 mm were included on the basis of inclusion and exclusion criteria. Patients were divided into two groups of ketamine-IN (0.4 mg/kg IN ketamine and an equal volume of placebo saline intravenously (IV)) and ketamine-IV (0.2 mg/kg ketamine IV with 0.5 ml saline IN) on the basis of balanced block randomization method. At 5, 10, 20, and 30 min, patients were assessed for VAS measurement and adverse events. Repeated measure ANOVA, independent t-test and chi square test were employed. The level of statistical significance was considered to be less than 0.05.
Results
Mean VAS in IN ketamine and IV group at minute 30 was 31.50 ± 13.40 and 29.35 ± 11.73, respectively. At minute 30, 31 patients (20.39%) required a low-dose of morphine as rescue analgesia (P = 0.427). The results showed that mean change score of VAS (difference of time 0 and time 30) in IN ketamine and IV ketamine VAS were 43.8 (95% confidence interval: 41.1–46.5) and 46.4 (95% confidence interval: 42.8–50.1) and there is no difference between two groups in case of score change of VAS (P = 0.245). Adverse events in nasal and intravenous ketamine in both groups were mild and transient.
Conclusion
IN ketamine is associated with few side effects and appropriate analgesic effects in isolated orthopedic trauma patients, and it may be used in cases where there is no need for venipuncture of peripheral vessels, especially in crowded EDs.
Keywords: Ketamine, Intranasal, Pain, Emergency
1. Background
Providing analgesia is one of the most important issues in patient's emergency care. Not good enough staff, and inadequate monitoring, in addition to lack of space in the emergency departments (EDs) is one of the most controversial management issues in overcrowded EDs that require analgesic medication without adverse events and without care requirement. IN fentanyl is routinely used in the ED for pediatric analgesia.1, 2
Ketamine is an N-Methyl-d-Aspartate antagonist receptor, with central and peripheral analgesic effects and its mechanism of action is different from opioids.3 Using IN ketamine with bioavailability of 45% provides acceptable analgesic blood levels.4 Ketamine is commonly utilized in anesthetic and sedation before surgical procedures3, 4 and acts as analgesic in smaller doses.3, 5 In addition, this drug can be used in combination with other opioids to reduce the dose of opioids.6, 7, 8 Little information is available on the ease of use, effects, and optimal dose of this drug as analgesic and few studies have been carried out in this regard, particularly in adults.9
In this study, we aimed to assess the effects of IN ketamine in patients with moderate to severe limb trauma (VAS > 60 mm) and if adequate analgesia without severe adverse events can be achieved, it can also be used in isolated traumatic adults even without venipuncture.
2. Materials and methods
2.1. Study design and setting
In a triple-blind randomized controlled clinical trial, all patients, aged 16 to 60 years, with isolated limb trauma and pain severity (visual analog scale (VAS)) over 60 mm were included based on the comparison of the analgesic effect of intranasal versus intravenous ketamine. The study was based on the principles of the Helsinki Declaration and approved by the Ministry of Health and Medical Sciences and Ethics Committee of Arak University of Medical Sciences, Arak, Iran (registered clinical trial number: IRCT2015061722777N1) as patients entered the study voluntarily with complete satisfaction. The information obtained from patients were kept confidential. Moreover, no cost was imposed on patients.
This study was carried out in …. Hospital ED, … in Vali-asr Hospital , Arak, Iran, …; a teaching general hospital with 600 visits per day in ED. Including criteria were 16–60 year old individuals with isolated limb trauma and pain severity (visual analog scale (VAS)) over 60 mm and exclusion criteria were as follows: lack of patient's consent to participate in the study and having a history of diseases, such as migraines, cardiac ischemia, head trauma with loss of consciousness, unstable vital signs, blood pressure over 180/100, schizophrenia, pregnancy, deformity, or nasal injury preventing administration of nasal medication, inability to express their pain for any cause during the study, a history of sensitivity to opioids and ketamine, taking strong painkillers such as tramadol, methadone, and opiates in the past 4 h, history of addiction and other trauma and instability of vital signs. Patients were not discharged in case of sedation and anesthesia until full consciousness.
The sample size was estimated at 70 patients in each group with respect to an alpha error of 5% and power of 90% with mean (and standard deviation) VAS in two groups of intravenous and subcutaneous ketamine at 1 (0.3), and 1.3 (0.7), respectively, using Stata statistical software and considering the probability of 10% lost to follow-up, 77 patients were considered in each group, resulting in a total of 154 patients.
Each patient was visited by a study investigator for acquisition of written informed consent and determined to meet study including criteria. After completing the questionnaire, including patients' demographic information and obtaining informed consent, in order to maintain a balance between the number of patients among groups, patients were divided into two groups of IN (A) and IV (B) based on balanced block randomization method by research supervisor. Four blocks were used, to conceal the process of patients' group allocation from the researcher and to balance the groups during the study. To achieve this, blocks (AABB, ABAB, ABBA, BBAA, BABA, and BAAB) were randomly selected and allocation pattern of patients was based on that block. The blocks were used as follows: for each 4 patients, one block was selected and nasal and intravenous drugs were given accordingly and to conceal the type of medications prescribed for patients, for the other four patients, another block was selected randomly and this technique continued until the sample size was completed.
The pain severity was assessed by the second physician who was unaware of the grouping and the type of prescribed medication (using a VAS measure) at 0, 5, 10, 20, and 30 min(s) and the data were recorded. A third person administered 0.4 mg/kg IN ketamine (50 mg/ml) to group A, sprayed equally by atomizer in each nostril, and simultaneously an equal volume of placebo saline was slowly injected IV. Group B received slow IV injection of 0.2 mg/kg ketamine (50 mg/ml) with 0.5 ml diluted in saline, while the same volume of 0.5 ml saline was equally sprayed in both nostrils. Drugs in group A were labeled with NA and VA, for IV and IN drugs; and drugs in group B were labeled with VB and NB, respectively, which was calculated by the supervisory assistance research for each patient based on weight and were labeled and given to nurses that were blinded to the study. At 5, 10, 20, and 30 min, patients were assessed for adverse events such as dizziness, dysphoria, nausea, etc. Patients with reduced VAS scale in T10 less than 13 mm (based the primary VAS) were excluded from the study and received routine morphine dose combined with routine monitoring. If the patient's pain level decreased significantly (at least 13 mm based primary VAS), but have not attained the minimal clinically significant change noticeable by patients (30 mm),10 half of the initial dose was given with placebo according to their group category (0.2 mg/kg IN and 0.1 mg/kg IV). In T20, if the decreasing pain based on initial pain score and patients' satisfaction was not at an acceptable level, half the initial dose (up to 0.8 mg/kg IN totally dose) for group A and a maximum total IV dose of 0.4 mg/kg was given IV for group B. All cases requiring the second and third doses were calculated by the supervisory assistance with labels A and B and given to the nurse; by the end of the study (T30) in the absence of acceptable pain relief with a maximum dose of IV or IN, 0.05 mg/kg dosage of IV morphine (rescue analgesic drug) combined with routine monitoring labeled Vm was given and the data was recorded.
The exact primary outcomes focused on pain reduction at 30 min; while secondary outcomes include assessment of adverse events e.g. nausea, vomiting, dizziness etc.
2.2. Data analysis
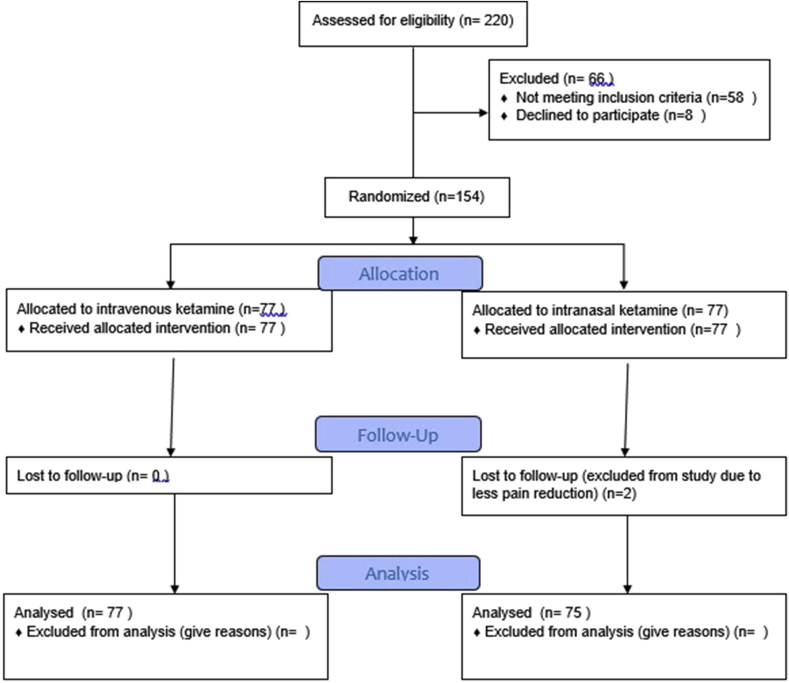
Data analysis was done in a blinded manner and the approach of analysis was not intention to treat analysis. Data of the subjects were presented as mean (SD) or frequency (percentage). Stata software, version 13 (Stata Corp, College Station, TX, USA) was used for statistical analyses. The data were analyzed by running repeated measure ANOVA, independent t-test and chi square test. The level of statistical significance was considered to be less than 0.05 (see Fig 1).
Fig. 1.
CONSORT Flow Diagram of study.
3. Results
In a randomized clinical trial, 154 patients with the complaint of isolated orthopedic trauma, including 91 males (59.09%) and 63 females (40.91%) in two group entered the study in two groups of IN Ketamine (KIN) and IV Ketamine (KIV) that were not significantly different in terms of gender (P = 0.870) (Table 1). Mean age of patients was generally 34.67 ± 12.17 years; that was 36.84 ± 10.84 years in KIN and 32.50 ± 13.08 years in KIV which was significantly different between groups (P = 0.026).
Table 1.
Demographic information and type of injury in patients with isolated orthopedic trauma.
| Characterize | Ketamine-IV | Ketamine-IN |
|---|---|---|
| age | 32.50 ± 13.08 | 36.84 ± 10.84 |
| Gender | ||
| Male | 31 (40.25%) | 45 (58.44%) |
| Injury | ||
| Upper limb | 52 (67.53%) | 47 (61.03%) |
| Lower limb | 25 (32.46%) | 30 (38.96%) |
Mean primary pain in patients according to VAS was generally 75.45 ± 13.91 mm in KIN and 75.06 ± 12.31 mm in KV. Mean pain was 75.84 ± 13.9 mm. There was no statistical significant difference between pain severity in two groups (P = 0.729).
Patients were categorized into KIN and KIV group. Using Repeated ANOVA test, there was no significant difference in mean pain between two groups. VAS was checked after 5 min which showed 49.22 mm reduction in KIV, and 24.28 in KIN group, which was statistically significant (P = 0.001). At 10, 20, and 30 min, the patients were assessed for pain severity, again. In KIN group at the 10th minute, 49 patients (63.64%) had clinically unacceptable pain reduction (less than 30 mm) and required a second dose of IN ketamine, but none of KIV needed a second dose. Moreover, two patients in KIN group had a less than 13 mm pain reduction (unacceptable pain reduction for patients), who were routinely treated with the conventional morphine therapy. Degree of pain relief in the fifth minute compared to the initial pain in KIN and KIV group was 24.28 and 49.22, respectively with a significant difference and the pain relief in intravenous ketamine group had a significant difference in comparison with nasal ketamine (P = 0.001). (Table 2, Table 3).
Table 2.
VAS reduction in patients with isolated orthopedic trauma.
| Time-Group | VAS reduction based primarily pain |
Total | |||
|---|---|---|---|---|---|
| 30< | 13-30 mm | <13 mm | |||
| T10 | Ketamine- IN [n (%)] | 26 (33.76%) | 49 (63.63%) | 2 (2.59%) | 77 |
| Ketamine- IV [n (%)] | 77 (100%) | 0 (0%) | 0 (0%) | 77 | |
| T20 | Ketamine- IN [n (%)] | 75 (100) | 0 | 0 | 75 |
| Ketamine- IV [n (%)] | 77 (100) | 0 | 0 | 77 | |
| T30 | Ketamine- IN [n (%)] | 58 (77.33%) | 17 (22.66%) | 0 (0%) | 75 |
| Ketamine- IV [n (%)] | 63 (81.82%) | 14 (18.18%) | 0 (0%) | 77 | |
T: time (minute), VAS: visual analog scale, mm: millimeter, IN: intranasal, IV: intravenous.
Table 3.
VAS in patients with isolated orthopedic trauma at T0-30.
| Group |
VAS in Ketamine- IN |
VAS in Ketamine- IV |
P Value* | ||
|---|---|---|---|---|---|
| Time | Mean ± SD | 95% Conf. Interval | Mean ± SD | 95% Conf. Interval | |
| T0 | 75.06 ± 12.31 | 72.34–79.34 | 75.84 ± 15.41 | 72.26–77.85 | 0.723 |
| T5 | 50.77 ± 11.89 | 24.48–28.75 | 26.62 ± 9.40 | 48.07–53.47 | 0.001 |
| T10 | 43.50 ± 12.54 | 16.95–20.96 | 18.96 ± 8.82 | 40.66–46.35 | 0.001 |
| T20 | 30.68 ± 11.34 | 18.87–23.46 | 21.16 ± 10.12 | 28.03–33.33 | 0.006 |
| T30 | 31.50 ± 13.40 | 26.68–32.01 | 29.35 ± 11.73 | 28.379–34.63 | 0.005 |
T: time (minute), VAS: visual analog scale, mm: millimeter, SD: standard deviation.
* Repeated ANOVA test.
The results showed that mean change score of VAS (difference of time 0 and time 30) in IN ketamine and IV ketamine were 43.8 (95% confidence interval: 41.1–46.5) and 46.4 (95% confidence interval: 42.8–50.1) respectively and there is no statistical significant difference between two groups in terms of score change of VAS (P = 0.245).
At the 30th minute, patients with less than 30 mm pain reduction than the initial pain received a low dose of morphine injection. A total of 31 patients (20.39%) required a low-dose of morphine injection. There was no significant difference between the two groups in terms of morphine requirement (P = 0.427). Mean dose of IV ketamine was 0.2 mg/kg and was 0.53 mg/kg in the nasal group (Table 4).
Table 4.
VAS changes less than 30 mm in T30 based on T0 in patients with isolated orthopedic trauma that required morphine.
| Morphine T30 | Ketamine- IN | Ketamine- IV | Total |
|---|---|---|---|
| Yes | 17 (22.66%) | 14 (18.18%) | 31 (20.39%) |
| No | 58 (77.33%) | 63 (81.82%) | 121 (79.60%) |
A total of 66 patients (43.42%) had adverse event such as mild dizziness and euphoria that were mild and transient and did not require treatment, while two patients were treated with midazolam due to agitation. There was no significant difference between the two groups in terms of overall adverse events (P = 0.458) (Table 5).
Table 5.
The number of patient with side effects after receiving ketamine in two groups.
| Side effect | Ketamine- IV | Ketamine- IN | P Value |
|---|---|---|---|
| yes | 37 (48.05%) | 29 (38.66%) | 0.458 |
| No | 40 (51.95%) | 46 (61.33%) | |
| Dizziness | 3 (3.89%) | 7 (9.09%) | 0.147 |
| euphoric | 14 (18.18%) | 8 (10.38%) | 0.475 |
| Drowsiness | 3 (3.89%) | 8 (10.38%) | 0.146 |
| Nausea | 2 (2.59%) | 3 (3.89%) | 0.582 |
| Vomiting | 0 (0%) | 0 (0%) | – |
| Agitation | 0 (0%) | 2 (2.59%) | 0.138 |
| Fatigue | 13 (16.88%) | 11 (14.28%) | 0.666 |
The patients' satisfaction of receiving nasal ketamine and not requiring venipuncture was asked at the 30th minute and 48 patients (62.34%) had full satisfaction of painlessness after receiving nasal drug.
4. Discussion
Ketamine is used in acute and chronic pain control in sickle cell anemia and cancer, and is a safe and effective drug for pain management in pre-hospital conditions and after surgery.11
In study of Yeaman et al. 56% of adult patients with orthopedic trauma had 20 mm pain reduction within 30 min using nasal dose of 1 mg/kg ketamine.9 In another study carried out on children with a dose of ketamine 1 mg/kg, about 82% of patients had more than 20 mm pain relief within 20 min and mean pain reduction was about 43 mm. In our study mean pain reduction was about 46–49 mm in our study, within 30 min, and 76.62% and 63.82% of patients had acceptable analgesia (pain reduction of more than 30 mm) in nasal and intravenous groups, respectively Mean dose of IV ketamine was 0.2 mg/kg while it was 0.53 mg/kg in the nasal group.12
Graudius et al. demonstrated equal analgesic efficacy of ketamine 1 mg/kg with nasal fentanyl 0.15 μg/kg in children aged 3–13 years with isolate limb trauma.13 Andolfattog et al. showed that ketamine is an effective drug in controlling pain and mean time needed for pain relief at 13 mm on VAS was 9.5 minutes.14
Johansson et al. examined the effects of nasal ketamine on 9 patients in 2013 with a dose of 0.45–25.1 mg/kg. VAS pain reduced from a mean of 8–10 to 2–4 at 30th minute.15
The results of this study are in line with the results of previous studies, although dosage and the study population are different among studies; and also criteria for adequacy of analgesia were not similar in various studies (13–30 mm); but generally, studies showed the analgesic effectiveness of nasal ketamine.9, 13, 14, 15, 16, 17
Adverse events in nasal and intravenous ketamine in both groups were mild and transient, although the rate of adverse events was higher in IV ketamine group with no statistically significant difference. The most frequent adverse events in patients included fatigue, euphoria, and dizziness. Two patients suffered from severe agitation in IV ketamine group, who were excluded, but no severe adverse events were observed in nasal group. In study of Sener et al. the incidences of adverse events e.g. nausea/vomiting and agitation, were mild with no respiratory events. Our results are in line with the results of other studies, reporting mild and transient adverse events.11, 12, 13, 14, 15, 16, 17, 18
One of the limitations of this study was no long-term follow-up and it was better that patients were followed for at least two hours, which was not possible due to the crowdedness of ED. The study included all cases of isolated limbs trauma, including long bone trauma and other bones such as hands, etc. The use of opioids before arriving at ED was considered based on the history taken and not approved by any tests. No sedation scale (eg. Ramsay Sedation Scale) was used to decide to discharge the patients.
5. Conclusion
Nasal ketamine is associated with few side effects and appropriate analgesic effects in isolated orthopedic trauma patients, and it may be used in cases where there is no need for venipuncture of peripheral vessels, especially in crowded EDs and also in prehospital situation that venipuncture is difficult. The limitations of the present study necessitate future high-quality trials on adults in different clinical conditions with a bigger sample size.
Conflict of interest
There is no conflict of interest.
Acknowledgment
This article was extracted from the residency thesis conducted by Dr Abdolghader Pakniyat. We appreciate the Research Council and Vice Chancellor for research of University of Arak University of Medicine, Arak, Iran, who were responsible for funding the project, and also the parents and children who worked on this project.
Footnotes
Peer review under responsibility of The Emergency Medicine Association of Turkey.
Contributor Information
Ramin Parvizrad, Email: rparvizrad@yahoo.com.
Abdolghader Pakniyat, Email: abdolghader.pakniyat@gmail.com.
Bita Malekianzadeh, Email: malekian.bita@yahoo.com.
Amir Almasi-Hashiani, Email: Amiralmasi2007@gmail.com.
References
- 1.Borland M., Jacobs I., King B., O'Brien D. A randomized controlled trial comparing intranasal fentanyl to intravenous morphine for managing acute pain in children in the emergency department. Ann Emerg Med. 2007;49:335–340. doi: 10.1016/j.annemergmed.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 2.Crellin D., Ling R.X., Babl F.E. Does the standard intravenous solution of fentanyl (50 microg/mL) administered intranasally have analgesic efficacy? Emerg Med Australas. 2010;22:62–67. doi: 10.1111/j.1742-6723.2010.01257.x. [DOI] [PubMed] [Google Scholar]
- 3.Woolf C.J., Thompson S.W. The induction and maintenance of central sensitization is dependent on N-methyl-n-aspartic acid receptor activation: implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- 4.Yanagihara Y., Ohtani M., Kariya S. Plasma concentration pro fi les of ketamine and norketamine after administration of various ketamine preparations to healthy Japanese volunteers. Biopharm Drug Dispos. 2003;24:37–43. doi: 10.1002/bdd.336. [DOI] [PubMed] [Google Scholar]
- 5.Punkett A., Turabi A., Wilkinson I. Battle field analgesia: current trends and concepts in pain treatment in US military casualties. Pain Manage. 2012;2:231–238. doi: 10.2217/pmt.12.18. [DOI] [PubMed] [Google Scholar]
- 6.Todd K.H., Funk K.G., Funk J.P., Bonacci R. Clinical significance of reported changes in pain severity. Ann Emerg Med. 1996;27:485–489. doi: 10.1016/s0196-0644(96)70238-x. [DOI] [PubMed] [Google Scholar]
- 7.Christenson K., Rogers E., Green G.A. Safety and effi cacy of intranasal ketamine for acute postoperative pain. Acute Pain. 2007;9:183–192. [Google Scholar]
- 8.Eide P.K., Jorum E., Stubhaug A., Bremnes J., Breivik H. Relief of post-herpetic neuralgia with the N-methyl-D-aspartic acid receptor antagonist ketamine: a double-blind, cross-over comparison with morphine and placebo. Pain. 1994;58:347–354. doi: 10.1016/0304-3959(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 9.Yeaman F.1, Meek R., Egerton-Warburton D., Rosengarten P., Graudins A. Sub-dissociative-dose intranasal ketamine for moderate to severe pain in adult emergency department patients. Emerg Med Australas. 2014;26(3):237–242. doi: 10.1111/1742-6723.12173. [DOI] [PubMed] [Google Scholar]
- 10.Ducharme J. Tintinalli's Emergency Medicine. seventh ed. 2011. Acute pain management in adults acute pain management in adults; pp. 259–264. [Google Scholar]
- 11.Kurdi Madhuri S., Theerth Kaushic A., Deva Radhika S. Ketamine: current applications in anesthesia, pain, and critical care. Anesth Essays Res. 2014;8(3):283–290. doi: 10.4103/0259-1162.143110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeaman F., Oakley E., Meek R., Graudins A. Sub-dissociative dose intranasal ketamine for limb injury pain in children in the emergency department: a pilot study. Emerg Med Australasia EMA. 2013;25(2):161–167. doi: 10.1111/1742-6723.12059. [DOI] [PubMed] [Google Scholar]
- 13.Graudins A., Meek R., Egerton-Warburton D., Oakley E., Seith R. The PICHFORK (pain in children fentanyl or ketamine) trial: a randomized controlled trial comparing intranasal ketamine and fentanyl for the relief of moderate to severe pain in children with limb injuries. Ann Emerg Med. 2015;65(3) doi: 10.1016/j.annemergmed.2014.09.024. 248–254.e1. [DOI] [PubMed] [Google Scholar]
- 14.Andolfatto G.1, Willman E., Joo D. Intranasal ketamine for analgesia in the emergency department: a prospective observational series. Acad Emerg Med. 2013;20(10):1050–1054. doi: 10.1111/acem.12229. [DOI] [PubMed] [Google Scholar]
- 15.Joakim J., Email Jonas S., Marie N., Erik S., Folke S., Henrik Z. Prehospital analgesia using nasal administration of S-ketamine – a case series. candinavian Journal of Trauma. Resusc Emerg Med. 2013;21:38. doi: 10.1186/1757-7241-21-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosseini Jahromi S.A., Hosseini Valami S.M., Adeli N., Yazdi Z. Comparison of the effects of intranasal midazolam versus different doses of intranasal ketamine on reducing preoperative pediatric anxiety: a prospective randomized clinical trial. J Anesth. 2012;26:878–882. doi: 10.1007/s00540-012-1422-6. [DOI] [PubMed] [Google Scholar]
- 17.Tran K.P., Nguyen Q., Truong X.N. A comparison of ketamine and morphine analgesia in prehospital trauma care: a cluster randomized clinical trial in rural Quang Tri province. Vietnam Prehosp Emerg Care. 2014;18(2):257–264. doi: 10.3109/10903127.2013.851307. [DOI] [PubMed] [Google Scholar]
- 18.Sener S., Eken C., Schultz C.H., Serinken M., Ozsarac M. Ketamine with and without midazolam for emergency department sedation in adults: a randomized controlled trial. Ann Emerg Med. 2011;57(2) doi: 10.1016/j.annemergmed.2010.09.010. 109–114.e2. [DOI] [PubMed] [Google Scholar]