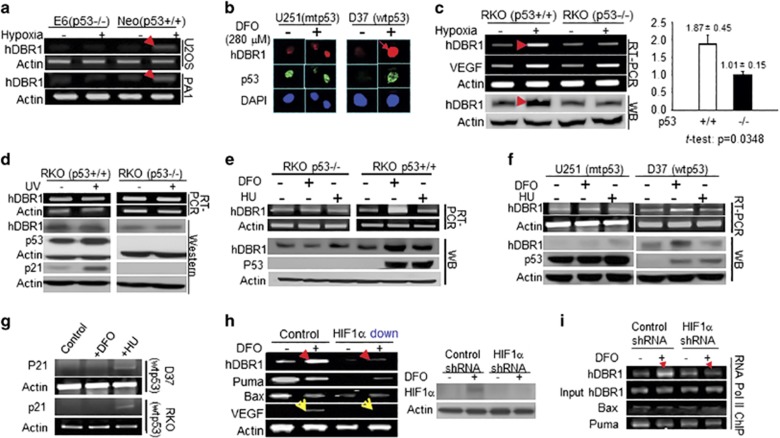
Figure 1.
hDBR1 expression is regulated by p53 in a hypoxic stress-dependent manner. (a) A higher level of hDBR1 expression was identified through RT–PCR in U2OS-neo and PA1-neo cells treated with 0.1% O2 (neo cells harbor a functional p53) than in similarly treated E6 cells, in which p53 function is deficient. (b) In situ immunofluorescent staining showed that the protein expression of hDBR1 correlated with the level of the functional p53 protein in the wtp53 (D37) and mtp53 (U251)-carrying brain tumor cell lines. The intensity of red fluorescence (anti-hDBR1 antibody) was high in treated D37 cells, but not in treated U251 cells (green fluorescence represents the mt or wtp53 protein (anti-p53 antibody)). In U251 cells, mtp53 accumulation was observed as bright green fluorescence under either normal or hypoxic conditions, whereas wtp53 was only highly expressed in D37 cells under hypoxic conditions and not in the normal control. Images were taken at a magnification of × 600. Western blotting and RT–PCR showed similar results in RKO cells (p53+/+ or p53-/-) exposed to hypoxia (c), but not in cells exposed to ultraviolet (UV) (d). (e, f) Western blotting and RT–PCR showed a substantially higher level of hDBR1 expression, but not DNA damage, in wtp53-containing cells than in mtp53 or p53 null cells under hypoxia. (g) RT–PCR showed that p21 expression was increased in wtp53 cells following exposure to hydroxyurea (HU) but was not clearly increased in cells under hypoxic stress. (h) The mRNA expression level of hDBR1, but not Bax or Puma, was dependent on the level of HIF1α (HIF1α levels are shown in the right panel). VEGF (a target of HIF1) expression was used as the positive control (yellow arrowheads). (i) RNA pol II ChIP analysis showed that HIF1 enhanced the recruitment of RNA pol II to the promoter regions of the hDBR1 gene, but not to the corresponding regions of the Bax and Puma genes.