Abstract
Early adversity, in the form of abuse, neglect, socioeconomic status, and other adverse experiences, is associated with poor physical and mental health outcomes. To understand the biologic mechanisms underlying these associations, studies have evaluated the relationship between early adversity and telomere length, a marker of cellular senescence. Such results have varied in regards to the size and significance of this relationship. Using meta-analytic techniques, we aimed to clarify the relationship between early adversity and telomere length while exploring factors affecting the association, including adversity type, timing, and study design. A comprehensive search in July 2016 of PubMed/MEDLINE, PsycINFO, and Web of Science identified 2 462 studies. Multiple reviewers appraised studies for inclusion or exclusion using a priori criteria; 3.9% met inclusion criteria. Data was extracted into a structured form; the Newcastle-Ottawa Scale assessed study quality, validity and bias. Forty-one studies (N =30 773) met inclusion criteria. Early adversity and telomere length were significantly associated (Cohen’s d effect size = −0.35; 95% CI, –0.46 to –0.24, p < 0.0001). Sensitivity analyses revealed no outlier effects. Adversity type and timing significantly impacted the association with telomere length (p < .0001 and p = .0025, respectively). Subgroup and meta-regression analyses revealed that medication use, medical or psychiatric conditions, case-control versus longitudinal study design, methodological factors, age and smoking significantly affected the relationship. Comprehensive evaluations of adversity demonstrated more extensive telomere length changes. These results suggest that early adversity may have long-lasting physiological consequences contributing to disease risk and biological aging.
Keywords: Telomere, meta-analysis, childhood maltreatment, early life stress, adversity, poverty, low socioeconomic status (SES), child abuse
Introduction
Early life adversity is a major public health problem experienced by over 19.4 million children1, 2. Children with a history of early adversity are at a greater risk of developing poor physical and mental health outcomes, including diabetes, asthma, depression, anxiety, and post-traumatic stress disorders3–5. These conditions are often chronic and severe, exacting costs in excess of $124 billion through suffering, disability, treatment, and loss of productivity over the lifespan6. Investigation into the biologic mechanisms by which early adversity increases risk for poor health outcomes provides evidence of accelerated biologic aging through shortened telomere length7–9.
Telomeres are DNA-protein complexes comprised of tandem TTAGGG repeats ranging from a few to 15 kilobases in length that are essential for maintaining chromosomal and genetic stability10. Telomeres shorten with each DNA replication cycle and, as such, telomere length serves as a biomarker of biological aging11. When telomeres become critically short, cells may enter senescence or undergo apoptosis9, 12. Telomere length is influenced by stress and inflammation9. Many chronic illnesses involve prolonged states of stress and/or inflammation, which may contribute associations between telomere length and somatic conditions, including heart disease, diabetes, asthma, obesity, chronic pain, irritable bowel syndrome, and neurodegenerative disorders11, 13–16. Proposed mechanisms underlying associations between stress and telomere length include mitochondrial dysfunction and telomerase inactivation due to heightened and prolonged stress signaling9, 17, 18. In addition to reflecting biologic stress, telomere attrition often precedes chronic disease development, suggesting that telomere erosion may be a causal link connecting early adversity and later disease12.
Telomere attrition early in life may be particularly detrimental19, leading to premature development of stress-related health disorders9, 12. Less than a decade ago, preliminary evidence suggested that childhood adversity was associated with telomere shortening20. Since then, numerous studies have examined associations between early adversity and telomere length9, 14. Shortened telomeres have been linked to adversity at multiple developmental stages18, 21–23 and after several types of adverse exposures24–26. Some investigations suggest a cumulative and dose-dependent negative relationship between early adversity and telomere length27, 28. However, numerous studies have not observed shorter telomeres after early adversity29–43.
Several issues arise when assessing the existing body of knowledge relating telomeres and early adversity. Most studies have modest sample sizes, limiting the ability to draw definitive conclusions. Additionally, variability in study design, methodology, subject characteristics, early adversity type and developmental timing limits the generalizability of available data. This meta-analysis aims to clarify the relationship between early adversity and telomere length by means of a systematic examination of the literature, comparing subjects with early adversity exposure to those without, and to identify moderators of the association with telomere length. We hypothesized that early adversity would be associated with reduced telomere length, and that this relationship would be modified by study and subject characteristics.
Methods and Materials
Protocol and Registration
A protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO, CRD42016035239). This study was designed, executed, and reported using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement44 and the Cochrane reporting items for meta-analyses45. The Meta-analysis Of Observational Studies in Epidemiology (MOOSE) Guidelines46 were also followed and adapted into PRISMA.
Study Eligibility
Included studies: 1) examined the effects of early adversity in the form of abuse, neglect, socioeconomic status (SES), or other adverse exposures on human subjects occurring prenatally up to age 18; 2) provided adequate description of adversity assessments; and 3) presented sufficient data to calculate effect sizes. Prospective, observational, and retrospective studies were considered. Studies using indirect proxies of early adversity, such as parental education alone, were excluded. When two manuscripts presented results from non-independent datasets, the manuscript with the larger number of subjects was included.
Information Sources and Search Strategy
A comprehensive electronic search in July 2016 identified English language studies indexed in PubMed/Medline, PsycINFO, and Web of Science; no publication date limitations were set. The search was performed by investigators with topic clinical and research experience (M.L., K.K.R.) in consultation with a librarian trained in systematic reviews. Investigators reviewed titles, abstracts, and articles; disagreements were settled by consensus. The search strategy included terms and combinations to identify early adversity and telomeres: “child neglect”, placenta, antenatal, prenatal, trauma, poverty, “child abuse”, “socioeconomic status”, “childhood maltreatment”, “parental loss”, environment, neighborhood, abuse, maltreatment, adversity, “early life stress”, telomerase, telomeres, telomere, telo* (Supplemental Table 1). Primary study and review article references were searched; studies were appraised for inclusion or exclusion using a priori criteria as described under study eligibility.
Data Extraction
Data were extracted independently (M.L., L.G., K.K.R.) using a structured form. Extractors were not blinded to study results, authors, or institutions; inter-rater reliability was high (>97%). Conflicts regarding data extraction were resolved by consensus with another reviewer (S.J.R.). Data extraction variables are listed in Table 1. When provided, data fully adjusted for potential covariates were abstracted rather than partially adjusted or unadjusted values. Study quality was assessed using Cochrane Review45 and Agency for Healthcare Research and Quality47 guidelines. The Newcastle-Ottawa Scales (NOS) for cross-sectional, case-control, or cohort designs48 were used to assess risk of bias for all studies (L.G.); blinded replications of these assessments had good reproducibility (97%; M.L.).
Table 1.
Characteristics of Included studies
| Study characteristics | Participant characteristics | Early adversity exposure | Telomere measurement | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| Author, year |
Study design/ Sample |
NOS | Study
N (exposed) |
Age (SD) |
Female | Medical condition |
Psychiatric condition |
Smoke | Medication use |
Type | Timing | Assessment Method |
Adversity and TL Association |
TL Measurement |
Tissue | Covariates |
| Adams, 2007 | Cross-sectional/Newcastle Thousand Families Study | 6/10 | 318 (2391) | 50 (NR) | 62.2% | NR | NR | NR | NR | SES | Birth | Registrar General’s social class | B=85.35, 95% CI −49.95 to 230.60, p>0.05 | qPCR | Leukocyte | Pack years smoking, mean rank of dietary antioxidants, BMI, units of alcohol per week and paternal age at birth |
| Asok, 2013 | Case-control/Low and High risk for maltreatment | 7/9 | 89 (51) | 4.9 (0.5) | 44.9% | NR | NR | – | NR | Other adversity | < 2 years old | Involvement in Child Welfare System | F(1,83)=4.37, p<0.05 | qPCR | Buccal cells | Income, birth weight, gender, and minority status |
| Bersani, 2016 | Cross-sectional/Combat-exposed male veterans | 7/10 | 76 (−) | 34.6 (9.1) | 0% | 6.5% mild asthma or allergies, 2.6% diabetes II, 2.6% stable angina, 11.8% clinical hypertension and 1.3% prostate cancer | 46% PTSD and 22.3% PTSD and MDD | 14.4% | 2.6% statins, 6.5% NSAID, 17.1% antidepressants, 1.3% antibiotics, 1.3% hormone drugs for prostate cancer and 1.3% analgesic | Abuse, neglect, and other adversity | < 18 years old | ETI | β=− 0.470, 95% CI −0.164 to −0.054, p<0.001 | PCR | Leukocyte | Age, BMI, ethnicity and antidepressant use |
| Boks, 2015 | Prospective cohort/Dutch military before and after deployed to Afghanistan | 6/9 | 96 (−) | 27 (9.2) | 0% | NR | 69.7% with high combat trauma exposure and PTSD symptoms | 19.7% | 4.1% antibiotics, 3.1% antihistamines and 1.1% benzodiazepine | Abuse, neglect, and other adversity | < 18 years old | ETI | B=0.16, p=0.95 | qPCR | Leukocyte | Baseline DNA methylation determined by Illumina 450K chip, time interval between assessment, baseline telomere length |
| Brody, 2015 | Cross-sectional/Adults in the Making (AIM) program | 7/10 | 216 (−) | 22 (1.1) | 59.1% | NR | NR | NR | NR | Other adversity | < 17 years old | IAI, DQS, CSS and FSI | -Non-supportive
parenting B=−0.074, SE=0.024, p<.01 |
qPCR | Lymphocyte | SES, life stress, and the use of alcohol and cigarettes at age 17, and blood pressure and BMI at age 22 |
| Cai, 2015 (a)(b) | Cross-sectional/Converge Study (Genital SA(a) or Intercourse SA(b) vs. No SA) | 5/10 | 11 670 | NR | 100% | NR | 45.7% depression | NR | 41.6% antidepressant | Narrowly defined abuse | < 16 years old | Semi-structured interview | -Genital SA t=−1.27, p=0.20 Characteristics of included studies -Intercourse SA t=−2.45, p=0.01 |
Low-coverage whole-genome Sequencing qPCR | Saliva | Genome wide variation in single nucleotide polymorphisms |
| Chen, 2014(a) | Cross-sectional/MDD patients | 6/10 | 20 | 37 (10.8) | 65% | none | 100% MDD | 30% | none | Abuse, neglect, and other adversity | < 18 years old | ACE scale | r=−.13, p>.10 | PCR | Leukocyte | Age and gender |
| Chen, 2014(b) | Cross-sectional/Healthy controls | 6/10 | 20 | 34.8 (9.6) | 67% | none | none | ~22% | none | Abuse, neglect, and other adversity | < 18 years old | ACE scale | r=−.61, p<.05 | PCR | Leukocyte | Age and gender |
| Drury, 2012 | Cross-sectional/Bucharest Early Intervention Project | 8/10 | 109 (109) | 6 to 10 | 43.1% | NR | NR | NR | NR | Other adversity | ≤ 22 months of age | Records | -Percent time institutionalized up to 22
months B=− 0.07027, SE= 0.03092, p<0.05 |
PCR | Buccal cells | Institutionalized versus foster care, ethnicity, age at telomere collection, low birth weight |
| Drury, 2014 | Case-control/Low vs. High Family Instability | 6/9 | 80 (46) | 10.2 (2.9) | 49% | NR | NR | NR | NR | Other adversity | < 15 years old | Questions extracted from the Preschool Age Psychiatric Assessment | Control: M 1.9, SD 0.5 Case: M 1.7, SD 0.3 t=2.51, p=.01 |
PCR | Buccal cell | Child gender, age, maternal and paternal age at conception, race, and maternal education |
| Entringer, 2011 | Case-control/Prenatal stress vs. Non-stress | 8/9 | 94 (45) | 24.4 (0.7) | 77.6% | none | none | none | none | Other adversity | Gestational period up to Birth | Semi-structured interview | B=−0.090, 95% CI = −0.179 to −0.001, p<0.05 | PCR | Leukocyte | Age, BMI, sex, birth weight, and early-life and concurrent stress level |
| Entringer, 2013 | Cross-sectional/Mother-newborn dyads | 7/10 | 27 dyads (−) | 0 | 52% | – | – | – | – | Other adversity | ≤ 9.2 weeks of gestation | Pregnancy-specific stress scale | B=−0.099, 95% CI −0.197 to −0.002, p=0.04 | PCR | Cord blood | Gestational age at birth, weight, sex, antepartum obstetric complications |
| Glass, 2010 | Case-control/Twins UK cohort with Abuse vs. no Abuse | 5/9 | 1 090 (PA n=20; SA n=34) | NR | NR | NR | NR | NR | NR | Narrowly defined abuse | NR | Two single questionnaire items assessed PA and two items assessed SA | -Physical Abuse Case: M 7.04, SD 0.58 Control: M 6.97, SD 0.67 p=0.61 -Sexual Abuse Case: M 6.86, SD 0.53 Control: M 7.05, SD 0.66 p=0.10 |
Southern blot | Leukocyte | Age, sex, BMI and smoking |
| Gotlib, 2015 | Case-control/daughters of mothers with MDD vs. Mothers with no psychiatric disorders | 6/9 | 97 (50) | 11.9 (1.5) | 100% | none | none | NR | NR | Maternal depression | ≤ 14 years old | SCID with mother | Case: M 1.524, SD 0.265 Control: M 1.754, SD 0.361 t(95)=3.582, p=0.001 |
PCR | Leukocyte | none |
| Jodczyk, 2014 | Cross-sectional/Christchurch Health and Development Study | 6/9 | 677 (SA n=83, PA n=112) | 28–30 | NR | none | 42.9% MDD, 33.7% Anxiety disorders and 28.7% Suicidal ideation/attempt | 31.4% | NR | Narrowly defined abuse | ≤ 16 years old | PBI, CTS and interview | -SA2 Case: M 1.24, SD 0.39 Control: M 1.17, SD 0.37 -PA2 Case: M 1.22, SD 0.51 Control: M 1.17, SD 0.33 |
PCR | Leukocyte | Sex, ethnic origin and family SES at birth |
| Kananen, 2010 | Cross-sectional/Health 2000 National cohort in Finland | 5/10 | 939 (552) | 49.8 (1.2) | 63% | NR | 32.9% anxiety; 28% of the cases have comorbid MDD and 22% a comorbid alcohol use disorder | NR | NR | Other adversity; SES | < 16 years old | Interview | -Number of childhood
adversities β= − 0.091, SE=0.32, p= 0.005 -Parental unemployment β=−0.157, p=0.008 |
PCR | Leukocyte | Age and sex |
| Kiecolt-Glaser, 2011 | Cross-sectional/community sample; caregiver vs. noncaregiver | 7/10 | 132 (42 Abuse and 74 Adversity) | 69.7 (10.1) | 72% | none | NR | 3.8% | Excluded patients using statins, systemic steroids, orantibiotics | Other adversity; Abuse and neglect | ≤ 16 years old | CTQ and semi-structured interview | -Other adversity t(73)=1.95, p=0.05 -Abuse and neglect t(74)=0.67, p=0.50 |
Southern blot | Leukocyte | Age, sex, BMI, exercise, sleep, alcohol use, caregiving status |
| Malan-Müller, 2013 (a)(b) | Case-control/ACE vs. No ACE (without(a) or with(b) HIV-positive) | 6/9 | 128 (66) | 29.8 | 100% | 64.84% HIV-positive | NR | NR | 16% antiretroviral treatment | Abuse and neglect | < 18 years old | CTQ | HIV(−) Control: M 1.02, SD
0.38 HIV(−) Case: M 0.92, SD 0.35 HIV(+) Control: M 0.59, SD 0.19 HIV(+) Case: M 0.62, SD 0.2 |
PCR | Leukocyte | none |
| Marchet-to, 2016 | Prospective cohort/Mother-newborn dyads | 7/9 | 24 dyads (6) | 0 | NR | – | – | – | – | Other adversity | ≤ third trimester of gestation | Holmes & Rahe Stress Scale | Case: M 6.98, SD 0.89 Control: M 8.74, SD 1.05 t=−3.99, p=0.003 |
TRF | Cord blood | Maternal age, gestational age at birth, and birth weight no different between groups |
| Mason, 2015 | Cross-sectional/Nurses Health Study II (NHSII) | 8/10 | 1 135 (PA −660; SA - 467) | 45.5 (4) | 100% | 86% type 2 diabetes or cardiovascular disease | NR | NR | NR | Narrowly defined abuse | ≤ 17 years old | Revised Conflict Tactics Scale (PA) and interview (SA) | -Sexual abuse Control: M −0.744, SD 0.3 Case: M −0.749, SD 0.3 |
PCR | Leukocyte | Age at blood draw, paternal age at participant’s birth, race, participant’s parental SES, |
| Mason, 2015 (cont.) | -Physical abuse Control: M −0.736, SD 0.3 Mild Case: M −0.752, SD 0.4 Moderate Case: M −0.773, SD 0.3 Severe Case: M −0.722, SD 0.4 |
childhood somatogram score, parental history of depression and medical issues | ||||||||||||||
| Mitchell, 2014 | Case-control/Harsh vs. Nurturing environment | 6/9 | 40 (20) | 9 | 0% | NR | NR | NR | NR | Abuse, neglect, and other adversity | ≤ 9 years old | Social disadvantage by survey Conflict Tactics Scale for physical and emotional abuse, CIDI for maternal depression | Control: M 10.3, SD 2.5 Case: 9.6, SD 4.1 |
PCR | Saliva | None |
| Need-ham, 2012 (a)(b) | Cross-sectional/AMERICO study: high(a) and low income(b) | 5/10 | 70 (14 <$40,000; 45 $40–$60,000) | 9.9 (1.5) | 52% | none | none | NR | NR | SES | ≤ 10 years old | SES Questionnaire | -Comparison with family income
≥$70,000 $40,000–$69,000 per year |
PCR | Leukocyte | Sex, age and race/ethnicity |
| Need-ham, 2012 (a)(b) (cont.) | OLS=−0.33, 95% CI
−0.68 to 0.03, p=0.07 −<$40,000 per year OLS=−0.38, 95% CI −0.82 to 0.06, p=0.09 |
|||||||||||||||
| O’Donovan, 2011 | Cross-sectional/ACE vs. No ACE (with or without PTSD) | 7/10 | 90 (18) | 30.55 (7.4) | 51.1% | none | 47.7% PTSD | 20.4% | none | Abuse and neglect | ≤ 14 years old | Life Stressor Checklist | r=− 0.42, p=0.003 | PCR | Leukocyte | Age |
| Révész, 2016 | Prospective/Netherlands Study of Depression and Anxiety (NESDA) | 6/9 | 2 936 (−) | 41, 47 | 66% | ~ 33.3% one or more somatic diseases | 74% current or remitted diagnosis of a depressive and/or anxiety disorder | 38.7% | NR | Abuse and neglect; SES3 | < 16 years old | Childhood Trauma Interview (CTI) and interview | Childhood trauma determinants of 6-year TL
attrition β=−23.81, SE=7.74, p=0.002 Neighborhood quality/SES3 B = −173.80, 95% CI: −298.80,− 49.01 p = 0.006) |
PCR | Leukocyte | Age and sex |
| Robertson, 2012 (a) | Cohort/The West of Scotland Twenty-07 Study- 1970s | 7/9 | 775 (−) | 35 | 53.3% | NR | NR | NR | NR | SES | ≤ 15 years old | Interview | -Parental class
“V” B=−0.772, SE=0.363, p=0.034 - Household financial difficulties B=−1.021, SE=0.817 p=0.212 |
PCR | Leukocyte | Gender and assay variation |
| Robertson, 2012 (b) | Cohort/The West of Scotland Twenty-07 Study- 1950s | 7/9 | 866 (−) | 55 | 54.2% | NR | NR | NR | NR | SES | ≤ 15 years old | Interview | -Parental class
“V” B=−0.153, SE=0.326, p=0.640 - Household financial difficulties B=−0.837, SE=1.057, p=0.429 |
PCR | Leukocyte | Gender and assay variation |
| Robertson, 2012 (c) | Cohort/The West of Scotland Twenty-07 Study- 1930s | 7/9 | 544 (−) | 75 | 56.8% | NR | NR | NR | NR | SES | ≤ 15 years old | Interview | -Parental class
“V” B=−0.286, SE=0.393, p=0.467 - Household financial difficulties B=−0.113, SE=0.490, p=0.818 |
PCR | Leukocyte | Gender and assay variation |
| Robles, 2016 | Cross-sectional/Children reporting warmth or conflict in the parent-child dyad | 6/10 | 39 (−) | 11 (1.5) | 59.3% | NR | NR | NR | NR | Other adversity | ≤ 11 years old | Youth Everyday Social Interaction, Mood measure and Child Home Data Questionnaire (marital interactions) | Parent–child
conflict B=−0.33, 95% CI −0.86, 0.19, p>0.05 Marital conflict B=−1.51, 95% CI−2.49 to −0.53, p<.001 |
PCR | Leukocyte | none |
| Savolainen, 2014 | Case-control/ACE vs. No ACE from Helsinki Birth Cohort Study | 6/9 | 1 486 (Separated, n=215; EA, EN or PA, n=953) | 61.5 (2.9) | 68.2% | 4.2% coronary heart disease, 2.6% stroke and 15.2% type 2 diabetes | 5,7% has been hospitalized for psychiatric disorder | 23.1% | NR | Other adversity; Abuse and neglect | < 10 | Finnish National Archives’ register for parental separation, retrospective question of trauma and TEC for lifespan experiences | -Non-separated vs.
Separated B=−0.108, 95% CI −0.255 to 0.039, p=0.414 -Abuse and neglect B=−0.033 CI=−0.0179, 0.110 (p=0.696) |
PCR | Leukocyte | Age, sex, DNA concentration, smoking, alcohol use, BMI, physical activity, any hospitalized mental disorders, medical issues, education, father’s occupation status in childhood, mother’s age at delivery. |
| Schaakxs, 2015 | Cross-sectional/Netherlands Study of Depression in Older Persons (NESDO) | 7/10 | 496 (336) | 70.6 (7.4) | 65.1% | Chronic diseases, 1.45 (1.17) median | 74,1% depressive disorder | NR | NR | Other adversity; Abuse and neglect | < 16 years old | CTI and interview | - Childhood other
adversity B=−79.88, SE=38.18, p=0.04 -Abuse and neglect B= −2.73, SE=8.01, p= 0.73 |
PCR | Leukocyte | Age, sex, and chronic diseases |
| Shalev, 2013 | Prospective/EnvironmentRisk Longitudinal Twin Study | 7/9 | 236 (108) | 5y then 10y | 49% | NR | NR | NR | NR | Other adversity; Narrowly defined abuse | ≤ 10 years old | CTS for WV, interview for bullying and PA | -Other
adversity B=−0.052, SE=0.021, p=0.015 -Physical abuse B= − 0.084 (SE=0.035, p= 0.018) |
PCR | Buccal cells | Baseline TL at 5 years age, sex, socioeconomic deprivation and BMI at age 10 |
| Surtees, 2011 | Cross-sectional/EPIC-Norfolk study | 8/10 | 4 441 (2 235) | 62 (−) | 100% | 9.8% diabetes or myocardial infarction or stroke | 19.2% MDD and 4.3% GAD | 40.6% | NR | Other adversity | < 17 years old | HLEQ | B=0.013, 95% CI 0.002 to
0.024, p=0.015 (positive B=shorter telomeres) |
PCR | Leukocyte | Age, physical health score, social class, obesity, smoking, self-reported health, disease |
| Theall, 2013 | Cross-sectional/Recruited through urban schools in New Orleans | 6/10 | 99 (36) | 4–14 | 52.9% | – | – | NR | SES | ≤ 14 years old | U.S. Census for economic deprivation, interview for neighborhood disorder | -Neighbor-hoods with high perceived
disorder β=−0.478, SD=0.184, p<0.05 -Percent below poverty line β=−0.010, SD=0.004, p<0.05 |
PCR | Saliva | Age, sex, household socioeconomic position, and years living in the neighborhood | |
| Tyrka, 2015(a) | Case-control/ACE vs. No ACE (with lifetime psychiatric disorder) | 8/9 | 111 (72) | 33.4 (11.4) | 66% | none | 11.7% MDD, 22.5% depressive, 2.7% PTSD and 11.7% anxiety | 13.5% | none | Other adversity; Abuse and neglect | < 18 years old | CTQ and interview | -Combined Case: M 5642.7, SD 4138.2 Control: M 7181.00, SD 4362.80 -Other adversity Case: M 6482.9, SD 3932.3 Control: M 7181.00, SD 4362.80 -Abuse and neglect Case: M 6034.1, SD 3784.0 Control: M 7181.0, SD 4362.8 |
PCR | Leukocyte | Age, gender, education, BMI, and childhood socioeconomic adversity |
| Tyrka, 2015(b) | Case-control/ACE vs. No ACE (Healthy sample) | 8/9 | 179 (66) | 29.5 (9.9) | 57.5% | none | none | 7.8% | none | Other adversity | < 18 years old | CTQ and interview | Case: M 6432.9, SD 3372.7 Control: M 7181.00, SD 4362.8 |
PCR | Leukocyte | Age, gender, education, BMI, and childhood SES |
| Tyrka, 2010 | Case-control/ACE vs. No ACE | 8/9 | 31 (10) | 26.9 (10.1) | 70.9% | none | none | 9.6% | none | Abuse and neglect | < 18 years old | CTQ | Case: M 0.70, SD 0.24 Control: M 1.02, SD 0.52 t(29) = 2.4, p=0.03 |
PCR | Leukocyte | Age, sex, smoking, OCP, BMI, race, SES, education,stress |
| Van Ockenburg, 2015 | Cross-sectional/Prevention of REnal and Vascular ENd stage Disease (PREVEND) | 9/10 | 1 094 (430) | 53.1 (11.4) | 53.7% | 19.1% chronic disease | NR | 23.9% | NR | Other adversity | < 12 years old | LTE | B=−0.001, SE=0.012, p=0.936 | PCR | Leukocyte | Age, sex, BMI, chronic diseases, frequency of sports, smoking, level of education |
| Wojcicki, 2015 | Cross-sectional Recruited prenatally at two hospitals in San Francisco | 8/10 | 203 (65) | 4 and 5 | NR | NR | Child behavior: 3.1% attention, 3.5% anxiety, 4.6% affective, 5.1% Developmental, 5.0% Oppositional defiant | – | NR | Maternal depression | ≤ 3 | Center for Epidemiologic Studies Depression Scale, Edinburgh Postpartum Depression Scale or major depressive episode/dysthymia per the MINI | β=− 363.99, p=0.01 | PCR | Leukocyte | Maternal TL, paternal age, child anxiety at 5 years and oppositional defiant behavior at 3, 4 or 5 years |
Note: BMI, body mass index. ETI, Early Trauma Inventory; MDD, Major depressive disorder; PCR, polymerase chain reaction; TRF, Telomere Restriction Fragment assay; PBI, Parental Bonding Instrument. SCID, Structured Clinical Interview for DSM-IV; CTQ, Childhood Trauma Questionnaire; WV, witnessing domestic violence; SL, separation and loss; NR, not reported; NSAID, nonsteroidal anti-inflammatory drugs; IAI, Ineffective arguing inventory; DQS, Discussion quality scale; CSS, Carver support scale; FSI, Family support inventory; ACE, Adverse Childhood Experiences. CTS, Conflict Tactics Scale; TEC, Traumatic Experiences Checklist; TL, Telomere length; HLEQ, Health and Life Experiences Questionnaire; M, mean; SD, standard deviation; SES, socio-economic status; LTE, List of Threatening Events; OCP; oral contraceptive pill use.
=based on percentages reported in Adams J et al, J Epidemiol Community Health 2004. Dec;58(12):1028–9;
=values obtained from systematic review reporting means and standard deviation;
=values obtained from Park M et al, PLoS One 2015. 10(6):e0128460, which utilizes the same dataset.
When studies did not report an overall effect but instead included data on various types of adversity exposures in the same group of subjects31, 33–35, 42, 43, 49, data were converted to standardized mean differences (SMDs) and pooled to allow comparison of a grouped early adversity value in the main meta-analysis50, unless the adversity grouping was not limited to early life36. When studies presented more than one independent dataset by one of the subgroups examined in this study (e.g., adversity type, medical/psychiatric condition), these were treated as separate datasets represented by the first author’s last name, publication date, and alpha character32, 34, 39, 40, 51, 52.
Statistical Analysis
Data were converted into SMDs using the effect size calculator53 and reported as Cohen’s d54. The SMD is the mean difference in telomere length between the early adversity-exposed and non-exposed groups divided by the pooled standard deviation, resulting in a unitless effect size measure comparable between studies. By convention, effect sizes of 0.2, 0.5, and 0.8 are small, medium and large, respectively54. If correlations (r) or odds ratios (OR) were reported, they were converted to Cohen’s d using the formulas d = 2r/(1−r2)1/2 or d = OR(31/2/π), respectively50 .
Analyses were performed using Comprehensive Meta-Analysis Software (V2.2.064 Biostat, Englewood, New Jersey). Heterogeneity was calculated using the I2 statistic, which provides a measure of the variance attributable to between-study differences55 (0% = none, 25% = low, 50% = moderate and 75% = high heterogeneity). A random effects model was utilized after initial fixed effects model analyses revealed high inter-study heterogeneity (I2 = 74%). Confidence intervals (CI; 95%) and p-values (α = .05) were calculated. Sensitivity analyses were performed utilizing the “leave-one-out” strategy56. Publication bias was assessed with Egger’s regression intercept57. Duval and Tweedie’s trim and fill analysis58 on both sides of the mean calculated effect size estimates accounting for reporting bias.
Moderator analyses
Meta-regression and subgroup moderator analyses were performed to examine potential sources of inter-study heterogeneity. The Benjamini and Hochberg59 method was used to control for multiple comparisons, with a false discovery rate (FDR) set to .05. Method of moments random-effects meta-regression50 was used for the moderators of mean age, percent cigarette users, percent females, NOS score, developmental exposure period, and time since exposure. Developmental stages were defined using the Center for Disease Control (CDC) definitions60 and neurodevelopmental data61; CDC stages were further collapsed to ensure a sufficient number of studies (k) for each grouping while maintaining developmental relevance. Groupings were: early development (prenatal-4 years; k = 7), childhood (up to12 years; k = 10), and adolescence (up to 18 years; k = 23); one study did not provide assessment age42. Time since exposure was defined as mean study population age minus the maximal age at adversity exposure.
Subgroups
For categorical moderators, subgroup analyses were conducted using a continuous random effects model62. Subgroups were delineated using the following criteria: Medical condition (some or all subjects had a current chronic medical condition; k = 8), psychiatric condition (some or all subjects had a current27, 28, 30, 39, 40, 49, 63–65 or current and/or past36, 43, 52, 66 psychiatric condition; k = 14), and medication use (some or all subjects were taking medication at the time of telomere measurement tissue collection; k = 5). Only studies specifying participant conditions and medication use were included in this subgroup analysis.
Telomere measurement technique
k = 36 studies examined the ratio of telomere repeat copy numbers to single-copy gene numbers (T/S ratio) using quantitative real-time polymerase chain reaction (qPCR). Two studies utilized Southern blot31, 42, one study used terminal restriction fragment (TRF) analysis67 and one study with two datasets used low-coverage whole-genome sequencing39. We compared qPCR to all other telomere measurement techniques.
Source tissue
Telomere measurement source tissues were: leukocyte (k = 31; included one study of lymphocytes), buccal cells (k = 4), saliva (k = 4), and cord blood (k = 2).
Study design
Study design was grouped as: case-control (k = 12), cross-sectional (k = 25), and prospective (k = 4).
Early adversity type and comprehensiveness of assessment
We wanted to examine if the comprehensiveness of adversity assessment impacted the relationship between telomeres and early adversity. As such, studies were grouped according to whether they narrowly assessed one or two forms of abuse (emotional, physical, sexual, or verbal abuse; k = 6), assessed all forms of abuse and neglect (k = 8), or assessed all forms of abuse, neglect, and other forms of adversity (k = 4). We also examined assessments of SES (k = 8) and maternal depression (k = 2). A broad category of other adversity (including child welfare involvement, non-supportive/conflict-driven parenting, institutionalization, family instability, domestic violence, psychosocial stress, bullying, divorce, parental separation, serious illness, or neighborhood disorder; k = 17) was created to include assessments of exposures not already categorized. For manuscripts assessing more than one type of adversity and presenting data on telomere length related to that adversity, effect sizes were calculated for each presented data and included in the appropriate subgroup. Supplemental Table 2 contains study descriptions of adversity assessment and resulting categorizations.
Results
After initial search and screening, 95 studies were assessed for eligibility. Thirty-four studies with 41 independent datasets met full inclusion criteria (N = 30 773, Figure 1). The cumulative average age at time of telomere measurement was 31 ± 22 years; 60 ± 25% were female. Thirty-eight percent of studies assessed other adversities, 18% abuse and neglect, 18% SES, 13% abuse, 9% abuse, neglect, and other adversities, and 4% maternal depression.
Figure 1. PRISMA flow diagram of included studies.
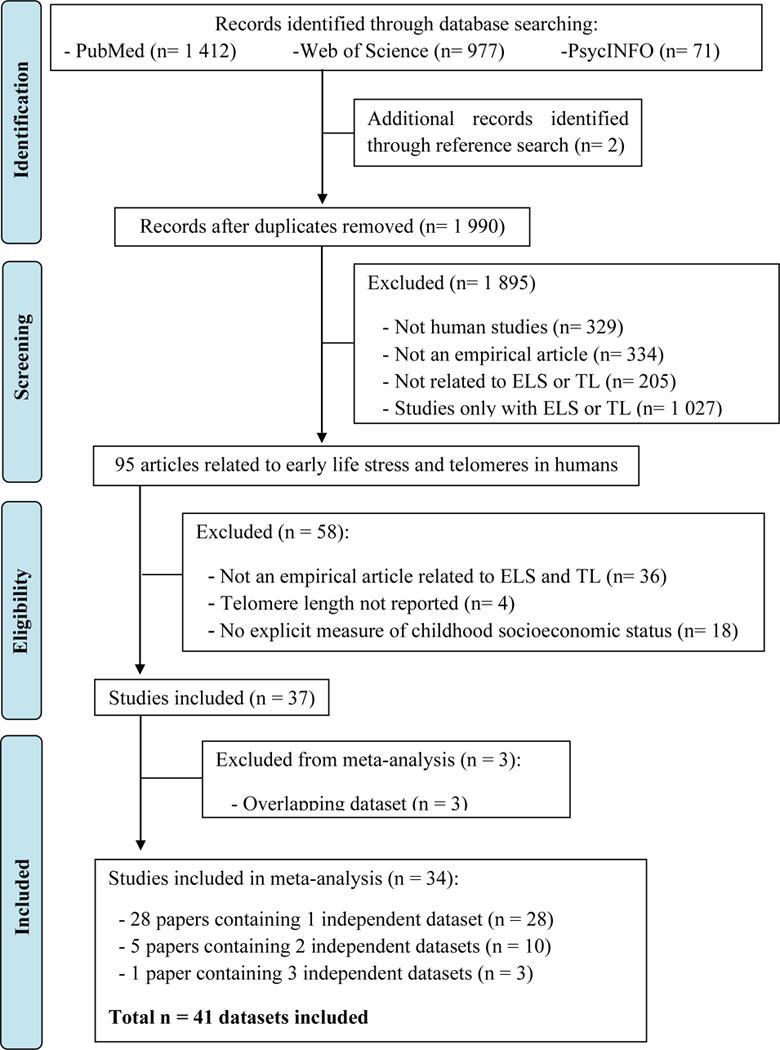
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for identification and inclusion of studies in the meta-analysis.
The overall association between early life adversity (k = 41) and telomere length as Cohen’s d was −0.35 (Figure 2; 95% CI, –0.46 to –0.24, p < .0001). Sensitivity analyses did not alter the overall Cohen’s d, suggesting the results were not driven by a single study. Egger’s regression suggested funnel plot asymmetry (Supplemental Figure 1; B = −2.04, 95% CI, −3.22 to −0.87, t = 3.51, p = .001), suggesting reporting bias and/or heterogeneity between studies68. Heterogeneity was detected in the primary meta-analysis (I2 = 42%); moderator analyses were performed to examine significant sources of this heterogeneity.
Figure 2. Forest plot of early life adversity and telomere length.
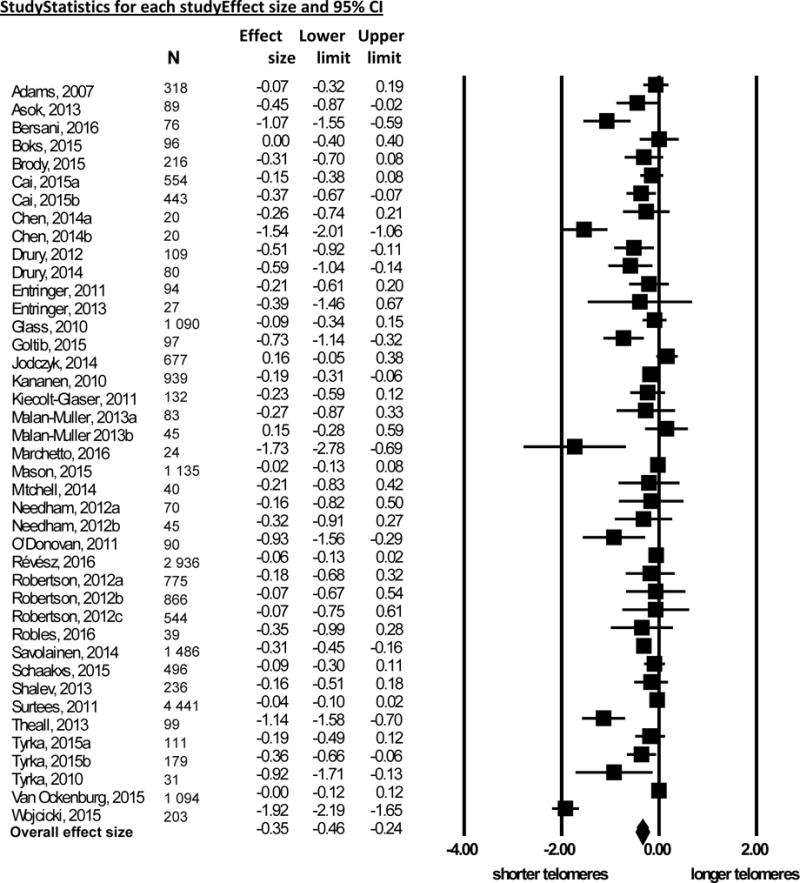
Forest plot of effect sizes reported as Cohen’s d (x-axis) evaluating early life adversity and telomere length using the random effects model. Points represent effect size; lines represent 95% confidence intervals (CI). Diamond indicates overall effect size and 95% CI.
Type of early life adversity, developmental timing, and telomere measurement
Moderator analysis by type of early adversity revealed a significant difference between groups (p < .0001, Figure 3, panel A). Studies with comprehensive adversity assessments (abuse, neglect and other adversities, d = −0.711) had negative effect sizes of greater magnitude than those with narrow adversity assessments (abuse and neglect or narrow focus on abuse; d = −0.13 and −0.055, respectively). Meta-regression of Cohen’s d versus developmental stage of adversity exposure revealed adversity in earlier developmental periods showed greater telomere shortening (B = 0.216, 95% CI, 0.076 to 0.356, p = .0025, R2 = .18; Figure 4, panel A). Increased temporal proximity to adversity exposure was also associated with shorter telomere length (B = 0.011, 95% CI 0.0045 to 0.0017, p = .0007, R2 = .10; Figure 4, panel B).
Figure 3. Subgroup moderator analyses.
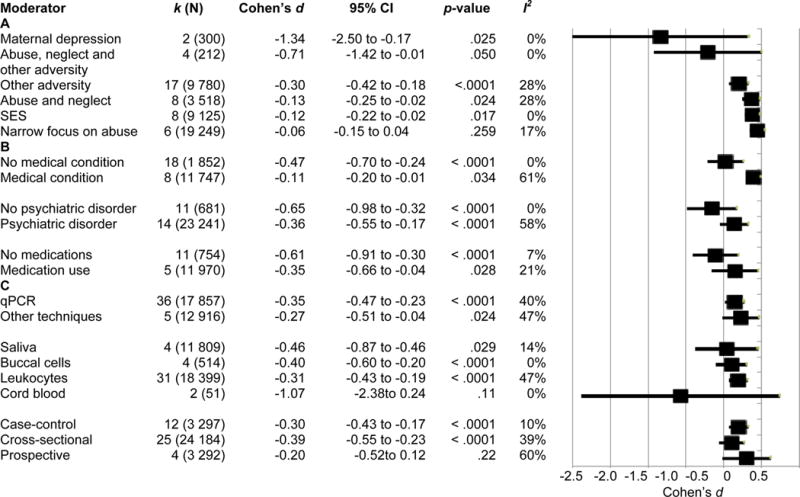
Subgroup analyses. Sub-group analyses were conducted using a continuous random effects model. Black squares represent the Cohen’s d effect size and lines represent 95% confidence interval (CI). A. Subgroup analysis by type of adversity exposure. B. Subgroup analysis by medical conditions, psychiatric disorders, and medication use. C. Subgroup analysis by study techniques. k = number of studies per group, N = total number of subjects from all studies; SES = socioeconomic status; qPCR = quantitative polymerase chain reaction.
Figure 4. Meta-regression analyses.
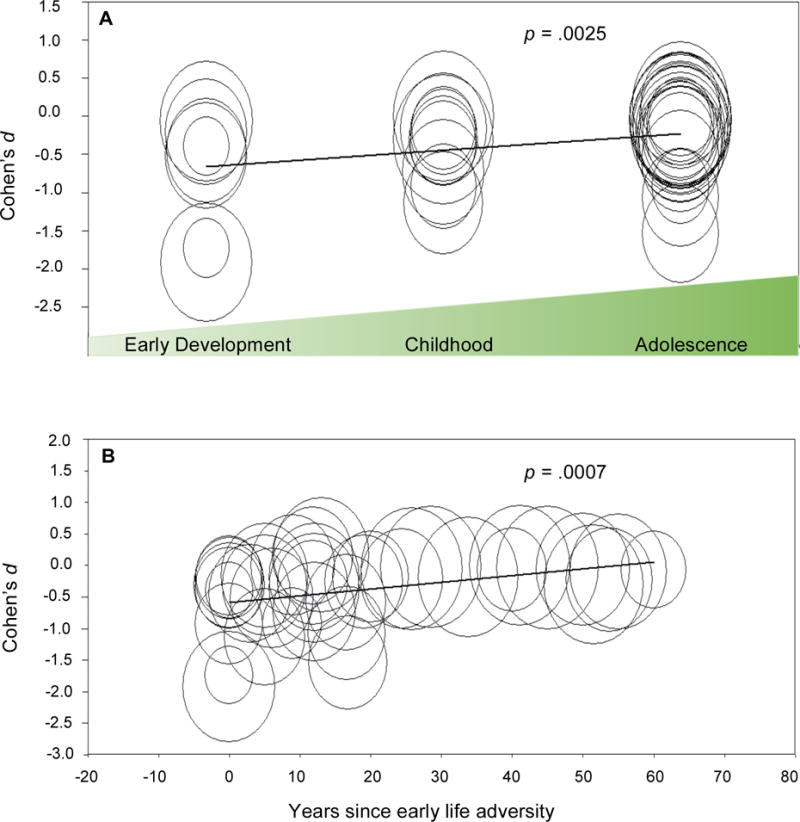
Meta-regression of early life adversity timing and telomere length. Each circle represents a study with size proportional to that study’s weight in the analysis. A. Developmental stage at age of adversity exposure versus Cohen’s d. Studies were grouped according to the reported age of adversity assessment. k = 7 studies assessed adversity during early development (prenatal-4 years), k = 10 during childhood (up to 12 years), and k = 23 during adolescence (up to 18 years). No studies are represented in more than one category. B. Years since adversity exposure versus Cohen’s d. X-axis values represent mean study population age minus the oldest age of reported adversity exposure.
Medical or psychiatric conditions, medication use, and demographics
Moderator analysis of studies including medical conditions vs. no medical condition and psychiatric conditions vs. no psychiatric condition were significant (p < .0001 for both). Studies with no medical or psychiatric conditions had negative effect sizes of greater magnitude than those with medical and psychiatric conditions, respectively (Figure 3 panel B). Similarly, moderator analysis of participant medication use was significant (p < .0001), with studies containing no participants on medications showing a negative Cohen’s d of greater magnitude (Figure 3 panel B).
Larger negative effects were seen in studies with younger participants (B = 0.010, 95%, CI 0.0049 to 0.0153, p < .0001, R2 = .19), and with less cigarette users (B = 0.009, 95% CI, 0.0005 to 0.0174, p = .038, R2 = .16). The relationship between percent female subjects and Cohen’s d was not significant (p = .098).
Telomere measurement technique
Studies grouped by telomere measurement technique were significantly different (p < .0001; Figure 3, panel C), with studies utilizing qPCR showing a significant and larger effect size (p < .0001). Studies utilizing other techniques also revealed a significant, negative effect size (p = .024).
Source tissue was a significant moderator of effect size (p < .0001; Figure 3, Panel C). Studies using leukocyte, buccal, or saliva cells showed a significant effect size (p < .0001, < .0001, and .029 respectively). Although the cord blood group Cohen’s d was largest in magnitude, it was not a significant grouping (p > .05).
Study design significantly moderated the relationship between early adversity and telomere length (p < .0001; Figure 3, panel C). Cross-sectional and case-control studies showed significant Cohen’s d (p < .0001 for both), but the effect size of the small number of prospective studies was not significant (p > .05). Risk of bias as determined by NOS score was not significantly associated with Cohen’s d (p > .05). All reported significant overall subgroup and regression analyses survived Benjamini-Hochberg correction for FDR.
Discussion
This meta-analysis supports an association between reduced telomere length and early life adversity. Using Cohen’s categorization54, the overall effect is between small to medium. Moderators can affect the relationship between telomere length and early adversity and reveal variables that have an additive or opposing effect. The Cohen’s d effect size magnitude was medium to large in some moderator analyses, including type of adversity, comorbidities, and medication use. Trim and fill analysis indicated asymmetry in the funnel plot. Cumulative effects analysis revealed the effect size approached that estimated by trim and fill analysis with the addition of the top six to seven weighted studies (data not shown), suggesting asymmetry due to heterogeneity between studies68. Our results help explain the existing literature, which includes mixed findings concerning the relationship between early adversity and telomeres.
The developmental timing of adversity exposure significantly influenced the effect size, with adversity earlier in development showing greater negative effects. This finding suggests that exposure to early adversity may impact a child’s developmental trajectory and health. Our finding that the magnitude of the negative association between early adversity and telomere length decreased with increasing years since exposure suggests that telomere shortening might be reversible over time, underscoring the fact that additional life experiences contribute to overall health. In our review of the literature on telomeres and early adversity, there was little data regarding consistency of care providers, nurturing relationships, and other resilience-associated factors69. To better understand the impact of all exposures, future studies would benefit from a comprehensive examination of both adversity and resilience factors.
The heterogeneity and magnitude of effect detected between early adversity and telomere length varied by the type of adversity exposure. Studies comprehensively assessing adversity, such as those examining abuse, neglect, and other adversities, revealed a negative Cohen’s d of greater magnitude than studies narrowly assessing only abuse. The abuse subgrouping was non-significant (p = .26), which may reflect undetected effects of neglect and other adversities occurring in both the abuse and comparison groups missed by the narrow assessment. The effect for the maternal depression grouping was large (d = −1.34), but included two studies with a total of 300 subjects. These two studies did not assess the influence of maternal depression on child experience; as such this finding warrants further investigation. Studies of SES reached significance (p =.017) with a small effect size. Only one SES study26 assessed SES as related to emotional stress 66; future investigations of environmental adversity and stress perception rather than SES alone may provide novel insights.
Mechanisms underlying early adversity and telomere length associations are largely unknown14, 25. Telomeres shorten after repeated cellular divisions and cellular stress exposures70. It has been speculated that early adversity directly activates or is associated with increased cellular stress and replication, resulting in accelerated telomere shortening8, 17. Telomerase activity, a key regulator of telomere length, is decreased with adversity exposure71. Telomere repair and lengthening strategies vary depending on the developmental phase of the cell72; it is possible these strategies are differentially responsive to adversity and may explain the relationship between developmental stage at adversity and impact on telomere length.
Previous meta-analyses reported a negative relationship between psychiatric disorders and telomere length73, 74. In this analysis, the Cohen’s d for studies including subjects with psychiatric disorders was of smaller magnitude than studies excluding psychiatric disorders. This finding likely reflects inclusion of subjects with psychiatric disorders in both the early stress cases and controls, confounding the ability to detect telomere shortening due to early stress alone. The inter-study heterogeneity was greater for the groups with psychiatric conditions compared to those without (I2 = 58% versus 0%), further suggesting that the relationship between psychiatric conditions and telomere length may confound the relationship between early adversity and telomeres. The analysis of medical conditions yielded similar results. Grouping studies based on subject medication use also impacted the magnitude of the relationship between early adversity and telomere length. This may reflect the underlying medical or psychiatric comorbidities or a direct effect of medication on telomere length.
Telomere measurement technique, source tissue, and study design all significantly moderated the association between early adversity and telomere length. qPCR showed a slightly larger Cohen’s d than other techniques combined, although both groupings were significant. As the effect size for studies using qPCR was in the same direction and of similar magnitude to both the overall effect size and other telomere measurement techniques, and given the comparable ease of qPCR compared to some telomere measurement techniques such as Southern blot, these results support the use of qPCR as valid technique. Studies using leukocytes, buccal cells and saliva all had significant relationships between early adversity and telomere shortening, suggesting that early adversity may involve systemic processes affecting multiple somatic tissues. The cord blood grouping was not significant. There were only two studies utilizing this source tissue; the magnitude of the effect was large (d = −1.07), but the confidence intervals were wide. These preliminary results suggest that further investigations utilizing cord blood are warranted, as definitive conclusions cannot be drawn without additional data.
Study design affected the relationship between telomere length and early adversity, with cross-sectional and case-control studies showing highly significant effects. Prospective studies did not have a significant effect and there was substantial heterogeneity in this group (I2 = 60%). Of the four studies in the prospective group, one examined newborns exposed to perinatal adversity67, one examined children22, and two examined adult populations with early adversity and psychiatric disorders30, 66. The effect sizes ranged from 0.003 to −1.73. The results of this meta-analysis suggest that differences in developmental timing of adversity exposure and comorbidities likely contributed to the heterogeneity. Further prospective studies are needed to clarify the relationship between early adversity and telomere length over time.
When examining the association between early adversity and telomere length by cigarette use, the Cohen’s d decreased in magnitude with an increasing percentage of smokers in the study (p = .038). Older subject age was also associated with effects that were smaller in magnitude (p < .0001). These findings may be influenced by the fact that studies of young children were assumed not to include smokers, and our finding that adversity at an earlier developmental stage was associated with a larger effect on telomere length.
The limitations of this meta analysis include the use of peer-reviewed, trial-level published data to increase confidence in the validity of the data, which is common practice in meta-analyses, but constrains Cohen’s d effect size calculations to data obtained from published studies. A pooled individual patient analysis approach could prove useful, especially for understanding moderator effects. Our analysis of developmental stage at adversity exposure is limited by the fact that many studies assessed adversity during large developmental timeframes rather than during discrete time periods. Most papers published were from developed nations; as such our ability to detect the long-term sequelae of poverty was limited due to the populations represented in the existing literature.
Telomere shortening may be a mechanism by which early adversity impacts disease risk. This may reflect underlying biological processes triggered by early life adversity, such as dysregulated stress signaling, altered metabolism and mitochondrial dysfunction, and increased inflammation and oxidative stress. Early adversity not only impacts children at an immediate, emotional and physical level75, but may have long-lasting health sequelae that are biologically-based as well. These results highlight the importance of preventing, recognizing and intervening on multiple forms of adversity including abuse, poverty, and caregiver loss and neglect. Heterogeneity within these results suggests that there are likely factors impacting individual susceptibility to telomere shortening after early life adversity exposure. Prospective studies with rich measures of exposures, medical and psychiatric conditions, and targeted interventions will help determine the causality and reversibility of the observed association between early adversity and telomere length, as well as help identify factors that may determine susceptibility or protection against early life adversity-associated telomere shortening. Additionally, individual-level patient analyses of these moderators may add to our understanding. Research examining the biological mechanisms by which early life adversity is associated with telomere attrition should focus on causal links, developmental stage of exposure, and interventions that may reverse these deleterious effects.
Supplementary Material
Supplemental Table 1. Full search strings
Supplemental Table 2. Included study adversity assessments and subgroup categorizations
SES = socioeconomic status. 1Data obtained from Park M et al, PLoS One 2015. 10(6):e0128460, which utilizes the same dataset.
Supplemental Figure 1. Funnel plot of all included studies
White data points = 41 included studies, black data points = imputed studies by Duval and Tweedie’s trim and fill analysis. White diamond represents the unadjusted effect size and 95% confidence interval (CI); black diamond represents the adjusted effect size and 95% CI using Duval and Tweedie’s trim and fill analysis. After adjustment, the calculated effect size increased to −0.46 (95% CI −0.60 to −0.32).
Footnotes
Conflicts of interest: All authors declare no potential conflicts of interest related to this manuscript.
All authors contributed to the conceptualization, design, and preparation of this work.
Supplementary information is available at Molecular Psychiatry’s website.
References
- 1.Children’s Bureau. Child Maltreatment. Human Health and Services; 2012. http://www.acf.hhs.gov/sites/default/files/cb/cm2012.pdf-page=20. [Google Scholar]
- 2.Child Abuse Statistics & Facts. 2016. Accessed Date Accessed 2016 Accessed. [Google Scholar]
- 3.Brown GR, Anderson B. Psychiatric morbidity in adult inpatients with childhood histories of sexual and physical abuse. The American journal of psychiatry. 1991;148(1):55–61. doi: 10.1176/ajp.148.1.55. [DOI] [PubMed] [Google Scholar]
- 4.Scott KM, Smith DA, Ellis PM. A population study of childhood maltreatment and asthma diagnosis: differential associations between child protection database versus retrospective self-reported data. Psychosomatic medicine. 2012;74(8):817–823. doi: 10.1097/PSY.0b013e3182648de4. [DOI] [PubMed] [Google Scholar]
- 5.Rich-Edwards JW, Spiegelman D, Lividoti Hibert EN, Jun HJ, Todd TJ, Kawachi I, et al. Abuse in childhood and adolescence as a predictor of type 2 diabetes in adult women. American journal of preventive medicine. 2010;39(6):529–536. doi: 10.1016/j.amepre.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang X, Brown DS, Florence CS, Mercy JA. The economic burden of child maltreatment in the United States and implications for prevention. Child Abuse Negl. 2012;36(2):156–165. doi: 10.1016/j.chiabu.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauer ME, Jeckel CM, Luz C. The role of stress factors during aging of the immune system. Annals of the New York Academy of Sciences. 2009;1153:139–152. doi: 10.1111/j.1749-6632.2008.03966.x. [DOI] [PubMed] [Google Scholar]
- 8.Wolkowitz OM, Epel ES, Reus VI, Mellon SH. Depression gets old fast: do stress and depression accelerate cell aging? Depress Anxiety. 2010;27(4):327–338. doi: 10.1002/da.20686. [DOI] [PubMed] [Google Scholar]
- 9.Ridout SJ, Ridout KK, Kao HT, Carpenter LL, Philip NS, Tyrka AR, et al. Telomeres, early-life stress and mental illness. Advances in psychosomatic medicine. 2015;34:92–108. doi: 10.1159/000369088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blackburn EH. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett. 2005;579(4):859–862. doi: 10.1016/j.febslet.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 11.Bernadotte A, Mikhelson VM, Spivak IM. Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging (Albany NY) 2016 doi: 10.18632/aging.100871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blackburn EH, Epel ES, Lin J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science. 2015;350(6265):1193–1198. doi: 10.1126/science.aab3389. [DOI] [PubMed] [Google Scholar]
- 13.Zhao J, Miao K, Wang H, Ding H, Wang DW. Association between telomere length and type 2 diabetes mellitus: a meta-analysis. PLoS One. 2013;8(11):e79993. doi: 10.1371/journal.pone.0079993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price LH, Kao HT, Burgers DE, Carpenter LL, Tyrka AR. Telomeres and early-life stress: an overview. Biol Psychiatry. 2013;73(1):15–23. doi: 10.1016/j.biopsych.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones GT, Power C, Macfarlane GJ. Adverse events in childhood and chronic widespread pain in adult life: Results from the 1958 British Birth Cohort Study. Pain. 2009;143(1–2):92–96. doi: 10.1016/j.pain.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305(5691):1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 17.Ridout KK, Carpenter LL, Tyrka AR. The Cellular Sequelae of Early Stress: Focus on Aging and Mitochondria. Neuropsychopharmacology. 2016;41(1):388–389. doi: 10.1038/npp.2015.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shalev I, Entringer S, Wadhwa PD, Wolkowitz OM, Puterman E, Lin J, et al. Stress and telomere biology: a lifespan perspective. Psychoneuroendocrinology. 2013;38(9):1835–1842. doi: 10.1016/j.psyneuen.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P. Telomere length in early life predicts lifespan. Proc Natl Acad Sci U S A. 2012;109(5):1743–1748. doi: 10.1073/pnas.1113306109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tyrka AR, Price LH, Kao HT, Porton B, Marsella SA, Carpenter LL. Childhood maltreatment and telomere shortening: preliminary support for an effect of early stress on cellular aging. Biol Psychiatry. 2010;67(6):531–534. doi: 10.1016/j.biopsych.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drury SS, Theall K, Gleason MM, Smyke AT, De Vivo I, Wong JY, et al. Telomere length and early severe social deprivation: linking early adversity and cellular aging. Mol Psychiatry. 2012;17(7):719–727. doi: 10.1038/mp.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shalev I, Moffitt TE, Sugden K, Williams B, Houts RM, Danese A, et al. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Mol Psychiatry. 2013;18(5):576–581. doi: 10.1038/mp.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Entringer S, Epel ES, Kumsta R, Lin J, Hellhammer DH, Blackburn EH, et al. Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proc Natl Acad Sci U S A. 2011;108(33):E513–518. doi: 10.1073/pnas.1107759108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell C, Hobcraft J, McLanahan SS, Siegel SR, Berg A, Brooks-Gunn J, et al. Social disadvantage, genetic sensitivity, and children’s telomere length. Proc Natl Acad Sci U S A. 2014;111(16):5944–5949. doi: 10.1073/pnas.1404293111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ridout SJ, Ridout KK, Kao H-T, Carpenter LL, Philip NS, Tyrka AR, Price LH. Telomeres, early-life stress, and mental illness. In: Balon RWT, editor. Clinical Challenges in the Biopsychosocial Interface: Update on Psychosomatics for the 21st Century Advances in Psychosomatic Medicine. Vol. 34. Karger AG; Basel, Switzerland: 2015. pp. 92–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theall KP, Brett ZH, Shirtcliff EA, Dunn EC, Drury SS. Neighborhood disorder and telomeres: connecting children’s exposure to community level stress and cellular response. Soc Sci Med. 2013;85:50–58. doi: 10.1016/j.socscimed.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kananen L, Surakka I, Pirkola S, Suvisaari J, Lonnqvist J, Peltonen L, et al. Childhood adversities are associated with shorter telomere length at adult age both in individuals with an anxiety disorder and controls. PLoS One. 2010;5(5):e10826. doi: 10.1371/journal.pone.0010826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Surtees PG, Wainwright NW, Pooley KA, Luben RN, Khaw KT, Easton DF, et al. Educational attainment and mean leukocyte telomere length in women in the European Prospective Investigation into Cancer (EPIC)-Norfolk population study. Brain Behav Immun. 2012;26(3):414–418. doi: 10.1016/j.bbi.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Adams J, Martin-Ruiz C, Pearce MS, White M, Parker L, von Zglinicki T. No association between socio-economic status and white blood cell telomere length. Aging Cell. 2007;6(1):125–128. doi: 10.1111/j.1474-9726.2006.00258.x. [DOI] [PubMed] [Google Scholar]
- 30.Boks MP, van Mierlo HC, Rutten BP, Radstake TR, De Witte L, Geuze E, et al. Longitudinal changes of telomere length and epigenetic age related to traumatic stress and post-traumatic stress disorder. Psychoneuroendocrinology. 2015;51:506–512. doi: 10.1016/j.psyneuen.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, Glaser R. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosomatic medicine. 2011;73(1):16–22. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malan-Muller S, Hemmings SM, Spies G, Kidd M, Fennema-Notestine C, Seedat S. Shorter telomere length - A potential susceptibility factor for HIV-associated neurocognitive impairments in South African women [corrected] PLoS One. 2013;8(3):e58351. doi: 10.1371/journal.pone.0058351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mason SM, Prescott J, Tworoger SS, DeVivo I, Rich-Edwards JW. Childhood Physical and Sexual Abuse History and Leukocyte Telomere Length among Women in Middle Adulthood. PLoS One. 2015;10(6):e0124493. doi: 10.1371/journal.pone.0124493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robertson T, Batty GD, Der G, Green MJ, McGlynn LM, McIntyre A, et al. Is telomere length socially patterned? Evidence from the West of Scotland Twenty-07 Study. PLoS One. 2012;7(7):e41805. doi: 10.1371/journal.pone.0041805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robles TF, Carroll JE, Bai S, Reynolds BM, Esquivel S, Repetti RL. Emotions and family interactions in childhood: Associations with leukocyte telomere length emotions, family interactions, and telomere length. Psychoneuroendocrinology. 2016;63:343–350. doi: 10.1016/j.psyneuen.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Savolainen K, Eriksson JG, Kananen L, Kajantie E, Pesonen AK, Heinonen K, et al. Associations between early life stress, self-reported traumatic experiences across the lifespan and leukocyte telomere length in elderly adults. Biol Psychol. 2014;97:35–42. doi: 10.1016/j.biopsycho.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 37.van Ockenburg SL, Bos EH, de Jonge P, van der Harst P, Gans RO, Rosmalen JG. Stressful life events and leukocyte telomere attrition in adulthood: a prospective population-based cohort study. Psychol Med. 2015;45(14):2975–2984. doi: 10.1017/S0033291715000914. [DOI] [PubMed] [Google Scholar]
- 38.Brody GH, Yu T, Beach SR, Philibert RA. Prevention effects ameliorate the prospective association between nonsupportive parenting and diminished telomere length. Prev Sci. 2015;16(2):171–180. doi: 10.1007/s11121-014-0474-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai N, Chang S, Li Y, Li Q, Hu J, Liang J, et al. Molecular signatures of major depression. Curr Biol. 2015;25(9):1146–1156. doi: 10.1016/j.cub.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen SH, Epel ES, Mellon SH, Lin J, Reus VI, Rosser R, et al. Adverse childhood experiences and leukocyte telomere maintenance in depressed and healthy adults. J Affect Disord. 2014;169:86–90. doi: 10.1016/j.jad.2014.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tyrka AR, Carpenter LL, Kao HT, Porton B, Philip NS, Ridout SJ, et al. Association of telomere length and mitochondrial DNA copy number in a community sample of healthy adults. Exp Gerontol. 2015;66:17–20. doi: 10.1016/j.exger.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glass D, Parts L, Knowles D, Aviv A, Spector TD. No correlation between childhood maltreatment and telomere length. Biol Psychiatry. 2010;68(6):e21–22. doi: 10.1016/j.biopsych.2010.02.026. author reply e23–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jodczyk S, Fergusson DM, Horwood LJ, Pearson JF, Kennedy MA. No association between mean telomere length and life stress observed in a 30 year birth cohort. PLoS One. 2014;9(5):e97102. doi: 10.1371/journal.pone.0097102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Bmj. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Higgins JPTGS. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; http://www.cochrane-handbook.org, updated March 2011. [Google Scholar]
- 46.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 47.Agency for Healthcare Research and Quality. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. 2015 http://effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/?pageaction=displayproduct&productid=318. [PubMed]
- 48.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European journal of epidemiology. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 49.Schaakxs R, Verhoeven JE, Oude Voshaar RC, Comijs HC, Penninx BW. Leukocyte telomere length and late-life depression. Am J Geriatr Psychiatry. 2015;23(4):423–432. doi: 10.1016/j.jagp.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 50.Bornstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. John Wiley & Sons, Ltd; Hoboken, New Jersey: 2009. [Google Scholar]
- 51.Needham BL, Fernandez JR, Lin J, Epel ES, Blackburn EH. Socioeconomic status and cell aging in children. Soc Sci Med. 2012;74(12):1948–1951. doi: 10.1016/j.socscimed.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 52.Tyrka AR, Parade SH, Price LH, Kao HT, Porton B, Philip NS, et al. Alterations of Mitochondrial DNA Copy Number and Telomere Length With Early Adversity and Psychopathology. Biol Psychiatry. 2016;79(2):78–86. doi: 10.1016/j.biopsych.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Effect Size Calculator. 2010 http://www.campbellcollaboration.org/escalc/html/EffectSizeCalculator-Home.php. Accessed Date Accessed 2010 Accessed.
- 54.Cohen J. Statistical Power Analysis for the Behavioral Sciences. second. Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 55.Thorlund K, Imberger G, Johnston BC, Walsh M, Awad T, Thabane L, et al. Evolution of heterogeneity (I2) estimates and their 95% confidence intervals in large meta-analyses. PloS one. 2012;7(7):e39471. doi: 10.1371/journal.pone.0039471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. International journal of epidemiology. 2008;37(5):1148–1157. doi: 10.1093/ije/dyn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 59.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995;B 57:289–300. [Google Scholar]
- 60.Child Development. 2016. Accessed Date Accessed 2016 Accessed. [Google Scholar]
- 61.Blakemore SJ. Imaging brain development: the adolescent brain. Neuroimage. 2012;61(2):397–406. doi: 10.1016/j.neuroimage.2011.11.080. [DOI] [PubMed] [Google Scholar]
- 62.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 63.Wojcicki JM, Heyman MB, Elwan D, Shiboski S, Lin J, Blackburn E, et al. Telomere length is associated with oppositional defiant behavior and maternal clinical depression in Latino preschool children. Transl Psychiatry. 2015;5:e581. doi: 10.1038/tp.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O’Donovan A, Epel E, Lin J, Wolkowitz O, Cohen B, Maguen S, et al. Childhood trauma associated with short leukocyte telomere length in posttraumatic stress disorder. Biol Psychiatry. 2011;70(5):465–471. doi: 10.1016/j.biopsych.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bersani FS, Lindqvist D, Mellon SH, Epel ES, Yehuda R, Flory J, et al. Association of dimensional psychological health measures with telomere length in male war veterans. J Affect Disord. 2016;190:537–542. doi: 10.1016/j.jad.2015.10.037. [DOI] [PubMed] [Google Scholar]
- 66.Revesz D, Milaneschi Y, Terpstra EM, Penninx BW. Baseline biopsychosocial determinants of telomere length and 6-year attrition rate. Psychoneuroendocrinology. 2016;67:153–162. doi: 10.1016/j.psyneuen.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 67.Marchetto NM, Glynn RA, Ferry ML, Ostojic M, Wolff SM, Yao R, et al. Prenatal stress and newborn telomere length. Am J Obstet Gynecol. 2016 doi: 10.1016/j.ajog.2016.01.177. [DOI] [PubMed] [Google Scholar]
- 68.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Bmj. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 69.Odgers CL, Jaffee SR. Routine versus catastrophic influences on the developing child. Annu Rev Public Health. 2013;34:29–48. doi: 10.1146/annurev-publhealth-031912-114447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blackburn EH, Greider CW, Szostak JW. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nature medicine. 2006;12(10):1133–1138. doi: 10.1038/nm1006-1133. [DOI] [PubMed] [Google Scholar]
- 71.Lindqvist D, Epel ES, Mellon SH, Penninx BW, Revesz D, Verhoeven JE, et al. Psychiatric disorders and leukocyte telomere length: Underlying mechanisms linking mental illness with cellular aging. Neurosci Biobehav Rev. 2015;55:333–364. doi: 10.1016/j.neubiorev.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Flores I, Blasco MA. The role of telomeres and telomerase in stem cell aging. FEBS Lett. 2010;584(17):3826–3830. doi: 10.1016/j.febslet.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 73.Ridout KK, Ridout SJ, Price LH, Sen S, Tyrka AR. Depression and telomere length: A meta-analysis. J Affect Disord. 2016;191:237–247. doi: 10.1016/j.jad.2015.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schutte NS, Malouff JM. The association between depression and leukocyte telomere length: a meta-analysis. Depress Anxiety. 2015;32(4):229–238. doi: 10.1002/da.22351. [DOI] [PubMed] [Google Scholar]
- 75.Zeanah CH, Gleason MM. Annual research review: Attachment disorders in early childhood–clinical presentation, causes, correlates, and treatment. J Child Psychol Psychiatry. 2015;56(3):207–222. doi: 10.1111/jcpp.12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Full search strings
Supplemental Table 2. Included study adversity assessments and subgroup categorizations
SES = socioeconomic status. 1Data obtained from Park M et al, PLoS One 2015. 10(6):e0128460, which utilizes the same dataset.
Supplemental Figure 1. Funnel plot of all included studies
White data points = 41 included studies, black data points = imputed studies by Duval and Tweedie’s trim and fill analysis. White diamond represents the unadjusted effect size and 95% confidence interval (CI); black diamond represents the adjusted effect size and 95% CI using Duval and Tweedie’s trim and fill analysis. After adjustment, the calculated effect size increased to −0.46 (95% CI −0.60 to −0.32).


