Abstract
This study aimed to investigate whether uric acid to creatinine (UA/Cr) ratio is associated with higher risk of metabolic syndrome (MetS) and its components. 332 adult Saudi type 2 diabetes mellitus (T2DM) patients were divided into UA/Cr tertiles. Risk for full MetS was significantly highest in individuals that constitutes the uppermost serum UA/Cr tertile [Odds ratio (OR): 1.80, 95% confidence interval (CI): 1.0–3.3; p < 0.001) after adjustment for age, gender and BMI. Similarly, risk for individual components of MetS like central obesity, hypertriglyceridemia, low HDL-cholesterol and hypertension was significantly highest in this tertile with OR’s of 2.61 (1.2–5.6), 1.42 (0.7–2.3), 1.45 (0.7–2.8) and 1.16 (0.6–2.2) respectively (all p-values < 0.001) after adjustment for age, gender, BMI and other components of MetS. Furthermore, serum UA/Cr levels increased with increasing number of MetS components (mean values of 4.44, 4.49, 4.64, 4.89 and 4.91 respectively for 1,2,3,4 and 5 MetS components, p-values < 0.001 after adjusting for age, gender and BMI). Our data suggest that serum UA/Cr in T2DM patients is strongly associated with full MetS as well as its individual components. These findings are of considerable clinical importance as serum UA/Cr may be used as a marker in the pathogenesis of MetS.
Introduction
Metabolic syndrome (MetS) is a major worldwide public health problem and is defined as a cluster of cardiovascular risk factors like obesity, hyperglycemia, hypertriglyceridemia, low high density lipoprotein cholesterol levels and hypertension1. In the Arabian Gulf region, the prevalence of MetS varies from 20.7–37.2% in males and from 32.1–42.7% in females2. In Saudi Arabia, the age-adjusted prevalence of MetS in males and females was reported to be 37.2% and 42% respectively3. The recent rapid economic growth and Westernization of lifestyle were partially blamed for the rise in MetS4. MetS is clinically important as it helps identify patients at high risk of developing cardiovascular disease (CVD), type 2 diabetes mellitus (T2DM)5,6 and chronic kidney disease (CKD)07.
There is a growing interest in the relationship of MetS with CKD. In a meta-analysis of 11 studies, MetS was reported to be associated with stage III CKD progression8. Both MetS and CKD are related to increased CVD events and the effect is cumulative if both are present9. However, the earliest stages of CKD are typically difficult to diagnose due to its asymptomatic nature. Biomarkers that could help identify the progression of different components of MetS could provide more effective prevention strategies and may unfold the relationship between these components and pathogenesis of CKD and incident CVD.
Serum creatinine (Cr) is a commonly used indicator for detecting small changes in glomerular filtration rate (GFR), hence a good biomarker of early stage CKD10. Raised circulating levels of Cr was found to be associated with increased risk of CVD, obesity and hypertension11–13. Similarly, elevated levels of serum uric acid (UA) is reported to be a marker for decreased renal function14 and a risk factor for hypertension and CVD15,16. Studies also suggest that serum UA is a strong indicator for type 2 diabetes mellitus (T2DM) independent of other confounding factors17. There is however limited evidence that these serum biomarkers could be used to detect pathogenesis of MetS. In this study, we combined these two biomarkers in ‘serum uric acid to creatinine ratio’ (UA/Cr). This biomarker is studied before18–20, however it is yet to be studied in relation to metabolic syndrome and in a population where the prevalence of MetS and T2DM are high21,22. This study aimed to fill that gap and investigate association of serum UA/Cr with MetS and its components in Saudi T2DM patients.
Results
General characteristics of subjects according to serum UA/Cr tertiles
A total of 332 Saudi subjects with T2DM were recruited for in this cross-sectional study. The mean age of the study population was 52.94 ± 11.4; 40.4% of whom were males. The overall prevalence of MetS was 56.3%. Subjects were divided into serum UA/Cr tertiles (Ter). Ter1 ranged from 2.1 to 4.0 [N = 110, mean ± standard deviation (SD) 3.24 ± 0.5], Ter2 ranged from 4.1 to 5.0 (110, 4.50 ± 0.3) and Ter3 ranged from 5.1 to 11.1 (112, 6.16 ± 1.0). The results are presented in Table 1. Individuals in the highest tertile of serum UA/Cr were significantly younger (50.1 years in Ter3, 53.92 in Ter2 and 54.84 in Ter1, p = 0.005) and had higher BMI (33.9 in Ter3, 32.17 in Ter2 and 30.41 in Ter1, p < 0.001)) than the ones in lowest tertile. The prevalence of MetS significantly increased in parallel with increasing tertiles, from 42.9% (Ter1) and 59.8% (Ter2) to 67.6% (Ter3) (p = 0.003). The differences in the prevalence of individual components of MetS from highest versus lowest tertiles of serum UA/Cr was statistically significant in central obesity (64.4% in Ter3 vs. 25.5% in Ter1, p < 0.001) and low HDL-cholesterol (64.8% in Ter3 vs. 48.6% in Ter1, p = 0.001). With respect to metabolic parameters, individuals in the higher serum UA/Cr tertile had significantly higher BMI, waist and hip circumferences, insulin, HOMA-IR and eGFR (all p < 0.05). In contrast, the individuals with higher serum UA/Cr tertile showed lower levels of fasting glucose, total cholesterol, HDL-cholesterol and urea (all p-values < 0.05).
Table 1.
General Characteristic of Subjects based on Serum UA/Cr Tertiles.
| Ter 1 (2.1–4.0) | Ter 2 (4.1–5.0) | Ter 3 (5.1–11.1) | P-Value | Total | |
|---|---|---|---|---|---|
| N | 110 | 110 | 112 | 332 | |
| UA/Cr | 3.2 ± 0.5 | 4.5 ± 0.3 | 6.2 ± 1.0 | <0.001 | 4.6 ± 1.3 |
| Clinical Characteristics | |||||
| Age (years) | 54.8 ± 11.4 | 53.9 ± 10.3 | 50.1 ± 12 | 0.005 | 52.9 ± 11.4 |
| BMI (kg/m²) | 26.8 ± 4.7 | 28.31 ± 4.3 | 29.8 ± 5.1 | <0.001 | 28.4 ± 4.9 |
| Waist (cm) | 94.5 ± 10.2 | 95.7 ± 9.1 | 96.55 ± 10.2 | 0.04 | 95.7 ± 9.8 |
| Hips (cm) | 97.2 ± 12.2 | 99.4 ± 11.9 | 104.4 ± 10.9 | 0.001 | 100.4 ± 11.9 |
| SBP (mmHg) | 135.5 ± 16.9 | 138.1 ± 16.5 | 139.0 ± 13.5 | 0.92 | 137.6 ± 15.7 |
| DBP (mmHg) | 79.8 ± 9.1 | 81.6 ± 8.9 | 82.12 ± 8.2 | 0.92 | 81.2 ± 8.8 |
| Glycemic Profile | |||||
| Fasting Glucose (mmol/l)# | 9.43 (7.8, 12.1) | 8.90 (7.2, 11.4) | 8.1 (6.6, 10.0) | 0.02 | 9.01 (7.1, 11.3) |
| Insulin (µlU/ml)# | 11.8 (6.6, 17.1) | 13.3 (6.7, 20.5) | 14.1 (8.9, 21) | 0.04 | 13.14 (7.2, 20.8) |
| HOMA-IR# | 4.5 (2.9, 7.6) | 4. 9 (2.7, 8.5) | 5.2 (2.9, 8.1) | 0.04 | 4.83 (2.9, 8.2) |
| Lipid Profile | |||||
| Total Chol (mmol/l) | 5.2 ± 1.1 | 4.8 ± 1 | 5.1 ± 1.1 | 0.03 | 5.05 ± 1.1 |
| Triglycerides (mmol/l) | 1.7 ± 0.9 | 1.7 ± 0.9 | 1.6 ± 0.7 | 0.81 | 1.66 ± 0.8 |
| HDL-Chol (mmol/l) | 1.2 ± 0.4 | 1.1 ± 0.3 | 1.1 ± 0.3 | 0.006 | 1.14 ± 0.3 |
| Renal Profile | |||||
| Calcium (mmol/l) | 2.5 ± 0.3 | 2.5 ± 0.3 | 2.5 ± 0.2 | 0.82 | 2.5 ± 0.3 |
| Albumin (g/l) | 42.0 ± 4.4 | 42.4 ± 5.2 | 41.0 ± 4.7 | 0.07 | 41.8 ± 4.8 |
| Uric Acid (µmol/l) | 239.5 ± 61.6 | 273.2 ± 62.5 | 338.3 ± 94.5 | 0.001 | 283.8 ± 84.9 |
| Creatinine (µmol/l) | 76.3 ± 22.6 | 61.4 ± 13.9 | 55.6 ± 12.6 | 0.001 | 64.39 ± 19 |
| eGFR (ml/min/1.73²) | 93.9 ± 24.3 | 110.8 ± 23.8 | 122.5 ± 30.3 | 0.001 | 109.1 ± 28.8 |
| UREA (mmol/l) | 5.2 ± 1.5 | 4.6 ± 1.7 | 4.6 ± 1.3 | 0.003 | 4.81 ± 1.5 |
| MetS Components (%) | |||||
| Central Obesity | 25.5 | 54.3 | 64.4 | <0.001 | 48.1 |
| Hyperglycemia | 100 | 100 | 100 | 1.0 | 100 |
| High TG | 29.9 | 32.7 | 34.3 | 0.853 | 32.9 |
| Low HDL-C | 48.6 | 62.6 | 64.8 | 0.001 | 58.1 |
| Hypertension$ | 49.5 | 48.6 | 54.7 | 0.749 | 51.4 |
| MetS | 42.9 | 59.8 | 67.6 | 0.003 | 56.3 |
Note: Data presented as mean ± standard deviation for normal variables and median (Q1, Q3) for non-normal variables (#). P-values derived from one way ANOVA (Kruskal-Wallis test for non-normal). SBP is systolic blood pressure, DBP is diastolic blood pressure, Chol is cholesterol and eGFR is estimated glomerular filtration rate.
Association between serum UA/Cr and other measured parameters
Correlations between serum UA/Cr and other measured parameters are summarized in Table 2. The first column shows unadjusted correlation coefficients and the corresponding p-value while ra in the second column shows correlation coefficients after adjustment with age, gender and BMI. Serum UA/Cr is inversely correlated with age (r = −0.175, p = 0.002), fasting glucose (r = −0.131, p = 0.02) and HDL-cholesterol (r = −0.175, p = 0.002).A significant positive correlation was found between serum UA/Cr and BMI (r = 0.214, p < 0.001), waist circumference (r = 0.135, p = 0.04), hip circumference (r = 0.234, p < 0.001) and eGFR (r = 0.359, p < 0.001).
Table 2.
Correlation between Serum UA/Cr and other measured parameters in T2DM.
| Parameters | r | p | ra | pa |
|---|---|---|---|---|
| Age (years) | −0.205 | <0.001 | −0.175 | 0.002 |
| BMI (kg/m2) | 0.324 | <0.001 | 0.214 | <0.001 |
| Waist (cm) | 0.146 | 0.05 | 0.135 | 0.04 |
| Hips (cm) | 0.319 | <0.001 | 0.234 | <0.001 |
| SBP (mmHg) | −0.017 | 0.76 | −0.030 | 0.60 |
| DBP (mmHg) | −0.001 | 0.99 | −0.017 | 0.77 |
| Fasting Glucose (mmol/l)# | −0.138 | 0.01 | −0.131 | 0.02 |
| Insulin (µlU/ml)# | 0.083 | 0.14 | 0.075 | 0.18 |
| HOMA-IR# | −0.017 | 0.76 | −0.024 | 0.67 |
| Total Cholesterol (mmol/l) | −0.067 | 0.23 | −0.091 | 0.10 |
| Triglycerides (mmol/l) | −0.030 | 0.59 | 0.003 | 0.95 |
| HDL-Cholesterol (mmol/l) | −0.133 | 0.02 | −0.175 | 0.002 |
| Calcium (mmol/l) | 0.005 | 0.92 | 0.060 | 0.29 |
| Albumin (g/l) | −0.121 | 0.03 | −0.044 | 0.44 |
| eGFR (ml/min/1.73²) | 0.412 | <0.001 | 0.359 | <0.001 |
| Urea (mmol/l) | −0.114 | 0.04 | −0.013 | 0.81 |
Note: r and p are the unadjusted correlation coefficients and associated p-value. ra and pa are values after adjustment with age, gender and BMI. SBP is systolic blood pressure, DBP is diastolic blood pressure and eGFR is estimated glomerular filtration rate. Non-normal variables (#) are log-transformed before analysis.
Association of serum UA/Cr with metabolic syndrome and its components
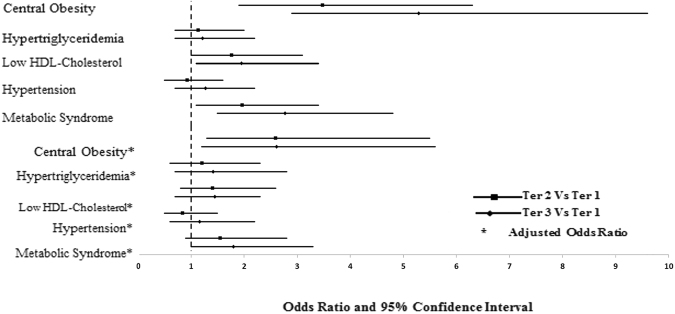
Table 3 presents the associations of serum UA/Cr levels and MetS risk and its components. Individuals with the highest serum UA/Cr tertile had higher odds of having full MetS compared with those in lowest tertile (Ter3 vs Ter1-OR: 1.80, 95% CI: 1.0 to 3.3; Ter2 vs Ter1-OR: 1.55, 95% C.I. 0.9 to 2.8; p for trend <0.001) after adjustment for age, gender and BMI. Associations of serum UA/Cr with individual components of MetS also revealed a similar trend. The odds ratio (OR.) of central obesity was 2.61 (C.I. 1.2–5.6) and 2.60 (1.3–5.5) with p < 0.001 for the trend in ter3 and ter2 respectively when compared to the lowest tertile (ter1) after adjustment with age, gender, BMI and rest of the components of MetS taken as dichotomized variables. Similarly, the odds ratios (OR) after adjustments for hypertriglyceridemia, low HDL-cholesterol and hypertension in ter3 vs. ter1 was 1.42 (0.7–2.8), 1.45 (0.7–2.3) and 1.16 (0.6–2.2) respectively; and ter2 vs. ter1 was 1.21 (0.6–2.3), 1.41 (0.8–2.6) and 0.84 (0.5–1.5), respectively (p-values < 0.001). Hyperglycemia, as a component of MetS, was excluded from the analysis since all subjects had fasting glucose >5.6 mmol/l. The odds ratio of MetS and its components in the higher tertiles (2 and 3) of serum UA/Cr compared to the lowest tertile (1) is shown in Fig. 1.
Table 3.
Adjusted Odds ratios for MetS and components in Serum UA/Cr Tertiles.
| Serum UA/Cr | OR (95% CI) | P-value | ||
|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | ||
| N | 107 | 107 | 108 | |
| Central Obesity | ||||
| Model a | Reference | 3.48 (1.9–6.3) | 5.29 (2.9–9.6) | <0.001 |
| Model b | Reference | 3.46 (1.9–6.2) | 5.12 (2.8–9.4) | <0.001 |
| Model c | Reference | 2.88 (1.4–5.7) | 3.28 (1.6–6.6) | <0.001 |
| Model d | Reference | 2.55 (1.2–5.3) | 2.54 (1.2–5.4) | <0.001 |
| Model e | Reference | 2.60 (1.3–5.5) | 2.61 (1.2–5.6) | <0.001 |
| Hypertriglyceridemia | ||||
| Model a | Reference | 1.14 (0.7–2.0) | 1.22 (0.7–2.2) | 0.787 |
| Model b | Reference | 1.14 (0.7–2.0) | 1.19 (0.7–2.1) | 0.026 |
| Model c | Reference | 1.19 (0.7–2.1) | 1.29 (0.7–2.4) | <0.001 |
| Model d | Reference | 1.21 (0.7–2.2) | 1.38 (0.8–2.6) | <0.001 |
| Model e | Reference | 1.21 (0.6–2.3) | 1.42 (0.7–2.8) | <0.001 |
| Low HDL-Cholesterol | ||||
| Model a | Reference | 1.77 (1.0–3.1) | 1.95 (1.1–3.4) | 0.033 |
| Model b | Reference | 1.76 (1.0–3.0) | 1.80 (1.0–3.1) | 0.003 |
| Model c | Reference | 1.63 (0.9–2.8) | 1.68 (0.9–2.9) | <0.001 |
| Model d | Reference | 1.58 (0.9–2.8) | 1.62 (0.9–2.7) | <0.001 |
| Model e | Reference | 1.41 (0.8–2.6) | 1.45 (0.7–2.3) | <0.001 |
| Hypertension | ||||
| Model a | Reference | 0.93 (0.5–1.6) | 1.27 (0.7–2.2) | 0.480 |
| Model b | Reference | 0.94 (0.6–1.6) | 1.42 (0.8–2.5) | 0.010 |
| Model c | Reference | 0.87 (0.5–1.5) | 1.23 (0.7–2.2) | <0.001 |
| Model d | Reference | 0.79 (0.4–1.4) | 1.05 (0.6–1.9) | <0.001 |
| Model e | Reference | 0.84 (0.5–1.5) | 1.16 (0.6–2.2) | <0.001 |
| MetS | ||||
| Model a | Reference | 1.97 (1.1–3.4) | 2.77 (1.5–4.8) | 0.001 |
| Model b | Reference | 1.96 (1.1–3.4) | 2.67 (1.5–4.7) | <0.001 |
| Model c | Reference | 1.65 (0.9–2.9) | 2.11 (1.1–3.6) | <0.001 |
| Model d | Reference | 1.55 (0.9–2.8) | 1.80 (1.0–3.3) | <0.001 |
Note: Significance was set at p < 0.05. Model ‘a’ is unadjusted (univariate). Each Model is adjusted for same set of variables in the previous model plus age (continuous), gender (male, female), BMI (continuous) and other components of MetS as present/absent in models ‘b’, ‘c’, ‘d’, and ‘e’ respectively.
Figure 1.
Odds ratio (OR) of MetS and its components in individuals with higher SrUa/Cr tertiles compared to lowest one. Central obesity [waist circumference >101.6 cm (males), >88.9 cm (females)], hypertriglyceridemia (triglycerides ≥1.7 mmol/l), low high-density lipoprotein cholesterol [HDL-cholesterol <1.03 (males), <1.30 (females)], hypertension (systolic blood pressure >130 mmHg and/or diastolic blood pressure >85 mmHg or current use of antihypertensive medications). Lowest serum UA/Cr tertile is taken as reference to calculate Odds Ratio. For each MetS component, values are adjusted for age, gender, BMI and other MetS components taken as dichotomized variable. The dotted line shows reference (lowest UA/Cr tertile).
The odds ratio of MetS and its components vis-à-vis different tertiles of serum UA/Cr after stratification for sex showed a similar trend observed in the overall data (Supplementary file 1).
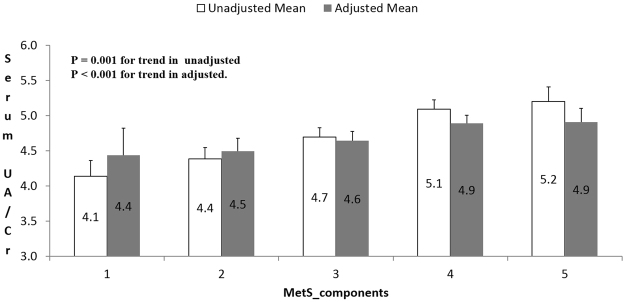
Interestingly, serum UA/Cr levels increased in parallel with the number of MetS components (Fig. 2). The mean (standard error of mean) serum UA/Cr value for the subjects with 1,2,3,4 and 5 MetS components was 4.44 (0.2), 4.49 (0.1), 4.64 (0.1), 4.89 (0.2) and 4.91 (0.4) respectively with p-value < 0.001 for the trend after adjustment with age, gender and BMI.
Figure 2.
Serum UA/Cr levels in individuals according to number of MetS components. Values are adjusted for age, gender and BMI. The adjusted mean (standard error of mean) serum UA/Cr value for the subjects with 1, 2, 3, 4 and 5 MetS components is 4.44 (0.2), 4.49 (0.1), 4.64 (0.1), 4.89 (0.2) and 4.91 (0.4) respectively.
Discussion
To the best of our knowledge, this is the first study to demonstrate the association of serum uric acid to creatinine ratio with MetS and its components. Earlier studies18,19 showed that higher serum UA/Cr correlated with lower levels of FEV1/FVC (Tiffeneau-Pinelli index) which is used in the diagnosis of chronic obstructive pulmonary disease (COPD). Another study by Liubao and colleagues20 suggested serum UA/Cr might be a better predictor of incident CKD than serum UA alone.
Higher serum UA/Cr levels correlated with an increased risk in all MetS components. The odds of having central obesity, hypertriglyceridemia, low HDL-cholesterol and hypertension for the highest serum UA/Cr tertile compared to the lowest were significant at 2.61, 1.42, 1.45 and 1.16 respectively (p < 0.001 in all) even after adjustment for age, gender, BMI and the rest of MetS components. Hyperuricemia was shown to be significantly associated with higher BMI, dyslipidemia and hypertension in earlier reports23,24. The underlying mechanism is still not well understood, though insulin resistance is suspected to be the mechanism interlinking hyperuricemia with the development of these metabolic disorders. Recently, the components of MetS were also found to be associated with high serum Cr levels25,26. In our study, higher BMI, waist and hip circumference was observed in the highest tertile of serum UA/Cr which was confirmed by a strong positive correlation of serum UA/Cr with BMI, waist and hips. In contrast, HDL-cholesterol was significantly lowest in the highest tertile of serum UA/Cr and confirmed by the significant inverse correlation of SrUa/Cr with HDL-cholesterol. Notably, levels of serum UA/Cr increased in subjects having more cradiometabolic risk factors and hence it may be useful in determining prognosis for MetS.
An interesting finding in this study is the inverse relation of fasting glucose with serum UA/Cr. Fasting glucose decreased from lower to higher serum UA/Cr tertiles and was also found to be inversely correlated with serum UA/Cr. Chronic high fasting glucose in T2DM subjects promotes hyper filtration state resulting in increased renal excretion of uric acid27 and this partially explains the inverse relationship, though we don’t have the data on the duration of diabetes in these subjects.
The association between serum UA and full MetS has been studied in other populations28,29. Similarly, a recent study30 showed serum Cr to be associated with MetS. In this study, we observed that having MetS was substantially higher in the higher tertiles of serum UA/Cr than the lower ones (Table 3). The prevalence of MetS also increased from lower to higher serum UA/Cr tertiles (Table 1). This can be partially explained by hyperuricemia derived endocrine imbalance in adipose tissue eventually resulting in low grade inflammation found in MetS31. It has also been observed that UA stimulates production of pro-inflammatory cytokines like C-reactive protein and tumor necrosis factor α32 and hence could potentially modulate chronic inflammatory processes. A noticeable increase in intake of fructose and purine-rich diet in the past few decades may contribute to raised serum uric acid levels and correlates well with rising prevalence of MetS33.
Interestingly, renal function is shown to be the main confounding factor for the association of serum UA with MetS and its components24,28,29. UA, which is a final product of purine metabolism, is mainly eliminated in the urine. Hence, impaired renal function associated with lower eGFR and higher serum creatinine levels correspond to higher levels of serum UA. Serum UA, in this study, is significantly and positively correlated with creatinine. Elevated serum UA may indicate an altered kidney function which itself has been found to be an independent predictor of MetS and CVD events34,35. Renal function-normalized serum UA such as serum UA/Cr, reflecting the net production of UA, may thus turn out to be a good marker in pathogenesis of MetS and related diseases.
The authors acknowledge some limitations. First, due to the cross-sectional nature of our study it is difficult to infer causality between risk of MetS and levels of serum UA/Cr. Secondly, although the data was adjusted for some confounding variables like age, gender, BMI and presence/absence of individual MetS components, we don’t have sufficient information on some other factors like duration of diabetes, menopausal status or dietary habits to include in our analysis. Residual confounding by these unknown factors is a possibility, although it seems unlikely that the strength of observed association between serum UA/Cr and MetS is completely nullified by these factors. Nevertheless, the study is the first to provide insights as to the role of serum UA/Cr in assessing MetS risk in a homogenous Arab population with T2DM. Future prospective studies taking into account the mentioned confounding factors should be designed to explore the causality and expand on the present findings.
Conclusion
In conclusion, our data suggest that levels of serum UA/Cr in T2DM are strongly associated with risk of MetS and its components even after adjusting for age, gender, and BMI. Consequently, lowering serum UA/Cr levels by adopting a healthier lifestyle may prove to be a useful strategy for lowering MetS burden. Further research is needed to address the causal relationship of serum UA/Cr in the pathogenesis of MetS.
Methods
Subjects, Anthropometry and Blood Collection
A total of 332 adult Saudi subjects with T2DM were selected randomly from Riyadh 2 cohort, whose details are previously published22. In brief, the subjects were recruited as a part of a nationwide screening project conducted by Biomarkers Research Program (BRP) at King Saud University, Riyadh, Saudi Arabia. A standardized manual of operation was followed for collecting fasting blood samples and the questionnaire data from each participant at the respective health center. Exclusion criteria include pregnant women and those with known complications such as renal, hepatic and cardiovascular diseases. Also, those taking medications related to these diseases and the ones that affect the circulating levels of uric acid like aspirin, thiazide diuretics etc. were excluded. Anthropometry included height, waist and hips (cm) and weight (kg) measured using Digital Pearson Scale (ADAM equipment Inc., USA). A standard procedure was utilized to measure resting blood pressure (measured twice by a qualified nurse). Blood samples were collected into a non-heparinized tube for centrifugation. The resulting serum samples were transferred immediately to the analysis facility; divided into several aliquots and stored at −80 °C until analysis.
Prior to inclusion, all the participants provided written and informed consent. The study was approved by the Ethics Committee of the College of Science, King Saud University, Riyadh, Saudi Arabia.
Biochemical measurements
All biochemical parameters except insulin were measured using a standard biochemical analyzer (Konelab, Thermo-Fisher Scientific, Finland). Serum insulin was quantified using fluorescent microbead technology by Luminex multiplex (Luminexcorp, TX, USA). Fasting glucose and lipid profile was quantified using routine biochemical tests in Konelab. The limit of detection for the assay was 0.02 mmol/l, 0.1 mmol/l, 0.04 mmol/l and 0.02 mmol/l for glucose, total cholesterol, HDL-cholesterol and triglyceride assays respectively. Calcium assay utilized Arsenazo III and albumin assay utilized a specific dye called bromocresol purple.
Cr, UA and urea assays were quantified using an enzymatic method utilizing creatinase, uricase and urease respectively supplied in ready to use reagents from Thermo-Fischer (reference numbers 981845,981788,981820 respectively). The limit of detection was 2μmol/l, 3.4 μmol/l and 1.1 mmol/l respectively. The imprecision of the creatinine, uric acid and urea assays was less than 1.4%, 4% and 6% respectively of the total CV. The calibrators and controls supplied were used routinely to check the performance of the assays. The QA department of King Saud University audits the BRP laboratory at regular intervals.
HOMA-IR was calculated as serum insulin (µlU/ml) x fasting glucose (mmol/l) /22.536. To estimate glomerular filtration rate (eGFR), we used modification of diet in renal disease (MDRD) equation which is:
where SCR is serum creatinine in μmol/l 37.
Definition of MetS
The National Cholesterol Education Programme Adult Treatment Panel III (NCEP ATP III) criteria was used to define MetS present if having three out of five components38. The five components of MetS tested in this study were as follows
Central Obesity: Waist circumference >101.6 cm (males), >88.9 cm (females).
Hyperglycemia: Fasting glucose >5.6 mmol/l or pharmacologic treatment for hyperglycemia.
Hypertriglyceridemia: Serum triglycerides ≥1.7 mmol/l.
Low HDL-Cholesterol: Serum HDL-cholesterol <1.03 mmol/l (males), <1.30 mmol/l (females).
Hypertension: Systolic blood pressure >130 mmHg and/or diastolic blood pressure >85 mmHg or current use of antihypertensive medications.
Data analysis
SPSS version 16.0 (Chicago, IL, USA) was used to analyze data. For statistical analysis, subjects were divided into serum UA/Cr tertiles. Tertiles 1, 2 and 3 ranged from 2.1 to 4.0 (N = 110), 4.1 to 5.0(N = 110) and 5.1 to 11.1(N = 112), respectively. Normal continuous variables were presented as mean ± standard deviation and non-normal variables were presented as median (first quartile, third quartile). Nominal variables were presented as percentages (%). Appropriate statistical tests were employed to check differences in central tendency as a trend between serum UA/Cr tertiles. Non-normal variables were log transformed before further analysis. Pearson correlation was used to determine associations between serum UA/Cr and other measured parameters controlling for age, gender and BMI. Multi-nominal logistic regression was done using serum UA/Cr tertiles as dependent variables (lowest tertile as reference) and MetS components (present versus absent) as independent factors with age, gender and BMI as covariates to calculate odds ratio. Significance was set at p < 0.05. Microsoft Excel 2010 was used to prepare figures.
Data Availability
The dataset analysed during the current study is available from the corresponding author on reasonable request.
Statement of Human and Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. The study was approved by the Ethics Committee of College of Science, King Saud University.
Electronic supplementary material
Acknowledgements
The project was supported by Deanship of Scientific Research, King Saud University and Prince Mutaib Bin Abdullah Chair for Biomarkers of Osteoporosis. The authors would like to acknowledge Mr. Hamza Saber for his efforts in maintaining/providing good quality samples for analysis during this study.
Author Contributions
N.M.A. and O.S.A. contributed in study conception and design, K.W. analyzed samples and wrote the manuscript, S.S. reviewed the manuscript and MSA supervised the study.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-12085-0.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome - a new world-wide definition. A consensus statement from the international diabetes federation. Diabetic Medicine. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 2.Bener A, Zirie M, Musallam M, Khader YS, Al-Hamaq AO. Prevalence of metabolic syndrome according to Adult Treatment Panel III and International Diabetes Federation criteria: a population-based study. Metab Syndr Relat Disord. 2009;7:221–9. doi: 10.1089/met.2008.0077. [DOI] [PubMed] [Google Scholar]
- 3.Al-Nozha M, et al. Metabolic syndrome in Saudi Arabia. Saudi Med J. 2005;26:1918–25. [PubMed] [Google Scholar]
- 4.Al-Qahtani DA, Imtiaz ML. Prevalence of metabolic syndrome in Saudi adult soldiers. Saudi Med J. 2005;26:1360–1366. [PubMed] [Google Scholar]
- 5.Wilson PW, D’Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 6.Gami AS, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 7.Kitiyakara C, et al. The metabolic syndrome and chronic kidney disease in a Southeast Asian cohort. Kidney Int. 2007;71:693–700. doi: 10.1038/sj.ki.5002128. [DOI] [PubMed] [Google Scholar]
- 8.Thomas G, et al. Metabolic syndrome and kidney disease: a systematic review and meta-analysis. Clin. J. of the Amer. Soc. of Neph. 2011;6:2364–2373. doi: 10.2215/CJN.02180311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prasad GR. Metabolic syndrome and chronic kidney disease:Current status and future directions. WJN. 2014;3:210–219. doi: 10.5527/wjn.v3.i4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalton RN. Serum creatinine and glomerular filtration rate: perception and reality. Clin. Chem. 2010;56:687–689. doi: 10.1373/clinchem.2010.144261. [DOI] [PubMed] [Google Scholar]
- 11.Culleton BF, et al. Prevalence and correlates of elevated serum creatinine levels: the Framingham Heart Study. Arch Intern Med. 1999;159:1785–1790. doi: 10.1001/archinte.159.15.1785. [DOI] [PubMed] [Google Scholar]
- 12.Coresh J, et al. Prevalence of high blood pressure and elevated serum creatinine level in the United States: findings from the third National Health and Nutrition Examination Survey (1988–1994) Arch Intern Med. 2001;161:1207–1216. doi: 10.1001/archinte.161.9.1207. [DOI] [PubMed] [Google Scholar]
- 13.Muntner P, He J, Hamm L, Loria C, Whelton PK. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. JASN. 2002;13:745–753. doi: 10.1681/ASN.V133745. [DOI] [PubMed] [Google Scholar]
- 14.Feig DI. Uric acid-a novel mediator and marker of risk in chronic kidney disease? Curr. Opin. Nephrol. Hypertens. 2009;18:526–530. doi: 10.1097/MNH.0b013e328330d9d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mankovsky B, Kurashvili R, Sadikot S. Is serum uric acid a risk factor for atherosclerotic cardiovascular disease? A review of the clinical evidence. Part 1. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2010;4:176–184. doi: 10.1016/j.dsx.2010.07.010. [DOI] [Google Scholar]
- 16.Johnson RJ, Feig DI, Herrara-Acosta J, Kang DH. Resurrection of uric acid as a causal risk factor in essential hypertension. Hypertension. 2005;45:18–20. doi: 10.1161/01.HYP.0000150785.39055.e8. [DOI] [PubMed] [Google Scholar]
- 17.Kodama S, et al. Association between serum uric acid and development of type 2 diabetes. Diabetes care. 2009;32:1737–1742. doi: 10.2337/dc09-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Pachon E, Padilla-Navas I, Shum C. Serum uric acid to creatinine ratio in patients with chronic obstructive pulmonary disease. Lung. 2007;185:21–24. doi: 10.1007/s00408-006-0076-2. [DOI] [PubMed] [Google Scholar]
- 19.Kocak ND, et al. Serum Uric Acid Levels and Uric Acid/Creatinine Ratios in Stable Chronic Obstructive Pulmonary Disease (COPD) Patients: Are These Parameters Efficient Predictors of Patients at Risk for Exacerbation and/or Severity of Disease? Med. Sci.Monit. 2016;22:4169–4176. doi: 10.12659/MSM.897759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu, L., Huang, L., Wu, H., Lou, Q. & Bian, R. Serum uric acid to creatinine ratio: A predictor of incident chronic kidney disease in type 2 diabetes mellitus patients with preserved kidney function. Diabetes and Vascular Disease Research 1–5 (2017). [DOI] [PubMed]
- 21.Aljohani NJ. Metabolic syndrome: Risk factors among adults in Kingdom of Saudi Arabia. J Family Community Med. 2014;21:170–175. doi: 10.4103/2230-8229.142971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Daghri NM, et al. Diabetes mellitus type 2 and other chronic non-communicable diseases in the central region, Saudi Arabia (Riyadh cohort 2): a decade of an epidemic. BMC medicine. 2011;9:76. doi: 10.1186/1741-7015-9-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rathmann W, Funkhouser E, Dyer AR, Roseman JM. Relations of hyperuricemia with the various components of the insulin resistance syndrome in young black and white adults: the CARDIA study. Ann Epidemiol. 1998;8:250–261. doi: 10.1016/S1047-2797(97)00204-4. [DOI] [PubMed] [Google Scholar]
- 24.Conen D, et al. Prevalence of hyperuricemia and relation of serum uric acid with cardiovascular risk factors in a developing country. BMC Public Health. 2004;4:9. doi: 10.1186/1471-2458-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen N, et al. Community-based study on CKD subjects and the associated risk factors. Nephrology Dialysis Transplantation. 2009;24:2117–2123. doi: 10.1093/ndt/gfn767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang IH, et al. Association between metabolic syndrome and chronic kidney disease in the Korean population. Nephrology. 2009;14:321–326. doi: 10.1111/j.1440-1797.2009.01091.x. [DOI] [PubMed] [Google Scholar]
- 27.Facchini F, Chen YDI, Hollenbeck CB, Reaven GM. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA. 1991;266:3008–3011. doi: 10.1001/jama.1991.03470210076036. [DOI] [PubMed] [Google Scholar]
- 28.Ishizaka N, Ishizaka Y, Toda EI, Nagai R, Yamakado M. Association between serum uric acid, metabolic syndrome, and carotid atherosclerosis in Japanese individuals. Arterioscler Thromb Vasc Biol. 2005;25:1038–1044. doi: 10.1161/01.ATV.0000161274.87407.26. [DOI] [PubMed] [Google Scholar]
- 29.Kim ES, et al. Serum uric acid level is associated with metabolic syndrome and microalbuminuria in Korean patients with type 2 diabetes mellitus. J Diabetes Complications. 2011;25:309–313. doi: 10.1016/j.jdiacomp.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, et al. Serum creatinine levels and risk of metabolic syndrome in a middle-aged and older Chinese population. Clinica Chimica Acta. 2015;440:177–182. doi: 10.1016/j.cca.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 31.Baldwin W, et al. Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes. 2011;60:1258–1269. doi: 10.2337/db10-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanellis J, Kang DH. Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Seminars in nephrology. 2005;25:39–42. doi: 10.1016/j.semnephrol.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 33.Cirillo P, et al. Uric acid, the metabolic syndrome, and renal disease. JASN. 2006;17:S165–S168. doi: 10.1681/ASN.2006080909. [DOI] [PubMed] [Google Scholar]
- 34.Ruilope LM, Garcia-Puig J. Hyperuricemia and renal function. Curr Hypertens Rep. 2001;3:197–202. doi: 10.1007/s11906-001-0038-2. [DOI] [PubMed] [Google Scholar]
- 35.Baker JF, Krishnan E, Chen L, Schumacher HR. Serum uric acid and cardiovascular disease: recent developments, and where do they leave us? Am J Med. 2005;118:816–826. doi: 10.1016/j.amjmed.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 36.Bonora E, et al. HOMA-estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects. Diabetes care. 2002;25:1135–1141. doi: 10.2337/diacare.25.7.1135. [DOI] [PubMed] [Google Scholar]
- 37.Levey AS, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 38.Huang PL. A comprehensive definition for metabolic syndrome. Disease models & mechanisms. 2009;2:231–237. doi: 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset analysed during the current study is available from the corresponding author on reasonable request.