Abstract
Purpose
Since the introduction of the first prosthetic mesh for abdominal hernia repair, there has been a search for the “ideal mesh.” The use of preclinical or animal models for assessment of necessary characteristics of new and existing meshes is an indispensable part of hernia research. Unfortunately, in our experience there is a lack of consensus among different research groups on which model to use. Therefore, we hypothesized that there is a lack of comparability within published animal research on hernia surgery due to wide range in experimental setup among different research groups.
Methods
A systematic search of the literature was performed to provide a complete overview of all animal models published between 2000 and 2014. Relevant parameters on model characteristics and outcome measurement were scored on a standardized scoring sheet.
Results
Due to the wide range in different animals used, ranging from large animal models like pigs to rodents, we decided to limit the study to 168 articles concerning rat models. Within these rat models, we found wide range of baseline animal characteristics, operation techniques, and outcome measurements. Making reliable comparison of results among these studies is impossible.
Conclusion
There is a lack of comparability among experimental hernia research, limiting the impact of this experimental research. We therefore propose the establishment of guidelines for experimental hernia research by the EHS.
Electronic supplementary material
The online version of this article (doi:10.1007/s10029-017-1605-z) contains supplementary material, which is available to authorized users.
Keywords: Hernia, Experimental research, Review, Animal models, Mesh
Introduction
Ever since the introduction of the first prosthetic mesh for reinforcement of abdominal hernia repair, there has been a search for the “ideal mesh” [1, 2]. After using meshes of silver and stainless steel for decades, the first “modern” synthetic polypropylene mesh was introduced in the 1950s [1–3]. Today, polypropylene mesh remains the most commonly used mesh worldwide in ventral and inguinal hernia repair [1, 2]. The ideal mesh, however, still has not been developed [1, 2, 4].
The ideal mesh must be tailored to each patient’s current needs in the current clinical situation [4, 5]. In order to provide a mesh for most patients, a continuing growth in variety of mesh concepts exists. For instance, meshes of various materials (from prosthetic or biologic origin), shapes (flat mesh, plugs, and 3D meshes), heavy and low weight, and with various coatings are available. Along with this is a growing body of data on assessing the feasibility of new meshes with the ultimate goal to improve patient outcomes [6].
Even though clinical research is the best method to really assess the outcome of new mesh concepts, preclinical animal models remain necessary for the assessment of biocompatibility and strength in the long run [7–9]. Especially since several important mesh characteristics, such as inflammation, shrinkage, ingrowth, remodeling, and adhesion formation to the mesh, can only be researched using experimental models, patients cannot be reoperated for evaluation of these key aspects [4]. However, in order to compare studies and to reproduce them, it is important that different research groups use comparable research methods. However, in our search for hernia models in the past, we came across a wide range of different models leading to the hypothesis that there is very little comparability within published animal research on hernia surgery [10, 11]. To support this hypothesis, we hereby present a systematic review of the literature on all available animal models for hernia research between 2000 and 2014.
Materials and methods
Literature search
A systematic search of the literature was performed using the “Excerpta Medica database” (Embase) and NCBI National Library of Medicine (PubMed). Search strategy was aimed at finding all literature concerning surgical meshes used for abdominal wall hernia in an animal model. Literature search was conducted as follows with aid of an experienced university librarian
Embase
(“surgical equipment”/de OR mesh*:ab,ti OR prothes*:ab,ti OR prosthet*:ab,ti) AND (herni*:ab,ti OR hernioplasty/de OR herniorrhaphy/de OR herniotomy/de OR hernia/de OR “abdominal wall hernia”/exp OR “incisional hernia”/de) AND [“experimental animal”/de OR “animal model”/de OR (vertebrate/exp NOT human/de) OR animal/de OR nonhuman/de OR rodent/exp OR (animal* OR nonhuman* OR rodent* OR rat OR rats OR mice OR mouse OR hamster* OR pigs OR porcine* OR swine* OR goat*)].
PubMed
{mesh*[tw] OR prothes*[tw] OR prosthet*[tw]) AND (herni*[tiab] OR hernia[mesh:noexp] OR Hernia, Abdominal[mesh] OR herniorrhaphy[mesh]).
AND ((animals[mesh] NOT humans[mesh]) OR (animal*[tw] OR nonhuman*[tw] OR rodent*[tw] OR rat[tw] OR rats[tw] OR mice[tw] OR mouse[tw] OR hamster*[tw] OR pigs[tw] OR porcine*[tw] OR swine*[tw] OR goat*[tw])}.
Study selection
Two independent researchers screened all titles and abstracts to select animal studies that were eligible for full-text review. Following primary screening, all full-text articles of the remaining studies were screened to identify studies using animal models aimed at mesh research.
We included all English, Dutch, and German literature using an animal model to study meshes designed for abdominal wall hernia repair published between January 01, 2000, and January 01, 2014. Clinical trials, abstracts, letters to the editor, or studies not primarily aimed at studying meshes were excluded from further analysis.
Study outcome
All included articles were read, and all relevant parameters concerning the studied animal models used were scored in a standardized scoring sheet. All scored parameters are mentioned in supplementary data (Table 1). First, parameters for the animal model were assessed, including subspecies. Sex, weight, and age of the animals were recorded when mentioned in the article. Also the use of a previously published model was scored; this was defined as a clear reference to a previously published use of the same animal model. Details of the model used were subsequently scored. This included the creation of a hernia defect and size of defect (when applicable), location of the mesh, and size of the implanted mesh. Thereafter, the use and type of control group were scored, and duration of follow-up was recorded. Finally, used outcome parameters were scored (mentioned in Table 1).
Table 1.
Scoring system for animal models
| Parameter | Outcome |
|---|---|
| Animal model | Pig |
| Rat | |
| Mice | |
| Rabbit | |
| Guinea pig | |
| Other: specify | |
| Subspecies | Free text |
| Sex | Male |
| Female | |
| Both | |
| Unknown/not specified | |
| Validated model | Yes |
| No (no reference to previous research) | |
| Infection model | Yes |
| No | |
| Unknown | |
| Defect | Yes, size (cm × cm) |
| No | |
| Unknown/not specified | |
| Mesh location | Intraperitoneal |
| Inlay | |
| Bridging | |
| Subcutaneous | |
| Preperitoneal | |
| Unknown/not specified | |
| Technique | Laparotomy |
| Laparoscopy | |
| Other: specify | |
| Unknown | |
| Mesh size | Size of mesh (cm × cm) |
| Control group | Yes: specify |
| No | |
| Unknown/not specified | |
| Follow-up | Duration of follow-up in days (1 month is scored as 30 days) |
| Outcome parameters | |
| Mesh ingrowth | Yes |
| No | |
| Adhesion quality | Yes |
| No | |
| Adhesion quantity | Yes |
| No | |
| Mechanical testing/tensiometry | Yes |
| No | |
| Mesh shrinkage | Yes |
| No | |
| Histology | Yes |
| No | |
| Immunohistochemistry | Yes |
| No | |
Statistics
When applicable, data were tested using the statistical package for the social sciences (SPSS) version 22 for normal distribution using the Kolmogorov–Smirnov test for normality. Normally distributed data were presented as mean and standard deviation. Not normally distributed data were presented as median with range. All other data were presented as a percentage.
Results
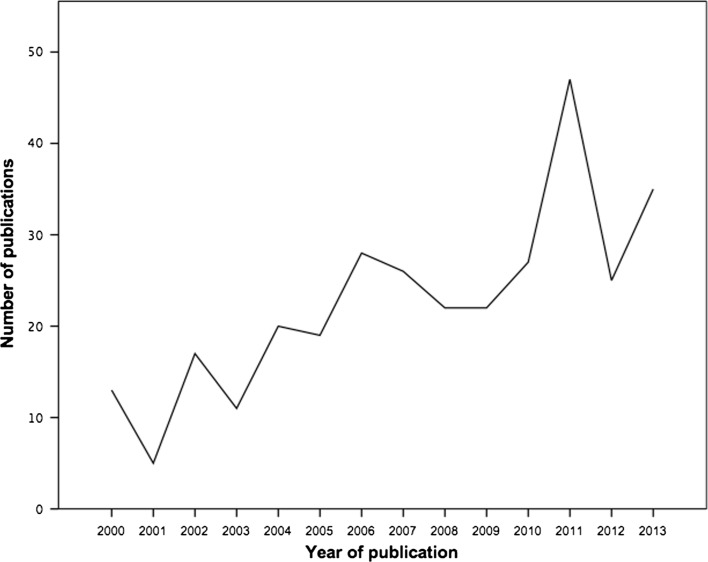
A total of 315 articles (supplementary data) were included in this study, of which 168 studied rats (53.3%), 66 studied rabbits (21.0%), and 53 studied pigs (16.8%). The remaining studies described use of mice, guinea pigs, primates, dogs, goats, sheep, and hamster models. A representation of the amount of publications per year showed an increase in yearly publications (Fig. 1). Due to the variety in animals used, and the even larger variety in different animal models, all further analyses were performed on the 168 articles using a rat model. All other animal types were excluded from further analysis. Results are mentioned in Table 2.
Fig. 1.
Number of publications per year since 2000
Table 2.
Outcome of all scored parameters
| Parameter | Outcome | |
|---|---|---|
| Animal model (%)a | Pig | 16.8% |
| Rat | 53.3% | |
| Mice | 3.5% | |
| Rabbit | 21.0% | |
| Guinea pig | 2.2% | |
| Other | 3.2% | |
| Subspecies (%) | Wistar | 46.4% |
| Sprague–Dawley | 46.4% | |
| Lewis | 4.1% | |
| Other | 1.9% | |
| Unspecified | 1.2% | |
| Sex (%) | Male | 66.7% |
| Female | 16.7% | |
| Both | 1.8% | |
| Unknown/unspecified | 15.4% | |
| Reference to previously used model (%) | Yes | 24.2% |
| No | 75.8% | |
| Number of meshes/animal (%) | 1 | 85.1% |
| 2 | 13.1% | |
| 3 | 0.6% | |
| Unspecified | 1.2% | |
| Defect (%) | Yes size (cm2) mean (range) | 72.0% 4.2 cm2 (0.5–18.0 cm2) |
| No | 27.4% | |
| Unknown | 0.6% | |
| Mesh location (%) | Intraperitoneal | 23.8% |
| Inlay | 11.9% | |
| Bridging | 20.0% | |
| Subcutaneous | 17.9% | |
| Preperitoneal | 5.4% | |
| Unknown | 11.9% | |
| Infection model | Yes | 9.5% |
| No | 90.5% | |
| Mesh size | Size of mesh (cm2) mean (Range) | 5.76 cm2 (0.8–20 cm2) |
| Unspecified (% of articles) | 17.3% | |
| Control group | Yes | 64.3% |
| Polypropylene mesh | 22.6% | |
| Sham | 22.6% | |
| Primary repair | 6.6% | |
| Other | 12.5% | |
| No/not described | 35.7% | |
| Antibiotics | Yes | 7.1% |
| No/not described | 92.9% | |
| Analgetics | Yes | 15.5% |
| No/not described | 84.5% | |
| Number of endpoints median (range) | 2 Time-points (1–6 time-points) undefined in 1 article | |
| Follow-up duration median (range) | 28 days (6 h–365 days) | |
| Outcome parameters used (%) | ||
| Mesh ingrowth | 10.1% | |
| Adhesions | Quality | 16.1% |
| Quantity | 10.7% | |
| Both | 24.4% | |
| Mechanical testing/tensiometry | 48.2% | |
| Mesh shrinkage | 17.3% | |
| Histology | 81.0% | |
| Immunohistochemistry | 23.2% | |
aFor this analysis, all animal types were scored; all other parameters only record results for rat studies
Rat models
A total of 168 articles described the use of a rat model, using a total of 9150 rats in 164 studies, 4 remaining studies did not define the amount of animals. Median number of animals used per study was 56 (range 10–218) with a median of three groups per study (mean 3.7, mode 2, range 1–20). Most articles described the use of either Sprague–Dawley (78 studies, 46.4%) or Wistar (78 studies, 46.4%); subspecies was not defined in two studies. Sex of animals was defined in 85.1% of studies with 112 (66.7%) using male rats, 28 (16.7%) female, and 3 (1.8%) using both sexes; sex was not defined in the remaining studies (14.9%). References, that indicated the use of an established and previously published model, were provided in only 24.2% of articles (41 studies). Frequently used models included those published by Alponat et al. (12 studies) [12], Peter-Puchner et al. (4 studies) [13], and Klinge et al. (3 studies) [14].
Methods
All rats underwent open surgery for mesh implantation, receiving 1 (85.1%), 2 (13.1%), or 3 (0.6%) meshes per animal. Most models included the creation of a true hernia defect model (121 articles, 72.0%), 1 study did not define the use of a defect, and the remainder (46 articles, 27.4%) did not create a hernia defect. Defect size varied between 0.5 and 18.0 cm2 with a mean of 4.2 cm2 (median 4.0, mode 6.0). Meshes were either placed as bridging within a defect (49 articles, 29.2%), intraperitoneal (40 articles, 23.8%), subcutaneous (30 articles, 17.9%), inlay (20 articles, 11.9%), or preperitoneal (9 articles, 5.4%). Mesh position was not specified in 11.9% of articles (20 studies). Models aimed at mesh infections were only used in 16 publications (9.5%).
Meshes were cut to size with a median size of 6 cm2 (mean 5.76 cm2, range 0.8–20 cm2); size of mesh was not defined in 29 articles (17.3%). Control groups were defined in 64.3% (108 articles). Most articles defined the use of a polypropylene control (including brand named polypropylene, e.g., Parietene) or sham operated animals (both 38 articles, 22.6%). Others included primary/suture repair (11 articles, 6.6%). Part of included articles compared mesh coatings instead of different meshes; this leads to uncoated meshes being control group in 17 studies (10.1%).
The use of perioperative antibiotics for infection prevention was only mentioned in 7.1% of articles (12 studies). Out of these studies, antibiotics used were from the penicillin group, gentamicin, and fluoroquinolone antibiotics (4 studies each). If animal models other than rats were added in the analysis, up to 20.6% of articles described the use of antibiotics, with cephalosporin-type antibiotics being used most.
Perioperative pain relief using analgesic medication has been mentioned in 15.5% of rat models (26 studies, versus 22.8% or 72 studies when reviewing all animal models). Within these 26 studies, opioid-type analgesics were used in majority of cases (17 articles, 10.1%), sometimes combined with NSAIDs (2 articles, 1.2%), followed by NSAID (7 articles, 4.2%) or local analgesics (5 articles, 3.0%).
Follow-up
Duration of follow-up was defined in 167 of 168 included articles. The number of endpoints ranged from 1 to 6 per article with a median of 2 time-points per article (mean 2.21, mode 1). Duration of follow-up ranged from 6 h to 365 days, with a median duration of 28 days. Time-points that were used most frequently were, respectively, 1 month (including follow-up defined as 4 weeks and 30 days), 3 months (or 90 days), and 1 or 2 weeks.
Outcome parameters
Outcome parameters were scored from all 168 articles. Histological examination of explanted meshes was performed in nearly all articles (81.0%, or 136 articles), 39 of these articles (23.2%) subsequently added immunohistochemical analysis. Strength of ingrowth was either defined as subjective macroscopic ingrowth (scored in 10.1%, 17 articles), or mechanical strength measured by tensiometry (scored in 48.2%, 81 articles). Adhesions were scored in 86 articles (51.1%), scored as adhesion quality (27 articles, 16.1%), adhesion quantity (18 articles, 10.7%), or both (41 articles, 24.4%). Mesh shrinkage was scored in only 17.3% of articles (29 articles). An analysis of the scoring systems used is presented in Table 3.
Table 3.
Overview of scoring systems used
| Different scoring systems (number of scoring systems) | Validated scoring system (number of scoring systems/% articles)a | (Semi-) quantitative or objective scoring (number of scoring systems/% articles) | New scoring system (% articles)b | Unknown or pure descriptive scoring (% of articles) | |
|---|---|---|---|---|---|
| Ingrowth | 12 | 3/5.9 | 4/76.4 | 5.9 | 11.8 |
| Adhesion quality | 24 | 19/75 | 16/73.5 | 14.7 | 7.3 |
| Adhesion quantity | 11 | 5/86.4 | 9/94.9 | 18.6 | 6.7 |
| Shrinkage | 9 | 3/89.6 | 29/100 | 20.7 | 0.0 |
| Histology | 47 | 13/47.1 | 7/36.8 | 22.8 | 30.1 |
| immunohistochemistry | 9 | 2/59.0 | 3/61.5 | 15.4 | 20.5 |
Number indicates the number of different scoring systems involved. The percentage is the percentage of articles involved
aValidated scoring system is defined as either a system with clear reference or an accepted system used in the same manner in multiple articles
bNew scoring systems are defined as scoring systems used only in one article
Discussion
Critical review of the literature revealed a large variety in mesh models; many different models, animal species, meshes, and parameters were assessed in the last decade leading to studies that were difficult to compare among each other.
Identical models including all parameters were not found to be implemented by different centers, in other words all centers apparently use their own specific models. Due to the growing variety in existing and new concepts of meshes, preclinical animal research is necessary to assess biocompatibility and effectiveness of new meshes before implementing them in clinical practice [7–9]. Furthermore, many of the important mesh characteristics are derived from and can only be properly researched using animal models [4]. However, for experimental research to have proper impact, research published by different research groups needs to be comparable and reproducible [3, 15].
In this study, we attempted to provide a systematic overview of all available animal models for mesh research. However, due to the large amount of different animals used we decided to focus on only one species. Although large animal models like pigs are supposed to resemble the human situation most, over 50% of all experimental hernia research focused on rat models [7, 16]. Therefore, we decided to limit this overview and only elaborate on rat models. We realize that limitation to one animal group might lead to bias in information leading to a possible underestimation or even overestimation of the problem. This could possibly be solved by using a combination of a small animal model for preliminary testing and immunohistochemistry, which might be followed by testing on a larger animal model, which will better resemble the anatomy of the human abdominal wall.
One of the first issues that needs to be addressed concerns the use of mostly young male rats. Although incisional hernias occur in both male and female patients, with some clinical studies even reporting female sex as an independent risk factor, almost all included experimental studies report the use of male rats only [17, 18]. Furthermore, more than one in every seven authors did not report the sex of the animal in their papers, even though there is an increasing amount of information on the effect of sex on the outcome [19, 20]. Therefore, we believe that in accordance to the ARRIVE guidelines and the recently published NIH policy there should be an effort to report on and also balance sex of animals in experimental hernia repair [20, 21]. Moreover, most studies rats used are of fairly young age, whereas most patients present with hernia’s later in life.
The results of our survey lead to the assumption that very few researchers make use of already published articles. Although this might be an underestimation due to the fact that not all researchers reference to previously published articles, there still seems to be a large variety in published models. This could lead to irreproducible results or results that cannot be compared between different publications [10, 11]. This also makes translation to clinical practice extremely difficult [7, 15, 16]. Hence, we think that limiting the range of mesh models to a smaller selection of models and clear referencing to standardized models could lead to increase the impact of future publications and in turn benefit hernia surgery [22–24].
We believe one important factor for the choice of hernia models should be that it closely resembles the human situation and follows the guidelines for hernia repair in humans. One discrepancy between human situation and most hernia models is the “hernia age.” Most animal models described use an acute hernia model, where the defect is created in the same procedure as the mesh is placed. In the human situation, hernias take time to mature, possibly altering postoperative results. Perhaps the use of a “mature hernia” model as proposed by Dubay et al. in 2006 would better resemble the clinical situation [25]. Furthermore, following the 2014 International Endohernia Society (IEHS) guidelines IntraPeritoneal Onlay Meshes (IPOM) with closed defects should be used [26].
Another point of interest is the mesh positioning. Although some mesh positions are considered outdated in the clinical setting, there is no decrease in the use of these models over the years. To further increase the impact of the animal studies on clinical practice, it might be good to translate guidelines for human hernia surgery to preclinical animal models. In particular, the IPOM with mesh augmentation, as is advocated in the recent IEHS guidelines, is only used in less than one-fourth of published studies [26]. Furthermore, since the preclinical studies are mostly aimed at investigating host response to meshes and mesh materials, the use of a standardized control group could improve reproducibility and could help put results in perspective.
Despite official guidelines on laboratory animal welfare in both Europe and the USA requiring the use of analgesics when pain is to be expected, analgesics are only reported in a minority of studies [27, 28]. Since hernia operations can be considered major abdominal surgery, pain is to be expected and use of analgesics and the reporting on their use should be promoted according to international regulations.
Despite the heterogeneity in the included studies, there already seems to be some degree in consensus for some aspects. For instance, most authors seem to agree that the creation of an abdominal wall defect is preferred above primary closure, be it with a large range in size of defects and meshes used. There also seems to be some degree of consensus for outcome parameters, whereas majority of studies use histological analysis and adhesion scoring as primary outcome. On the other hand, up to one-fifth of these articles seem to introduce new scoring systems to evaluate these outcome measurements instead of using readily available validated methods.
Hence, we believe guidelines for publishing and reporting of experimental research for hernia research need to be put in place. Different aspects of hernia research need to be standardized in order to increase impact of experimental research. Furthermore, standardization should lead to a reduction in the discrepancy between results in animal research and clinical research, as is often seen in many fields of medicine [23, 29]. Additionally, standardization would make definitive statements on new mesh products easier, as they can easily be compared to results from well-known materials.
Furthermore, the standardization of mesh research should be extended to the industry. The current regulations for approval of a new mesh concept by the FDA require the material only to be “substantially equivalent” to readily available materials, leaving interpretation of this equivalency open to interpretation of the manufacturer [30, 31]. The manufacturer does have to compare the new device to similar devices. However, new guidance documents from the FDA do note that any change to direct or indirect tissue-contacting products should be evaluated using biocompatibility analysis. We believe there should be standardized requirements set by the hernia societies for any new hernia devices introduced on the market.
One of the limitations of this study could be the lack of information on the quality of the animal models, preferably using the ARRIVE guidelines for animal research as proposed by Kilkenny et al. in 2010 [21]. However, we believe this does not aid the aim of our study. Furthermore, we believe the quality assessment of hernia research deserves a separate review additionally assessing the implementation of the ARRIVE guidelines within the hernia research.
Therefore, following the consensus for clinical research as published by Muysoms et al., we believe guidelines and recommendations for experimental mesh research need to be put in place or at least start a discussion on the consensus within animal hernia research models [32]. We therefore propose the establishment of an EHS (European Hernia Society) chapter for experimental research.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Authors contributions
R. Vogels was involved in the brainstorm session preliminary to the review process, decision on inclusion and exclusion criteria, literature search and selection of articles, reading of included articles, setting up the database with all parameters included in this review, and writing and proofreading the submitted article. R. Kaufmann was involved in the brainstorm session preliminary to the review process, decision on inclusion and exclusion criteria, literature search and selection of articles, reading of included articles, setting up the database with all parameters included in this review, and writing and proofreading the submitted article. L van den Hil aided with the database including all article parameters and aided in writing process and proofreading the article. S. van Steensel aided with the database including all article parameters and aided in writing process and proofreading the article. M. Schreinemacher was involved in the preliminary brainstorm session and aided in setting in- and exclusion criteria and relevant scoring parameters. Furthermore, he aided in writing and proofreading process and is involved in setting up an experimental chapter for EHS J. Lange was involved in the preliminary brainstorm session and aided in setting in- and exclusion criteria and relevant scoring parameters. Furthermore, he aided in writing and proofreading process and is involved in setting up an experimental chapter for EHS N. Bouvy was involved in the preliminary brainstorm session and aided in setting in- and exclusion criteria and relevant scoring parameters. Furthermore, he aided in writing and proofreading process and is involved in setting up an experimental chapter for EHS
Compliance with ethical standards
Conflict of interest
RV, RK, LH, SS, MS, JL, NB declare no conflict of interest.
Statement of human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
No informed consent was necessary for the study.
Footnotes
R. R. M. Vogels and R. Kaufmann contributed equally to this work.
Electronic supplementary material
The online version of this article (doi:10.1007/s10029-017-1605-z) contains supplementary material, which is available to authorized users.
Contributor Information
R. R. M. Vogels, Email: r.vogels@maastrichtuniversity.nl
N. D. Bouvy, Email: n.bouvy@mumc.nl
References
- 1.Basile F, Biondi A, Donati M. Surgical approach to abdominal wall defects: history and new trends. Int J Surg. 2013;11:S20–S23. doi: 10.1016/S1743-9191(13)60008-4. [DOI] [PubMed] [Google Scholar]
- 2.Shankaran V, Weber DJ, Reed RL, 2nd, Luchette FA. A review of available prosthetics for ventral hernia repair. Ann Surg. 2011;253(1):16–26. doi: 10.1097/SLA.0b013e3181f9b6e6. [DOI] [PubMed] [Google Scholar]
- 3.Cobb WS, Peindl RM, Zerey M, Carbonell AM, Heniford BT. Mesh terminology 101. Hernia. 2009;13(1):1–6. doi: 10.1007/s10029-008-0428-3. [DOI] [PubMed] [Google Scholar]
- 4.Eriksen JR, Gogenur I, Rosenberg J. Choice of mesh for laparoscopic ventral hernia repair. Hernia. 2007;11(6):481–492. doi: 10.1007/s10029-007-0282-8. [DOI] [PubMed] [Google Scholar]
- 5.Bilsel Y, Abci I. The search for ideal hernia repair; mesh materials and types. Int J Surg. 2012;10(6):317–321. doi: 10.1016/j.ijsu.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Burger JWA, Luijendijk RW, Hop WCJ, Halm JA, Verdaasdonk EGG, Jeekel J. Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Trans Meet Am Surg Assoc. 2004;240:176–183. doi: 10.1097/01.sla.0000141193.08524.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bittner R, Bingener-Casey J, Dietz U, Fabian M, Ferzli GS, Fortelny RH, et al. Guidelines for laparoscopic treatment of ventral and incisional abdominal wall hernias (International Endohernia Society [IEHS])-Part 2. Surg Endosc. 2014;28(2):353–379. doi: 10.1007/s00464-013-3171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bringman S, Conze J, Cuccurullo D, Deprest J, Junge K, Klosterhalfen B, et al. Hernia repair: the search for ideal meshes. Hernia. 2010;14(1):81–87. doi: 10.1007/s10029-009-0587-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schreinemacher M, Henatsch D, van Barneveld K, Bouvy N. The need for standardised animal models and scoring systems in assessing mesh biocompatibility. Hernia. 2010;14(3):335–336. doi: 10.1007/s10029-010-0642-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deeken CR, Faucher KM, Matthews BD. A review of the composition, characteristics, and effectiveness of barrier mesh prostheses utilized for laparoscopic ventral hernia repair. Surg Endosc. 2012;26(2):566–575. doi: 10.1007/s00464-011-1899-3. [DOI] [PubMed] [Google Scholar]
- 11.Sanders DL, Kingsnorth AN. Prosthetic mesh materials used in hernia surgery. Expert Rev Med Devices. 2012;9(2):159–179. doi: 10.1586/erd.11.65. [DOI] [PubMed] [Google Scholar]
- 12.Alponat A, Lakshminarasappa SR, Teh M, Rajnakova A, Moochhala S, Goh PM, et al. Effects of physical barriers in prevention of adhesions: an incisional hernia model in rats. J Surg Res. 1997;68(2):126–132. doi: 10.1006/jsre.1996.4979. [DOI] [PubMed] [Google Scholar]
- 13.Petter-Puchner AH, Fortelny R, Mittermayr R, Ohlinger W, Redl H. Fibrin sealing versus stapling of hernia meshes in an onlay model in the rat. Hernia. 2005;9(4):322–329. doi: 10.1007/s10029-005-0009-7. [DOI] [PubMed] [Google Scholar]
- 14.Klinge U, Junge K, Spellerberg B, Piroth C, Klosterhalfen B, Schumpelick V. Do multifilament alloplastic meshes increase the infection rate? Analysis of the polymeric surface, the bacteria adherence, and the in vivo consequences in a rat model. J Biomed Mater Res. 2002;63(6):765–771. doi: 10.1002/jbm.10449. [DOI] [PubMed] [Google Scholar]
- 15.Kimmelman J, Mogil JS, Dirnagl U. Distinguishing between exploratory and confirmatory preclinical research will improve translation. PLoS Biol. 2014;12(5):e1001863. doi: 10.1371/journal.pbio.1001863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Penttinen R, Gronroos JM. Mesh repair of common abdominal hernias: a review on experimental and clinical studies. Hernia. 2008;12(4):337–344. doi: 10.1007/s10029-008-0362-4. [DOI] [PubMed] [Google Scholar]
- 17.Itatsu K, Yokoyama Y, Sugawara G, Kubota H, Tojima Y, Kurumiya Y, et al. Incidence of and risk factors for incisional hernia after abdominal surgery. Br J Surg. 2014;101(11):1439–1447. doi: 10.1002/bjs.9600. [DOI] [PubMed] [Google Scholar]
- 18.Sorensen LT, Hemmingsen UB, Kirkeby LT, Kallehave F, Jorgensen LN. Smoking is a risk factor for incisional hernia. Arch Surg. 2005;140(2):119–123. doi: 10.1001/archsurg.140.2.119. [DOI] [PubMed] [Google Scholar]
- 19.Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35(3):565–572. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509(7500):282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8(6):e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hackam DG. Translating animal research into clinical benefit. BMJ. 2007;334(7586):163–164. doi: 10.1136/bmj.39104.362951.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henderson VC, Kimmelman J, Fergusson D, Grimshaw JM, Hackam DG. Threats to validity in the design and conduct of preclinical efficacy studies: a systematic review of guidelines for in vivo animal experiments. PLoS Med. 2013;10(7):e1001489. doi: 10.1371/journal.pmed.1001489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490(7419):187–191. doi: 10.1038/nature11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DuBay DA, Wang X, Adamson B, Kuzon WM, Jr, Dennis RG, Franz MG. Mesh incisional herniorrhaphy increases abdominal wall elastic properties: a mechanism for decreased hernia recurrences in comparison with suture repair. Surgery. 2006;140(1):14–24. doi: 10.1016/j.surg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Bittner R, Bingener-Casey J, Dietz U, Fabian M, Ferzli GS, Fortelny RH, et al. Guidelines for laparoscopic treatment of ventral and incisional abdominal wall hernias (International Endohernia Society (IEHS)-part 1. Surg Endosc. 2014;28(1):2–29. doi: 10.1007/s00464-013-3170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes In: UNION TEPATCOTE, ed. 20-10-2010. Official Journal of the European Union 2010:33–79
- 28.Public Health Service Policy on Humane Care and Use of Laboratory Animals In: Services USDoHaH, Health NIo, Welfare OoLA, eds. 2015
- 29.van der Worp HB, Howells DW, Sena ES, Porritt MJ, Rewell S, O’Collins V, et al. Can animal models of disease reliably inform human studies? PLoS Med. 2010;7(3):e1000245. doi: 10.1371/journal.pmed.1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harth KC, Rosen MJ. Major complications associated with xenograft biologic mesh implantation in abdominal wall reconstruction. Surg Innov. 2009;16(4):324–329. doi: 10.1177/1553350609353609. [DOI] [PubMed] [Google Scholar]
- 31.Segan RD. A response to “Major complications associated with xenograft biologic mesh implantation in abdominal wall reconstruction” (Harth KC, Rosen MJ. Surg Innov. 2009;16:324-329) and discussion of the MAUDE (manufacturer and user facility device experience) database, FDA regulation of biologic implants, and evidence-based medicine. Surg Innov. 2010;17(3):273–275. doi: 10.1177/1553350610371214. [DOI] [PubMed] [Google Scholar]
- 32.Muysoms FE, Deerenberg EB, Peeters E, Agresta F, Berrevoet F, Campanelli G, et al. Recommendations for reporting outcome results in abdominal wall repair: results of a Consensus meeting in Palermo, Italy, 28–30 June 2012. Hernia. 2013;17(4):423–433. doi: 10.1007/s10029-013-1108-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.