Abstract
Outcomes of psychotic disorders are associated with high personal, familiar, societal and clinical burden. There is thus an urgent clinical and societal need for improving those outcomes. Recent advances in research knowledge have opened new opportunities for ameliorating outcomes of psychosis during its early clinical stages. This paper critically reviews these opportunities, summarizing the state‐of‐the‐art knowledge and focusing on recent discoveries and future avenues for first episode research and clinical interventions. Candidate targets for primary universal prevention of psychosis at the population level are discussed. Potentials offered by primary selective prevention in asymptomatic subgroups (stage 0) are presented. Achievements of primary selected prevention in individuals at clinical high risk for psychosis (stage 1) are summarized, along with challenges and limitations of its implementation in clinical practice. Early intervention and secondary prevention strategies at the time of a first episode of psychosis (stage 2) are critically discussed, with a particular focus on minimizing the duration of untreated psychosis, improving treatment response, increasing patients’ satisfaction with treatment, reducing illicit substance abuse and preventing relapses. Early intervention and tertiary prevention strategies at the time of an incomplete recovery (stage 3) are further discussed, in particular with respect to addressing treatment resistance, improving well‐being and social skills with reduction of burden on the family, treatment of comorbid substance use, and prevention of multiple relapses and disease progression. In conclusion, to improve outcomes of a complex, heterogeneous syndrome such as psychosis, it is necessary to globally adopt complex models integrating a clinical staging framework and coordinated specialty care programmes that offer pre‐emptive interventions to high‐risk groups identified across the early stages of the disorder. Only a systematic implementation of these models of care in the national health care systems will render these strategies accessible to the 23 million people worldwide suffering from the most severe psychiatric disorders.
Keywords: Psychosis, schizophrenia, psychosis risk, clinical high risk, first episode psychosis, universal prevention, selective prevention, indicated prevention, outcomes, clinical staging
Psychotic disorders such as schizophrenia are common, with 23.6 million prevalent cases worldwide in 20131. One in two people living with schizophrenia does not receive care for the condition2. The recovery rates (one in seven3) and associated disability (11th cause of disability worldwide in 20131) following a first episode of psychosis have not improved over the past seventy years under routine clinical care1, 3. Although existing psychopharmacological treatments alone can reduce some symptoms, they have little impact on the outcome of the illness4.
The annual national costs for the schizophrenia population ranged from US$94 million to US$102 billion worldwide, up to 1.65% of the gross domestic product5. Furthermore, risk of all‐cause mortality for psychotic disorders is twice (risk ratio 2.54) that of the general population6. There is thus an urgent clinical and societal need for improving outcomes of psychosis.
Recent advances in research knowledge have opened new opportunities for ameliorating outcomes of psychosis during the critical periods surrounding the first episode of the illness (about 2 years before7 and 3 years after8 the onset). In this paper, we critically review these opportunities, summarizing the state‐of‐the‐art knowledge and focusing on recent discoveries and future avenues for first episode research and clinical interventions.
As a conceptual framework we will adopt a revised version of the clinical staging model9 (Table 1). We will mostly focus on non‐affective psychoses, although some issues can also be applied to the other types of psychoses.
Table 1.
Revised clinical staging model for psychotic disorders and interventions for improving the outcomes of first‐episode psychosis (FEP)
| Clinical stage | Definition | Definition in clinical staging model | Intervention |
|---|---|---|---|
| 0 | Asymptomatic genetic risk | Premorbid | Selective primary prevention |
| Improved mental health literacy | |||
| Family psychoeducation | |||
| 1a | Negative and cognitive symptoms | CHR‐P | Indicated primary prevention |
| Formal mental health literacy | |||
| Family psychoeducation | |||
| Active reduction of substance misuse | |||
| 1b | Attenuated psychotic symptoms | CHR‐P | Indicated primary prevention |
| Family and individual psychoeducation | |||
| Active reduction of substance misuse | |||
| Vocational support | |||
| Psychological therapies | |||
| 1c | Short‐lived remitting psychotic episodes | CHR‐P | Indicated primary prevention |
| As for 1b | |||
| Close‐in monitoring | |||
| 2 | Full‐threshold FEP | Early full recovery | Early intervention and secondary prevention |
| Family and individual psychoeducation | |||
| Psychological therapies | |||
| Active reduction of substance misuse | |||
| Atypical antipsychotics and other medications | |||
| Vocational rehabilitation | |||
| 3a | Single relapse of psychotic disorder | Late/incomplete recovery | Early intervention and tertiary prevention |
| As for 2, but with emphasis on relapse prevention and early warning signs | |||
| 3b | Multiple relapses | Late/incomplete recovery | Early intervention and tertiary prevention |
| As for 2, but with emphasis on long‐term stabilization | |||
| 3c | Incomplete recovery from first episode | Late/incomplete recovery | Early intervention and tertiary prevention |
| As for 3a; clozapine in case of treatment resistance | |||
| 4 | Severe, persistent or unremitting illness | Chronicity | Maintenance intervention |
| As for 3a‐c, but with emphasis on social participation despite ongoing disability |
CHR‐P – clinical high risk for psychosis
PRIMARY PREVENTION
Mental health promotion aims to promote positive mental health by increasing psychological well‐being, competence and resilience, and by creating supporting living conditions and environments. It is not addressed in the present paper.
Primary prevention aims to reduce the incidence of symptoms and ultimately of mental disorders10. The three categories of primary prevention identified by the World Health Organization (WHO)11 are: universal prevention, targeting the general public or a whole population group that has not been identified on the basis of individual risk; selective prevention, targeting individuals or subgroups of the population whose risk of developing a mental disorder is significantly higher than the rest of the population; and indicated prevention, targeting high‐risk individuals who are identified as having minimal but detectable signs or symptoms foreshadowing mental disorders.
Universal prevention of psychosis
Universal primary prevention must take the form of a safe population‐wide intervention that promotes normal development. Research in this area is still in its infancy, because no established pathophysiological mechanisms to be targeted have been validated12.
A recent pioneering, randomized placebo‐controlled clinical trial of dietary phosphatidylcholine supplementation was conducted in a small sample of healthy pregnant women, starting in the second trimester and continuing through the third postnatal month13. The intervention aimed at correcting delays in cerebral inhibition that may develop perinatally, as indexed by electrophysiological biomarkers. The intervention was free of significant side effects and showed proof of concept efficacy.
Although larger studies need to be conducted to validate these initial findings, future research in this field is warranted over the next decade. Promising research candidates for the universal prevention of psychosis and the supporting evidence, which awaits future replication, are listed in Table 2.
Table 2.
Candidate universal interventions for primary prevention of psychosis
| Intervention | Supporting evidence | Target |
|---|---|---|
| Perinatal phosphatidylcholine | Randomized controlled trial13 | Electrophysiological biomarkers of neonatal development |
| School‐based interventions | Randomized controlled trials14, 15 | Bullying, victimization, pro‐bullying attitudes, pro‐victim attitudes, empathy toward victims |
| Fetal and neonatal N‐acetylcysteine | Randomized controlled trial16 | Biomarkers of neuroinflammation and neuroprotection |
| N‐3 polyunsaturated fatty acids | Review17 | Biomarkers of neuroinflammation |
| Vitamins A, D, B‐group, folic acid | Original study, meta‐analysis18, 19 | Biomarkers of neuroinflammation |
| Sulphoraphane | Review20 | Biomarkers of oxydative stress |
| Prebiotics | Review21 | Microbiota dysbiosis |
| School‐based interventions | Randomized controlled trial, review22, 23 | Substance abuse |
| Exercise training | Original studies24, 25, 26, 27 | Brain plasticity, structure, connectivity, cognitive functioning |
Asymptomatic genetic risk (stage 0)
The staging perspective (Table 1) provides a framework for research and conceptualization of earlier premorbid interventions to alter the developmental pathway to first‐episode psychosis. Selective interventions in this stage could target parental, perinatal, social or later environmental risk factors before symptoms and help‐seeking behaviour manifest28, such as those listed in Table 3.
Table 3.
Some environmental risk factors for psychosis supported by meta‐analytical level of evidence in the current literature
| Type of environmental risk factor | Meta‐analytical association with psychosis |
Association measure type: mean (95% CI) |
|---|---|---|
| Parental risk factors | Parental psychosis29 | RR: 7.87 (4.14‐14.94) |
| Parental affective disorder29 | RR: 6.42 (2.20‐18.78) | |
| Old paternal age30 | RR: 2.22 (1.46‐3.37)[Link] | |
| Perinatal risk factors | Complications of pregnancy31, 32, 33 | OR: 2.44 (1.13‐5.26)b |
| Abnormal foetal growth and development31, 32 | OR: 3.89 (1.40‐10.84)c | |
| Complications of delivery31, 32 | OR: 2.21 (1.38‐3.54)d | |
| Gestational influenza33 | RR: 1.56 (1.05‐2.32) | |
| Season of birth34 | OR: 1.07 (1.05, 1.08) | |
| Social risk factors | Ethnic minority35, 36, 37 | RR: 4.7 (3.3‐6.8)e |
| First and second generation immigrant status38 | IRR: 2.3 (2.0‐2.7)f | |
| Urbanicity39 | OR: 2.37 (2.01‐2.81) | |
| Later risk factors | Infections40, 41, 42 | OR: 2.70 (1.34‐4.42)g |
| Traumatic brain injury43 | OR: 1.65 (1.17‐2.32) | |
| Vitamin D deficiency44 | OR: 2.16 (1.32‐3.56) | |
| Daily tobacco use45 | OR: 2.18 (1.23‐3.85) | |
| Cannabis heavy abuse46 | OR: 3.90 (2.84‐5.34) | |
| Childhood trauma and adversity47 | OR: 2.75 (2.17‐3.47) | |
| Adult life events48 | OR: 3.19 (2.15‐4.75) | |
| Premorbid IQ49, 50 | OR: 4.78 (3.19‐7.13)h |
RR – risk ratio, OR – odds ratio, IRR – incidence rate ratio
aage >55, bgestational age <37 weeks, cbirth weight <2000g, dincubator or resuscitator, eBlack African vs. White British, ffirst generation migrants, gToxoplasma gondii, hIQ<70. Some of these risk factors may also include a genetic component.
Although this is an exciting area for future research, currently there are no robust and effective preventive strategies to reduce the risk of psychosis in asymptomatic individuals exposed to these environmental risk factors51. For now, the primary viable strategy is to use the family high‐risk approach (selecting offspring of individuals with schizophrenia), even though this approach will only yield roughly 10% of the individuals from these families who will develop psychosis51.
Improving mental health literacy in these at‐risk populations may represent an effective pragmatic strategy to help prevent or facilitate earlier intervention in psychosis (Table 1).
Clinical high risk for psychosis (CHR‐P, stage 1a‐c)
State of the art
The introduction of specific semi‐structured interviews52, 53, 54, about two decades ago55, for the ascertainment of signs and symptoms suggestive of psychosis risk states has allowed the identification of individuals at clinical high risk for the development of psychosis (CHR‐P) before full symptoms manifest56. These individuals are functionally impaired in comparison with matched controls at baseline57 and have an up to 20% 2‐year risk (95% CI: 17%‐25%) of developing psychosis58.
Their risk peaks in the first two years59 and is specific for the development of psychotic disorders but not for emerging non‐psychotic disorders60, 61. However, less than half of those who will not develop psychosis will eventually remit (35% of the baseline cohort)62, since persistent comorbidities (that were already present at baseline63, 64, 65) and functional impairment are frequently observed at follow‐up64.
Indicated interventions through specialist CHR‐P provision have been recognized as an important component of clinical services for early psychosis intervention66, 67, 68 – see, for instance, the guidelines of the UK National Institute for Health and Care Excellence (NICE)69, and the Access and Waiting Time (AWT) standards of the UK National Health Service67.
Conceptually, although most of CHR‐P individuals (73%) would present with some comorbid DSM‐IV diagnosis at baseline63, 70, the intervention is still considered preventive71 (indicated) since these individuals are selected on the basis of having early signs or symptoms of psychosis risk.
Indicated interventions in CHR‐P people may improve the outcome of first‐episode psychosis through the following mechanisms: a) delayed or prevented onset of a first episode; b) better engagement with services and reduced comorbidity; c) reduced duration of untreated psychosis (DUP); and d) improved early detection and amelioration of the severity of first‐episode cases (secondary prevention).
Meta‐analysis of randomized controlled trials in CHR‐P individuals suggests that short‐term (6‐12 months) psychological interventions can halve the risk of illness onset at 12 months72. However, the preventive effect is not sustained over a longer period of time (24 months and longer); so, these findings should be interpreted cautiously and may indicate a delayed rather than prevented psychosis onset. No trials have investigated whether long‐term provision of focused interventions may result in sustained benefits. Furthermore, the three largest studies of preventive interventions in individuals at ultra‐high risk for psychosis have turned out to be negative, possibly because of low power73, 74, 75. At the moment, there are no approved interventions that have been shown to reliably alter the long‐term course of the disorder12.
CHR‐P services are effective in improving trust and engagement76, with high satisfaction of users. Furthermore, since most CHR‐P people present with comorbid disorders that are not severe enough to be accepted and treated by generic mental health services, CHR‐P services may also improve these problems as well as provide vocational support and reduce family stress.
Patients who engage with CHR‐P services and who will later develop the disorder show a substantial reduction of their DUP (11 days on average) compared to patients who do not present to clinical services until the first episode (approximately 1 year on average)77. Compared to patients accessing first episode services, patients who presented in the CHR‐P stage are also less likely to require admission following the onset of psychosis (46% vs. 68%) and less likely to require a compulsory admission in the short term (30% vs. 62%)77.
Finally, the presence of CHR‐P services may have extended benefits for the identification of first‐episode cases and for secondary prevention. In fact, about one‐third of patients referred to CHR‐P services have already developed a first episode of psychosis at the time of initial contact78. First‐episode patients presented to CHR‐P service spent fewer days in hospital (less than 17), had a shorter referral to diagnosis time (–74.5 days), a lower frequency of admission (incidence rate ratio = 0.49), and a lower likelihood of compulsory admission (odds ratio = 0.52) compared to patients who were first diagnosed by first‐episode services78. However, these findings may be confounded by a selection bias, which is discussed below here.
Challenges and future advancements
Even assuming that an effective preventive treatment altering the course of the illness may be discovered in the next generation of interventional studies, the overall impact of treating CHR‐P individuals on the outcomes of first‐episode psychosis is still undetermined. This is mostly due to the fact that the potential benefits of the primary prevention during the CHR‐P stage are practically limited by the difficulty to identify and treat all the individuals who are at risk of developing the disorder.
How should CHR‐P individuals be recruited from secondary mental health services?
Current guidelines recommend that the CHR‐P assessment should be primarily offered to individuals who are “already distressed by mental problems and seeking help for them”79. These individuals represent an exceptional window of opportunity for preventive interventions as they are already in contact with secondary mental health services. Unfortunately, only 5.19% of the total cases of emerging first‐episode psychosis among patients accessing secondary mental health services are detected and under the care of CHR‐P services that had been well established (several years before) in the local national health system80.
This result is highly disturbing, as it indicates that the overall real‐world impact of CHR‐P detection and treatment for improving the outcomes of first‐episode psychosis is minimal, missing 95% of individuals who will eventually develop psychosis. Thus, it seems crucial to optimize the proportion of individuals at risk of developing psychosis who are referred to CHR‐P services. Individualized risk estimation e‐tools that are based on easily collectable variables have recently been developed and externally validated (www.psychosis-risk.net)80. Since the vast majority (91%) of patients referred to first‐episode services had a first point of contact within secondary mental health care81, the use of these tools can substantially extend the benefits of preventive interventions to most at‐risk individuals and eventually result in a massive impact for the improvement of first‐episode psychosis outcomes.
How should CHR‐P individuals be recruited outside clinical samples?
The use of the CHR‐P approach outside clinical samples or for screening purposes is not recommended, because its low ability to rule in psychosis52 produces a substantial dilution of risk enrichment82, leading to underpowered clinical trials75 and questionable clinical relevance for preventive interventions52, 83, 84, 85. For example, using CHR‐P assessment in the general non‐help‐seeking adolescent population is associated with a 2.5‐year risk of psychosis onset of 2% only86.
At the same time, it seems important to continue exploring the usefulness of an extended use of CHR‐P assessment to populations not accessing mental health services in order to improve detection of at‐risk cases. Possible solutions may include the use of meta‐analytical Fagan's nomogram52 or stratification models84 that have recently been made available to estimate the overall risk enrichment of samples undergoing CHR‐P assessment.
A complementary approach may be based on the use of sequential testing methods87. The sequential use of screening instruments and CHR‐P assessment in non‐help‐seeking adolescents from the general population may identify individuals who are at potential risk of developing psychosis in the following years88. Sequential testing is in line with the clinical staging model and can be further enhanced by front‐line primary care youth mental health models developed to facilitate the access of young people from the school and community (see https://www.headspace.org.au).
Innovative strategies to identify non‐help‐seeking individuals at risk of psychosis can also involve the use of e‐health technologies, for example based on semantic analysis of social media postings.
Can we provide stratified treatments to the CHR‐P subgroups?
Future advances could also develop stratified preventive treatments targeting the different CHR‐P clinical stages (a, b or c), that may have different characteristics with respect to underlying disease processes and prognosis89. On the basis of the increasing risk (clinical stage 1a: 3% at 2 years58; clinical stage 1b: 19% at 2 years58; clinical stage 1c: 39% at 2 years58 and 51% at more than 3 years90), and symptoms severity91 (individuals in the clinical stage 1c would formally meet the ICD criteria for a brief psychotic disorder92), preventive interventions for the clinical stage 1a can be supplemented by specific psychological therapies and individual psychoeducation for the clinical stage 1b.
These treatments may be further supported by a more intensive or close‐in monitoring for the clinical stage 1c, which is characterized by short‐lived and self‐remitting psychotic episodes lasting few weeks only (e.g., less than 4 weeks)90. In line with the clinical staging model, the stage 1c is less severe compared to patients experiencing a first episode of schizophrenia (clinical stage 2), who do not spontaneously remit from their symptoms without antipsychotic treatment and who show substantial higher risk of relapses90.
EARLY INTERVENTION AND SECONDARY/TERTIARY PREVENTION
Full threshold first‐episode psychosis with early recovery (stage 2)
State of the art
The stage 2 encompasses the acute phase or crisis, that is characterized by florid psychotic symptoms (sustained symptoms lasting four weeks or more as suggested by the NICE Quality Standard 10293), followed by an early recovery phase or post‐acute phase observed in the first 6‐12 months following the acute episode.
Recovery is usually operationalized as concurrent clinical remission – less than mild symptoms at the Positive and Negative Syndrome Scale (PANSS) (≤3), the Scale for the Assessment of Positive Symptoms (SAPS)/Scale for the Assessment of Negative Symptoms (SANS) (<3), or the Brief Psychiatric Rating Scale (BPRS) (≤3), sustained for at least 6 months94 – and functional remission (proper social functioning in the main domains of everyday life)95. Early interventions and secondary preventive interventions during stage 2 may improve the outcome of first‐episode psychosis through the following mechanisms: a) DUP reduction; b) improvement of treatment response; c) improved well‐being, functioning and social skills with reduction of burden on the family; d) treatment of comorbid substance use; e) secondary prevention of disease progression.
A long DUP is associated with poor general symptomatic outcome, more severe positive and negative symptoms, lesser likelihood of remission, and poor social functioning and global outcome, but not employment, quality of life or hospital treatment96. The meta‐analytical correlations are small in magnitude (r = 0.13‐0.18), yet robust96. Since the majority of DUP is accounted for by delays in accessing early intervention services and help seeking97, at least in the UK, it is a modifiable factor even during the clinical stage 2. Community psychosis awareness campaigns, including publicity and community engagement integrated with a specific youth mental health direct care pathway, can halve the DUP compared to detection as usual (mean 104 vs. 285 days)97.
Beyond the impact on DUP, intervention in the clinical stage 2 can be associated with substantial improvements in treatment response. A systematic research of the literature summarizing the results of randomized controlled trials of integrated multicomponent early intervention services for patients experiencing a first episode of psychosis is presented in Table 4. The multicomponent interventions were mostly based on the comprehensive use of antipsychotics98, 99, 100, 102, 105, 106, 107, 108, individual psychological treatments98, 99, 100, 105, 106, 107, 108, family98, 99, 100, 102, 105, 106, 107 and vocational98, 99, 102, 105, 107 support. Small trials showed minimal beneficial effects or no effects at all on clinical outcomes99, 100, 110. Larger trials showed a significant short‐term (i.e., up to 24 months) improvement of treatment response under specialized integrated early interventions compared to standard community care. The improved response to the comprehensive treatments was characterized by lower disengagement from care98, 102, 105; reduction of positive100, 102, 107, negative100, 102 and total105, 106, 107 psychotic symptoms; reduced hospitalization98, 107, lower dosages of antipsychotic medications102, and improved functioning106.
Table 4.
Randomized controlled trials of the effectiveness of specialized integrated early intervention services for first‐episode psychosis
| Study | Intervention | Control | Treatment group (N) | Control group (N) | Follow‐up (months) | Outcome |
|---|---|---|---|---|---|---|
| Craig et al98 | Specialized integrated early intervention (antipsychotics, cognitive behaviour therapy, family counselling, vocational help) | Treatment as usual in community care | 71 | 73 | 18 | No difference in relapse, reduced psychiatric hospitalization and disengagement |
| Kuipers et al99 | Specialized integrated early intervention (atypical antipsychotics, cognitive behaviour therapy, family intervention, vocational help) | Treatment as usual in community care | 32 | 27 | 12 | No significant benefits including psychiatric hospitalization |
| Grawe et al100 Sigrúnarson et al101 | Specialized integrated early intervention (family psychoeducation and therapy, home crisis management, cognitive behaviour therapy, antipsychotics) | Treatment as usual in community care | 30 | 20 | 24 168 | At 24 months, reduced negative and positive symptoms; no benefits on psychiatric hospitalization or recurrences. No substantial long‐term effects. |
| Petersen et al102 Bertelsen et al103 Secher et al104 | Specialized integrated early intervention (family psychoeducation, social skills training, antipsychotics) | Treatment as usual in community care | 275 | 272 | 12, 24 60 120 | At 12 months, reduced hospitalization. At 24 months, improvement on positive and negative symptoms, substance abuse, treatment adherence; lower dosage of antipsychotic medication, higher satisfaction with treatment, reduced burden to the family; no effect on psychiatric hospitalization. At 60 months, many positive effects disappeared; more patients living independently. At 120 months, most positive effects had diminished or vanished. |
| Kane et al105 | Specialized integrated early intervention (family psychoeducation, resilience‐focused individual therapy, supported employment and education, antipsychotics) | Treatment as usual in community care | 223 | 131 | 24 | Reduced disengagement, greater improvement in quality of life, well‐being and total psychopathology, greater involvement in work and school, no effect on psychiatric hospitalization |
| Ruggeri et al106 | Specialized integrated early intervention (cognitive behaviour therapy, family intervention, case management, antipsychotics) | Treatment as usual in community care | 272 | 172 | 9 | Reduced total symptom severity, improved functioning and emotional well‐being; no effect on psychiatric hospitalization or disengagement |
| Srihari et al107 | Specialized integrated early intervention (antipsychotics, family education, cognitive behaviour therapy, vocational support) | Treatment as usual in community care | 60 | 57 | 24 | Reduced psychiatric hospitalization, positive and total psychotic symptoms, improved vocational engagement, no effect on functioning |
| Chang et al108 Chang et al109 | 3‐year specialized integrated early intervention (psychosocial interventions, cognitive behaviour therapy, antipsychotics) | 2‐year specialized integrated early intervention and 1‐year step‐down care | 82 | 78 | 12 | Better functioning, reduced negative and depressive symptoms and disengagement, no effect on psychiatric hospitalization |
| Ando et al110 | Specialized integrated early intervention | Treatment as usual in community care | 34 | 34 | 9 | No effects on disengagement, functional remission, psychiatric hospitalization, self‐harm, suicide attempt, social relationship |
Specialized interventions during the clinical stage 2 are associated with higher patients’ satisfaction with treatment102 and improved personal well‐being105, 106, characterized by better sense of purpose, motivation, curiosity and emotional engagement105. These improvements translated into better quality of life105 and greater involvement in school and work105, 107, with an overall reduced burden to the family102. Family interventions for first‐episode psychosis are an integral component of treatment, but they can have beneficial effects even as standalone treatment, with greater 12‐month improvements in family burden and caregiving experience, reductions in severity of psychotic symptoms and duration of re‐hospitalizations111.
The detrimental impact of illicit substance abuse on the long‐term outcome of psychosis is well known, with a dose‐dependent association112. Available trials confirm that it is possible to reduce substance abuse in first‐episode psychosis through specialized integrated early intervention services102. Randomized controlled trials are directly investigating the effectiveness of a behavioural intervention for reducing cannabis use among young people receiving treatment from early intervention services113, 114.
Finally, interventions in this phase are crucial for the secondary prevention of illness progression to clinical stage 3, in particular to prevent relapse into a second episode of psychosis (3a). This is significant, because relapse interferes with the social and vocational development of individuals suffering from a first episode of psychosis, which has an impact on long‐term outcomes115.
Challenges and future advancements
Although specialized first episode services that provide a comprehensive care can significantly improve outcomes of first‐episode psychosis, and their implementation is overall recommended116, there are some significant challenges.
Are specialized integrated early intervention services effective in preventing relapses?
Despite the benefits yielded by specialized integrated early intervention services, many patients still have an increased risk of relapsing into a second episode of psychosis following an initial recovery (clinical stage 3a). Criteria for relapse vary across studies, but readmission to a psychiatric hospital is the most common definition of psychotic relapse in the existing literature117.
Since randomized controlled trials provide the gold standard methodology for evaluating interventions for relapse prevention, we have updated an earlier meta‐analysis that included only three trials investigating the risk of relapse/admission to psychiatric hospital under specialized early intervention services, compared to standard care118. We now include 12 trials stratified for different time points, as indicated in Table 4.
We found that mean relapse rates under treatment as usual were 14% (95% CI: 10%‐20%) at 9 months, 49% (95% CI: 29%‐69%) at 24 months, and 76% (95% CI: 53%‐90%) at more than 10 years, while under the specialized integrated early intervention services they were 17% (95% CI: 13%‐21%) at 9 months, 38% (95% CI: 14%‐66%) at 24 months and 54% (95% CI: 36%‐70%) at more than 10 years.
Figure 1 shows that there was no meta‐analytical evidence that specialized integrated early intervention services can substantially improve the odds ratio for having a relapse compared to standard care, at any time points. These negative findings are in line with naturalistic studies, showing that about 50% of cases of first‐episode non‐affective psychosis relapse at least once (clinical stage 3a), while 34% have multiple relapses (clinical stage 3b). Adherence (odds ratio 2.9) and schizophrenia diagnosis (odds ratio 2.2) were the most robust predictors of the first relapse119.
Figure 1.
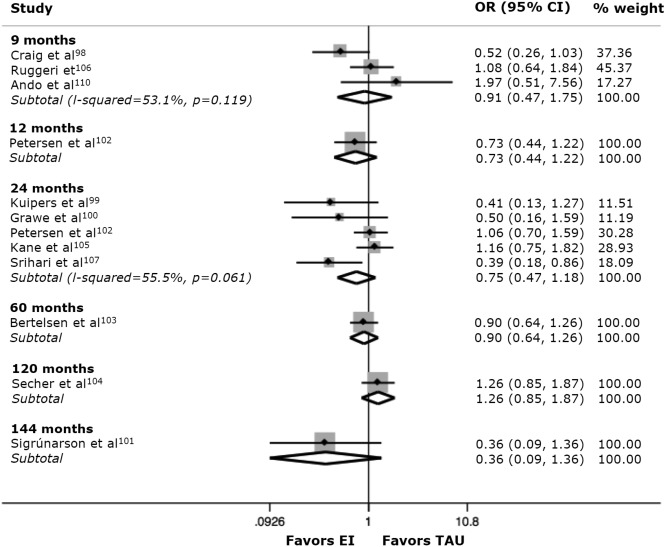
Meta‐analytical odds for relapses (hospital readmission) with specialized integrated early intervention services (EI) compared to standard care (TAU) in the community. Odds ratios smaller than 1 indicate an association of reduced relapses with EI, while odds ratios greater than 1 indicate an association of reduced relapses with TAU. Weights are from random effects analysis.
These findings are also in line with the lack of stringent evidence for a robust effect of antipsychotics on relapse prevention in the long term and with meta‐analyses indicating that the overall rate of long‐term recovery following a first episode of psychosis has not improved much worldwide over the past decades3. There is still much to be done to develop effective integrated treatments for tertiary relapse prevention in early psychosis.
Should we use long‐acting injectable antipsychotics earlier?
International treatment guidelines for first‐episode psychosis recommend antipsychotic medication maintenance for at least 1‐2 years to prevent relapse120. The most robust meta‐analysis of randomized controlled trials of antipsychotics in first‐episode patients showed 26% risk of relapse in the treatment group at 1 year, compared to 61% in the placebo group at 1 year (risk ratio = 0.47)121.
Since antipsychotics are effective in the short term to prevent relapse, and non‐adherence is a modifiable risk factor, it seems justifiable to introduce the use of long‐acting injectable antipsychotics (LAIs) earlier in the treatment of psychosis, during the clinical stage 2122. LAIs are superior to placebo not only for the prevention of relapse but also for the reduction of symptoms in acutely ill patients with established psychosis122.
However, seven independent meta‐analyses of available randomized controlled trials, including one conducted in recent‐onset psychosis (including only three trials enrolling patients with a diagnosis of psychosis within 1‐5 years)123, found no evidence that LAIs are associated with better efficacy on relapse prevention, compared to oral antipsychotics124, 125, 126, 127, 128, 129.
It is possible that randomized controlled trials enrol patient samples that are not representative of real‐world clinical practice. In fact, meta‐analyses of studies comparing LAIs vs. oral antipsychotics in the same patients, that better reflect real‐world efficacy, found strong evidence for LAIs superiority on preventing hospital admission (risk ratio = 0.43)130. Furthermore, since the available trials have been mostly conducted in chronic patients or in patients with some years of active psychosis, the actual efficacy of LAIs in patients with a first episode of psychosis (clinical stage 2) is undetermined. In general, LAIs are similar to one another in terms of relapse prevention122.
Using LAIs in first‐episode patients with clear risk factors for relapse – such as a diagnosis of schizophrenia, non‐adherence to oral antipsychotics, comorbid substance misuse and poor insight – may thus substantially improve outcomes of first‐episode psychosis.
For how long should early intervention services be offered?
Beyond relapse prevention, most trials indicate that the benefits provided by early intervention services are attenuated over the long term101, 103, 104, at more than 2‐year follow‐up, although these findings may be due to insufficient power. It is likely that the positive effects of intensive early treatment are sustained only if patients continue to receive specialized services (though at what intensity/frequency remains a question).
A recent trial compared a 3‐year provision of specialized services versus a 2‐year provision of the same. The extended year was associated with significant benefits on negative and positive symptoms, as well as on functioning108. This also aligns with the clinical staging model, wherein symptom resolution and clinical stabilization take place at an earlier stage followed by gradual functional improvement, which occurs later and requires substantially longer to achieve.
Discharging first‐episode patients back to primary care or poor morale generic mental health services that focus heavily on patients with persistent illness, after 1‐2 years of specialized early intervention care, is likely to result in the erosion of the initial advantages and gains and is thus unlikely to change their long‐term recovery outcomes.
Longer‐term early intervention services spanning the entire critical period of 5 years8 are under development131. A subset of cases will almost certainly need longer‐term expert care. In the context of competing demands and budgetary constraints, it is important to note that the costs for comprehensive specialized integrated care are exceeded by its benefits, relative to standard community care132, 133, 134.
Schizophrenia spectrum vs. affective spectrum first‐episode psychosis: does it make any difference?
Formulating a specific ICD or DSM diagnosis of psychosis at the time of the first contact with the first‐episode services is challenging, because the clinical features are relatively non‐specific. However, the NICE recommendation 1.3.4.3 for first‐episode psychosis clearly indicates that if the patient's presentation suggests an affective rather than schizophrenia spectrum psychosis, different clinical guidelines (e.g., those for bipolar disorder or for depression) should be followed at least for psychopharmacological treatments120.
A meta‐analysis conducted in 14,484 first‐episode patients, with an average follow‐up of 4.5 years, found a high prospective diagnostic stability for schizophrenia spectrum psychoses (0.93; 95% CI: 0.89‐0.97) and for affective spectrum psychoses (0.84; 95% CI: 0.79‐0.89), which is comparable to other clinical diagnoses in medicine135. In line with the clinical staging model, the retrospective diagnostic stability was low for both spectra (0.60), indicating that many first‐episode patients who receive a non‐specific diagnosis of psychosis (e.g., psychosis not otherwise specified) will eventually develop schizophrenia or affective psychoses135. Therefore, having a baseline diagnosis of schizophrenia spectrum or affective spectrum psychotic disorder may still have significant clinical impacts136.
Schizophrenia features are strong predictors of poor long‐term outcomes (e.g., at 3 years137 and 10 years138, 139, 140) in first‐episode patients, with odds ratio ranging from 5.70 to 8.86140. An initial diagnosis of schizophrenia has been associated with higher risk of relapse at 3 years (odds ratio 2.7)119. The worse prognostic outcome of an initial schizophrenia diagnosis has been confirmed even in modern specialized integrated early intervention services that were offering state‐of‐the‐art treatments to improve outcome for first‐episode psychosis119, 140, 141. However, when communicating with patients, it may be preferable to use the broader term psychosis rather than schizophrenia, to fully reflect the possibility of plastic and heterogeneous outcomes.
For how long should we treat remitted patients with antipsychotics?
Because evidence is robust for the effectiveness of antipsychotic medication in reducing the short‐term risk of relapse, it would seem reasonable to recommend medication maintenance for all first‐episode individuals. However, the long‐term efficacy of antipsychotics for relapse prevention is less established. Furthermore, since treatment disengagement is common early in the illness and is largely patient‐driven142, more effective alternatives could be considered143. Finally, there is increasing concern that cardiometabolic risk factors and abnormalities are present early in the illness, and related to the underlying mental disorder, unhealthy lifestyle and antipsychotic medications144, as well as subtle extrapyramidal symptoms145.
As a consequence of these considerations, the long‐term use of antipsychotic medications has been recently questioned146 and discontinuation of antipsychotic medication after 1‐2 years is partially recommended by some clinical guidelines147. Two recent trials have investigated this issue, comparing treatment maintenance versus reduction/discontinuation strategies. In the short term (within the first 3 years), the risk of relapse was twice in the reduction/discontinuation group compared to the maintenance group145, 148. However, in the longer term (at 7 years), the risk of relapse was comparable (62% in the reduction/discontinuation group vs. 69% in the maintenance group)145.
Despite some important methodological limitations136, it was additionally found that recovery and functional remission rates in the reduction/discontinuation group were twice those seen in the non‐dose reduction/discontinuation group145. Importantly, the patients included in these trials had all experienced a clinical or functional remission that was sustained for six145 or 18148 months (i.e., clinical stage 2). Discontinuing antipsychotic treatment before remission is achieved (e.g., for the clinical stage 3) is associated with higher time to remission and later risk of relapse149, 150.
Overall, these findings indicate that the effect of antipsychotics is mostly symptomatic and unlikely to change the underlying course of the disorder, raising suspicion that these drugs may delay but not actually prevent relapses12. In fact, longer treatment periods with antipsychotics before withdrawal are not associated with reduced risk of relapse143, with a rapid return of symptoms in the relapse episode to severity levels similar to those in the first psychotic episode143.
On the basis of the existing conflicting evidence, treatment reduction may be a stage 2 specific option only for the subset of patients who had achieved a clinical remission94 and are not at high risk of relapse. The challenge would be to identify these low‐risk individuals prior to considering treatment reduction151. Future research is thus needed to develop reliable stratification models for these patients according to the most robust risk factors for relapse: longer duration of untreated psychosis, male gender, poor baseline functioning and educational status, and a diagnosis of schizophrenia152, 153.
A recent meta‐analysis indicated that the risk of relapse in patients diagnosed with schizophrenia who have achieved a clinical remission and then discontinued antipsychotic medications was 78% at 24 months and 84% at more than 36 months90. Accordingly, it has been suggested to exclude from treatment discontinuation/reduction strategies first‐episode patients who have been diagnosed with schizophrenia at baseline152.
However, future replication trials are required before treatment discontinuation/reduction can be safely implemented in clinical practice. A viable solution could be to use psychological treatments rather than placebo in both arms of a future discontinuation/reduction vs. maintenance trial, which may be an acceptable and effective alternative for patients who have chosen not to take antipsychotic drugs154.
Incomplete recovery from first episode of psychosis (stage 3)
State of the art
The critical period after the onset of psychosis extends to the clinical stage 3. There are three forms of incomplete recovery: a) recovery is initially achieved but then followed by a relapse (clinical stage 3a); b) initial recovery is followed by multiple relapses (clinical stage 3b); c) premorbid functional or symptoms levels are never fully reached (clinical stage 3c).
Early interventions and tertiary preventive interventions during stage 3 may improve the outcome of first‐episode psychosis through the following mechanisms: a) addressing treatment resistance; b) improving well‐being and social skills with reduction of burden on the family; c) treatment of comorbid substance use; d) prevention of multiple relapses and disease progression.
The failure to respond to two different antipsychotics, at therapeutic doses and for a sufficient duration155, means that a person meets the criteria for treatment resistance, and may thus be in the clinical phase 3c. Approximately 30% of patients with first‐episode psychosis manifest a minimal response to antipsychotics156. Recognizing treatment resistance earlier and treating these cases with clozapine157 at this stage could produce larger benefits in several domains of outcomes, because of the greater retention of patients' personal and social agency114, 158, 159.
Early interventions that can improve the well‐being, functioning and social skills with reduction of burden on the family as well as treating comorbid substance use are similar to those described for the clinical stage 2.
Although it has been suggested that acute psychotic exacerbations represent active periods of a morbid process that leads to disease progression (the “neurotoxic hypothesis of psychosis”), to date there is limited empirical evidence to support illness progression after each relapse143. The mechanisms of toxicity have not been described160 and supporting evidence is conflicting161. On the one hand, based on limited data, times to remission are significantly longer for the second and third episodes162; treatment discontinuation163 and the effective dose164 are higher during the subsequent episodes compared to the first one (suggesting reduced effectiveness of antipsychotics when reintroduced after illness recurrence); and relapse duration (but not frequency) is associated with gray matter alterations165. On the other hand, patients’ symptoms return to baseline with resumption of antipsychotic medication after the relapse148, and the pattern of treatment response across single episode and multiple episodes patients is not different and highly variable163, 166. For example, emergent treatment failure after relapse is evident in 16% of the first‐episode and 14% of the multi‐episode samples respectively163, 166, replicating an earlier finding that 1 in 6 patients failed to recover from each of their first four relapses, irrespective of which relapse it was167. Finally, a subset of patients (23%) can even be treatment resistant at the time of illness onset, even before the first relapse168.
It is important to note that, beyond the controversies regarding disease progression after each relapse, it is clear that each relapse is a traumatic experience associated with potentially serious psychosocial and functional consequences that are impacting the quality of life of the patient and the caregiver. Unfortunately, no clear interventions have been developed and validated for the tertiary prevention of disease progression from stage 3a to stage 3b (prevention of relapse recurrences), because second relapses are not consistently associated with robust modifiable risk factors such as non‐adherence119. Similarly, there are no approved treatments to prevent progression to clinical stage 4. Overall, these data are in line with the limited evidence for substantial protective effects of antipsychotics on relapse prevention in the long term and highlight a clear need for further prospective research elucidating the role of relapse on illness progression in early psychosis.
Challenges and future directions
A new test to identify non‐response to antipsychotics and reduce delay to clozapine usage
Recent studies suggest that, among treatment‐resistant first‐episode schizophrenia patients, 70% never experienced any symptomatic remission from the time of their first presentation, while 30% had achieved a symptomatic remission before developing treatment resistance during the first 5 years of illness168. Therefore, for the majority of cases, treatment resistance could be most appropriately addressed with clozapine at an early stage of its presentation, particularly given that early treatment with clozapine is effective157, and that worse outcomes are seen with a delayed use of the drug169. In standard mental health services, the mean delay in initiating clozapine is 4 years170.
A further possibility to accelerate the use of clozapine for treatment‐resistant patients may be to use a diagnostic test to predict non‐response to antipsychotics. A meta‐analysis of 34 studies (N = 9,460) found that a <20% PANSS or BPRS reduction at week 2 of antipsychotic treatment predicted non‐response at 12 weeks, with a specificity of 86% and a positive predictive value of 90%171. The use of this test in early intervention services can facilitate the switch to a second antipsychotic (ideally LAIs in patients with risk factors for relapse) and therefore minimize the delay to clozapine.
Another possibility could be to identify treatment‐resistant patients at baseline. Research in this field is in its infancy, but a recent study suggested that it is possible to identify specific predictors of treatment‐resistant schizophrenia172.
Can we prevent negative symptoms?
The presence of prominent negative symptoms at baseline is one of the strongest predictors of poor outcome in first‐episode patients173, 174. Negative symptoms are twice as likely to become non‐responsive to treatments than positive symptoms140. A recent meta‐analysis found that no available treatment for negative symptoms reached the threshold for robust clinically meaningful improvement175.
Poor social functioning, disorganized symptoms and schizophrenia diagnosis are baseline risk factors that can be used to identify first‐episode patients at risk of developing negative symptoms140. Negative symptoms are also predicted by longer DUP176, suggesting that programmes aimed at shortening DUP might reduce the prevalence of negative symptoms and improve prognosis of first‐episode psychosis177.
LIMITATIONS OF THE CLINICAL STAGING MODEL
Staging models have been widely adopted in oncology, because stages are defined by clear pathophysiological boundaries associated with discrete changes in mortality risk and treatment choices 174, 178. On the contrary, the example of ventricular enlargements highlights the lack of utility of current neurobiological measures to inform prognosis and treatment decisions in psychosis179. Translation from clinical to pathophysiological staging is not yet available in psychosis.
Variation in cancer severity within a stage (e.g., tumor size or number of metastases) has fewer implications for prognosis and treatment than variation between stages. This is not the case for psychosis, where high heterogeneity and variations within each stage (e.g., stage 2)58 play a substantial role. Additional robust evidence is needed to support the incremental clinical utility of the discrete stages proposed (e.g., from stage 3 to stage 4)178, 180.
TOWARDS AN INTERNATIONAL COORDINATED SPECIALTY PROGRAMME FOR EARLY PSYCHOSIS
In conclusion, we show here that to improve outcomes of a complex, heterogeneous syndrome such as psychosis, it is necessary to globally adopt complex models integrating a clinical staging framework and coordinated specialty care programmes133 that offer pre‐emptive interventions to high‐risk groups identified across the early stages of the disorder181.
It is possible to improve outcomes of first‐episode psychosis using stage‐specific interventions that are comprehensive182, i.e. ranging from the universal prevention of psychosis to strategies for overcoming treatment‐resistant psychosis, and transdiagnostic, i.e. spanning broader spectra during the clinical stage 1 and the psychosis spectrum during the clinical phase 2.
Although we have detailed the key clinical strategies for improving outcomes at each clinical stage, it is clear that only a systematic implementation of these cost‐effective132 models of care in the national health care systems will render these strategies accessible to the 23 million people worldwide suffering from the most severe psychiatric disorders.
REFERENCES
- 1. Global Burden of Disease Study 2013 Collaborators . Global, regional, and national incidence, prevalence, and years lived with dis ability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386:743‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Schizophrenia. www.who.int/mental_health/management/schizophrenia/en/.
- 3. Jaaskelainen E, Juola P, Hirvonen N et al. A systematic review and meta‐analysis of recovery in schizophrenia. Schizophr Bull 2013;39:1296‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Millan MJ, Andrieux A, Bartzokis G et al. Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov 2016;5:485‐51. [DOI] [PubMed] [Google Scholar]
- 5. Chong HY, Teoh SL, Wu DB et al. Global economic burden of schizophrenia: a systematic review. Neuropsychiatr Dis Treat 2016;12:357‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta‐analysis. JAMA Psychiatry 2015;72:334‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McGlashan TH. Early detection and intervention of schizophrenia: rationale and research. Br J Psychiatry 1998;172(Suppl. 33):3‐6. [PubMed] [Google Scholar]
- 8. Birchwood M, Todd P, Jackson C. Early intervention in psychosis. The critical period hypothesis. Br J Psychiatry 1998;172(Suppl. 33):53‐9. [PubMed] [Google Scholar]
- 9. McGorry PD, Hickie IB, Yung AR et al. Clinical staging of psychiatric disorders: a heuristic framework for choosing earlier, safer and more effective interventions. Aust N Z J Psychiatry 2006;40:616‐22. [DOI] [PubMed] [Google Scholar]
- 10. Gordon R. An operational classification of disease prevention. Publ Health Rep 1983;98:107‐9. [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization. Prevention of mental disorders. Effective interventions and policy options. Geneva: World Health Organization, 2004. [Google Scholar]
- 12. Millan MJ, Andrieux A, Bartzokis G et al. Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov 2016;15:485‐515. [DOI] [PubMed] [Google Scholar]
- 13. Ross RG, Hunter SK, McCarthy L et al. Perinatal choline effects on neonatal pathophysiology related to later schizophrenia risk. Am J Psychiatry 2013;170:290‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Waasdorp TE, Bradshaw CP, Leaf PJ. The impact of schoolwide positive behavioral interventions and supports on bullying and peer rejection: a randomized controlled effectiveness trial. Arch Pediatr Adolesc Med 2012;166:149‐56. [DOI] [PubMed] [Google Scholar]
- 15. Nocentini A, Menesini E. KiVa Anti‐Bullying Program in Italy: evidence of effectiveness in a randomized control trial. Prev Sci 2016;17:1012‐23. [DOI] [PubMed] [Google Scholar]
- 16. Jenkins DD, Wiest DB, Mulvihill DM et al. Fetal and neonatal effects of N‐acetylcysteine when used for neuroprotection in maternal chorioamnionitis. J Pediatr 2016;168:67‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pusceddu MM, Kelly P, Stanton C et al. N‐3 Polyunsaturated fatty acids through the lifespan: implication for psychopathology. Int J Neuropsychopharmacol (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dawson SL, Bowe SJ, Crowe TC. A combination of omega‐3 fatty acids, folic acid and B‐group vitamins is superior at lowering homocysteine than omega‐3 alone: a meta‐analysis. Nutr Res 2016;36:499‐508. [DOI] [PubMed] [Google Scholar]
- 19. Kurtys E, Eisel UL, Verkuyl JM et al. The combination of vitamins and omega‐3 fatty acids has an enhanced anti‐inflammatory effect on microglia. Neurochem Int 2016;99:206‐14. [DOI] [PubMed] [Google Scholar]
- 20. Do KQ, Cuenod M, Hensch TK. Targeting oxidative stress and aberrant critical period plasticity in the developmental trajectory to schizophrenia. Schizophr Bull 2015;41:835‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fond G, Boukouaci W, Chevalier G et al. The “psychomicrobiotic”: targeting microbiota in major psychiatric disorders: a systematic review. Pathol Biol 2015;63:35‐42. [DOI] [PubMed] [Google Scholar]
- 22. Patnode CD, O'Connor E, Rowland M et al. Primary care behavioral interventions to prevent or reduce illicit drug use and nonmedical pharmaceutical use in children and adolescents: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med 2014;160:612‐20. [DOI] [PubMed] [Google Scholar]
- 23. Vogl LE, Newton NC, Champion KE et al. A universal harm‐minimisation approach to preventing psychostimulant and cannabis use in adolescents: a cluster randomised controlled trial. Subst Abuse Treat Prev Policy 2014;9:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci 2002;25:295‐301. [DOI] [PubMed] [Google Scholar]
- 25. Draganski B, Gaser C, Busch V et al. Neuroplasticity: changes in grey matter induced by training. Nature 2004;427:311‐2. [DOI] [PubMed] [Google Scholar]
- 26. Douw L, Nieboer D, van Dijk BW et al. A healthy brain in a healthy body: brain network correlates of physical and mental fitness. PLoS One 2014;9:e88202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee TM, Wong ML, Lau BW et al. Aerobic exercise interacts with neurotrophic factors to predict cognitive functioning in adolescents. Psychoneuroendocrinology 2014;39:214‐24. [DOI] [PubMed] [Google Scholar]
- 28. Fusar‐Poli P, Tantardini M, De Simone S et al. Deconstructing vulnerability for psychosis: meta‐analysis of environmental risk factors for psychosis in subjects at ultra high‐risk. Eur Psychiatry 2016;40:65‐75. [DOI] [PubMed] [Google Scholar]
- 29. Rasic D, Hajek T, Alda M et al. Risk of mental illness in offspring of parents with schizophrenia, bipolar disorder, and major depressive disorder: a meta‐analysis of family high‐risk studies. Schizophr Bull 2014;40:28‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Torrey EF, Buka S, Cannon TD et al. Paternal age as a risk factor for schizophrenia: how important is it? Schizophr Res 2009;114:1‐5. [DOI] [PubMed] [Google Scholar]
- 31. Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta‐analytic review. Am J Psychiatry 2002;159:1080‐92. [DOI] [PubMed] [Google Scholar]
- 32. Geddes JR, Verdoux H, Takei N et al. Schizophrenia and complications of pregnancy and labor: an individual patient data meta‐analysis. Schizophr Bull 1999;25:413‐23. [DOI] [PubMed] [Google Scholar]
- 33. Cai L, Wan CL, He L et al. Gestational influenza increases the risk of psychosis in adults. Med Chem 2015;11:676‐82. [DOI] [PubMed] [Google Scholar]
- 34. Davies G, Welham J, Chant D et al. A systematic review and meta‐analysis of Northern Hemisphere season of birth studies in schizophrenia. Schizophr Bull 2003;29:587‐93. [DOI] [PubMed] [Google Scholar]
- 35. Bosqui TJ, Hoy K, Shannon C. A systematic review and meta‐analysis of the ethnic density effect in psychotic disorders. Soc Psychiatry Psychiatr Epidemiol 2014;49:519‐29. [DOI] [PubMed] [Google Scholar]
- 36. Tortelli A, Errazuriz A, Croudace T et al. Schizophrenia and other psychotic disorders in Caribbean‐born migrants and their descendants in England: systematic review and meta‐analysis of incidence rates, 1950–2013. Soc Psychiatry Psychiatr Epidemiol 2015;50:1039‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kirkbride JB, Errazuriz A, Croudace TJ et al. Incidence of schizophrenia and other psychoses in England, 1950–2009: a systematic review and meta‐analyses. PLoS One 2012;7:e31660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bourque F, van der Ven E, Malla A. A meta‐analysis of the risk for psychotic disorders among first‐ and second‐generation immigrants. Psychol Med 2011;41:897‐910. [DOI] [PubMed] [Google Scholar]
- 39. Vassos E, Pedersen CB, Murray RM et al. Meta‐analysis of the association of urbanicity with schizophrenia. Schizophr Bull 2012;38:1118‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Khandaker GM, Zimbron J, Dalman C et al. Childhood infection and adult schizophrenia: a meta‐analysis of population‐based studies. Schizophr Res 2012;139:161‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Arias I, Sorlozano A, Villegas E et al. Infectious agents associated with schizophrenia: a meta‐analysis. Schizophr Res 2012;136:128‐36. [DOI] [PubMed] [Google Scholar]
- 42. Sutterland AL, Fond G, Kuin A et al. Beyond the association. Toxoplasma gondii in schizophrenia, bipolar disorder, and addiction: systematic review and meta‐analysis. Acta Psychiatr Scand 2015;132:161‐79. [DOI] [PubMed] [Google Scholar]
- 43. Molloy C, Conroy RM, Cotter DR et al. Is traumatic brain injury a risk factor for schizophrenia? A meta‐analysis of case‐controlled population‐based studies. Schizophr Bull 2011;37:1104‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Valipour G, Saneei P, Esmaillzadeh A. Serum vitamin D levels in relation to schizophrenia: a systematic review and meta‐analysis of observational studies. J Clin Endocrinol Metab 2014;99:3863‐72. [DOI] [PubMed] [Google Scholar]
- 45. Gurillo P, Jauhar S, Murray RM et al. Does tobacco use cause psychosis? Systematic review and meta‐analysis. Lancet Psychiatry 2015;2:718‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Marconi A, Di Forti M, Lewis CM et al. Meta‐analysis of the association between the level of cannabis use and risk of psychosis. Schizophr Bull 2016;42:1262‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Varese F, Smeets F, Drukker M et al. Childhood adversities increase the risk of psychosis: a meta‐analysis of patient‐control, prospective‐ and cross‐sectional cohort studies. Schizophr Bull 2012;38:661‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Beards S, Gayer‐Anderson C, Borges S et al. Life events and psychosis: a review and meta‐analysis. Schizophr Bull 2013;39:740‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: a meta‐analytic review. Am J Psychiatry 2008;165:579‐87. [DOI] [PubMed] [Google Scholar]
- 50. Khandaker GM, Barnett JH, White IR et al. A quantitative meta‐analysis of population‐based studies of premorbid intelligence and schizophrenia. Schizophr Res 2011;132:220‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Seidman LJ, Nordentoft M. New targets for prevention of schizophrenia: is it time for interventions in the premorbid phase? Schizophr Bull 2015;41:795‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fusar‐Poli P, Cappucciati M, Rutigliano G et al. At risk or not at risk? Meta‐analysis of the prognostic accuracy of psychometric interviews for psychosis prediction. World Psychiatry 2015;14:322‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fusar‐Poli P, Cappucciati M, Rutigliano G et al. Towards a standard psychometric diagnostic interview for subjects at ultra high risk of psychosis: CAARMS versus SIPS. Psychiatry J 2016:7146341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fusar‐Poli P, Schultze‐Lutter F. Predicting the onset of psychosis in patients at clinical high risk: practical guide to probabilistic prognostic reasoning. Evidence‐Based Mental Health 2016;19:10‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yung AR, McGorry PD, McFarlane CA et al. Monitoring and care of young people at incipient risk of psychosis. Schizophr Bull 1996;22:283‐303. [DOI] [PubMed] [Google Scholar]
- 56. Fusar‐Poli P, Borgwardt S, Bechdolf A et al. The psychosis high‐risk state: a comprehensive state‐of‐the‐art review. JAMA Psychiatry 2013;70:107‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fusar‐Poli P, Rocchetti M, Sardella A et al. Disorder, not just a state of risk: meta‐analysis of functioning and quality of life in subjects at high clinical risk for psychosis. Br J Psychiatry 2015;207:198‐206. [DOI] [PubMed] [Google Scholar]
- 58. Fusar‐Poli P, Cappucciati M, Borgwardt S et al. Heterogeneity of risk for psychosis within subjects at clinical high risk: meta‐analytical stratification. JAMA Psychiatry 2016;73:113‐20. [DOI] [PubMed] [Google Scholar]
- 59. Kempton M, Bonoldi I, Valmaggia L et al. Speed of psychosis progression in people at ultra high clinical risk: a complementary meta‐analysis. JAMA Psychiatry 2015;72:622‐3. [DOI] [PubMed] [Google Scholar]
- 60. Fusar‐Poli P, Rutigliano G, Stahl D et al. Long‐term validity of the at risk mental state (ARMS) for predicting psychotic and non‐psychotic mental disorders. Eur Psychiatry 2017;42:49‐54. [DOI] [PubMed] [Google Scholar]
- 61. Webb JR, Addington J, Perkins DO et al. Specificity of incident diagnostic outcomes in patients at clinical high risk for psychosis. Schizophr Bull 2015;41:1066‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Simon AE, Borgwardt S, Riecher‐Rössler A et al. Moving beyond transition outcomes: meta‐analysis of remission rates in individuals at high clinical risk for psychosis. Psychiatry Res 2013;209:266‐72. [DOI] [PubMed] [Google Scholar]
- 63. Fusar‐Poli P, Nelson B, Valmaggia L et al. Comorbid depressive and anxiety disorders in 509 individuals with an at‐risk mental state: impact on psychopathology and transition to psychosis. Schizophr Bull 2014;40:120‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rutigliano G, Valmaggia L, Landi P et al. Persistence or recurrence of non‐psychotic comorbid mental disorders associated with 6‐year poor functional outcomes in patients at ultra high risk for psychosis. J Affect Disord 2016;203:101‐10. [DOI] [PubMed] [Google Scholar]
- 65. Lin A, Wood SJ, Nelson B et al. Outcomes of nontransitioned cases in a sample at ultra‐high risk for psychosis. Am J Psychiatry 2015;172:249‐58. [DOI] [PubMed] [Google Scholar]
- 66. National Health Service England . Mental health access and waiting time standards. London: National Health Service England, 2014. [Google Scholar]
- 67.National Health Service England. Achieving better access to mental health services by 2020. London: National Health Service England, 2014.
- 68. Fusar‐Poli P, Carpenter WT, Woods SW et al. Attenuated psychosis syndrome: ready for DSM‐5.1? Annu Rev Clin Psychol 2014;10:155‐92. [DOI] [PubMed] [Google Scholar]
- 69.National Institute for Health and Care Excellence. Psychosis and schizophrenia in children and young people: recognition and management. www.nice.org.uk. [PubMed]
- 70. Nelson B, Yuen HP, Wood SJ et al. Long‐term follow‐up of a group at ultra high risk (“prodromal”) for psychosis: the PACE 400 study. JAMA Psychiatry 2013;70:793‐802. [DOI] [PubMed] [Google Scholar]
- 71. O'Connell M, Boat T, Warner K. (eds). Preventing mental, emotional, and behavioral disorders among young people: progress and possibilities. Washington: National Academies Press, 2009. [PubMed] [Google Scholar]
- 72. van der Gaag M, Smit F, Bechdolf A et al. Preventing a first episode of psychosis: meta‐analysis of randomized controlled prevention trials of 12 month and longer‐term follow‐ups. Schizophr Res 2013;149:56‐62. [DOI] [PubMed] [Google Scholar]
- 73. McGorry P, Nelson B, Markulev C et al. Effect of ω‐3 polyunsaturated fatty acids in young people at ultrahigh risk for psychotic disorders. JAMA Psychiatry 2017;74:19‐27. [DOI] [PubMed] [Google Scholar]
- 74. Morrison AP, French P, Stewart SL et al. Early detection and intervention evaluation for people at risk of psychosis: multisite randomised controlled trial. BMJ 2012;344:e2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. McFarlane WR, Levin B, Travis L et al. Clinical and functional outcomes after 2 years in the early detection and intervention for the prevention of psychosis multisite effectiveness trial. Schizophr Bull 2015;41:30‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fusar‐Poli P, Byrne M, Badger S et al. Outreach and support in south London (OASIS), 2001–2011: ten years of early diagnosis and treatment for young individuals at high clinical risk for psychosis. Eur Psychiatry 2013;28:315‐26. [DOI] [PubMed] [Google Scholar]
- 77. Valmaggia LR, Byrne M, Day F et al. Duration of untreated psychosis and need for admission in patients who engage with mental health services in the prodromal phase. Br J Psychiatry 2015;207:130‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fusar‐Poli P, Diaz‐Caneja CM, Patel R et al. Services for people at high risk improve outcomes in patients with first episode psychosis. Acta Psychiatr Scand 2016;133:76‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Schultze‐Lutter F, Michel C, Schmidt SJ et al. EPA guidance on the early detection of clinical high risk states of psychoses. Eur Psychiatry 2015;30:405‐16. [DOI] [PubMed] [Google Scholar]
- 80. Fusar‐Poli P, Rutigliano G, Stahl D et al. Development and validation of a clinically based risk calculator for the transdiagnostic prediction of psychosis. JAMA Psychiatry 2017;74:493‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Birchwood M, Connor C, Lester H et al. Reducing duration of untreated psychosis: care pathways to early intervention in psychosis services. Br J Psychiatry 2013;203:58‐64. [DOI] [PubMed] [Google Scholar]
- 82. Fusar Poli P. Why ultra high risk criteria for psychosis prediction do not work well outside clinical samples and what to do about it. World Psychiatry 2017;16:212‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fusar‐Poli P, Schultze‐Lutter F, Cappucciati M et al. The dark side of the moon: meta‐analytical impact of recruitment strategies on risk enrichment in the clinical high risk state for psychosis. Schizophr Bull 2016;42:732‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Fusar‐Poli P, Rutigliano G, Stahl D et al. Deconstructing pretest risk enrichment to optimize prediction of psychosis in individuals at clinical high risk. JAMA Psychiatry 2016;73:1260‐7. [DOI] [PubMed] [Google Scholar]
- 85. Fusar‐Poli P, Schultze‐Lutter F, Addington J. Intensive community outreach for those at ultra high risk of psychosis: dilution, not solution. Lancet Psychiatry 2016;3:18. [DOI] [PubMed] [Google Scholar]
- 86. Michel C, Schimmelmann BG, Schultze‐Lutter F. What becomes of risk symptoms in the community? 2.5 year follow‐up findings of the Bern Epidemiological At‐Risk (BEAR) Study. Early Interv Psychiatry 2016;10(S1):129. [Google Scholar]
- 87. Schmidt A, Cappucciati M, Radua J et al. Improving prognostic accuracy in subjects at clinical high risk for psychosis: systematic review of predictive models and meta‐analytical sequential testing simulation. Schizophr Bull 2017;43:375‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Calkins M, Moore T, Satterthwaite T et al. Persistence of psychosis spectrum symptoms in the Philadelphia Neurodevelopmental Cohort: a prospective two year follow‐up. World Psychiatry 2017;16:62‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Fusar‐Poli P. The Clinical High‐Risk State for Psychosis (CHR‐P), Version II. Schizophr Bull 2017;43:44‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Fusar‐Poli P, Cappucciati M, Bonoldi I et al. Prognosis of brief psychotic episodes: a meta‐analysis. JAMA Psychiatry 2016;73:211‐20. [DOI] [PubMed] [Google Scholar]
- 91. Carrion R, Correll C, Auther A et al. A severity‐based clinical staging model for the psychosis prodrome: longitudinal findings from New York RAP study. Schizophr Bull 2017;43:64‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Fusar‐Poli P, Cappucciati M, De Micheli A et al. Diagnostic and prognostic significance of brief limited intermittent psychotic symptoms (BLIPS) in individuals at ultra high risk. Schizophr Bull 2017;43:48‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.National Institute for Health and Care Excellence. Bipolar disorder, psychosis and schizophrenia in children and young people. www.nice.org.uk. [PubMed]
- 94. Andreasen NC, Carpenter WT Jr, Kane JM et al. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry 2005;162:441‐9. [DOI] [PubMed] [Google Scholar]
- 95. Wunderink L, Sytema S, Nienhuis FJ et al. Clinical recovery in first‐episode psychosis. Schizophr Bull 2009;35:362‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Penttila M, Jaaskelainen E, Hirvonen N et al. Duration of untreated psychosis as predictor of long‐term outcome in schizophrenia: systematic review and meta‐analysis. Br J Psychiatry 2014;205:88‐94. [DOI] [PubMed] [Google Scholar]
- 97. Connor C, Birchwood M, Freemantle N et al. Don't turn your back on the symptoms of psychosis: the results of a proof‐of‐principle, quasi‐experimental intervention to reduce duration of untreated psychosis. BMC Psychiatry 2016;16:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Craig TK, Garety P, Power P et al. The Lambeth Early Onset (LEO) Team: randomised controlled trial of the effectiveness of specialised care for early psychosis. BMJ 2004;329:1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kuipers E, Holloway F, Rabe‐Hesketh S et al. An RCT of early intervention in psychosis: Croydon Outreach and Assertive Support Team (COAST). Soc Psychiatry Psychiatr Epidemiol 2004;39:358‐63. [DOI] [PubMed] [Google Scholar]
- 100. Grawe RW, Falloon IR, Widen JH et al. Two years of continued early treatment for recent‐onset schizophrenia: a randomised controlled study. Acta Psychiatr Scand 2006;114:328‐36. [DOI] [PubMed] [Google Scholar]
- 101. Sigrúnarson V, Grawe RW, Morken G. Integrated treatment vs. treatment‐as‐usual for recent onset schizophrenia; 12 year follow‐up on a randomized controlled trial. BMC Psychiatry 2013;13:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Petersen L, Jeppesen P, Thorup A et al. A randomised multicentre trial of integrated versus standard treatment for patients with a first episode of psychotic illness. BMJ 2005;331:602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bertelsen M, Jeppesen P, Petersen L et al. Five‐year follow‐up of a randomized multicenter trial of intensive early intervention vs standard treatment for patients with a first episode of psychotic illness: the OPUS trial. Arch Gen Psychiatry 2008;65:762‐71. [DOI] [PubMed] [Google Scholar]
- 104. Secher RG, Hjorthoj CR, Austin SF et al. Ten‐year follow‐up of the OPUS specialized early intervention trial for patients with a first episode of psychosis. Schizophr Bull 2015;41:617‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kane JM, Robinson DG, Schooler NR et al. Comprehensive versus usual community care for first‐episode psychosis: 2‐year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry 2016;173:362‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ruggeri M, Bonetto C, Lasalvia A et al. Feasibility and effectiveness of a multi‐element psychosocial intervention for first‐episode psychosis: results from the cluster‐randomized controlled GET UP PIANO trial in a catchment area of 10 million inhabitants. Schizophr Bull 2015;41:1192‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Srihari VH, Tek C, Kucukgoncu S et al. First‐episode services for psychotic disorders in the U.S. public sector: a pragmatic randomized controlled trial. Psychiatr Serv 2015;66:705‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Chang WC, Chan GH, Jim OT et al. Optimal duration of an early intervention programme for first‐episode psychosis: randomised controlled trial. Br J Psychiatry 2015;206:492‐500. [DOI] [PubMed] [Google Scholar]
- 109. Chang WC, Kwong VW, Chan GH et al. Prediction of functional remission in first‐episode psychosis: 12‐month follow‐up of the randomized‐controlled trial on extended early intervention in Hong Kong. Schizophr Res 2016;173:79‐83. [DOI] [PubMed] [Google Scholar]
- 110. Ando S, Nishida A, Koike S et al. Comprehensive early intervention for patients with first‐episode psychosis in Japan (J‐CAP): nine‐month follow‐up of randomized controlled trial. Early Interv Psychiatry 2016;8(S1):1‐180. [Google Scholar]
- 111. Chien WT, Thompson DR, Lubman DI et al. A randomized controlled trial of clinician‐supported problem‐solving bibliotherapy for family caregivers of people with first‐episode psychosis. Schizophr Bull 2016;42:1457‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Schoeler T, Petros N, Di Forti M et al. Association between continued cannabis use and risk of relapse in first‐episode psychosis: a quasi‐experimental investigation within an observational study. JAMA Psychiatry 2016;73:1173‐9. [DOI] [PubMed] [Google Scholar]
- 113. Johnson S, Sheridan Rains L, Marwaha S et al. A randomised controlled trial of the clinical and cost‐effectiveness of a contingency management intervention compared to treatment as usual for reduction of cannabis use and of relapse in early psychosis (CIRCLE): a study protocol for a randomised controlled trial. Trials 2016;17:515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Edwards J, Elkins K, Hinton M et al. Randomized controlled trial of a cannabis‐focused intervention for young people with first‐episode psychosis. Acta Psychiatr Scand 2006;114:109‐17. [DOI] [PubMed] [Google Scholar]
- 115. Penn DL, Waldheter EJ, Perkins DO et al. Psychosocial treatment for first‐episode psychosis: a research update. Am J Psychiatry 2005;162:2220‐32. [DOI] [PubMed] [Google Scholar]
- 116. Nordentoft M, Rasmussen JO, Melau M et al. How successful are first episode programs? A review of the evidence for specialized assertive early intervention. Curr Opin Psychiatry 2014;27:167‐72. [DOI] [PubMed] [Google Scholar]
- 117. Gleeson JF, Alvarez‐Jimenez M, Cotton SM et al. A systematic review of relapse measurement in randomized controlled trials of relapse prevention in first‐episode psychosis. Schizophr Res 2010;119:79‐88. [DOI] [PubMed] [Google Scholar]
- 118. Alvarez‐Jimenez M, Parker AG, Hetrick SE et al. Preventing the second episode: a systematic review and meta‐analysis of psychosocial and pharmacological trials in first‐episode psychosis. Schizophr Bull 2011;37:619‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Pelayo‐Teran JM, Gajardo Galán VG, de la Ortiz‐ Garcĺa de la Foz V et al. Rates and predictors of relapse in first‐episode non‐affective psychosis: a 3‐year longitudinal study in a specialized intervention program (PAFIP). Eur Arch Psychiatry Clin Neurosci 2017;267:315‐23. [DOI] [PubMed] [Google Scholar]
- 120.National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management. www.nice.org.uk. [PubMed]
- 121. Leucht S, Tardy M, Komossa K et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta‐analysis. Lancet 2012;379:2063‐71. [DOI] [PubMed] [Google Scholar]
- 122. Correll CU, Citrome L, Haddad PM et al. The use of long‐acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry 2016;77(Suppl. 3):1‐24. [DOI] [PubMed] [Google Scholar]
- 123. Kishi T, Oya K, Iwata N. Long‐acting injectable antipsychotics for the prevention of relapse in patients with recent‐onset psychotic disorders: a systematic review and meta‐analysis of randomized controlled trials. Psychiatry Res 2016;246:750‐5 [DOI] [PubMed] [Google Scholar]
- 124. Ostuzzi G, Bighelli I, So R et al. Does formulation matter? A systematic review and meta‐analysis of oral versus long‐acting antipsychotic studies. Schizophr Res 2017;183:10‐21. [DOI] [PubMed] [Google Scholar]
- 125. Misawa F, Kishimoto T, Hagi K et al. Safety and tolerability of long‐acting injectable versus oral antipsychotics: a meta‐analysis of randomized controlled studies comparing the same antipsychotics. Schizophr Res 2016;176:220‐30. [DOI] [PubMed] [Google Scholar]
- 126. Kishi T, Matsunaga S, Iwata N. Mortality risk associated with long‐acting injectable antipsychotics: a systematic review and meta‐analyses of randomized controlled trials. Schizophr Bull 2016;42:1438‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kishimoto T, Robenzadeh A, Leucht C et al. Long‐acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta‐analysis of randomized trials. Schizophr Bull 2014;40:192‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Fusar‐Poli P, Kempton MJ, Rosenheck RA. Efficacy and safety of second‐generation long‐acting injections in schizophrenia: a meta‐analysis of randomized‐controlled trials. Int Clin Psychopharmacol 2013;28:57‐66. [DOI] [PubMed] [Google Scholar]
- 129. Haddad PM, Taylor M, Niaz OS. First‐generation antipsychotic long‐acting injections v. oral antipsychotics in schizophrenia: systematic review of randomised controlled trials and observational studies. Br J Psychiatry 2009;195(Suppl. 52):S20‐8. [DOI] [PubMed] [Google Scholar]
- 130. Kishimoto T, Nitta M, Borenstein M et al. Long‐acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta‐analysis of mirror‐image studies. J Clin Psychiatry 2013;74:957‐65. [DOI] [PubMed] [Google Scholar]
- 131. Lutgens D, Iyer S, Joober R et al. A five‐year randomized parallel and blinded clinical trial of an extended specialized early intervention vs. regular care in the early phase of psychotic disorders: study protocol. BMC Psychiatry 2015;15:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Rosenheck R, Leslie D, Sint K et al. Cost‐effectiveness of comprehensive, integrated care for first episode psychosis in the NIMH RAISE Early Treatment Program. Schizophr Bull 2016;42:896‐906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Csillag C, Nordentoft M, Mizuno M et al. Early intervention services in psychosis: from evidence to wide implementation. Early Interv Psychiatry 2016;10:540‐6. [DOI] [PubMed] [Google Scholar]
- 134. Park AL, McCrone P, Knapp M. Early intervention for first‐episode psychosis: broadening the scope of economic estimates. Early Interv Psychiatry 2016;10:144‐51. [DOI] [PubMed] [Google Scholar]
- 135. Fusar‐Poli P, Cappucciati M, Rutigliano G et al. Diagnostic stability of ICD/DSM first episode psychosis diagnoses: meta‐analysis. Schizophr Bull 2016;42:1395‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Catts SV, O'Toole BI. The treatment of schizophrenia: can we raise the standard of care? Aust N Z J Psychiatry 2016;50:1128‐38. [DOI] [PubMed] [Google Scholar]
- 137. Chang WC, Lau ES, Chiu SS et al. Three‐year clinical and functional outcome comparison between first‐episode mania with psychotic features and first‐episode schizophrenia. J Affect Disord 2016;200:1‐5. [DOI] [PubMed] [Google Scholar]
- 138. Friis S, Melle I, Johannessen JO et al. Early predictors of ten‐year course in first‐episode psychosis. Psychiatr Serv 2016;67:438‐43. [DOI] [PubMed] [Google Scholar]
- 139. Heslin M, Lappin JM, Donoghue K et al. Ten‐year outcomes in first episode psychotic major depression patients compared with schizophrenia and bipolar patients. Schizophr Res 2016;176:417‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Austin SF, Mors O, Budtz‐ Jorgensen E et al. Long‐term trajectories of positive and negative symptoms in first episode psychosis: a 10 year follow‐up study in the OPUS cohort. Schizophr Res 2015;168:84‐91. [DOI] [PubMed] [Google Scholar]
- 141. Morgan C, Lappin J, Heslin M et al. Reappraising the long‐term course and outcome of psychotic disorders: the AESOP‐10 study. Psychol Med 2014;44:2713‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Dixon LB, Holoshitz Y, Nossel I. Treatment engagement of individuals experiencing mental illness: review and update. World Psychiatry 2016;15:13‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Emsley R, Chiliza B, Asmal L et al. The nature of relapse in schizophrenia. BMC Psychiatry 2013;13:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Correll CU, Robinson DG, Schooler NR et al. Cardiometabolic risk in patients with first‐episode schizophrenia spectrum disorders: baseline results from the RAISE‐ETP study. JAMA Psychiatry 2014;71:1350‐63. [DOI] [PubMed] [Google Scholar]
- 145. Wunderink L, Nieboer RM, Wiersma D et al. Recovery in remitted first‐episode psychosis at 7 years of follow‐up of an early dose reduction/discontinuation or maintenance treatment strategy: long‐term follow‐up of a 2‐year randomized clinical trial. JAMA Psychiatry 2013;70:913‐20. [DOI] [PubMed] [Google Scholar]
- 146. Murray RM, Quattrone D, Natesan S et al. Should psychiatrists be more cautious about the long‐term prophylactic use of antipsychotics? Br J Psychiatry 2016;209:361‐5. [DOI] [PubMed] [Google Scholar]
- 147. Takeuchi H, Suzuki T, Uchida H et al. Antipsychotic treatment for schizophrenia in the maintenance phase: a systematic review of the guidelines and algorithms. Schizophr Res 2012;134:219‐25. [DOI] [PubMed] [Google Scholar]
- 148. Mayoral‐van Son J, de la Foz VO, Martinez‐Garcia O et al. Clinical outcome after antipsychotic treatment discontinuation in functionally recovered first‐episode nonaffective psychosis individuals: a 3‐year naturalistic follow‐up study. J Clin Psychiatry 2016;77:492‐500. [DOI] [PubMed] [Google Scholar]
- 149. Winton‐Brown TT, Elanjithara T, Power P et al. Five‐fold increased risk of relapse following breaks in antipsychotic treatment of first episode psychosis. Schizophr Res 2017;179:50‐6. [DOI] [PubMed] [Google Scholar]
- 150. Karson C, Duffy RA, Eramo A et al. Long‐term outcomes of antipsychotic treatment in patients with first‐episode schizophrenia: a systematic review. Neuropsychiatr Dis Treat 2016;12:57‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. McGorry P, Alvarez‐Jimenez M, Killackey E. Antipsychotic medication during the critical period following remission from first‐episode psychosis: less is more. JAMA Psychiatry 2013;70:898‐900. [DOI] [PubMed] [Google Scholar]
- 152. Alvarez‐Jimenez M, O'Donoghue B, Thompson A et al. Beyond clinical remission in first episode psychosis: thoughts on antipsychotic maintenance vs. guided discontinuation in the functional recovery era. CNS Drugs 2016;30:357‐68. [DOI] [PubMed] [Google Scholar]
- 153. Di Capite S, Upthegrove R, Mallikarjun P. The relapse rate and predictors of relapse in patients with first‐episode psychosis following discontinuation of antipsychotic medication. Early Interv Psychiatry (in press). [DOI] [PubMed] [Google Scholar]
- 154. Morrison AP, Turkington D, Pyle M et al. Cognitive therapy for people with schizophrenia spectrum disorders not taking antipsychotic drugs: a single‐blind randomised controlled trial. Lancet 2014;383:1395‐403. [DOI] [PubMed] [Google Scholar]
- 155. Howes OD, McCutcheon R, Agid O et al. Treatment‐resistant schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) Working Group Consensus Guidelines on Diagnosis and Terminology. Am J Psychiatry 2017;174:216‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Harvey PD, Rosenthal JB. Treatment resistant schizophrenia: course of brain structure and function. Prog Neuropsychopharmacol Biol Psychiatry 2016;70:111‐6. [DOI] [PubMed] [Google Scholar]
- 157. Agid O, Arenovich T, Sajeev G et al. An algorithm‐based approach to first‐episode schizophrenia: response rates over 3 prospective antipsychotic trials with a retrospective data analysis. J Clin Psychiatry 2011;72:1439‐44. [DOI] [PubMed] [Google Scholar]
- 158. Kreyenbuhl JA, Medoff DR, McEvoy JP et al. The RAISE Connection Program: psychopharmacological treatment of people with a first episode of schizophrenia. Psychiatr Serv 2016;67:1300‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Williams R, Malla A, Roy M et al. What is the place of clozapine in the treatment of early psychosis in Canada? Can J Psychiatry 2017;62:109‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Zipursky RB, Agid O. Recovery, not progressive deterioration, should be the expectation in schizophrenia. World Psychiatry 2015;14:94‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Emsley R, Chiliza B, Asmal L. The evidence for illness progression after relapse in schizophrenia. Schizophr Res 2013;148:117‐21. [DOI] [PubMed] [Google Scholar]
- 162. Lieberman JA, Alvir JM, Koreen A et al. Psychobiologic correlates of treatment response in schizophrenia. Neuropsychopharmacology 1996;14(Suppl. 3):13S‐21S. [DOI] [PubMed] [Google Scholar]
- 163. Emsley R, Oosthuizen P, Koen L et al. Comparison of treatment response in second‐episode versus first‐episode schizophrenia. J Clin Psychopharmacol 2013;33:80‐3. [DOI] [PubMed] [Google Scholar]
- 164. McEvoy JP, Hogarty GE, Steingard S. Optimal dose of neuroleptic in acute schizophrenia. A controlled study of the neuroleptic threshold and higher haloperidol dose. Arch Gen Psychiatry 1991;48:739‐45. [DOI] [PubMed] [Google Scholar]
- 165. Andreasen NC, Liu D, Ziebell S et al. Relapse duration, treatment intensity, and brain tissue loss in schizophrenia: a prospective longitudinal MRI study. Am J Psychiatry 2013;170:609‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Emsley R, Nuamah I, Hough D et al. Treatment response after relapse in a placebo‐controlled maintenance trial in schizophrenia. Schizophr Res 2012;138:29‐34. [DOI] [PubMed] [Google Scholar]
- 167. Wiersma D, Nienhuis FJ, Slooff CJ et al. Natural course of schizophrenic disorders: a 15‐year followup of a Dutch incidence cohort. Schizophr Bull 1998;24:75‐85. [DOI] [PubMed] [Google Scholar]
- 168. Lally J, Ajnakina O, Di Forti M et al. Two distinct patterns of treatment resistance: clinical predictors of treatment resistance in first‐episode schizophrenia spectrum psychoses. Psychol Med 2016;46:3231‐40. [DOI] [PubMed] [Google Scholar]
- 169. Ucok A, Cikrikcili U, Karabulut S et al. Delayed initiation of clozapine may be related to poor response in treatment‐resistant schizophrenia. Int Clin Psychopharmacol 2015;30:290‐5. [DOI] [PubMed] [Google Scholar]
- 170. Howes OD, Vergunst F, Gee S et al. Adherence to treatment guidelines in clinical practice: study of antipsychotic treatment prior to clozapine initiation. Br J Psychiatry 2012;201:481‐5. [DOI] [PubMed] [Google Scholar]
- 171. Samara MT, Leucht C, Leeflang MM et al. Early improvement as a predictor of later response to antipsychotics in schizophrenia: a diagnostic test review. Am J Psychiatry 2015;172:617‐29. [DOI] [PubMed] [Google Scholar]
- 172. Wimberley T, Stovring H, Sorensen HJ et al. Predictors of treatment resistance in patients with schizophrenia: a population‐based cohort study. Lancet Psychiatry 2016;3:358‐66. [DOI] [PubMed] [Google Scholar]
- 173. Diaz‐Caneja CM, Pina‐Camacho L, Rodriguez‐Quiroga A et al. Predictors of outcome in early‐onset psychosis: a systematic review. NPJ Schizophr 2015;1:14005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. McGorry P, Keshavan M, Goldstone S et al. Biomarkers and clinical staging in psychiatry. World Psychiatry 2014;13:211‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Fusar‐Poli P, Papanastasiou E, Stahl D et al. Treatments of negative symptoms in schizophrenia: meta‐analysis of 168 randomized placebo‐controlled trials. Schizophr Bull 2015;41:892‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Galderisi S, Mucci A, Bitter I et al. Persistent negative symptoms in first episode patients with schizophrenia: results from the European First Episode Schizophrenia Trial. Eur Neuropsychopharmacol 2013;23:196‐204. [DOI] [PubMed] [Google Scholar]
- 177. Melle I, Larsen TK, Haahr U et al. Prevention of negative symptom psychopathologies in first‐episode schizophrenia: two‐year effects of reducing the duration of untreated psychosis. Arch Gen Psychiatry 2008;65:634‐40. [DOI] [PubMed] [Google Scholar]
- 178. Mathalon DH. Challenges associated with application of clinical staging models to psychotic disorders. Biol Psychiatry 2011;70:600‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Fusar‐Poli P, Meyer‐Lindenberg A. Forty years of structural imaging in psychosis: promises and truth. Acta Psychiatr Scand 2016;134:207‐24. [DOI] [PubMed] [Google Scholar]
- 180. Duffy A, Malhi GS, Grof P. Do the trajectories of bipolar disorder and schizophrenia follow a universal staging model? Can J Psychiatry 2017;62:115‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. McGorry PD. Pre‐emptive intervention in psychosis: agnostic rather than diagnostic. Aust N Z J Psychiatry 2011;45:515‐9. [DOI] [PubMed] [Google Scholar]
- 182. Leguay D. Advocacy for the establishment of a comprehensive strategy to reduce the “burden” of schizophrenic disorders. Encephale 2016;42:476‐83. [DOI] [PubMed] [Google Scholar]


