Abstract
This study aimed to determine if, following two years of early intervention service for first‐episode psychosis, three‐year extension of that service was superior to three years of regular care. We conducted a randomized single blind clinical trial using an urn randomization balanced for gender and substance abuse. Participants were recruited from early intervention service clinics in Montreal. Patients (N=220), 18‐35 years old, were randomized to an extension of early intervention service (EEIS; N=110) or to regular care (N=110). EEIS included case management, family intervention, cognitive behaviour therapy and crisis intervention, while regular care involved transfer to primary (community health and social services and family physicians) or secondary care (psychiatric outpatient clinics). Cumulative length of positive and negative symptom remission was the primary outcome measure. EEIS patients had a significantly longer mean length of remission of positive symptoms (92.5 vs. 63.6 weeks, t=4.47, p<0.001), negative symptoms (73.4 vs. 59.6 weeks, t=2.84, p=0.005) and both positive and negative symptoms (66.5 vs. 56.7 weeks, t=2.25, p=0.03) compared to regular care patients. EEIS patients stayed in treatment longer than regular care patients (mean 131.7 vs. 105.3 weeks, t=3.98, p<0.001 through contact with physicians; 134.8 ± 37.7 vs. 89.8 ± 55.2, t=6.45, p<0.0001 through contact with other health care providers) and received more units of treatment (mean 74.9 vs. 39.9, t=4.21, p<0.001 from physicians, and 57.3 vs. 28.2, t=4.08, p<0.001 from other health care professionals). Length of treatment had an independent effect on the length of remission of positive symptoms (t=2.62, p=0.009), while number of units of treatment by any health care provider had an effect on length of remission of negative symptoms (t=−2.70, p=0.008) as well as total symptoms (t=−2.40, p=0.02). Post‐hoc analysis showed that patients randomized to primary care, based on their better clinical profile at randomization, maintained their better outcome, especially as to remission of negative symptoms, at the end of the study. These data suggest that extending early intervention service for three additional years has a positive impact on length of remission of positive and negative symptoms compared to regular care. This may have policy implications for extending early intervention services beyond the current two years.
Keywords: First‐episode psychosis, extension of early intervention service, regular care, positive symptoms, negative symptoms, outcome, remission
Psychotic disorders, comprised primarily of schizophrenia spectrum and affective psychoses, have a lifetime median prevalence of 4%1, 2 and enormous negative personal, social and economic consequences3, 4.
Outcome trajectories are generally established during the “critical period” (i.e., the early years of psychosis)5, 6, 7. This has fuelled the development of specialized early intervention services in many parts of the world8, 9. Such services are characterized by comprehensive, multi‐modal and phase‐specific treatment of patients with a first episode of psychosis, typically centred around assertive case management with access to multiple psychosocial interventions in addition to use of medications9 and, in some cases, efforts at reducing delay in treatment10.
The short‐term benefits of early intervention services compared to regular care for treatment of first‐episode psychosis have been reported in a number of studies measuring syndromal and functional outcomes as well as substance abuse, aggression and/or suicidal behaviour, re‐hospitalization and cost‐effectiveness10, 11, 12, 13. While these studies are very encouraging, these gains may not be retained once patients are transferred to regular care after the first two years of early intervention services14, as reported by the OPUS I study15.
Another uncontrolled trial, using a substantially lower intensity of early intervention service following two years of full intensity service, produced more encouraging results. This study showed higher rates of full remission of positive symptoms for the last two of five years of follow‐up than the OPUS I study (54.3% vs. 41.3%, respectively)16.
Two recent studies have produced mixed results. A study from Hong Kong reported benefits of a third year extension of early intervention service17. Another randomized controlled trial, just published from Denmark (OPUS II study), failed to find any benefit of extending early intervention service from two to five years when compared to two years of early intervention service followed by three years of regular care, using severity of negative symptoms as the primary outcome18.
The objective of the present trial, similar to the OPUS II study just published, was to examine if extending treatment in an early intervention service over the entire five‐year “critical period” produces better outcomes than two years of early intervention service followed by regular care, using a randomized controlled single blind design.
METHODS
Design and participants
The central postulate tested in this study was that the experimental group, that is, individuals receiving early intervention service for an extended period (five years), will show a significantly longer remission of positive and negative symptoms than the control group (individuals receiving early intervention service for two years followed by regular care for three years).
The study was carried out between 2008 and 2015. We used an open‐label randomized controlled design. Prior to randomization, all patients had received two years of treatment for their first episode of psychosis in one of the early intervention services included within the McGill University network. These services follow guidelines incorporating modified assertive case management, lowest effective dose pharmacotherapy, family intervention, group interventions to facilitate recovery, cognitive behaviour therapy when indicated, and crisis intervention9, 19, 20.
We included patients able to provide informed consent, meeting DSM‐IV criteria for a psychotic disorder (schizophrenia spectrum psychoses or affective psychosis) confirmed with the Structured Clinical Interview for DSM‐IV Axis I Disorders, Patient Edition21, and having completed 24 (±3) months of treatment in one of the above‐mentioned early intervention services. Patients were included irrespective of their remission status and presence or not of comorbid substance abuse.
Exclusion criteria were inability to provide informed consent or to speak either English or French fluently, and an IQ below 70 as assessed using the short form of the Wechsler Adult Intelligence Scale22.
Randomization and patient allocation
All patients receiving treatment for first‐episode psychosis in an early intervention service of the McGill University Network were approached for participation in the study, usually following the 18‐month review. At month 24 (±3 months), patients who met inclusion and exclusion criteria and signed an informed consent were allocated to either the experimental or the control intervention using a computerized urn randomization protocol23 carried out by a trial statistician not connected with any of the services. This procedure improves upon chance allocation by adjusting assignment probabilities based on key intake characteristics (gender and comorbid diagnosis of substance abuse) that could influence outcomes. Group allocation was concealed in sealed opaque envelopes.
Randomization results were revealed to the patient and, if he/she was randomized to regular care, the transfer process was initiated within two weeks. Baseline assessments were conducted by the research coordinator prior to randomization. Outcome evaluations were carried out in a setting different from the clinical ones by a trained researcher who was blinded to treatment assignment, was not involved in patient care and did not have access to patients’ clinical records. Patients were instructed and reminded not to reveal the nature of treatment they were receiving or the name and location of their treating clinicians. In addition, data collected from each patient's case files by the project co‐ordinator were re‐coded to remove any information that would identify the treatment allocation.
Primary outcome measure
Clinical remission is among the most desirable outcomes and is also strongly associated with functional recovery24, 25, 26. Length of remission of positive, negative and both positive and negative symptoms (i.e., total remission) is reported here as the primary outcome as per the trial registration. We also report the proportion of patients who were in remission for at least three months during the follow‐up period.
Remission was measured by administering the Scale for Assessment of Positive Symptoms (SAPS)27 and the Scale for Assessment of Negative Symptoms (SANS)28 every three months. Patients scoring 2 or less on all of the global (subscale) items of either scale were considered to be in remission for that scale, and those with scores of 2 or less on all global items of both scales were considered to be in total remission.
Demographic and clinical data at the time of randomization were obtained from the program database and confirmed with patients during the baseline interview. Treatment contact was defined as face‐to‐face professional interventions by either a physician or another health care provider (e.g., case manager). Second generation antipsychotic medications were used invariably and the dosage was expressed as chlorpromazine equivalents29. Adherence to antipsychotic medications was self‐reported and not confirmed with any assays or through verification with treating clinicians in order not to break the blind assessment. Attempts were made to verify with the dispensing pharmacy whenever possible.
The study was approved by McGill University Human Ethics Committee. Patients in both conditions were paid compensations for travel expenses ($20) for each study assessment.
Sample size was calculated based on findings of the previous uncontrolled study of extension of early intervention service16 for length of positive symptom remission. Taking into consideration attrition over time, we estimated that a sample of 220 patients randomized to the two treatment conditions and 167 evaluable patients would have sufficient power to detect significant group differences on the primary outcome measure.
Trial interventions
Experimental intervention
The experimental intervention – extended early intervention service (EEIS) for three years following two years of early intervention service – comprised the elements detailed below.
Modified assertive case management tailored to meet the needs of younger patients in the early phase of illness9 was continued as the primary mode of service delivery, with a case load of 20‐22 cases per case manager. During this extended phase, the case manager provided continued emphasis on appropriate treatment goals, such as adherence to treatment, reintegration into employment and/or educational activities, improving patients’ understanding about their illness, reducing dependence on hospital services, providing crisis intervention and promoting independence.
The case manager continued to facilitate maintenance of remission primarily through encouraging adherence to medication, controlling substance use, and teaching skills for identifying early warning signs of relapse. Based on each patient's profile of early (prodromal) signs observed prior to onset of first episode and over the first 24 months of treatment, a signature profile of early warning signs30 was created. At each contact, case managers evaluated the status of the early warning signs, and patients were trained by the case manager to monitor these signs to prevent future relapses. Use of relapse prevention strategy and early warning signs was monitored continuously through monthly meetings between the case managers and the research team.
Families of EEIS patients were offered booster sessions of structured family education and multiple family group interventions31. A family self‐help support group was active throughout the study period.
Cognitive behaviour therapy was provided using the same criteria as in the pre‐randomization phase (a major depressive episode, anxiety disorder or residual psychotic and/or negative symptoms). Therapists received weekly peer supervision, and recordings from the sessions were reviewed for quality assurance.
Severity of alcohol and drug consumption in the previous six months was assessed with the timeline follow‐back procedure and followed by brief intervention to reduce substance abuse, if indicated32. Interventions lasting up to 20 minutes were undertaken, using motivational interviewing principles, Case managers had received training and ongoing supervision from one of the co‐investigators.
Control intervention
The control intervention – early intervention service for two years followed by regular care for three years – was implemented as follows.
Patients randomized to regular care received treatment in general medical or regular psychiatric services available to them in the absence of participation in the trial. Transfers were made to two levels of regular care in the community: “primary care” (which in Québec includes community health and social service centres and family physicians with variable support from psychiatric services) or “secondary care” (through outpatient services attached to a hospital where most of the care is provided by psychiatrists often with nursing or other professional involvement).
Prior to randomization, clinicians – in collaboration with patients and their families – decided on the best choice within the regular care system based on the complexity of patient's needs as emerging during the two years of initial treatment in the early intervention service. Those with a more complex course were recommended follow‐up with secondary care, while patients who had been stable for a lengthy period of time were advised transfer to primary care. Each patient randomized to regular care was transferred to the new service with a personalized meeting involving the patient, his/her early intervention service case manager, and the new clinician taking charge of the patient's care, accompanied by relevant documentation.
Data analysis
We estimated the length of time patients stayed in treatment with their respective services and compared the number of total treatment exposures received by patients for both groups.
Multiple regression was the main approach to analysis. The length of remission of (positive, negative and total) symptoms was the principal variable of interest. Site and number (and length) of treatments received from any health care provider were tested as possible covariates and entered in that order. Because of high co‐linearity between number and length of treatments, these were entered alternately.
These covariates were selected because of their potential to confound the primary outcome. For example, greater frequency of treatment interventions is expected in EEIS, as case managers are required to have contact with their patients with a minimum frequency of once per month and usually every two weeks, and to increase this frequency if the clinical condition so requires. The frequency of treatment may, therefore, have a confounding effect on outcome irrespective of the treatment model. Given the difference in the length of stay in the study across the two conditions, it was important also to determine if the length of exposure to treatment had an independent effect.
We also compared the proportion of patients who were in a state of remission for a minimum of three months (over one period of assessment) at any time during the study between those randomized to EEIS and regular care. This analysis was performed in all patients who had a minimum of one assessment post‐randomization.
Information on the primary outcome variable, if missing as a result of patients not completing some interviews, was supplemented by clinical data derived from case files from all services within regular care as well as those in EEIS. An experienced research assistant was trained to reconstruct remission of positive and negative symptoms from the case files. Ratings were then reviewed with the project coordinator. Data were included in the analysis until time of completion of the study or withdrawal.
RESULTS
The patient recruitment, randomization process and patient allocation to treatment group have been described in the paper presenting the study protocol33. An updated consort flow diagram (Figure 1) shows patient allocation on randomization, number of patients receiving treatment in the assigned condition (EEIS vs. regular care), study withdrawals and number of patients included in the analyses.
Figure 1.
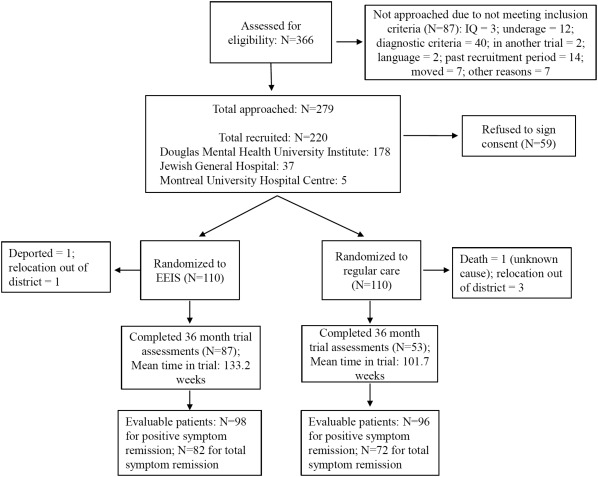
CONSORT flow diagram. EEIS – extended early intervention service
Table 1 shows that there were no significant differences between the two groups – EEIS (N=110) and regular care (N=110) – on any demographic or clinical variables, including remission status, at the time of randomization.
Table 1.
Demographic and clinical characteristics at randomization
|
Total (N=220) |
EEIS (N=110) |
Regular care (N=110) |
|
|---|---|---|---|
| Age at onset of first‐episode psychosis (years, mean±SD) | 22.4 ± 4.4 | 21.9 ± 4.1 | 22.9 ± 4.7 |
| Gender (N male, %) | 151 (68.6%) | 75 (68.2%) | 76 (69.1%) |
| Marital status (N single, %) | 200 (90.9%) | 103 (93.6%) | 97 (88.2%) |
| Education (N high school or less, %) | 103 (46.8%) | 53 (48.2%) | 50 (45.4%) |
| Duration of untreated psychosis (weeks, mean±SD) | 49.3 ± 123.6 (median=11.6 weeks) | 52.4 ± 148.8 (median=8.3 weeks) | 46.3 ± 92.7 (median=12.7 weeks) |
| Primary diagnosis of schizophrenia spectrum (N, %) | 143 (65.0%) | 74 (67.3%) | 69 (62.7%) |
| Secondary diagnosis of substance abuse/dependence (N, %) | 105 (47.7%) | 52 (47.3%) | 53 (48.2%) |
| Antipsychotic dose in chlorpromazine equivalents (mg, mean±SD) | 314.6 ± 332.6 | 299.9 ± 350.1 | 329.7 ± 342.9 |
| SAPS total score (mean±SD) | 6.5 ± 9.7 (N=216) | 7.1 ± 10.4 (N=107) | 6.0 ± 8.9 (N=109) |
| SANS total score (mean±SD) | 13.8 ± 11.6 (N=204) | 13.6 ± 10.4 (N=103) | 14.0 ± 12.8 (N=101) |
| Positive symptom remission (N, %) | 161 (73.2%) | 81 (73.6%) | 80 (72.7%) |
| Negative symptom remission (N, %) | 107 (48.6%) | 53 (48.2%) | 54 (49.1%) |
| Total symptom remission (N, %) | 92 (41.8%) | 45 (40.9%) | 47 (42.7%) |
EEIS – extended early intervention service, SAPS – Scale for the Assessment of Positive Symptoms, SANS – Scale for the Assessment of Negative Symptoms
Patients randomized to regular care were transferred in almost equal proportions to “primary care” (N=51, 46.4%) and “secondary care” (N=48, 43.6%), with 11 (10%) patients dropping out after randomization before they could be transferred. While transfer to regular care was started within two weeks of randomization, it was dependent on the ability and policies of receiving services and often involved considerable delays (mean 25.7 ± 16.1 weeks). EEIS patients, on the other hand, continued with their previous case managers and psychiatrists in the same early intervention service as prior to randomization.
Over the course of the study, one patient randomized to regular care died of unknown causes (at 30 weeks), one patient randomized to the EEIS got deported (at 23.2 weeks), and four patients (three on regular care, at 84.0, 139.2 and 91.5 weeks, and one on EEIS, at 50.7 weeks, respectively) moved out of town. No suicides were reported from either group. Data on these patients were included in the analyses until the time of withdrawal from the study.
The mean dose of antipsychotic medication was comparable (299.9 ± 350.1 and 329.7 ± 342.9 chlorpromazine equivalent mg/day, respectively, for EEIS and regular care). Nine and seven patients were prescribed clozapine, respectively, in the EEIS and regular care groups over the course of the study. Self‐reported adherence rates, based on 103 patients for EEIS and 73 patients for regular care groups, were extremely high (95% and 97%, respectively).
At the end of the study, 49 patients had lost their blind assessment status. Most of them (N=40) were from the EEIS group, almost invariably as a result of patients inadvertently stating their place of treatment or the name of their case manager during their assessment by the research staff.
Patients were considered withdrawn from the study if they missed three consecutive assessments. The number of patients who completed research assessments as per the protocol for the entire 36‐month period was significantly higher in the EEIS than the regular care group (N=87, 79.1% and N=53, 48.2%, respectively, χ2=22.7, p<0.001). The length of stay in the study (time to withdrawal) was significantly higher for EEIS (mean 133.2 ± 43.4 weeks) than for regular care (mean 101.7 ± 53.9 weeks, t=4.76, df=218, p<0.001). Complete data for the primary outcome (remission), from the time of randomization to end of study (or withdrawal), was available in 98 (89.1%) patients for positive symptom remission, and 82 (74.5%) patients for both positive and negative symptom remission for the EEIS group. The respective numbers for the regular care group were 96 (87.2%) and 72 (65.5%).
Table 2 shows that patients treated in EEIS stayed in treatment for significantly longer time than patients in regular care (131.7 ± 37.4 vs. 105.3 ± 51.5 weeks through contact with physicians, t=3.98, p<0.001; 134.8 ± 37.7 vs. 89.8 ± 55.2 weeks through contact with other health care providers, t=6.45, p<0.0001). Patients in EEIS received a significantly higher number of interventions either from a physician or another health care provider compared to the regular care group (74.9 ± 43.6 vs. 39.9 ± 69.1, t=4.21, p<0.001; and 57.3 ± 37.3 vs. 28.2 ± 59.6, t=4.08, p<0.001, respectively).
Table 2.
Clinical care received during follow‐up
| Number of interventions (mean±SD) |
Length of treatment (weeks, mean±SD) |
|||
|---|---|---|---|---|
| EEIS | Regular care | EEIS | Regular care | |
| Physicians | 74.9 ± 43.6* | 39.9 ± 69.1 | 131.7 ± 37.4* | 105.3 ± 51.5 |
| Other health care providers | 57.3 ± 37.3* | 28.2 ± 59.6 | 134.8 ± 37.7** | 89.8 ± 55.2 |
EEIS – extended early intervention service
*p<0.001, **p<0.0001
Remission status
Patients in the EEIS experienced remission of positive symptoms for a significantly longer period than patients in regular care (mean 92.5 ± 41.9 vs. 63.6 ± 46.7 weeks, standardized beta=0.34, t=4.47, p<0.001). Neither site nor number of times seen by any health care provider added any significant effect. However, length of treatment showed an independently significant effect on length of positive symptom remission (standardized beta=0.20; t=2.62, p=0.009), suggesting that longer stay in treatment was associated with longer remission of positive symptoms (Table 3).
Table 3.
Variables affecting length of remission (regression analysis)
| Beta | SE | Standardized beta | t | p | |
|---|---|---|---|---|---|
| Positive symptom remission | |||||
| Treatment group | 31.58 | 7.06 | 0.34 | 4.47 | <0.001 |
| Site | −4.35 | 9.82 | −0.03 | −0.44 | 0.66 |
| Length of treatment | 0.20 | 0.08 | 0.20 | 2.62 | 0.009 |
| Negative symptom remission | |||||
| Treatment group | 13.79 | 6.98 | 0.15 | 2.84 | 0.005 |
| Site | −9.18 | 8.00 | −0.08 | −1.65 | 0.10 |
| Number of interventions | 0.25 | 0.09 | −0.25 | −2.70 | 0.008 |
| Positive and negative symptom remission | |||||
| Treatment group | 19.80 | 8.80 | 0.23 | 2.25 | 0.03 |
| Site | −10.40 | 11.03 | −0.08 | −0.94 | 0.35 |
| Number of interventions | 0.28 | 0.12 | −0.25 | −2.40 | 0.02 |
For negative symptom remission, the effect of treatment condition was significant favouring EEIS (mean 73.4 ± 43.7 vs. 59.6 ± 47.0 weeks, standardized beta=0.15, t=2.84, p=0.005). While site had no independent effect, the number of units of treatment with any health care provider showed a significant effect (standardized beta=−0.25, t=−2.70, p=0.008), suggesting that higher number of interventions was associated with shorter length of remission (Table 3). The length of treatment had no effect (standardized beta=0.12, t=1.46, p=0.15).
For total remission (of both positive and negative symptoms), treatment group (EEIS vs. regular care) showed a statistically significant difference (mean 66.5 ± 41.6 vs. 56.7 ± 45.0 weeks, standardized beta=0.23, t=2.25, p=0.03). While site had no effect on the outcome, number of treatment interventions did (standardized beta=−0.25, t=−2.40, p=0.02), suggesting that higher number of treatment encounters was associated with shorter length of total remission of both positive and negative symptoms (Table 3). The length of treatment had no such effect (standardized beta=−0.01, t=−0.12, p=0.90).
The proportion of patients who met criteria for positive, negative and total symptom remission (extending a minimum of three months) at any time during the study was not significantly different between the two groups (see Table 4). It is important to note that at randomization (Table 1) the proportion of patients allocated to EEIS and regular care who were in remission for positive symptoms (73.6% and 72.7%), negative symptoms (48.2% and 49.1%) and both positive and negative symptoms (40.9% and 42.7%) were lower than that reported at the end of the study (82.7% and 78.1%, 62.5% and 60.5%, 58.5% and 58.3%, respectively). However, these differences were not statistically significant.
Table 4.
Proportion in remission at any time during the trial
| EEIS | Regular care | X2 | p | |
|---|---|---|---|---|
| Positive symptom remission | 81/98 (82.7%) | 75/96 (78.1%) | 0.63 | 0.47 |
| Negative symptoms remission | 55/88 (62.5%) | 49/81 (60.5%) | 0.07 | 0.87 |
| Total remission | 48/82 (58.5%) | 42/72 (58.3%) | 0.01 | 1.00 |
EEIS – extended early intervention service
Post‐hoc analyses
As indicated above, patients randomized to regular care were transferred either to primary care (N=51) or secondary care (N=48). This selection of type of care was made very carefully with the intention of matching patients’ needs to the level of care in order to maximize the benefits of treatment.
At baseline (time of randomization), patients transferred to primary care had a higher level of education, while patients transferred to secondary care had a higher level of positive and negative symptoms, a lower rate of positive, negative and total symptom remission, and a more common comorbid diagnosis of substance abuse (Table 5). There were no other differences between the two groups on any other characteristics, including duration of untreated psychosis.
Table 5.
Post‐hoc analyses in patients transferred to primary or secondary care
| Primary (N=51) | Secondary (N=48) | Test | p | |
|---|---|---|---|---|
| Baseline | ||||
| Post‐secondary education (N, %) | 31 (60.8%) | 18 (39.1%) | χ2=4.53 | 0.03 |
| Substance abuse (N, %) | 20 (46.5%) | 28 (68.3%) | χ2=4.06 | 0.05 |
| SAPS total score (mean±SD) | 2.4 ± 3.5 | 9.7 ± 10.1 | z =−4.37 | <0.001 |
| SANS total score (mean±SD) | 10.7 ± 10.4 | 19.9 ± 14.4 | t =−3.39 | <0.001 |
| Positive symptom remission (N, %) | 45 (88.2%) | 26 (54.2%) | χ2=14.15 | <0.001 |
| Negative symptom remission (N, %) | 32 (62.7%) | 16 (33.3%) | χ2=8.54 | <0.001 |
| Total symptom remission (N, %) | 31 (60.8%) | 10 (20.8%) | χ2=16.26 | <0.001 |
| Follow‐up and outcome | ||||
| Number of treatment interventions (mean±SD) | 20.8 ± 24.8 | 60.1 ± 94.9 | z =3.90 | <0.001 |
| Length of treatment (weeks, mean±SD) | 102.3 ± 55.3 | 107.7 ± 48.8 | t =−0.47 | 0.64 |
| Positive symptom remission length (weeks, mean±SD) | 75.2 ± 48.6 | 57.2 ± 42.2 | t =1.90 | 0.07 |
| Negative symptom remission length (weeks, mean±SD) | 73.9 ± 47. 8 | 47.0 ± 41.6 | t=2.52 | <0.01 |
| Total symptom remission length (weeks, mean±SD) | 66.1 ± 46.4 | 46.9 ± 40.6 | t=1.66 | 0.10 |
| Positive symptom remission at any time (N, %) | 44 (86.3%) | 24 (50.0%) | χ2 =15.12 | <0.001 |
| Negative symptom remission at any time (N, %) | 33 (64.7%) | 11 (22.9%) | χ2=17.49 | <0.001 |
| Total symptom remission at any time (N, %) | 31 (60.8%) | 7 (14.6%) | χ2=22.32 | <0.001 |
SAPS – Scale for the Assessment of Positive Symptoms, SANS – Scale for the Assessment of Negative Symptoms
During follow‐up, secondary care patients received a significantly higher number of treatment interventions from either a physician or another health care provider (p<0.001). There was no difference in the overall length of time patients stayed in treatment, but secondary care patients received treatment from other health care providers for longer periods (mean 101.4 ± 49.5 vs. 76.5 ± 58.8 weeks, t=−2.08, p=0.04) and more frequently (mean 45.5 ± 84.0 vs. 12.1 ± 13.9 weeks, t=−2.48, p=0.01). This most likely reflects a combination of greater clinical needs as well as availability of other health care providers for those in secondary care.
At the end of follow‐up, primary care patients had been in negative symptom remission for significantly longer periods (p<0.01). The differences on positive symptom remission, although in the same direction, did not reach statistical significance. A significantly higher proportion of primary care patients had met criteria for positive, negative and total symptom remission at any time during the course of follow‐up (p<0.001) (Table 5).
DISCUSSION
The principal finding of this study is that, following two years of early intervention service, patients with first‐episode psychosis randomized to continue in that service (EEIS) were in remission of positive, negative and total (both positive and negative) symptoms for significantly longer time during the subsequent three‐year period than were patients randomized to regular care.
The longer periods of remission of both positive and negative symptoms in EEIS is likely related to significant efforts by case managers to keep patients engaged in treatment, follow them closely with a flexible approach including community and clinic based appointments, involve them in monitoring their own risk of relapse, provide access to psychosocial interventions when needed (e.g., family intervention, cognitive behaviour therapy), and include management of substance abuse in the treatment program. In addition, patients had ready access to the assigned psychiatrist, often facilitated by their respective case managers, for unscheduled appointments. The extra effort involved in early intervention services in considering patient's psychosocial needs and the ready access to psychosocial interventions may have led to sustained negative symptom remission, given the documented, albeit modest, impact of psychosocial interventions on negative symptoms34, 35. The inverse association between number of treatment interventions and length of remission of negative symptoms, as well as total remission, most likely reflects the need for greater frequency of treatment contacts for patients who were not in remission.
It appears that, over the study period, patients randomized to both interventions not only maintained the status of remission, but that rates of all types of remission increased (see Tables 1 and 4). This suggests that even for patients transferred to regular care some gains from the first two years of early intervention service may be maintained. However, what seems particularly relevant is how long such remission of positive or negative symptoms was sustained, given the strong association of the length of remission with functional outcome24, 25. Results from a previous study of patients with first‐episode psychosis (N=159) showed that, at the end of two years, the length of positive and negative symptom remission had contributed 15% and 13%, respectively, of the 38% of explained variance in functional outcome (employment and social relationships)27.
A comparison with a previous study of patients with first‐episode psychosis, followed up in a low intensity early intervention service after two years of full intensity early intervention service, may provide some context for the relevance of the findings reported here16. Although the measures of outcome are not identical, the results of the current study confirm the superior outcome on positive symptoms in EEIS reported in that study16. However, our study also shows an advantage of EEIS in its impact on length of negative symptom remission and, as a consequence, on total remission of both positive and negative symptoms. The previous study did not have a control sample of an alternate service. A recent study from Hong Kong also showed an independent effect of an extension of one year following the initial two years of early intervention service17, although there are significant differences in the cultural and resource contexts between that study and the current one.
The most recent trial from the OPUS program in Denmark has reported the absence of any significant differences in the level of negative symptoms between a three‐year extension of early intervention service and regular care following two years of early intervention service18. The differences in the results of our study and the new OPUS study can be explained at several levels. The OPUS study assessed the level of negative symptoms only at two time points, post‐randomization and the end of the study, while we used three‐monthly assessments of positive and negative symptoms over the study period. The differences cannot be attributed to a higher intensity of treatment in our EEIS, given our case manager to patient ratio of 22:1, compared to 15:1 in the OPUS trial. However, there are likely differences in the intensity of care available in regular care between the two studies, with the OPUS study reporting a higher intensity of services such as case management provided in regular care in Denmark. Last, but not least, length of remission of symptoms may be a more robust measure of outcome because of its strong association with functional outcome than level of symptoms at any given time.
One of the limitations in a trial with a long follow‐up is attrition rate, which was greater for regular care (51.8%) than for EEIS (20.9%) in our study. While adding methodological rigour, thirteen detailed evaluations may have increased the risk of attrition due to burden of repeated assessments, as well as led to loss of blinding of assessments over time. Despite this, our completion rate at 3‐year post‐randomization is comparable to the new OPUS study (66% vs. 71%). Further, patients in each treatment condition stayed in the research protocol for considerable time (mean 133.2 weeks for EEIS and 101.7 for regular care). We were able to utilize data for 65‐89% of patients for evaluation of the primary outcome measures through additional data being derived from the clinical files. Since the quality of records available varied across services and was likely better in the EEIS, this may have biased some of the results. Every attempt was made by the project coordinator to verify the accuracy of the data retrieved by the research staff. This additional information was only required for one third of the cases. This may be more accurate than imputing data for a missing assessment from a previous one conducted three months earlier. Such imputation may not capture the change in symptoms occurring over such a long period.
The lower attrition rate of patients in EEIS may reflect a higher level of engagement for patients compared to regular care, one of the objectives of most early intervention services. This is likely explained by the central role of case management and continuity of care in the EEIS. Patients randomized to regular care often had to make a difficult transition to another service, despite strong efforts by the early intervention service staff to assertively engage with regular care to facilitate such transfers. Sustained engagement in treatment may be an important outcome in itself. It is possible, therefore, that continuity of care was an important ingredient for the superiority of outcome in EEIS.
Although our study was conducted within a network of three early intervention services that used an identical service model, it is still possible that differences in staffing and culture of treatment may have had an effect on outcome. However, our results show a lack of any effect of site on outcome. For regular care, all patients had access to the same system of care across the three clinical sites.
While the results of this study suggest an overall superior outcome measured by the length of symptomatic remission for patients treated in an EEIS, it is likely that extension of early intervention service may be particularly beneficial to certain patients and that such an extension may not be necessary to all. In other words, some patients with a better prognostic profile may do well if transferred to an appropriate level of regular care. In order to examine this possibility, we have reported post‐hoc analyses on patients who were transferred to regular care. Our results suggest that after careful matching, achieved through consensus with patients and their families and based on their progress over the preceding two years of early intervention service, a substantial proportion of patients did well following transfer to primary care. The latter patients had a higher education level, a lower rate of substance abuse and were clinically stable at the time of randomization. As expected these patients received lower intensity of care, while those transferred to specialist regular care received higher frequency of care from psychiatrists and other health care providers. It should, however, be emphasized that the transition of patients to a different form and level of care needs very careful management and requires considerable effort by the early intervention service, as was done in this study.
In conclusion, in this randomized controlled trial, we explored whether an extension of early intervention service beyond the first two years is likely to provide greater benefits than transfer to regular care. Our results suggest that, for the entire group of patients with first‐episode psychosis receiving care in an early intervention service, an extension of additional three years is beneficial to obtain better clinical outcomes. However, as suggested by our post‐hoc analysis, a subgroup of patients with good prognostic characteristics achieved following two years of early intervention service may do well in a lower intensity system of care.
Our findings have potential significance for policies regarding length of early intervention services to be recommended for patients with first‐episode psychosis beyond the first two years. This will, however, need to be supported by sound health economic data, which will be examined in a subsequent report.
ACKNOWLEDGEMENTS
This study was supported by an operational grant from the Canadian Institutes of Health Research (grant MCT 94189; registration CCT‐NAPN‐18590). A. Malla is supported by the Canada Research Chairs Program. The authors also acknowledge the assistance of M.‐C. Rondeau, N. Pawliuk, A. Rho and K. MacDonald and thank all the participants who agreed to participate in the study.
REFERENCES
- 1. McGrath J, Saha S, Chant D et al. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev 2008;30:67‐76. [DOI] [PubMed] [Google Scholar]
- 2. Proctor S, Mitford E, Paxton R. First episode psychosis: a novel methodology reveals higher than expected incidence; a reality‐based population profile in Northumberland, UK. J Eval Clin Pract 2004;10:539‐47. [DOI] [PubMed] [Google Scholar]
- 3. Osby U, Correia N, Brandt L et al. Time trends in schizophrenia mortality in Stockholm county, Sweden: cohort study. BMJ 2000;321:483‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rössler W, Salize HJ, van Os J et al. Size of burden of schizophrenia and psychotic disorders. Eur Neuropsychopharmacol 2005;15:399‐409. [DOI] [PubMed] [Google Scholar]
- 5. Birchwood M, Todd P, Jackson C. Early intervention in psychosis. The critical period hypothesis. Br J Psychiatry 1998;172(Suppl. 33):53‐9. [PubMed] [Google Scholar]
- 6. Harrison G, Hopper K, Craig T et al. Recovery from psychotic illness: a 15‐ and 25‐year international follow‐up study. Br J Psychiatry 2001;178:506‐17. [DOI] [PubMed] [Google Scholar]
- 7. Harrow M, Grossman LS, Jobe TH et al. Do patients with schizophrenia ever show periods of recovery? A 15‐year multi‐follow‐up study. Schizophr Bull 2005;31:723‐34. [DOI] [PubMed] [Google Scholar]
- 8. McGorry PD, Edwards J, Mihalopoulos C et al. EPPIC: an evolving system of early detection and optimal management. Schizophr Bull 1996;22:305‐26. [DOI] [PubMed] [Google Scholar]
- 9. Malla A, Norman R, McLean T et al. A Canadian programme for early intervention in non‐affective psychotic disorders. Aust N Z J Psychiatry 2003;37:407‐13. [DOI] [PubMed] [Google Scholar]
- 10. Marshall M, Rathbone J. Early intervention for psychosis. Cochrane Database Syst Rev: 2006;4:CD004718. [DOI] [PubMed] [Google Scholar]
- 11. Harvey P‐O, Lepage M, Malla A. Benefits of enriched intervention compared with standard care for patients with recent‐onset psychosis: a metaanalytic approach. Can J Psychiatry 2007;52:464‐72. [DOI] [PubMed] [Google Scholar]
- 12. McCrone P, Craig TK, Power P et al. Cost‐effectiveness of an early intervention service for people with psychosis. Br J Psychiatry 2010;196:377‐82. [DOI] [PubMed] [Google Scholar]
- 13. Petersen L, Jeppesen P, Thorup A et al. A randomised multicentre trial of integrated versus standard treatment for patients with a first episode of psychotic illness. BMJ 2005;331:602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Linszen D, Dingemans P, Lenior M. Early intervention and a five year follow up in young adults with a short duration of untreated psychosis: ethical implications. Schizophr Res 2001;51:55‐61. [DOI] [PubMed] [Google Scholar]
- 15. Bertelsen M, Jeppesen P, Petersen L et al. Five‐year follow‐up of a randomized multicenter trial of intensive early intervention vs standard treatment for patients with a first episode of psychotic illness: the OPUS trial. Arch Gen Psychiatry 2008;65:762‐71. [DOI] [PubMed] [Google Scholar]
- 16. Norman RM, Manchanda R, Malla AK et al. Symptom and functional outcomes for a 5 year early intervention program for psychoses. Schizophr Res 2011;129:111‐5. [DOI] [PubMed] [Google Scholar]
- 17. Chang WC, Kwong VWY, Chan GHK et al. Prediction of functional remission in first‐episode psychosis: 12‐month follow‐up of the randomized‐controlled trial on extended early intervention in Hong Kong. Schizophr Res 2016;173:79‐83. [DOI] [PubMed] [Google Scholar]
- 18. Albert N, Melau M, Jensen H et al. Five years of specialised early intervention versus two years of specialised early intervention followed by three years of standard treatment for patients with a first episode psychosis: randomised, superiority, parallel group trial in Denmark (OPUS II). BMJ 2017;356:i6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Royal Australian and New Zealand College of Psychiatrists Clinical Practice Guidelines Team for the Treatment of Schizophrenia and Related Disorders . Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the treatment of schizophrenia and related disorders. Aust N Z J Psychiatry 2005;39:1‐30. [DOI] [PubMed] [Google Scholar]
- 20. Iyer S, Jordan G, MacDonald K et al. Early intervention for psychosis: a Canadian perspective. J Nerv Ment Dis 2015;203:356‐64. [DOI] [PubMed] [Google Scholar]
- 21. First MB, Spitzer RL, Gibbon M et al. Structured Clinical Interview for DSM‐IV Axis I Disorders – Patient Edition (SCID‐I/P, Version 2.0). New York: New York State Psychiatric Institute, 1995. [Google Scholar]
- 22. Christensen BK, Girard TA, Bagby RM. Wechsler Adult Intelligence Scale ‐ Third Edition short form for index and IQ scores in a psychiatric population. Psychol Assess 2007;19:236‐40. [DOI] [PubMed] [Google Scholar]
- 23. Stout RL, Wirtz PW, Carbonari JP et al. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol Suppl 1994;12:70‐5. [DOI] [PubMed] [Google Scholar]
- 24. Malla A, Norman R, Manchanda R et al. Symptoms, cognition, treatment adherence and functional outcome in first‐episode psychosis. Psychol Med 2002;32:1109‐19. [DOI] [PubMed] [Google Scholar]
- 25. Cassidy CM, Norman R, Manchanda R et al. Testing definitions of symptom remission in first‐episode psychosis for prediction of functional outcome at 2 years. Schizophr Bull 2010;36:1001‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jordan G, Lutgens D, Joober R et al. The relative contribution of cognition and symptomatic remission to functional outcome following treatment of a first episode of psychosis. J Clin Psychiatry 2014;75:566‐72. [DOI] [PubMed] [Google Scholar]
- 27. Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS). Iowa City: University of Iowa, 1984. [Google Scholar]
- 28. Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS). Iowa City: University of Iowa, 1983. [Google Scholar]
- 29. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry 2003;64:663‐7. [DOI] [PubMed] [Google Scholar]
- 30. Birchwood M, Spencer E, McGovern D. Schizophrenia: early warning signs. Adv Psychiatr Treat 2000;6:93‐101. [Google Scholar]
- 31. McFarlane WR, Lukens E, Link B et al. Multiple‐family groups and psychoeducation in the treatment of schizophrenia. Arch Gen Psychiatry 1995;52:679‐87. [DOI] [PubMed] [Google Scholar]
- 32. Brown TG, Dongier M, Ouimet MC et al. Brief motivational interviewing for DWI recidivists who abuse alcohol and are not participating in DWI intervention: a randomized controlled trial. Alcohol Clin Exp Res 2010;34:292‐301. [DOI] [PubMed] [Google Scholar]
- 33. Lutgens D, Iyer S, Joober R et al. A five‐year randomized parallel and blinded clinical trial of an extended specialized early intervention vs. regular care in the early phase of psychotic disorders: study protocol. BMC Psychiatry 2015;15:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fusar‐Poli P, Papanastasiou E, Stahl D et al. Treatments of negative symptoms in schizophrenia: meta‐analysis of 168 randomized placebo‐controlled trials. Schizophr Bull 2015;41:892‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lutgens D, Gariepy G, Malla A. Psychological and psychosocial interventions for negative symptoms in psychosis: systematic review and meta‐analysis. Br J Psychiatry 2017;210:324‐32. [DOI] [PubMed] [Google Scholar]


