Abstract
The rapid advances and adoption of smartphone technology presents a novel opportunity for delivering mental health interventions on a population scale. Despite multi‐sector investment along with wide‐scale advertising and availability to the general population, the evidence supporting the use of smartphone apps in the treatment of depression has not been empirically evaluated. Thus, we conducted the first meta‐analysis of smartphone apps for depressive symptoms. An electronic database search in May 2017 identified 18 eligible randomized controlled trials of 22 smartphone apps, with outcome data from 3,414 participants. Depressive symptoms were reduced significantly more from smartphone apps than control conditions (g=0.38, 95% CI: 0.24‐0.52, p<0.001), with no evidence of publication bias. Smartphone interventions had a moderate positive effect in comparison to inactive controls (g=0.56, 95% CI: 0.38‐0.74), but only a small effect in comparison to active control conditions (g=0.22, 95% CI: 0.10‐0.33). Effects from smartphone‐only interventions were greater than from interventions which incorporated other human/computerized aspects along the smartphone component, although the difference was not statistically significant. The studies of cognitive training apps had a significantly smaller effect size on depression outcomes (p=0.004) than those of apps focusing on mental health. The use of mood monitoring softwares, or interventions based on cognitive behavioral therapy, or apps incorporating aspects of mindfulness training, did not affect significantly study effect sizes. Overall, these results indicate that smartphone devices are a promising self‐management tool for depression. Future research should aim to distil which aspects of these technologies produce beneficial effects, and for which populations.
Keywords: Smartphone technology, mental health interventions, depression, e‐health, mhealth, apps, cognitive training, mood monitoring, cognitive behavioral therapy, mindfulness training
Depression is now recognized as a leading cause of global disability, impacting over 300 million people around the world1. In countries like the US, 9% of the population may have depression at any one time2. Beyond the personal suffering, depression is associated with unemployment, poor physical health, impaired social functioning and, in its most severe forms, suicide3. Thus, the disorder carries a high cost for both the individual and the society, particularly when considering the economic burden incurred through clinical care and lost productivity4.
Depression is a potentially treatable condition, with a range of available medications and psychological interventions that are supported by robust clinical evidence. While the choice of pharmacotherapy or psychotherapy depends on many factors, for most individuals with mild or moderate depression they may be nearly equivalent5.
However, there are many barriers towards both of these treatment methods. For instance, access to mental health care remains limited, as almost half of the world's population lives in countries where there is less than one psychiatrist per 100,000 people6, and continued shortage in mental health care staff is expected for both the near and long term future7, 8. Additionally, medications and psychotherapies may carry some level of stigma (particularly among younger people), which further limits their effectiveness9, 10.
Furthermore, although these therapies demonstrate high clinical efficacy for reducing symptoms, they may not always bring about full and sustained remission in those treated. Finally, many people experience either subclinical depression or residual depressive symptoms even after achieving clinical response to treatment. Therefore, novel primary and/or adjunctive methods for reducing depression on a population scale are urgently needed.
Digital technologies may represent a novel and viable solution. Mobile phones are among the most rapidly adopted innovations in recent history, and smartphone ownership continues to increase in both developed and developing countries11. Through providing ubiquitous Internet connectivity, along with the capacity to download and run externally created applications (“apps”), smartphone technology presents an opportunity to transform mobile phones into devices which could provide global, cost‐effective and evidence‐based mental health services on demand and in real time12.
This clear therapeutic potential has triggered a wave of interest and investment in mental health apps from governments, technology companies, advocacy groups, and research groups internationally13, 14. But in the enthusiasm to realize the potential of apps for depression, it has become difficult to separate actual efficacy from overzealous aspirational claims15. With thousands of mental health apps readily available through Apple or Google marketplaces, finding a useful tool supported by robust evidence to manage one's depression is clearly a challenge for a lay person16, 17. The increasing media promotion and accessibility of apps for mental health now presents a “duty of care” issue towards ensuring that people have information and understanding of evidence‐based digital treatments for depression.
Recent meta‐analyses have documented that various smartphone interventions can have positive effects on physical diseases, such as diabetes18, and mental health conditions, such as anxiety19. However, the clinical effect of smartphone interventions on symptoms of depression has yet to be established. Thus, our aim was to examine the efficacy of delivering mental health interventions via smartphones for reducing depressive symptoms in both clinical and non‐clinical populations. We also sought to use subgroup and meta‐regression analyses in order to explore which aspects of smartphone interventions are associated with greater or lesser efficacy for depressive symptoms. The results of these meta‐analyses provide the first overall estimate of effects from such interventions, along with informing treatment choices and future research in this area.
METHODS
This systematic review and meta‐analysis followed the PRISMA statement for transparent and comprehensive reporting of methodology and results20. In order to eliminate researcher bias, the search strategy, inclusion criteria and data extraction, as well as the overall and pre‐planned subgroup analyses, strictly adhered to those adopted in a previous systematic review of smartphone interventions for anxiety19, as specified in a registered online protocol (CRD42017064882).
Search strategy
We conducted an electronic search of the following databases: Cochrane Central Register of Controlled Trials, Health Technology Assessment Database, Allied and Complementary Medicine (AMED), Health Management Information Consortium (HMIC), Ovid MEDLINE, Embase, and PsycINFO, from inception to May 1, 2017. The search applied the PICO framework21, using a range of relevant terms to capture all potentially eligible results relating to smartphone mental health interventions for depressive symptoms. An additional search of Google Scholar was implemented, and reference lists of retrieved articles were checked to identify any further eligible studies.
Eligibility criteria
Only English‐language articles were included. Eligible studies were all randomized controlled trials (RCTs) examining the effects of mental health interventions delivered via smartphone devices with at least one outcome measure for depressive symptoms. We aimed to examine the effects of smartphone interventions on primary depression, comorbid depression and subclinical depressive symptoms. No restrictions were placed on diagnosis or any other clinical or demographic characteristics of eligible samples.
Three independent investigators judged article eligibility (JF, JN and JT), with any disagreements resolved through discussion. “Smartphones” were defined as mobile phones with 3G or 4G Internet connectivity, along with the ability to download, install and run external applications (“apps”). RCTs of interventions delivered solely or in part via smartphone devices matching this definition, aimed at improving mental health or well‐being (with depression as a primary or secondary outcome), were included in the review.
Studies using either “inactive” or “active” control groups were eligible for inclusion. “Inactive” control groups were classified as those in which participants received no intervention during the trial period (or were put into a waitlist until pre‐and‐post measures had been collected from both groups). “Active” control groups were categorized as those which attempted to control for the time and attention given to people in the smartphone intervention condition, by using apps not aimed at treating depression, in‐person interventions, or other forms of activities or patient contact. RCTs comparing smartphone interventions to antidepressant medications were also eligible for inclusion. All eligible studies had a duration of at least one week (thus excluding studies measuring changes in mood following a single use of smartphone apps).
Data extraction
A systematic extraction form was used for each article to collect the following data: a) study information (sample size, mean age of participants, diagnostic information or relevant inclusion criteria, study length and trial quality); b) intervention features (app/program name, regularity of instructed use, smartphone program summary, any additional intervention components, details of the control condition); c) effects on depressive symptoms (changes in total depressive symptoms scored before and after smartphone and control interventions using any clinically validated rating scale). For studies which used more than one measure of depression, a mean total change was calculated by pooling outcomes from each measure.
Statistical analyses
All analyses were conducted by Comprehensive Meta‐Analysis 2.022, using a random‐effects model23 to account for between‐study heterogeneity. The total difference in changes in depressive symptoms between smartphone interventions and control conditions were pooled to compute the overall effect size of the former (as Hedges’ g), with 95% confidence intervals (CI). For RCTs comparing smartphone interventions to both inactive and active control conditions, the comparative effects with active control groups were used in the primary analysis. After computing main effects, a sensitivity analysis was applied to investigate effects of smartphone interventions in RCTs which used intention‐to‐treat analyses or had complete outcome data.
To quantify the degree to which statistical heterogeneity in the meta‐analyses arose due to between‐study differences, rather than due to chance, Cochran's Q (with p value) and I² were used. Included studies were also assessed using the Cochrane Collaboration's Risk of Bias tool. This examined study quality in six areas of trial design (sequence generation, allocation sequence concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting), ranking each area as high, low or unknown for risk of bias24.
Risk of publication bias was examined using a funnel plot of study effect sizes, and Egger's regression test was applied to all aforementioned analyses. Furthermore, a Duval and Tweedie's trim‐and‐fill analysis was conducted to re‐calculate the pooled effect size after removing any studies which may introduce publication bias (i.e., small studies with large effect sizes from the positive side of the funnel plot). Additionally, a “fail‐safe N” was used to account for the file draw problem25, estimating the number of non‐significant unpublished trials which would be needed to cause the observed p value to exceed 0.05.
Pre‐planned subgroup analyses were conducted to examine whether effects of smartphone interventions differed when comparing them to inactive or active control conditions. Additionally, we carried out a range of exploratory post‐hoc subgroup and meta‐regression analyses in order to examine which factors may impact the effectiveness of smartphone interventions, particularly with regards to sample details (i.e., clinical population, age, gender) and treatment characteristics (i.e., psychological basis, technological features and length of smartphone interventions).
RESULTS
The search returned a total of 1,517 records; 981 after duplicates were excluded. Title and abstract screening removed a further 913 articles. Full versions were retrieved for 68 papers, of which 16 met eligibility criteria. Two further articles were retrieved following an additional search of Google Scholar. Thus, 18 unique RCTs were included in the meta‐analysis, assessing the effects of 22 different smartphone‐delivered mental health interventions. The article inclusion/exclusion process is shown in Figure 1.
Figure 1.
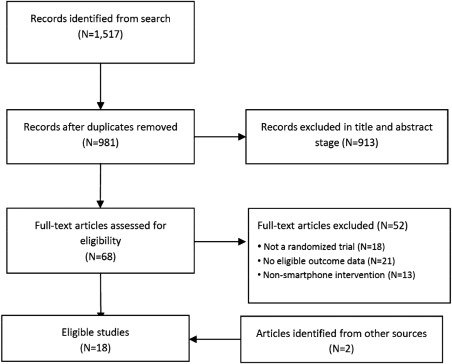
PRISMA flow chart of study selection
Characteristics of included studies
Full details of each study are displayed in Table 1. Outcome data were available from 18 RCTs. Two papers reported outcome data in a format not suited for meta‐analysis, but the corresponding authors provided the raw data to enable inclusion26, 30. Mean sample ages ranged from 18 to 59 years (median 39 years). All but two studies32, 34 used some indication of mental health issues as inclusion criteria. For clinical populations, two studies recruited people with major depression36, 43, two individuals with bipolar disorder28, 30, one young people in primary care with any mental health condition40. Others recruited individuals from the general population with self‐reported mild‐to‐moderate depression26, 27, 39, 41, suicidal thoughts/tendencies42, probable attention‐deficit/hyperactivity disorder (ADHD)37, anxiety disorders29, 33, insomnia31, or symptoms of post‐traumatic stress disorder (PTSD)35. One further study examined older adults with memory complaints38.
Table 1.
Details of included studies
| Study | Sample type | N (each condition) | Age (years, mean) | Design | Other intervention aspects | Outcome measure |
|---|---|---|---|---|---|---|
| Arean et al26 | Self‐reported mild‐to‐moderate depression | 211,209,206 | 33.9 | 12 weeks of Project EVO (cognitive training app) vs. iPST (problem‐solving therapy app) vs. Health Tips control app | None | PHQ‐9 |
| Birney et al27 | Self‐reported mild‐to‐moderate depression | 150,150 | 40.7 | 6 weeks of MoodHacker (CBT‐based depression app) vs. links to approved depression websites | Daily e‐mails to provide additional digital content and prompt engagement | PHQ‐9 |
| Depp et al28 | DSM‐IV bipolar disorder | 41,41 | 47.5 | 10 weeks of PRISM (mood monitoring and self‐management app) vs. paper and pencil equivalent | Both groups received four sessions of individual therapy | MADRS |
| Enock et al29 | Self‐reported high social anxiety | 158,141 | 34.8 | 4 weeks of CBM Active (cognitive bias modification training app) vs. inactive training or waitlist control | None | DASS |
| Faurholt‐Jepsen et al30 | ICD‐10 bipolar disorder | 33,34 | 29.3 | 6 months of MONARCA (self‐monitoring app) vs. regular smartphone use | Patients could also contact their clinicians directly using the smartphone, in case of deterioration | HAM‐D |
| Horsch et al31 | Self‐reported mild insomnia | 74,77 | 39.7 | 6 to 7 weeks of Sleepcare (CBT‐based insomnia app) vs. waitlist control | None | CES‐D |
| Howells et al32 | General population | 57,64 | 40.3 | 10 days of Headspace (mindfulness app) vs. list‐making app control | None | CES‐D |
| Ivanova et al33 | Self‐reported social anxiety | 50,51,51 | 35.3 | 10 weeks of guided ACTsmart (acceptance and commitment therapy app) vs. unguided ACTsmart vs. waitlist control | Participants also provided with pen‐and‐paper booklet for completing written assignments and a CD with ACT exercises | PHQ‐9 |
| Kahn et al34 | US veterans | 44, 41,42, 46 | NA | 16 weeks of Mission Reconnect program (using mindfulness and awareness techniques) vs. Prevention and Relationship Enhancement program vs. both programs together vs. waitlist control | Strategies for applying learnt techniques in challenging situations, and additional audio exercises | BDI‐II |
| Kuhn et al35 | Self‐reported traumatic event + PTSD symptoms | 62,58 | 39 | 3 months of PTSD Coach (app providing psychoeducation, symptom tracking and self‐management strategies) vs. waitlist control | None | PHQ‐8 |
| Ly et al36 | DSM‐IV major depression | 46,47 | 30.6 | 10 weeks of Behavioral Activation app plus 4 face‐to‐face behavioral activation sessions vs. 10 face‐to‐face behavioral activation sessions | None | BDI‐II |
| Moell et al37 | Self‐reported data to diagnose ADHD | 26,27 | 36.8 | 6 weeks of LivingSMART (app facilitating life organization and improving attentional control) vs. waitlist control | Computer‐aided training on how to use the apps; participants were also allocated a coach to help with app usage | HADS |
| Oh et al38 | Older adults with self‐reported memory complaints | 18,19,16 | 59.3 | 8 weeks of SMART vs. Fit Brains (two cognitive training apps) vs. waitlist control | None | CES‐D |
| Proudfoot et al39 | Self‐reported mild‐to‐moderate depression | 126,195,198 | 39 | 7 weeks of MyCompass (app enabling self‐monitoring of problematic moods, thoughts and behaviors, tracking their severity, and receiving feedback advice and mental health management tips by SMS) vs. attention‐matched and waitlist control | Computer modules provided to deliver evidence‐based interventions | DASS |
| Reid et al40 | Youth mental health patients | 68,46 | 18 | 2 to 4 weeks of MobileType (app tracking mental health relevant thoughts and behaviors) vs. using a control app which tracks irrelevant behaviors | Participants reviewed information gathered by MobileType with their general practitioner, and were given guides for managing mental health | DASS |
| Roepke et al41 | Clinically significant depression | 93,97,93 | 40.2 | 1 month of SuperBetter (app supporting self‐esteem and self‐acceptance) vs. SuperBetter Plus (app adopting principles of CBT and positive psychology) vs. waitlist control | None | CES‐D |
| Tighe et al42 | Recent suicidal thoughts | 31,30 | 26.3 | 6 weeks of ibobbly (app based on acceptance and commitment therapy principles) vs. waitlist control | 24‐hour helpline details available through the app in case of suicidality | PHQ‐9 |
| Watts et al43 | DSM‐IV major depression | 10,15 | 41 | 8 weeks of Get Happy (CBT‐based depression app) vs. computerized CBT program | Clinician contact during first two weeks to check and promote adherence | BDI‐IIPHQ‐9 |
CBT – cognitive behavioral therapy, PTSD – post‐traumatic stress disorder, ADHD – attention‐deficit/hyperactivity disorder, PHQ – Patient Health Questionnaire, MADRS – Montgomery‐Åsberg Depression Rating Scale, DASS – Depression Anxiety Stress Scale, HAM‐D – Hamilton Rating Scale for Depression, CES‐D – Center for Epidemiological Studies – Depression, BDI‐II – Beck Depression Inventory II, HADS – Hospital Anxiety Depression Scale, NA – not available
Smartphone interventions lasted between 4 and 24 weeks. Depressive symptoms were measured as a primary outcome in 12 studies, and as a secondary outcome in six. The following tools were used: the Depression Anxiety Stress Scale44 depression subscale in three studies29, 39, 40; the Center for Epidemiological Studies Depression scale45 in four31, 32, 38, 41; the Beck Depression Inventory II46 in three34, 36, 43; the Patient Health Questionnaire47 in six26, 27, 33, 35, 42, 43; the Hamilton Rating Scale for Depression48 in one30; the Hospital Anxiety Depression Scale49 in one37; and the Montgomery‐Åsberg Depression Rating Scale50 in one28.
The results from the Cochrane Risk of Bias assessments are displayed in Table 2. This shows that the most frequent risk factor for bias was inadequate blinding of participants, with only five of 18 studies using intervention‐matched comparators for which the participants would not be aware of their treatment/control status or of the hypothesized outcomes of the trial.
Table 2.
Quality assessment in included studies
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Arean et al26 | + | + | + | + | + | + | – |
| Birney et al27 | + | + | – | + | + | + | – |
| Depp et al28 | + | + | + | + | + | + | |
| Enock et al29 | + | + | + | + | + | ||
| Faurholt‐Jepsen et al30 | + | + | – | + | + | + | + |
| Horsch et al31 | + | + | – | – | + | + | – |
| Howells et al32 | + | + | + | + | – | + | |
| Ivanova et al33 | + | + | + | + | – | ||
| Kahn et al34 | + | + | + | + | – | ||
| Kuhn et al35 | + | – | – | + | + | ||
| Ly et al36 | + | + | + | + | + | + | |
| Moell et al37 | – | + | + | + | |||
| Oh et al38 | – | – | + | + | |||
| Proudfoot et al39 | + | + | + | + | + | + | |
| Reid et al40 | + | + | + | + | + | + | + |
| Roepke et al41 | + | + | – | + | + | + | – |
| Tighe et al42 | + | + | – | – | + | + | + |
| Watts et al43 | + | + | – | + |
1 – random sequence generation, 2 – allocation concealment, 3 – blinding of participants and personnel, 4 – blinding of outcome assessment, 5 – incomplete outcome data, 6 – selective outcome reporting, 7 – other bias
Overall effects of smartphone interventions on depressive symptoms
Figure 2 displays the pooled effect size from smartphone interventions on depressive symptoms, along with individual effects from each app trialled. A random‐effects meta‐analysis revealed a small‐to‐moderate positive effect size of smartphone mental health interventions for reducing depressive symptoms in comparison to control conditions (18 studies, N=3,414, g=0.383, 95% CI: 0.24‐0.52, p<0.001).
Figure 2.
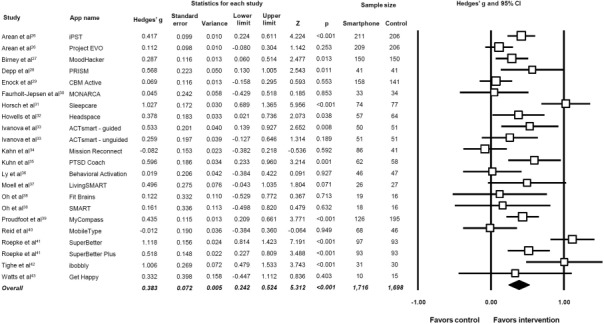
Meta‐analysis of the effects of smartphone interventions on depressive symptoms. Box size represents study weighting. Diamond represents overall effect size and 95% CI.
Although there was heterogeneity across the study data (Q=80.8, p<0.01, I²=74.0%), there was no evidence of publication bias (p=0.255 in Egger's regression test), and the fail‐safe N was 567 (estimating that 567 unpublished “null” studies would need to exist for the actual p value to exceed 0.05). A trim‐and‐fill analysis identified no outlier studies, and thus did not change the observed effect size.
When considering only the studies which used intention‐to‐treat analyses and/or reported complete outcome data, we found a similar effect of smartphone interventions on depressive symptoms (16 studies, N=3,320, g=0.399, 95% CI: 0.25‐0.55, p<0.001; Q=80.0, I2=77.5%).
In our pre‐planned subgroup analyses, we found that effect sizes were significantly greater when comparing smartphone interventions to inactive conditions than when using active control conditions (Q=9.76, p=0.002; Figure 3). Compared to inactive control conditions, the pooled effect size across 13 smartphone interventions (N=1,674) was g=0.558 (95% CI: 0.38‐0.74), indicating a moderate effect on depressive symptoms. However, when compared to active control conditions, smartphone interventions had only a small effect size on depressive symptoms (12 studies, N=2,381, g=0.216, 95% CI: 0.10‐0.33). Both studies with active and inactive controls had significant heterogeneity, but no evidence of publication bias (Table 3).
Figure 3.
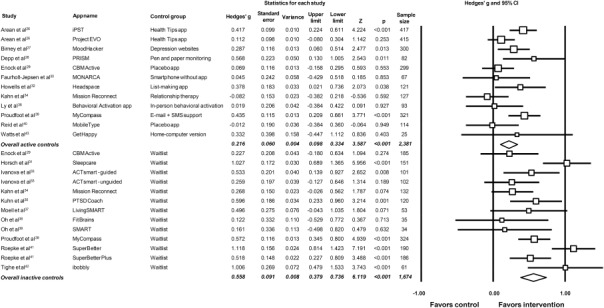
Meta‐analysis showing effects of smartphone interventions on depressive symptoms in comparison to active and inactive controls. Box size represents study weighting. Diamonds represent overall effect size and 95% CI.
Table 3.
Effects of smartphone‐delivered mental health interventions on depressive symptoms: pre‐planned subgroup analyses
| Studies | Sample size (smartphone/control) | Meta‐analysis | Heterogeneity | Publication bias (Egger's regression) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hedges' g | 95% CI | p | Q | p | I2 | Intercept | p | ||||
| Main analysis | 18 | 1,716/1,698 | 0.383 | 0.242 | 0.524 | <0.001 | 80.8 | <0.01 | 74.0 | 0.80 | 0.26 |
| Intent‐to‐treat or complete outcome data | 16 | 1,669/1,651 | 0.399 | 0.248 | 0.550 | <0.001 | 80.0 | <0.01 | 77.5 | 1.68 | 0.15 |
| Smartphone vs. active control | 12 | 1,195/1,186 | 0.216 | 0.098 | 0.334 | <0.001 | 20.8 | 0.03 | 47.2 | −0.49 | 0.34 |
| Smartphone vs. inactive control | 13 | 891/783 | 0.558 | 0.379 | 0.736 | <0.001 | 34.9 | <0.01 | 65.6 | 0.25 | 0.25 |
Significant values are highlighted in bold prints
Population characteristics and effects on depressive symptoms
We also applied post‐hoc subgroup analyses to studies that had used mood disorder inclusion criteria, in order to explore which populations smartphone interventions may be most effective for. As shown in Table 4, the only populations in which smartphone interventions significantly reduced depressive symptoms were those with self‐reported mild‐to‐moderate depression (5 studies, N=1,890, g=0.518, 95% CI: 0.28‐0.75, p<0.001; Q=36.6, I2=83.6). There was no significant effect among the smaller samples with major depressive disorder, bipolar disorder and anxiety disorders (two studies each).
Table 4.
Post‐hoc analyses: mood disorder samples
| Studies | Sample size (smartphone/control) | Meta‐analysis | Heterogeneity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Hedges’ g | 95% CI | p | Q | p | I2 | ||||
| Self‐reported mild‐to‐moderate depression | 5 | 917/973 | 0.518 | 0.282 | 0.754 | <0.001 | 36.6 | <0.001 | 83.6 |
| Major depressive disorder | 2 | 56/62 | 0.085 | −0.273 | 0.443 | 0.642 | 0.49 | 0.484 | 0.00 |
| Bipolar disorder | 2 | 74/75 | 0.314 | −0.198 | 0.827 | 0.229 | 2.53 | 0.112 | 60.4 |
| Anxiety disorders | 2 | 259/242 | 0.250 | −0.023 | 0.523 | 0.073 | 4.13 | 0.127 | 51.6 |
Significant values are highlighted in bold prints
Mixed‐effects meta‐regressions were applied to explore whether continuous moderators of average age, gender distribution and sample size affected study findings, but found no indication that these factors influenced observed effect sizes (all p>0.2).
Intervention characteristics and effects on depressive symptoms
In order to gain insight into which aspects of smartphone interventions make them effective for depressive symptoms, we performed further comparative subgroup analyses after separating studies on the basis of common characteristics, such as intervention components, feedback types, and therapeutic approaches applied. The common features examined, and the results of all subgroup comparisons, are detailed in full in Table 5.
Table 5.
Post‐hoc analyses: intervention features
| Studies | Sample size (smartphone/control) | Meta‐analysis | Heterogeneity | Between groups tests | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hedges’ g | 95% CI | p | Q | p | I2 | Q | p | ||||
| Delivered solely via smartphone | 10 | 1,103/1,075 | 0.479 | 0.271 | 0.687 | <0.001 | 62.05 | <0.01 | 80.66 | ||
| Not delivered solely via smartphone | 8 | 613/623 | 0.241 | 0.088 | 0.394 | 0.002 | 13.38 | <0.01 | 40.22 | 3.277 | 0.070 |
| In‐app feedback | 8 | 750/816 | 0.534 | 0.258 | 0.810 | <0.001 | 54.41 | <0.01 | 85.02 | ||
| No in‐app feedback | 11 | 966/882 | 0.266 | 0.143 | 0.389 | <0.001 | 18.95 | <0.01 | 36.68 | 3.02 | 0.082 |
| In‐person feedback | 6 | 309/246 | 0.137 | −0.079 | 0.353 | 0.214 | 8.66 | 0.12 | 42.25 | ||
| No in‐person feedback | 13 | 1,407/1,452 | 0.465 | 0.302 | 0.627 | <0.001 | 61.6 | <0.01 | 75.645 | 5.654 | 0.017 |
| Mental health focused apps | 15 | 1,286/1,292 | 0.438 | 0.276 | 0.601 | <0.001 | 2.09 | 0.72 | 0.00 | ||
| Cognitive training apps | 4 | 430/406 | 0.123 | −0.012 | 0.258 | 0.074 | 63.6 | <0.01 | 74.83 | 8.517 | 0.004 |
| Mood monitoring features | 9 | 653/709 | 0.336 | 0.182 | 0.489 | <0.001 | 16.6 | 0.06 | 82.81 | ||
| No mood monitoring | 9 | 1,063/989 | 0.418 | 0.191 | 0.645 | <0.001 | 64.0 | <0.01 | 45.71 | 0.348 | 0.555 |
| CBT‐based intervention | 7 | 541/615 | 0.531 | 0.339 | 0.722 | <0.001 | 13.5 | 0.04 | 55.58 | ||
| Not CBT‐based | 12 | 1,175/1,083 | 0.311 | 0.130 | 0.493 | 0.001 | 59.0 | <0.01 | 76.26 | 2.661 | 0.103 |
| Mindfulness aspects | 6 | 615/573 | 0.487 | 0.214 | 0.760 | <0.001 | 38.3 | <0.01 | 81.716 | ||
| No mindfulness aspects | 12 | 1,101/1,125 | 0.321 | 0.160 | 0.482 | <0.001 | 38.9 | <0.01 | 66.549 | 1.049 | 0.306 |
CBT – cognitive behavioral therapy
Significant values are highlighted in bold prints
These analyses showed that smartphone interventions which involved “in‐person” (i.e., human) feedback had small, non‐significant effects on depressive symptoms (g=0.137, 95% CI: −0.08 to 0.35, p=0.214), whereas those which did not use in‐person feedback had moderate positive effects (g=0.465, 95% CI: 0.30‐0.63, p<0.001). The difference between these subgroups was statistically significant (p=0.017).
Additionally, the effects of smartphone interventions which were delivered entirely via the smartphone device (10 studies, N=2,178, g=0.479, 95% CI: 0.27‐0.69, p<0.001) appeared larger than those which were not self‐contained smartphone‐only interventions (8 studies, N=1,236, g=0.241, 95% CI: 0.09‐0.39, p=0.002), although the difference between these subgroups fell short of significance (p=0.07).
Similarly, interventions which provided “in‐app feedback”, such as summary statistics and progress scores, had greater effect sizes (g=0.534, 95% CI: 0.26‐0.81, p<0.001) than those which did not have in‐app feedback (g=0.266, 95% CI: 0.14‐0.39, p<0.001), although again the difference between subgroups was non‐significant (p=0.082).
The only other notable finding was that the studies of cognitive training apps had a significantly (p=0.004) smaller effect size on depression outcomes (four studies, N=836, g=0.123, 95% CI: −0.012 to 0.26, p=0.074) than those which focused on mental health (15 studies, N=2,578, g=0.438, 95% CI: 0.28‐0.60, p<0.001).
The use of mood‐monitoring softwares, cognitive behavioral therapy (CBT)‐based interventions and mindfulness training did not appear to influence study effect sizes (all p>0.1 between subgroups with vs. without these features).
A mixed‐effects meta‐regression of study effect size with intervention length (in weeks) found indication of a slight negative relationship between the two, with smaller effects observed from longer interventions, although this correlation fell short of statistical significance (B=–0.025, SE=0.014, Z=−1.72, p=0.086).
DISCUSSION
To our knowledge, this is the first meta‐analysis to examine the efficacy of smartphone interventions for depressive symptoms. Our systematic search identified 18 RCTs, examining 22 mental health interventions delivered via smartphone devices, across a total of 3,414 participants. Thus, the literature base for this particular area has evolved swiftly, and is considerably larger than that found for smartphone interventions in other conditions. Around twice the number of eligible interventions and participants were identified compared to recent meta‐analyses of smartphone interventions for diabetes and anxiety18, 19. Furthermore, 14 of the 18 eligible studies were published within the last two years, which may reflect both the increased research interest in using apps for mental health13 and the increased ownership, access and use of mental health apps by patients and health care organizations.
The main analysis found that smartphone interventions had a moderate positive effect on depressive symptoms, with no indication of publication bias affecting these findings. However, our subgroup analyses found that the effects of smartphone interventions were substantially larger when compared to inactive (g=0.56) than active (g=0.22) control conditions. The same pattern of effect sizes was observed in our meta‐analysis of smartphone interventions for anxiety19. Previous reviews of other technological interventions for mental health conditions have reported similar findings, as a meta‐analysis of virtual reality interventions for treating anxiety found significant effects in comparison to inactive controls, but no difference from traditional psychological treatments51. The extent to which the observed effects on depressive symptoms arise from using the device itself, rather than the psychotherapeutic components of the intervention, should be examined and quantified in future research, to further explore the notion of a “digital placebo” influencing findings52.
We also explored other factors which may drive the effects of smartphone interventions for depressive symptoms, using a range of post‐hoc subgroup analyses. With regards to population type, significant benefits of smartphone apps were only found for those with self‐reported mild‐to‐moderate depression. This may be due to variations in subgroup sample sizes, as the majority of studies were conducted in non‐clinical populations, thus leaving the analyses for major depression and bipolar disorder underpowered to detect significant effects. Nonetheless, the nature of smartphone interventions does appear to position them as an ideal self‐management tool for those with less severe levels of depression. The observed effects indicate that these interventions are well‐placed for delivering low‐intensity treatment within a stepped‐care approach53, or even prevention of mild‐to‐moderate depression among the millions of people affected by subclinical symptoms54. The findings that neither age nor gender had any relationship with study effect size indicate that smartphone interventions may be applicable to a broad range of individuals.
With regards to intervention features, we found that those delivered entirely via smartphone devices had significantly greater effects than those which also involved other human/computerized aspects. Similarly, those using “in‐person feedback” components had significantly smaller effects than those which did not. It seems counterintuitive that additional features/human feedback would decrease smartphone effectiveness. However, this relationship is likely due to the fact that apps not relying on external components have been designed as more comprehensive and self‐contained tools. Indeed, we found some indication that studies which provided in‐app feedback were more effective than those without. It should also be noted that the single study which compared a therapist‐guided smartphone intervention to the same intervention without therapist support found equal effects across the two groups33.
Smartphone interventions based on CBT significantly reduced depressive symptoms, as did those which incorporated aspects of mindfulness training or mood monitoring. However, we were not able to elucidate which of the features were most effective. A previous study which directly compared smartphone apps based on principles of either behavioral activation or mindfulness also found no overall difference between the two approaches55. Nonetheless, results showed that those with more severe depression experienced greater benefits from the behavioral activation app, whereas those with mild depression benefitted more from the mindfulness app. Understanding both which psychological interventions are best delivered via a smartphone and which patient populations will most benefit from smartphone‐based interventions will require further research. As smartphone apps for mental health are becoming easier to create, focusing research on specific populations will enable more personalized and likely effective uses.
The trend‐level negative correlation between effectiveness and length of intervention indicates that another factor to consider when designing optimal apps is user engagement56. Lower rates of user engagement over time have been found in numerous other mental health app studies57, 58, 59. Higher rates of engagement have also been associated with those apps designed for brief interactions60, suggesting the need to customize interventions to the ways people use smartphones. While there is early research on the optimal design and presentation of telehealth platforms61, 62, the impact on patient engagement and outcomes remains an area of nascent exploration. Understanding other factors related to app use, such as socioeconomic status, health literacy63, technology literacy and health status64, 65, also remain important targets for further research.
A major strength of this meta‐analysis is the strict adherence to a registered protocol which exactly described the search strategy, inclusion criteria, data extraction and analytic procedures. However, one drawback is that we only included smartphone interventions which have been evaluated in RCTs. Given the wide availability of mental health apps, ensuring that consumers and clinicians have access to evidence‐based interventions is vital for informed decision making. While the sheer number of apps available, and their frequent updating14, 66, makes rating each impossible, research elucidating the components of effective apps and highlighting best practices may offer information immediately useful for clinical care. Of note, future studies must identify and report safety concerns regarding the use of smartphone interventions67. The ability of smartphones to immediately register entered mood data, compute if responses exceed a certain threshold, and if so activate emergency response systems, offer real time safety monitoring absent from traditional depression treatment.
Another limitation is the significant heterogeneity found across the analyses. Although this heterogeneity was statistically accounted for by the random‐effects models when computing the effect size and respective p values, this still does indicate that significant between‐study differences existed, even when subgrouping by sample/intervention type. Due to the extent of differences between studies, it was difficult to establish the single most effective components of smartphone interventions, or determine which populations these interventions are best suited for. Future studies which directly test alternative approaches against each other in non‐inferiority controlled trials, while assessing outcome variation between subsamples of participants55, would add great value to our understanding of what would constitute the optimal smartphone app for depressive symptoms, and in which populations these methods may be most effective.
In conclusion, the evidence to date indicates that mental health interventions delivered via smartphone devices can reduce depressive symptoms. However, delivering treatments via a smartphone introduces several new aspects which need to be considered, beyond the platform change alone. Specifically, we have yet to establish the ways in which user engagement, feedback loops, expectancy effects, and individual patient characteristics influence intervention outcomes. Rather than a barrier, these variables represent new opportunities for further research to optimize and personalize smartphone‐based interventions.
Given the early indication of efficacy, and rapidly growing empirical research base, it is possible to envisage that continued technological advances will ultimately lead to scalable and cost‐effective digital treatments for depressive symptoms56, 68. Thus, along with continuing to design and evaluate optimal apps, further research should also be dedicated towards establishing feasible methods for implementing smartphone‐based interventions within health care systems.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the kind assistance of J. Anguera (Neuroscape, University of California San Francisco), K. Hallgren (Behavioral Research in Technology & Engineering Center, University of Washington) and M. Faurholt‐Jepsen (Psychiatric Center Copenhagen, Rigshospitalet, Copenhagen) who agreed to share study data necessary for the meta‐analysis. J. Firth is funded by a Blackmores Institute Fellowship and a Medical Research Council doctoral training grant; J. Torous by a National Library of Medicine T15 training grant (4T15LM007092‐25) and the Natalia Mental Health Foundation; S. Rosenbaum by a University of New South Wales Scientia & National Health and Medical Research Council (NHMRC) Early Career Fellowship (APP1123336); J. Nicholas by an Australian Postgraduate Award, and the NHMRC Centre for Research Excellence in Suicide Prevention (APP1042580); R. Carney by an Economic and Social Research Council grant (E SJ5000991); J. Sarris by an NHMRC Research Fellowship (APP1125000). The first two authors contributed equally to this work.
REFERENCES
- 1. World Health Organization. Depression and other common mental disorders: global health estimates. Geneva: World Health Organization, 2017. [Google Scholar]
- 2. Center for Disease Control and Prevention . Current depression among adults – United States, 2006 and 2008. Morbidity and Mortality Weekly Report 2010;59:1229‐35. [PubMed] [Google Scholar]
- 3. Hawton K, Casañas i Comabella C, Haw C et al. Risk factors for suicide in individuals with depression: a systematic review. J Affect Disord 2013;147:17‐28. [DOI] [PubMed] [Google Scholar]
- 4. McCrone PR, Dhanasiri S, Patel A et al. Paying the price: the cost of mental health care in England to 2026. London: King's Fund, 2008. [Google Scholar]
- 5. Cuijpers P, Sijbrandij M, Koole SL et al. The efficacy of psychotherapy and pharmacotherapy in treating depressive and anxiety disorders: a meta‐analysis of direct comparisons. World Psychiatry 2013;12:137‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization . Global health workforce, finances remain low for mental health. www.who.int.
- 7. Liu JX, Goryakin Y, Maeda A et al. Global health workforce labor market projections for 2030. Human Resources for Health 2017;15:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fricchione GL, Borba CP, Alem A et al. Capacity building in global mental health: professional training. Harv Rev Psychiatry 2012;20:47‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gulliver A, Griffiths KM, Christensen H. Perceived barriers and facilitators to mental health help‐seeking in young people: a systematic review. BMC Psychiatry 2010;10:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pedersen ER, Paves AP. Comparing perceived public stigma and personal stigma of mental health treatment seeking in a young adult sample. Psychiatry Res 2014;219:143‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Poushter J. Smartphone ownership and internet usage continues to climb in emerging economies. Pew Research Center 2016;22. [Google Scholar]
- 12. Aboujaoude E, Salame W, Naim L. Telemental health: a status update. World Psychiatry 2015;14:223‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Firth J, Torous J, Yung AR. Ecological momentary assessment and beyond: the rising interest in e‐mental health research. J Psychiatr Res 2016;80:3‐4. [DOI] [PubMed] [Google Scholar]
- 14. Larsen ME, Nicholas J, Christensen H. Quantifying app store dynamics: longitudinal tracking of mental health apps. JMIR mHealth uHealth 2016;4:e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Torous J, Firth J. Bridging the dichotomy of actual versus aspirational digital health. World Psychiatry (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shen N, Levitan M‐J, Johnson A et al. Finding a depression app: a review and content analysis of the depression app marketplace. JMIR mHealth uHealth 2015;3:e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Powell AC, Torous J, Chan S et al. Interrater reliability of mHealth app rating measures: analysis of top depression and smoking cessation apps. JMIR mHealth uHealth 2016;4:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cui M, Wu X, Mao J et al. T2DM self‐management via smartphone applications: a systematic review and meta‐analysis. PLoS One 2016;11:e0166718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Firth J, Torous J, Nicholas J et al. Can smartphone mental health interventions reduce symptoms of anxiety? A meta‐analysis of randomized controlled trials. J Affect Disord 2017;218:15‐22. [DOI] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schardt C, Adams MB, Owens T et al. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak 2007;7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Borenstein M, Hedges L, Higgins J et al. Comprehensive Meta‐Analysis Version 2.0. Englewood: Biostat, 2005. [Google Scholar]
- 23. DerSimonian R, Kacker R. Random‐effects model for meta‐analysis of clinical trials: an update. Contemp Clin Trials 2007;28:105‐14. [DOI] [PubMed] [Google Scholar]
- 24. Higgins JP, Altman DG, Gøtzsche PC et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Orwin RG. A fail‐safe N for effect size in meta‐analysis. J Educ Stat 1983;8:157‐9. [Google Scholar]
- 26. Arean PA, Hallgren KA, Jordan JT et al. The use and effectiveness of mobile apps for depression: results from a fully remote clinical trial. J Med Internet Res 2016;18:e330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Birney AJ, Gunn R, Russell JK et al. MoodHacker mobile web app with email for adults to self‐manage mild‐to‐moderate depression: randomized controlled trial. JMIR mHealth uHealth 2016;4:e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Depp CA, Ceglowski J, Wang VC et al. Augmenting psychoeducation with a mobile intervention for bipolar disorder: a randomized controlled trial. J Affect Disord 2015;174:23‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Enock PM, Hofmann SG, McNally RJ. Attention bias modification training via smartphone to reduce social anxiety: a randomized, controlled multi‐session experiment. Cogn Ther Res 2014;38:200‐16. [Google Scholar]
- 30. Faurholt‐Jepsen M, Frost M, Ritz C et al. Daily electronic self‐monitoring in bipolar disorder using smartphones ‐ the MONARCA I trial: a randomized, placebo‐controlled, single‐blind, parallel group trial. Psychol Med 2015;45:2691‐704. [DOI] [PubMed] [Google Scholar]
- 31. Horsch CH, Lancee J, Griffioen‐Both F et al. Mobile phone‐delivered cognitive behavioral therapy for insomnia: a randomized waitlist controlled trial. J Med Internet Res 2017;19:e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Howells A, Ivtzan I, Eiroa‐Orosa FJ. Putting the 'app' in happiness: a randomised controlled trial of a smartphone‐based mindfulness intervention to enhance wellbeing. J Happiness Stud 2016;17:163‐85. [Google Scholar]
- 33. Ivanova E, Lindner P, Ly KH et al. Guided and unguided Acceptance and Commitment Therapy for social anxiety disorder and/or panic disorder provided via the Internet and a smartphone application: a randomized controlled trial. J Anxiety Disord 2016;44:27‐35. [DOI] [PubMed] [Google Scholar]
- 34. Kahn JR, Collinge W, Soltysik R. Post‐9/11 veterans and their partners improve mental health outcomes with a self‐directed mobile and Web‐based wellness training program: a randomized controlled trial. J Med Internet Res 2016;18:18‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kuhn E, Kanuri N, Hoffman JE et al. A randomized controlled trial of a smartphone app for posttraumatic stress disorder symptoms. J Consult Clin Psychol 2017;85:267‐73. [DOI] [PubMed] [Google Scholar]
- 36. Ly KH, Topooco N, Cederlund H et al. Smartphone‐supported versus full behavioural activation for depression: a randomised controlled trial. PLoS One 2015;10:e0126559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moell B, Kollberg L, Nasri B et al. Living smart ‐ a randomized controlled trial of a guided online course teaching adults with ADHD or sub‐clinical ADHD to use smartphones to structure their everyday life. Internet Interv 2015;2:24‐31. [Google Scholar]
- 38. Oh SJ, Seo S, Lee JH et al. Effects of smartphone‐based memory training for older adults with subjective memory complaints: a randomized controlled trial. Aging Ment Health 2017:1‐9. [DOI] [PubMed] [Google Scholar]
- 39. Proudfoot J, Clarke J, Birch M‐R et al. Impact of a mobile phone and web program on symptom and functional outcomes for people with mild‐to‐moderate depression, anxiety and stress: a randomised controlled trial. BMC Psychiatry 2013;13:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reid SC, Kauer SD, Hearps SJ et al. A mobile phone application for the assessment and management of youth mental health problems in primary care: health service outcomes from a randomised controlled trial of mobiletype. BMC Fam Pract 2013;14:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Roepke AM, Jaffee SR, Riffle OM et al. Randomized controlled trial of SuperBetter, a smartphone‐based/internet‐based self‐help tool to reduce depressive symptoms. Games Health J 2015;4:235‐46. [DOI] [PubMed] [Google Scholar]
- 42. Tighe J, Shand F, Ridani R et al. ibobbly mobile health intervention for suicide prevention in Australian Indigenous youth: a pilot randomised controlled trial. BMJ Open 2017;7:e013518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Watts S, Mackenzie A, Thomas C et al. CBT for depression: a pilot RCT comparing mobile phone vs. computer. BMC Psychiatry 2013;13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Henry JD, Crawford JR. The short‐form version of the Depression Anxiety Stress Scales (DASS‐21): construct validity and normative data in a large non‐clinical sample. Br J Clin Psychol 2005;44:227‐39. [DOI] [PubMed] [Google Scholar]
- 45. Radloff LS. The CES‐D scale: a self‐report depression scale for research in the general population. Appl Psychol Meas 1977;1:385‐401. [Google Scholar]
- 46. Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory‐II. San Antonio: Psychological Corporation, 1996. [Google Scholar]
- 47. Kroenke K, Spitzer RL, Williams JB. The PHQ‐9. J Gen Intern Med 2001;16:606‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983;67:361‐70. [DOI] [PubMed] [Google Scholar]
- 50. Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382‐9. [DOI] [PubMed] [Google Scholar]
- 51. Opriş D, Pintea S, García‐Palacios A et al. Virtual reality exposure therapy in anxiety disorders: a quantitative meta‐analysis. Depress Anxiety 2012;29:85‐93. [DOI] [PubMed] [Google Scholar]
- 52. Torous J, Firth J. The digital placebo effect: mobile mental health meets clinical psychiatry. Lancet Psychiatry 2016;3:100‐2. [DOI] [PubMed] [Google Scholar]
- 53. Andrews G, Cuijpers P, Craske MG et al. Computer therapy for the anxiety and depressive disorders is effective, acceptable and practical health care: a meta‐analysis. PLoS One 2010;5:e13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cuijpers P, Smit F. Subclinical depression: a clinically relevant condition? Tijdschrift voor Psychiatrie 2008;50:519‐28. [PubMed] [Google Scholar]
- 55. Ly KH, Truschel A, Jarl L et al. Behavioural activation versus mindfulness‐based guided self‐help treatment administered through a smartphone application: a randomised controlled trial. BMJ Open 2014;4:e003440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Anguera JA, Jordan JT, Castaneda D et al. Conducting a fully mobile and randomised clinical trial for depression: access, engagement and expense. BMJ Innovations 2016;2:14‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lattie EG, Schueller SM, Sargent E et al. Uptake and usage of IntelliCare: a publicly available suite of mental health and well‐being apps. Internet Interv 2016;4:152‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Owen JE, Jaworski BK, Kuhn E et al. mHealth in the wild: using novel data to examine the reach, use, and impact of PTSD coach. JMIR Mental Health 2015;2:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Frisbee KL. Variations in the use of mHealth tools: the VA Mobile Health Study. JMIR mHealth uHealth 2016;4:e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mohr DC, Tomasino KN, Lattie EG et al. IntelliCare: an eclectic, skills‐based app suite for the treatment of depression and anxiety. J Med Internet Res 2017;19:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Alfonsson S, Olsson E, Linderman S et al. Is online treatment adherence affected by presentation and therapist support? A randomized controlled trial. Comput Human Behav 2016;60:550‐8. [Google Scholar]
- 62. Sarkar U, Gourley GI, Lyles CR et al. Usability of commercially available mobile applications for diverse patients. J Gen Intern Med 2016;31:1417‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mackert M, Mabry‐Flynn A, Champlin S et al. Health literacy and health information technology adoption: the potential for a new digital divide. J Med Internet Res 2016;18:e264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. de Alva FEM, Wadley G, Lederman R. (eds). It feels different from real life: users' opinions of mobile applications for mental health. Proceedings of the Annual Meeting of the Australian Special Interest Group for Computer Human Interaction, Parkville, December 2015.
- 65. Ancker JS, Witteman HO, Hafeez B et al. “You Get Reminded You're a Sick Person”: personal data tracking and patients with multiple chronic conditions. J Med Internet Res 2015;17:e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nicholas J, Larsen ME, Christensen H et al. Systematic assessment of mobile apps for bipolar disorder: features and content. Bipolar Disord; 2015;17:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Faurholt‐Jepsen M, Munkholm K, Frost M et al. Electronic self‐monitoring of mood using IT platforms in adult patients with bipolar disorder: a systematic review of the validity and evidence. BMC Psychiatry 2016;16:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hallgren KA, Bauer AM, Atkins DC. Digital technology and clinical decision making in depression treatment: current findings and future opportunities. Depress Anxiety 2017;34:494‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]


