SUMMARY
Cytauxzoonosis is an emerging infectious disease that affects wild felids as well as the domestic cat; it is caused by the apicomplexan protozoan parasites belonging to the genus Cytauxzoon. Cytauxzoon felis is the species of major concern, whose transmission occurs via the bite of an infected tick. Cytauxzoonosis of the domestic cat has historically been considered uniformly fatal, with a short course of illness, and most domestic cats die within 9 to 15 days postinfection. However, increasing evidence of domestic cats surviving C. felis infection suggests the existence of different strains with various levels of pathogenicity. Although wild felids are considered natural reservoirs for this parasite, a number of studies suggest that domestic cats that have survived nonlethal infections may serve as an additional reservoir. The current article comprehensively reviews the parasite and its life cycle, geographic distribution, genetic variability, and pathogenesis, as well as host immunology and the diagnosis, treatment, and prevention of infection in the domestic cat. This information should provide a basis for better understanding the parasite as well as the pathogenesis of the disease.
KEYWORDS: Cytauxzoon, bobcat, cytauxzoonosis, domestic cat, wild felid
INTRODUCTION
Cytauxzoonosis, an emerging tick-borne disease of domestic cats and wild felids, is caused by the protozoa belonging to the genus Cytauxzoon. Cytauxzoon felis, the agent of cytauxzoonosis of major concern, belongs to the phylum Apicomplexa, class Sporozoasida, order Piroplasmorida, and family Theileriidae (1–8). For many years, it was endemic exclusively to North America, i.e., to the southern, southeastern, and mid-Atlantic states of the United States (9–16). Only recently has it been reported in South America, i.e., in Brazil, and a few other identified species have been reported in Europe and other geographic regions (17–28). The most common natural host for C. felis is the bobcat (Lynx rufus). Upon infection, bobcats generally experience a short course of non-life-threatening illness followed by a full recovery. They serve as a natural reservoir for the pathogen (10, 29, 30). In contrast, infected domestic cats (Felis catus) usually succumb to infections within 9 to 15 days postinfection (p.i.). Infected domestic cats show clinical signs such as pyrexia, anorexia, dehydration, depression, icterus, and hepatosplenomegaly. They usually die within 24 to 48 h upon presentation to veterinarians, in the presence or absence of supportive therapy and experimental therapeutic regimens against this protozoan (2–6, 11–13, 15, 31, 32). However, recent research has indicated that this scenario can also present the other way around. That is, some bobcats suffer from severe acute cytauxzoonosis, sometimes even leading to death, and in contrast, the domestic cat can be clinically normal or carry the parasite for a long period upon recovery from acute cytauxzoonosis (14, 17, 33–37). It appears that the more we learn about the parasite and the disease, the more questions are being raised and need to be answered.
Due to the severity of feline cytauxzoonosis and its geographic expansion, it is crucially important to raise awareness of the disease among veterinarians, pet owners, and the general public. Thus, the current article reviews the parasite and its life cycle, geographic distribution, genetic variability, and pathogenesis, as well as host immunology and the diagnosis, treatment, and prevention of infection in the domestic cat.
THE PARASITES AND THEIR LIFE CYCLE
Cytauxzoon Species
Feline cytauxzoonosis was first reported in 1976 in Missouri, with four fatal cases (1). The disease has been endemic mainly in the domestic cat in the southern and southeastern United States, which is considered ground zero of the disease (2–6). The disease has also been reported from other geographic regions, such as South America, Europe, Africa, and Asia (17–28). The species and nomenclature of the causative protozoa have been a topic of debate for over a decade due to the indistinguishable morphological features of the intraerythrocytic merozoites, commonly called piroplasms. In general, intraerythrocytic piroplasms have a diameter of 1 to 2 μm and may appear in several different forms (2–6, 38). The most common form is a round “signet ring” form (Fig. 1A) (16). Bipolar oval “safety pin” and round anaplasmoid bodies are less common, whereas tetrads are occasionally discovered in Giemsa-stained blood smears (2–6, 38). However, genomic data (http://piroplasmadb.org/piro/) and DNA sequences have revealed differences that are sufficient enough to differentiate the causative protozoan agents at the species level and finally to allow for species classification within the genus (7, 8, 39–43).
FIG 1.
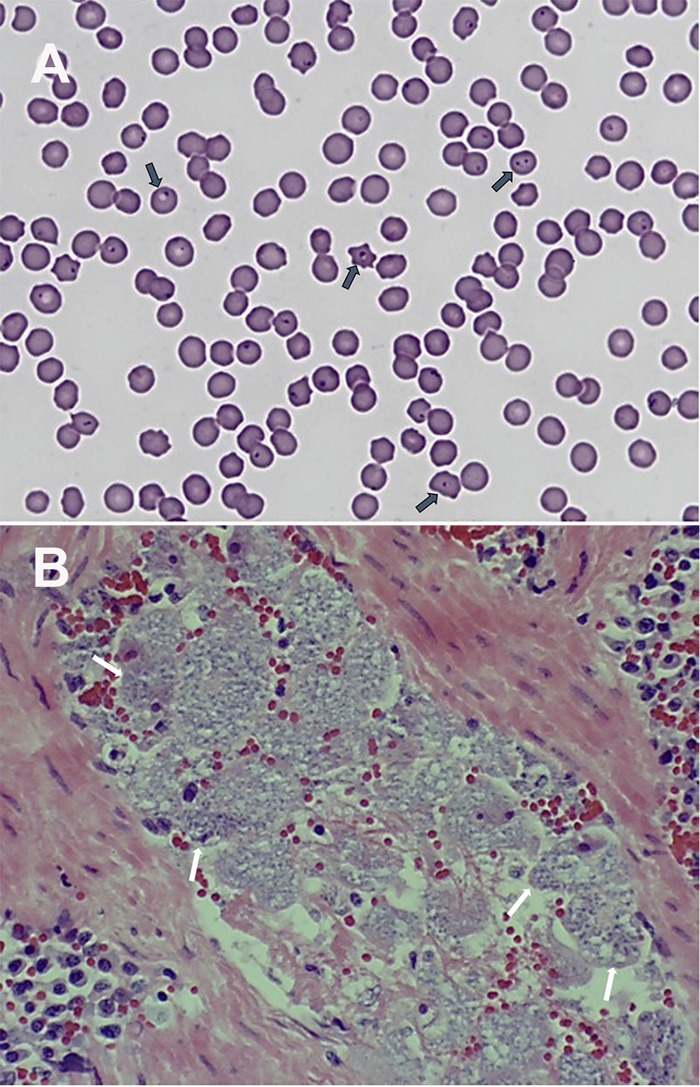
(A) Intraerythrocytic piroplasms (dark arrows) in a blood smear from an infected domestic cat. (B) Numerous schizont-laden macrophages (white arrows) in a splenic blood vessel. Both original micrographs were taken with a 100× oil immersion lens. (Reprinted from reference 16 with permission of Elsevier.)
The genus Cytauxzoon is closely related to Theileria (43). The piroplasm stages in the erythrocytes of mammalian hosts are morphologically similar, making it very difficult, if not impossible, to distinguish them. The two were originally thought to differ from one another by the location of schizogony within the host cells. Schizogony of Cytauxzoon spp. occurs in macrophages, whereas that of Theileria spp. takes place in lymphocytes (2–6, 38, 44). However, Theileria spp. were later found to also infect macrophages (45, 46). Macrophages containing mature Cytauxzoon schizonts are greatly increased in size, to up to 250 μm in diameter, and are often associated with endothelial cells of capillaries of many internal organs (Fig. 1B) (16), which contributes to capillary occlusion, resulting in tissue damage and organ failure (2–6, 15, 32, 44).
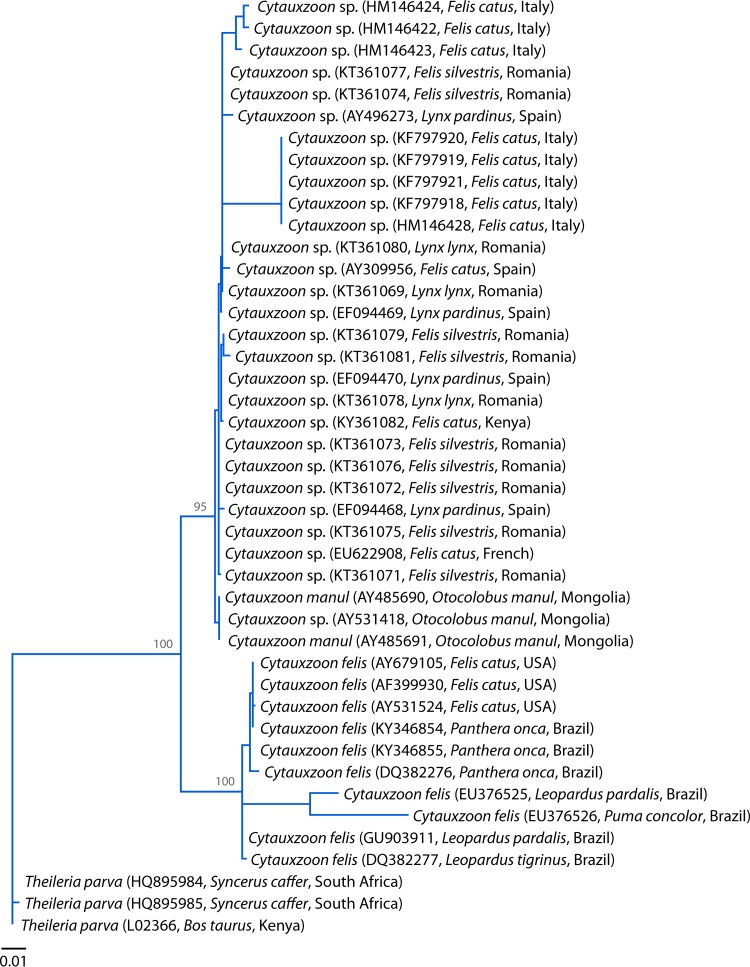
Cytauxzoon species harbored by the domestic cat and wild felids include C. felis, C. manul, and a Cytauxzoon species of undetermined status (Fig. 2). In the present review, the latter is referred to as the Cytauxzoon sp. European strain. C. manul naturally occurs in free-ranging Pallas's cats (Otocolobus manul) in Mongolia (19, 20). The Cytauxzoon sp. European strain was first found in a domestic cat with clinical signs in Spain (8). It was also discovered in France and Italy, again in the domestic cat (22, 47, 48). It was further detected in Iberian lynxes (Lynx pardinus) in Spain (23, 49, 50). More recently, the protozoan was found in Romania, in four Eurasian lynxes (Lynx lynx) and 12 wild cats (Felis silvestris), with a collective prevalence of 62.5% (51).
FIG 2.
Phylogenetic analysis based on the 18S rRNA gene sequences of C. felis, C. manul, and the Cytauxzoon sp. European strain from domestic and wild animals. The sequences of the 18S rRNA genes were aligned using MAFFT 7.122, and the phylogenetic tree topology was obtained from a maximum likelihood analysis by use of PhyML 3.1, with the GTR + G substitution model selected by jModelTest 2.0.2. The strain name, GenBank accession number, host, and area of origin are listed. Numbers at the nodes indicate bootstrap support obtained by repeating the analysis 100 times. Theileria parva was used as an outgroup.
The 18S rRNA genes of these protozoa have been sequenced. The difference between C. felis and C. manul is 1.49%. The difference between C. felis and the Cytauxzoon sp. European strain is 1.73%. The difference between C. manul and the Cytauxzoon sp. European strain is 0.39% (19, 20). Therefore, it is plausible that the latter two in the Old World may represent the same species, which would carry the name C. manul if this is supported by more evidence (Fig. 2) (51). Nevertheless, the current review focuses on C. felis in the New World.
Parasite Life Cycle
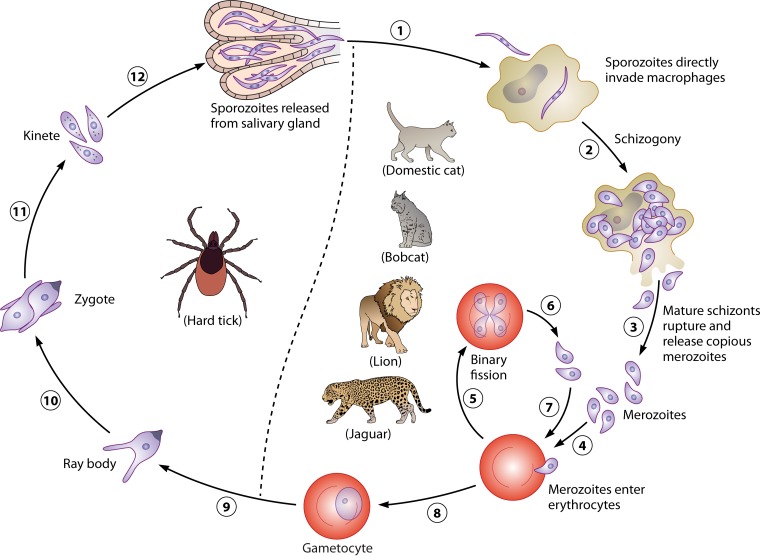
The life cycle of C. felis requires a tick vector and a felid host, in which the sexual and asexual reproduction processes, respectively, occur. These processes are not yet fully understood, especially the sexual reproduction within the tick vector. Bobcats (Lynx rufus) serve as a natural reservoir host in the southern and southeastern United States. They generally experience non-life-threatening illness and recover fully from the infection (30). They often carry intraerythrocytic merozoites, commonly known as piroplasms, after recovering from infection. Upon ingestion by a suitable vector, i.e., Amblyomma americanum, the lone star tick, or Dermacentor variabilis, the American dog tick, gametocytes are released into the tick's gut after digestion of erythrocytes and develop into ray bodies, also known as gametes (52). A male gamete fertilizes a female one, resulting in the formation of a diploid zygote. Zygotes mature into motile haploid kinetes that invade the salivary gland, in which sporozoites form as a result of sporogony (52). These sporozoites are infective to a feline host, such as a bobcat or a domestic cat. Therefore, these hard tick vectors serve as the definitive (final) hosts (Fig. 3) (52). Only nymphs and adults are infective to felids, since there is no evidence of transovarial transmission. Upon inoculation into a domestic cat or a wild felid during a blood meal by a tick vector, sporozoites directly enter host macrophages by a mechanism that has yet to be elucidated. They undergo asexual reproduction by schizogony, resulting in formation of mature schizonts. The latter release numerous merozoites upon rupture of the infected macrophages (52). Subsequently, merozoites infect erythrocytes and undergo asexual reproduction by binary fission, yielding merozoites in the red blood cells (RBCs). Merozoites egress from the erythrocytes and invade new ones, followed by asexual reproduction. This cycle continues repeatedly in the feline host. Therefore, the domestic cat and wild felids serve as intermediate hosts for Cytauxzoon spp. (Fig. 3) (52).
FIG 3.
Schematic life cycle of Cytauxzoon felis. The life cycle of C. felis requires a hard tick vector as a definitive host and a felid host as an intermediate host. Both Dermacentor variabilis and Amblyomma americanum are competent vectors. Since there is no transovarial transmission, only nymphs and adults are infective to felids. All felids are capable hosts, but no other mammals are. Bobcats and domestic cats are the most common hosts. After inoculation by a tick vector during a blood meal (1), sporozoites directly enter macrophages of a felid host, in which they undergo schizogony (2), resulting in formation of mature schizonts that rupture the infected macrophages and release copious merozoites (3). Numerous enlarged schizont-laden macrophages occlude small blood vessels of various organs, which is responsible for acute cytauxzoonosis. Merozoites enter erythrocytes (4), undergo binary fission (5), and ultimately kill their host cells, resulting in release of merozoites (6). Merozoites enter other erythrocytes (7), and the asexual cycle continues. Some intraerythrocytic merozoites differentiate into gametocytes, which are infectious for a tick vector (8). In the tick host, the gametocytes develop into ray bodies (9) that fuse to form a diploid zygote (10) in the gut. Zygotes mature into motile haploid kinetes (11) that enter the tick's salivary glands, where they undergo multiple fission to produce numerous sporozoites that are infectious for a felid host (12).
Transmission
Most studies on transmission were originally performed by inoculation of animals with peripheral blood and/or tissue homogenates of the lymph nodes, liver, spleen, or lungs of C. felis-infected animals. In an experiment aimed to study interspecies transmission of cytauxzoonosis, both domestic and wild animals were used. These included 9 species of laboratory animals (mouse, Mus musculus [ICR]; nude mouse, M. musculus [BALB/c-nu]; rat, Rattus norvegicus [Sprague-Dawley]; gerbil, Meriones unguiculatus [Mongolian]; hamster, Mesocricetus auratus [golden]; guinea pig, Cavia porcehus [Hartley]; chinchilla, Chinchilla laniger [mixed colors]; rabbit, Oryctolagus cuniculus [NZW]; and squirrel monkey, Saimiri sciureus [Bolivian]), 4 species of domestic farm animals (cattle, Bos taurus [Angus]; sheep, Ouis aries [mixed breed]; goat, Capra hircus [mixed breed]; and swine, Sus scrofa [mixed breed]), 17 wildlife species (coyote, Canis latrans [southern Missouri]; red fox, Vulpes fulua [northern Missouri]; striped skunk, Mephitis mephitis [northwestern Missouri]; raccoon, Procyon lotor [central Missouri]; woodchuck, Marmota monax [central Missouri]; yellow-bellied marmot, Marmota flaviventris [central Missouri]; opossum, Didelphis marsupialis [central Missouri]; ground squirrel, Citellus tridecemlineatus [eastern Kansas]; gray squirrel, Sciurus carolinensis [central Missouri]; prairie meadow vole, Microtus ochrogaster [central Missouri]; white-footed mouse, Peromyscus maniculatus [central Missouri]; little brown bat, Myotis lucifugus [central Missouri]; eastern cottontail rabbit, Sylvilagus floridanus [central Missouri]; white-tailed deer, Odocoileus virginianus [central Missouri]; ocelot, Felis pardalis; mountain lion, Felis concolor; and bobcat, Lynx rufus rufus [eastern bobcat]), cat (Felis domesticus [mixed breed]), and dog (Canis familiaris [mixed breed]) (53). These animals were individually inoculated with blood and/or tissue homogenates from domestic cats which were experimentally infected with C. felis and were euthanatized due to severe cytauxzoonosis. A Florida bobcat died 2 weeks after inoculation, with typical clinical signs of cytauxzoonosis prior to its death. An eastern bobcat developed no overt clinical signs of cytauxzoonosis, although it carried a persistent parasitemia. No animals of the other species, except a sheep with persistent low parasitemia, developed cytauxzoonosis clinically or subclinically, as confirmed by blood smear or histopathologic or necropsy examinations. Nevertheless, domestic cats inoculated with the blood from this sheep did not develop any clinical cytauxzoonosis or parasitemia (53). A subsequent study showed that splenectomized sheep inoculated with blood and tissue homogenates prepared from a fatally C. felis-infected cat did not develop any clinical signs of disease or parasitemia (54). These studies collectively demonstrated that sheep are not susceptible to C. felis infection. One plausible explanation for the sheep with persistent parasitemia is a concomitant infection with another species of piroplasm that is morphologically indistinguishable from C. felis.
It is worthwhile to include a brief discussion of the schizogonous phase in bobcats, the natural reservoir of C. felis. In one experiment, two C. felis-free bobcats were each fed 400 adult D. variabilis ticks. These ticks were infected with C. felis in the nymphal stage by taking a blood meal on a naturally infected splenectomized bobcat. Numerous schizont-filled macrophages were observed in the prescapular lymph node of one bobcat 11 days after tick attachment. This bobcat died 8 days later, with typical clinical signs and microscopic evidence of cytauxzoonosis. However, no schizonts were found in the same lymph node of the second bobcat on the 30th day after tick attachment. Furthermore, 10 free-ranging bobcats with natural infection by C. felis were confined for 1 to 12 months before euthanasia for microscopic examination of schizonts in the liver, spleen, lungs, and lymph nodes. No schizonts were found in any examined tissues of any bobcats. Therefore, only a limited schizogony appears to occur in bobcats (30). Even this brief schizogony presented in the blood mononuclear cells may lead to death of the domestic cat after inoculation into the peripheral blood from clinically normal infected bobcats (Lynx rufus) or other wild felines. For example, among four domestic cats that received a parenteral inoculation of blood from C. felis-infected but clinically normal bobcats, one domestic cat died of a cytauxzoonosis, 18 days after inoculation with schizont-laden macrophages, that was mainly located in lymph nodes and lungs. The other three developed parasitemia 5 to 14 days after inoculation, as shown by microscopy of blood smears, but they remained clinically normal. No schizonts were found in any tissues of two of the three clinically normal cats during necropsy 2 months after inoculation (10). The third cat was subsequently challenged parenterally on day 40 postinoculation with the blood of a domestic cat dying of experimentally induced cytauxzoonosis. This domestic cat showed manifestation of cytauxzoonosis and was euthanized at 9 days postchallenge (10). Another similar study also showed that one domestic cat died 12 days after inoculation with the blood of a Florida panther (Felis concolor coryi) infected with C. felis (55).
Several studies have shown that inoculation of peripheral blood from a parasitemic cat into an uninfected domestic cat can result only in a detectable erythroparasitemia in the recipient, with no clinical signs. For example, after inoculation of an uninfected domestic cat with the whole blood of an infected domestic cat, the recipient showed a detectable parasitemia in erythrocytes starting at 8 days postinoculation that was persistent for at least 18 months. Furthermore, PCR and sequencing confirmed the identity of the parasites as C. felis. Nevertheless, the domestic cat was clinically normal throughout this entire course (14). In another study, six domestic cats were randomly divided into two groups that were subcutaneously injected with either dexamethasone or sterile water. Afterwards, they were inoculated intravenously (i.v.) with C. manul in the whole blood of a Pallas's cat (Otocolobus manul) (56). Although they were clinically healthy, these domestic cats harbored low levels of parasites in the blood by 9 days postinoculation. They were further challenged with C. felis schizonts to study whether they were protected by the prior exposure to C. manul. A seventh healthy domestic cat, serving as a control receiving no inoculation of C. manul, was injected only with sterile water prior to the challenge with C. felis schizonts. All seven domestic cats developed cytauxzoonosis with typical clinical signs at 5 days postchallenge, and the diagnosis was further confirmed by histopathology at necropsy (56).
Taken together, these transmission studies indicate that inoculation of uninfected domestic cats with C. felis schizonts causes cytauxzoonosis and death of recipient cats. In contrast, inoculation of peripheral blood containing only piroplasms does not result in schizogony or illness. Additionally, domestic cats receiving intraerythrocytic piroplasms did not have adequate protection against a subsequent challenge with schizonts from an infected felid or sporozoites from a tick vector. This has practical value for vaccine development, i.e., determining which parasitic life cycle stage should be targeted for vaccination.
Can the parasite be transmitted congenitally from queens to kittens? Two clinically normal queens who had lived mostly outdoors in rural Arkansas were found to be positive for the parasite by microscopic review of blood smears and PCR. One queen delivered one live kitten, and the other queen gave birth to two litters, with seven and six live-born kittens. Both microscopy and PCR were performed to detect C. felis parasites in these 14 apparently healthy kittens before 12 weeks of age. All kittens tested negative for the parasites. This experiment indicates that C. felis failed to be transmitted vertically from mother to offspring (57).
Host Specificity
In addition to infections in domestic cats and bobcats, C. felis infection has also been reported for other wild felines. A survey in Florida showed that the prevalences of C. felis in Texas cougars (Puma concolor stanleyana) and Florida panthers (Puma concolor coryi) were 39% and 36%, respectively. The cougars and panthers were asymptomatic but carried intraerythrocytic piroplasms, and infection with C. felis did not appear to have a negative impact on their hematologic parameters (58). Furthermore, C. felis infection has also been diagnosed for ocelots (Leopardus paradise), jaguars (Panthera onca), lions (Panthera leo), and tigers (Panthera tigris) (34, 35, 58–63).
Among other Cytauxzoon species, C. manul has been detected in Pallas's cats (Otocolobus manul) and lions (Panthera leo) (19, 20, 27). The Cytauxzoon sp. European strain has been found in domestic cats, meerkats (Suricata suricatta), wild cats (Felis silvestris), Eurasian lynxes (Lynx lynx), and Iberian lynxes (Lynx pardinus) (21, 23, 28, 64, 65). Collectively, only the domestic cat and wild felids are suitable hosts of Cytauxzoon spp. (2–6).
Tick Vectors
Members of the two other genera in the order Piroplasmorida, i.e., Theileria and Babesia, use a tick vector in their life cycle. Therefore, it is reasonable to assume this to be true for C. felis as well. Laboratory-raised D. variabilis nymphs were allowed to feed on a bobcat (Lynx rufus) naturally infected with C. felis under controlled laboratory conditions. Three and 8 weeks after molting, the adult ticks fed on splenectomized domestic cats. The cats died at 13 to 17 days p.i., with confirmed cytauxzoonosis (29). As mentioned in the life cycle section, two C. felis-negative bobcats were fed on by C. felis-infected adult D. variabilis ticks. The data from the experiment indicated that D. variabilis ticks are capable of transmitting C. felis to bobcats (30). A similar experiment was carried out with domestic cats. In this case, the D. variabilis adults arose from nymphs that had fed on domestic cats with experimental infection by C. felis. They then were allowed to feed on C. felis-negative domestic cats. The latter developed cytauxzoonosis (44). Collectively, these experiments unequivocally showed that D. variabilis is a vector of C. felis that is capable of transmitting the protozoan from bobcats to domestic cats, between bobcats, and between domestic cats, at least under experimental conditions. Currently, little is known about the tick vector of C. manul and the Cytauxzoon sp. European strain. It is almost certain that those parasites do not use D. variabilis as a vector, because its geographic distribution is limited to North America (66).
The effectiveness of D. variabilis as a vector in the areas of endemicity under natural conditions cannot be determined from such experiments. The natural vector capacity of arthropod vectors is influenced by many factors, such as vector species, vector population, vector susceptibility to a pathogen, the pathogen's effect on the vector, vector life span, and vector blood preference, to name a few. A confirmed vector under laboratory conditions does not always hold up as a natural vector.
An epidemiological study of ticks was conducted by means of PCR and sequencing. In total, 1,631 ticks were collected in 2007 to 2009 from hosts, such as humans, domestic animals, and wildlife, in areas where cytauxzoonosis is endemic, including Kentucky, Georgia, Tennessee, and Texas. Nevertheless, bobcats (Lynx rufus) and domestic cats were not included, except for one feral domestic cat with only one D. variabilis tick. An overall prevalence of 1.3% was found for C. felis in D. variabilis ticks (9/702 ticks). In contrast, no C. felis-positive ticks were found among 743 A. americanum, 99 Amblyomma cajennense, 16 Amblyomma maculatum, 4 Ixodes scapularis, and 1 Ixodes woodi tick and 64 unidentified Amblyomma sp. nymphs (67).
Similarly, a total of 1,362 ticks were collected either individually or in pools from dogs and domestic cats in Missouri, where feline cytauxzoonosis is endemic. The ticks were analyzed by PCR for the 18S rRNA gene and internal transcribed spacer 1 (ITS-1) sequence of C. felis. Three A. americanum nymphs were positive, which yielded a prevalence of 0.9% and indicated that A. americanum could be a potential vector for the protozoan, while no other tick species, including D. variabilis, were found to be positive for C. felis (68). Further, A. americanum nymphs were allowed to feed on a domestic cat with a clinically normal infection by C. felis that was confirmed by a blood smear showing intraerythrocytic piroplasms, PCR amplification of the 18S rRNA gene, and sequencing. The resulting adult ticks, after molting, were allowed to feed on a C. felis-negative domestic cat. The cat developed typical clinical signs of cytauxzoonosis at 11 days p.i. However, it resolved the clinical signs by 23 days p.i. and remained clinically normal until day 74 p.i., when the experiment ended. Interestingly, the domestic cats that were fed on by three other tick species, namely, D. variabilis, Ixodes scapularis, and Rhipicephalus sanguineus, never became clinically ill. Necropsy of the domestic cat that was fed on by D. variabilis revealed no evidence of C. felis infection (69). Later, the same authors performed another, similar experiment with an expanded number of domestic cats. Four domestic cats each were used in the A. americanum and D. variabilis groups this time. Again, all four cats fed on by A. americanum ticks became sick, with clinical signs of cytauxzoonosis, and survived the infection. In contrast, none of the four cats fed on by D. variabilis became ill or PCR positive (70). Further, a cross-sectional study was carried out to determine the minimum infection rate of C. felis in ticks by collecting unengorged A. americanum and D. variabilis ticks from wild habitats in an area of endemicity. A. americanum ticks were positive for C. felis in both the adult and nymphal stages, with 0.6% of males (1/178 ticks), 1.5% of females (3/197 ticks), and 0.8% of nymphs (3/393 nymphs) being positive. No D. variabilis ticks were found to be positive for C. felis (70). These data confirm that A. americanum is a primary vector of C. felis, although it does not rule out D. variabilis as a vector.
In a survey of bobcat infections in association with tick density in 13 U.S. states, Shock et al. found that high densities of A. americanum were associated with higher prevalences of C. felis. In contrast, no such association existed between the density of D. variabilis and C. felis prevalence (71). Altogether, the data presented above confirm that both D. variabilis and A. americanum are suitable vectors for C. felis in North America. It appears that the latter has a higher vector capacity in nature. Nevertheless, their exact roles in maintaining and transmitting C. felis infection in areas where it is enzootic remain to be explored.
How long does it take for a tick vector to transmit C. felis to a domestic cat upon its attachment? Can C. felis be transmitted by the host ingesting a C. felis-positive tick? A recent study aimed to answer these questions. In the study, 49 domestic cats were randomly divided into seven groups, with seven cats per group. Five groups of domestic cats were each infested with 25 male-female pairs of C. felis-positive A. americanum adults per domestic cat for 12, 18, 24, 36, or 48 h. In the sixth group, each domestic cat was fed the same number of C. felis-infected adult ticks embedded in canned cat food. In the seventh group, 50 C. felis-infected ticks were allowed to feed on each cat until repletion. Six of seven domestic cats in group 7 were determined to be positive for C. felis between 8 and 12 days after infestation. One of seven cats in the 48-h group, i.e., group 5, was determined to be positive 12 days after infestation. None of the domestic cats in the other groups were found to be positive for C. felis. These data indicate that transmission of C. felis by an A. americanum tick requires a minimum of 36 h of attachment and that transmission cannot be achieved by the host's ingestion of C. felis-infected ticks (72). Further studies are needed for other Cytauxzoon species and other ticks.
Can tick attachment and transmission of C. felis to a domestic cat be prevented by a collar, and how effective is it? In an experiment, 10 domestic cats were each fitted with a 10% imidacloprid and 4.5% flumethrin collar (Seresto; Bayer Animal Health), and another 10 domestic cats were used as untreated controls. All cats were intact females of 6 months of age and were randomly assigned to either the control or treatment group. They were each exposed to 25 A. americanum adult ticks that were experimentally infected by C. felis. No ticks were found on any of the treated cats. In contrast, they attached to every domestic cat in the nontreated control group, with geometric means of 15.3 and 14.2 ticks at 24 and 48 h, respectively. Zero (0%) and 9 of 10 (90%) domestic cats in the collar-treated and nontreated control groups, respectively, were C. felis positive. It was concluded that the 10% imidacloprid and 4.5% flumethrin collar effectively prevented ticks from attaching, feeding, and transmitting C. felis to domestic cats (73).
PHYLOGENETIC CLASSIFICATION
Parasites in the order Piroplasmorida are blood parasites of vertebrates, and some of them cause serious diseases in domestic animals (2–6, 74–78). The taxonomy of the order Piroplasmorida is debatable, with considerable confusion about the relationships among the piroplasmid species and the classification method (7, 8, 39–42, 77). Three genera, i.e., Babesia, Theileria, and Cytauxzoon, are established based on morphological, ultrastructural, and parasite life cycle characteristics, such as host preference and the cell type(s) infected (4–8, 39–42, 77). All species of Babesia, Theileria, and Cytauxzoon are transmitted by a tick vector. However, the mechanisms of transmission are different (2–6, 52, 74–78). During a blood meal by a suitable tick vector, Babesia sp. sporozoites directly enter the host erythrocytes and develop into merozoites. Merozoites undergo binary fission in the erythrocytes, resulting in two or sometimes four daughter cells, and the erythrocytes break apart. Each daughter enters another red blood cell, and this cycle repeats (74–77). In contrast, Cytauxzoon and Theileria protozoa first infect macrophages and/or lymphocytes, in which they undergo schizogony, resulting in rupture of the infected host cells and release of numerous merozoites (2–6, 52, 78). The merozoites enter erythrocytes and undergo asexual binary fission, yielding merozoites. Upon rupture of infected erythrocytes, these merozoites infect other red blood cells, and the intraerythrocytic cycle continues (2–6, 52, 78).
There are differences in the development of Babesia, Cytauxzoon, and Theileria in a tick vector. The zygotes of Babesia species undergo multiplication and invade multiple organs of the tick, including the salivary glands and ovaries. Thus, parasites are passed from mother ticks to their offspring by transovarial transmission. The sporozoites in the larvae of these offspring ticks are infectious for mammalian hosts (74–77). However, Cytauxzoon and Theileria species are limited to transstadial transmission. Their zygotes invade only the salivary glands to develop into infectious sporozoites. Consequently, the larvae are not infective to mammalian hosts due to a lack of sporozoites (2–6, 52, 78). These life cycle characteristics make it possible to distinguish Babesia from Cytauxzoon and Theileria species. Although a previous study indicated that Theileria species invade lymphocytes, whereas Cytauxzoon species enter macrophages, more studies show that Theileria can also infect macrophages (45, 46). Therefore, this classification scheme for Cytauxzoon and Theileria species has limited value.
In an attempt to resolve the controversy, several studies have used molecular methodology, such as 18S rRNA gene sequencing, to classify the order Piroplasmorida (39–42, 77). Unfortunately, they have made it no clearer, if not more confusing, because Cytauxzoon species have been put into different phylogenetic positions within the order Piroplasmorida (39–42, 77). Recently, the taxonomy of the order Piroplasmorida was revisited based on the mitochondrial genome sequences and structures (43). Five distinct lineages within the order Piroplasmorida were identified based on phylogenetic analysis of the nucleotide sequences of the concatenated mitochondrial genome and the 18S rRNA gene. The five lineages were further confirmed by analysis of cox1-encoded amino acid sequences and the mitochondrial genome structure as well as the biology of Piroplasmorida organisms. The Cytauxzoon (Cytauxzoon felis) and Theileria (Theileria annulata, Theileria parva, and Theileria orientalis) species were grouped into a single lineage, and the four previously identified lineages were the Babesia sensu stricto lineage (Babesia sp. Coco, Babesia bigemina, Babesia bovis, Babesia caballi, Babesia canis, Babesia vogeli, Babesia rossi, and Babesia gibsoni), the western Babesia lineage (Babesia conradae), the Babesia microti lineage (Babesia microti-like species, Babesia microti, and Babesia rodhaini), and the Theileria equi lineage (Theileria equi) (43). Although the currently assigned nomenclature provides important new evidence for elucidation of the evolutionary relationships of the order Piroplasmorida, it has room for reconsideration and improvement. Some clades may even have their nomenclature changed due to the limited number of samples and species used in the recent phylogenetic analysis (43). So far, only the C. felis mitochondrial genome sequence and structure have been characterized, whereas those of the other Cytauxzoon species, such as the Cytauxzoon sp. European strain, remain unknown. Thus, further studies using larger numbers of samples and representative species within the order Piroplasmorida are warranted to refine their clade taxonomy.
EPIDEMIOLOGY: WORLDWIDE DISTRIBUTION, SEASONALITY, AND RISK FACTORS
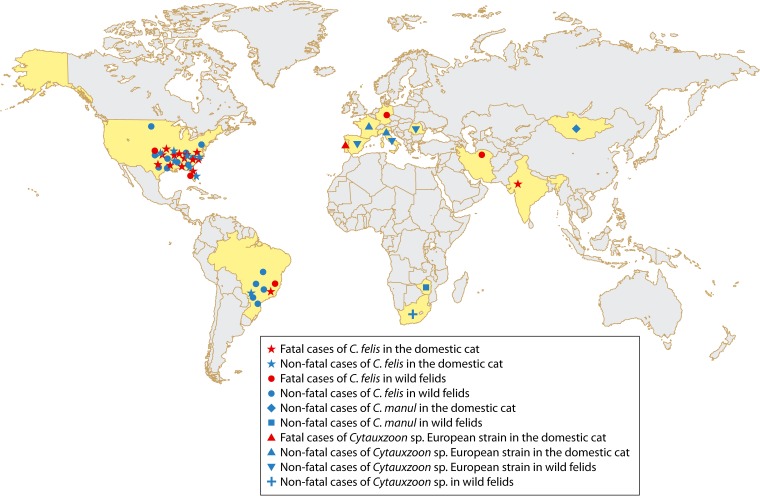
The first fatal case of feline cytauxzoonosis in India was reported in 2009. The diagnosis was based solely on clinical signs and microscopic demonstration of intraerythrocytic piroplasms. The domestic cat was a 4-month-old male kitten with anorexia, prostration, anemia, and pyrexia. It collapsed on the 3rd day of reference, even with application of a broad spectrum of antibiotics and supportive therapy (79). According to the authors of the study, this was also the first case of feline cytauxzoonosis caused by C. felis in Asia, which has yet to be confirmed, since no molecular data were presented. The next reports in Asia were from Iran, where a stray cat and a wild cat (Felis silvestris) were infected with C. felis, as confirmed by blood smear and molecular analyses, respectively (25, 26). The stray cat exhibited clinical signs of cytauxzoonosis, with moderate anemia, and the wild cat died after 4 days of treatment with piperacillin-tazobactam (Tazocin) and clindamycin (11 mg/kg of body weight per os [p.o.] every 12 h [q12h]) (25, 26). As mentioned later in this review, C. felis has been found in Brazil, in domestic cats (18, 80) and zoo lions (Panthera leo) (17). In addition, it was also found in a zoo tiger (Panthera tigris) in Germany (35). Therefore, so far, cytauxzoonosis caused by C. felis has been found in North America (United States), South America (Brazil), Europe (Germany), and Asia (India and Iran) (Fig. 4).
FIG 4.
Geographic distribution of Cytauxzoon infections in domestic cats and wild felids. The countries where cases of cytauxzoonosis have been reported are highlighted in yellow.
A retrospective study of feline cytauxzoonosis in domestic cats between 1998 and 2006, in North Carolina (28 cases), South Carolina (3 cases), and Virginia (3 cases), determined that 32 of 34 cases were diagnosed between April and September (12). A similar study of 56 cases of feline cytauxzoonosis in western Kentucky between 2001 and 2011 revealed a single peak in May, with diagnoses made between March and October (16). A total of 232 cases from two veterinary hospitals in Stillwater, OK, from 1995 to 2006 were collectively analyzed. First, there was no significant fluctuation in annual numbers of diagnosed cytauxzoonosis cases over the entire period. Second, the number of cases peaked in April, May, and June, with a minor peak in August and September (81). Clearly, the peak time of diagnosis in the U.S. regions of endemicity is May and June.
In an effort to determine different environmental conditions as risk factors for feline cytauxzoonosis, Raghavan and colleagues used geographic information systems (GIS) (82). They used a cohort of 69 confirmed cases of cytauxzoonosis and 123 control cases with negative microscopic findings for piroplasms yet similar clinical signs. Significant risk factors for cytauxzoonosis in the study region included total edge contrast index, grassland coverage, humidity during the 9th week prior to case arrival, and an interaction variable, i.e., diurnal temperature range × percent mixed-forest area. Raghavan et al. concluded that land-cover areas and climatic conditions favorable for tick populations were strong risk factors for feline cytauxzoonosis (82). Further analysis of the potential ecological distribution of C. felis in Missouri, Oklahoma, and Arkansas showed once again that environmental niches suitable for tick breeding were more likely related to transmission of the parasite in the domestic cat (83). Environmental analysis showed that cytauxzoonosis cases were more likely to occur in low-density residential areas and were significantly associated with more wooded cover and closer proximity to natural or unmanaged areas (81). Considering that C. felis requires a tick vector in its life cycle, these results confirm what would have been expected.
LETHAL INFECTIONS IN BOBCATS AND OTHER WILD FELIDS
As mentioned earlier, bobcats, unlike the domestic cat, often experience non-life-threatening illness followed by a recovery from C. felis infections, thereby serving as a natural reservoir (29, 30). That being said, severe and fatal cases of cytauxzoonosis in bobcats have been recorded in the literature. A free-ranging bobcat cub (Lynx rufus) presenting to a veterinary clinic in Kansas was euthanized due to a moribund state. During necropsy, multifocal atelectasis, splenomegaly, and pericardial effusion were evident. Microscopy of hematoxylin and eosin (H&E)-stained tissue sections revealed subacute pulmonary thrombosis and greatly enlarged macrophages filled with schizonts within blood vessels in many tissues. Pathological changes at both the gross and microscopic levels confirmed that cytauxzoonosis was the cause of the bobcat cub death (36). In addition, a 7-year-old female white tiger (Panthera tigris) died of cytauxzoonosis in Florida. The tiger presented with a history of a 2-day duration of anorexia, lethargy, mild dehydration, fever, and anemia. She progressed rapidly the following day, to recumbency, coma, and death after developing icterus. Detailed microscopy analysis detected merozoites in RBCs and schizonts of C. felis in macrophages of various tissues. Again, clinical signs and intraerythrocytic piroplasms confirmed cytauxzoonosis as the cause of death (34).
Cases of fatal cytauxzoonosis in wild felids occur not only in the United States but also in other geographic regions. Fatal cases of cytauxzoonosis occurred in a lioness (Panthera leo) raised in captivity and her 6-month-old cub in a Brazilian zoo. The lioness showed weight loss, depression, anemia, alopecia, dark discolored urine, tachypnea, nystagmus, deafness, and a staggering gait. Necropsy revealed marked pulmonary edema, red and semiliquid intestinal content, and slight gelatinous translucent edema in the mediastinum. The cub presented with endocardial and pulmonary edema, hemothorax, and hepatic and splenic congestion and died soon afterwards. Histopathological examination revealed schizont-filled macrophages in numerous tissues and organs. These were the first confirmed cases of fatal cytauxzoonosis in South America (17). In 1984, a young Bengal tiger (Panthera tigris) in a German zoo died of an unknown protozoal infection. The tiger was born and raised in the zoo. The infection was retrospectively confirmed as fatal cytauxzoonosis by histologic and microscopic examination (35).
Although the last two case reports are interesting, caution should be taken in interpretation of their significance. Both cases were from wild felids in zoos. Zoos often obtain exotic animals from other geographic regions, including from North America, where C. felis is endemic. Although in both cases the wild felids were born and raised in the countries where the cases were reported, it seems premature to conclude that cytauxzoonosis is endemic in Brazil and Germany. There is a possibility that these zoos might have imported C. felis-infected animals from areas of endemicity and that these cases were merely a result of tick transmissions that occurred on site. Indeed, the German zoo directly imported three young bobcats from the United States before the Bengal tiger infection (4). However, this might not be the case for Brazil, as described below.
NONFATAL AND CLINICALLY NORMAL INFECTIONS IN THE DOMESTIC CAT
Although it is considered uniformly fatal (2–6), feline cytauxzoonosis in the domestic cat turns out to have a wide spectrum of clinical signs, ranging from clinically normal through nonfatal to fatal manifestations. In this section, only clinically normal and nonfatal infections are reviewed. A 1-year-old spayed female domestic cat with a history of 2 days of fever, lethargy, and anorexia was diagnosed with C. felis infection by demonstration of intraerythrocytic piroplasms consistent with C. felis at a level of 0.5% parasitemia. The cat received supportive therapy yet developed severe icterus and had dark brown urine within 24 h. Nevertheless, the cat recovered fully, was confined indoors afterwards, and was clinically normal for 2.5 years post-initial diagnosis. This was apparently the first documented case of a domestic cat recovering from a natural infection of C. felis in Oklahoma (84). Since then, more recovery cases of feline cytauxzoonosis have been reported in the literature. These include 3 cases in Arkansas and Georgia and 18 cases in northwestern Arkansas and northeastern Oklahoma. All these cases were confirmed by PCR and DNA sequencing (14, 85).
Furthermore, cross-sectional surveys of the domestic cat in the southern and southeastern United States have revealed many clinically normal infections in the domestic cat. In a survey of domestic cats in Florida (494), North Carolina (392), and Tennessee (75), using PCR and DNA sequencing, 0.4%, 0.0%, and 1.3% of cats, respectively, were positive for C. felis, with an overall prevalence of 0.3% (85). In addition, a real-time PCR analysis of blood samples collected from clinically healthy domestic cats with a high risk of C. felis infections in Arkansas and Georgia showed that the prevalence rate of the parasite was 30.3% (27/89 cats) (33). More recently, a study of 902 domestic cats in Arkansas, Missouri, and Oklahoma by PCR yielded a 6.2% prevalence (37). A summary of data on nonfatal and clinically normal infections of the domestic cat and other felids is shown in Table 1. Collectively, these data unequivocally demonstrate that these infections are anything but rare events in the regions of endemicity of the southern and southeastern United States.
TABLE 1.
Cross-sectional studies of Cytauxzoon infections in asymptomatic domestic cats and other felids
| Continent and country | Study period (yr)a | Animal species | Test method | Cytauxzoon speciesb | No. of positive animals/no. of animals tested (%) | Reference |
|---|---|---|---|---|---|---|
| North America | ||||||
| USA | 1999–2000 | Domestic cat (Felis catus) | PCR | C. felis | 3/961 (0.31) | 85 |
| USA | 2008–2010 | Domestic cat (Felis catus) | PCR | C. felis | 27/89 (30.3) | 33 |
| USA | 2008–2012 | Domestic cat (Felis catus) | PCR | C. felis | 56/902 (6.2) | 37 |
| USA | 2002 | Bobcat (Lynx rufus) | PCR | C. felis | 10/30 (33.3) | 107 |
| USA | 2004–2006 | Bobcat (Lynx rufus) | PCR | C. felis | 5/69 (7.2) | 107 |
| USA | 1999–2010 | Bobcat (Lynx rufus) | PCR | C. felis | 138/696 (19.8) | 71 |
| USA | 2008–2010 | Bobcat (Lynx rufus) | PCR | C. felis | 34/133 (25.6) | 33 |
| USA | 2003–2015 | Bobcat (Lynx rufus) | PCR | C. felis | 88/125 (70.4) | 121 |
| USA | 1983–1997 | Cougar (Puma concolor) | Blood smear | C. felis | 33/91 (36.3) | 58 |
| USA | 1989–2005 | Cougar (Puma concolor) | PCR | C. felis | 5/41 (12.2) | 60 |
| USA | 1999–2010 | Cougar (Puma concolor) | PCR | C. felis | 1/7 (14.3) | 71 |
| South America | ||||||
| Brazil | 2013 | Domestic cat (Felis catus) | PCR | C. felis | 1/151 (0.66) | 80 |
| Brazil | 2001–2004 | Ocelot (Leopardus pardalis) | PCR | C. felis | 1/16 (6.3) | 61 |
| Brazil | 2006 | Jaguar (Panthera onca) | PCR | C. felis | 1/9 (11.1) | 62 |
| Brazil | 2006 | Ocelot (Leopardus pardalis) | PCR | C. felis | 6/29 (20.7) | 62 |
| Brazil | 2006 | Puma (Puma concolor) | PCR | C. felis | 2/9 (22.2) | 62 |
| Brazil | 2000–2009 | Jaguar (Panthera onca) | PCR | C. felis | 29/30 (96.7) | 63 |
| Africa | ||||||
| South Africa | 2011 | Meerkat (Suricata suricatta) | PCR | Cytauxzoon sp. | 26/46 (56.5) | 28 |
| Zimbabwe | NA | Lion (Panthera leo) | PCR | C. manul | 2/86 (2.3) | 27 |
| Europe | ||||||
| Spain | 2005–2008 | Domestic cat (Felis catus) | PCR | Cytauxzoon sp. | 8/644 (1.24) | 122 |
| Spain | 2003–2007 | Iberian lynx (Lynx pardinus) | PCR | Cytauxzoon sp. | 24/77 (31.2) | 50 |
| Spain | 2004–2006 | Iberian lynx (Lynx pardinus) | PCR | Cytauxzoon sp. | 3/20 (15) | 23 |
| Spain | NA | Wild cat (Felis silvestris) | PCR | Cytauxzoon sp. | 4/6 (66.7) | 64 |
| France | 2006–2007 | Domestic cat (Felis catus) | PCR | Cytauxzoon sp. | 1/116 (0.8) | 47 |
| Italy | 2007–2008 | Domestic cat (Felis catus) | PCR | Cytauxzoon sp. | 27/118 (22.9) | 22 |
| Italy | 2011–2014 | Wild cat (Felis silvestris) | PCR | Cytauxzoon sp. | 3/21 (14.3) | 65 |
| Romania | 2014 | Wild cat (Felis silvestris) | PCR | Cytauxzoon sp. | 6/12 (50) | 51 |
| Romania | 2014 | Eurasian lynx (Felis silvestris) | PCR | Cytauxzoon sp. | 4/4 (100) | 51 |
NA, not available.
The Cytauxzoon species found in Europe is defined as the Cytauxzoon sp. European strain in the text and in Fig. 4.
Unexpectedly, nonfatal and clinically normal infections of the domestic cat by C. felis occur in geographic regions other than the United States as well. In Brazil, 0.7% of 151 cats (86 owned and 65 stray) were positive by PCR and DNA sequencing. This Brazilian strain clustered in the same clade as that for other C. felis strains by phylogenetic analysis (80). Another survey showed that 96.7% (29/30 animals) of wild jaguars (Panthera onca) in Brazil were positive for C. felis by PCR and DNA sequencing (63). These data, along with the cases presented earlier for wild zoo felids (62), confirm that C. felis and cytauxzoonosis occur in Brazil (17). Consequently, both North and South America are regions of endemicity for C. felis.
Similarly, the Cytauxzoon sp. European strain was reported in clinically normal domestic cats, with prevalences ranging from 0% to 22.9%. For example, a study in northeastern Italy showed that the prevalence of Cytauxzoon sp. infection was 22.9% (27/118 animals) for detection using PCR amplification of the 18S rRNA gene and 15.3% (18/118 animals) by microscopic examination of blood smear (22). In France, 0.8% of 116 domestic cats were positive for Cytauxzoon sp. by PCR and DNA sequencing (47).
A recent study showed that subclinically infected domestic cats could transmit clinical cytauxzoonosis to a domestic cat, with A. americanum as a vector (69, 70). This is very important because naturally infected domestic cats serving as an additional reservoir would greatly increase the risk of exposure for domestic cats in general.
PATHOGENESIS AND CLINICAL FEATURES
The pathogenesis of C. felis infection is associated with the various life cycle stages of the parasite and the host species (2–6). A host acquires an infection by the bite of an infected tick. During a blood meal, an infected tick inoculates the infectious sporozoites into the dermis of a host, where they invade endothelium-associated mononuclear cells and progress to form schizonts (Fig. 1B) (2–6, 16). These large, schizont-filled macrophages occlude small vessels in various organs, especially in the lungs, spleen, kidneys, and liver (2–6, 11–13, 15, 32, 44, 86–91). Vascular obstruction is one of the major pathophysiologic mechanisms that lead to considerable pathological changes, such as circulatory impairment, hemolytic anemia, and multiorgan dysfunction (2–6, 11–13, 15, 32, 44, 86–91).
An infected domestic cat first shows clinical signs at approximately 5 to 14 days p.i., with these signs including fever, inappetence, depression, lethargy, dyspnea, vomiting, icterus, tachycardia, generalized pain, and vocalization. The sick cat experiences a nonregenerative anemia, thrombocytopenia, and lymphopenia (2–6, 92–95). Moribund cats commonly manifest recumbency, hypothermia, and vocalization in the terminal stages of disease. Most of them appear to die from a shock-like state (2–6).
A 1-year-old neutered male domestic shorthair cat was euthanized due to rapidly worsening clinical signs the day after being evaluated for a sudden onset of inappetence and lethargy. Necropsy findings included noncollapsing lungs with multiple loci of hemorrhage, a brownish yellow liver, and an enlarged spleen with a meaty consistency (95). In the brain, schizont-laden macrophages of increased size were found occluding leptomeningeal and parenchymal arterioles and venules, as well as in the small capillaries throughout the gray and white matter and the choroid plexus. Necrosis, including necrotic neurons, was found in the gray or white matter, with marked vacuolation (95). In a retrospective study of 148 cases archived between January 1995 and June 2005 in the Oklahoma Animal Disease Diagnostic Laboratory, Snider and colleagues found overall moderate interstitial pneumonia, mild alveolar macrophage numbers, mild intra-alveolar hemorrhage, moderate neutrophil infiltration, and moderate to severe vascular occlusion (89).
Although schizogony of C. felis appears to be short, most domestic cats die in less than 1 week from the onset of signs. In contrast, most bobcats survive through this stage. These findings suggest that schizogony of this parasite is responsible for the clinical manifestations of cytauxzoonosis in the domestic cat (2–6, 29, 30, 86–95).
Mature schizonts rupture the enlarged macrophages and release large numbers of merozoites, which infect erythrocytes, resulting in the formation of the ring-shaped piroplasms (Fig. 1A) (16). In general, clinical signs occur prior to the appearance of blood piroplasms. At the erythrocytic stage, the piroplasm undergoes multiplication, and dividing forms may be observed. Although it is low and difficult to detect in early infection, parasitemia typically increases as the disease progresses, with values ranging from <1% to as high as 4% (2–6). It may reach as high as 50% in some unusual cases (2–6). In the acute phase, parasitemia revealed by blood smears is commonly detected in the late stage of cytauxzoonosis, usually 1 to 3 days before death (2–6, 15, 86–95).
Domestic cats that have recovered from the acute infection frequently maintain intraerythrocytic piroplasms for years. In this chronic stage, piroplasms themselves appear to be relatively harmless and do not cause clinical signs. Nevertheless, these domestic cats may serve as additional reservoir hosts. Cats with subclinical cytauxzoonosis usually have lower parasitemia than that of cats with acute infections, with values ranging from 0.045% to 1.27% (2–6, 96). Host immune status may affect parasitemia, since immunosuppression of a host increased its parasitemia (97).
IMMUNOLOGY
Despite decades of research, very limited information has been gained regarding the feline immune response to C. felis infection. A previous study showed that domestic cats inoculated intraperitoneally with bobcat peripheral blood containing the nonpathogenic piroplasms generated anti-Cytauxzoon antibodies. Antibody levels tended to be related to the degree of parasitemia. High antibody titers were observed at or near the peak of parasitemia (97). Another study demonstrated that domestic cats inoculated with Pallas's cat blood containing C. manul piroplasms did not develop fatal cytauxzoonosis. These cats were not completely protected from a challenge with C. felis schizonts (56). On the other hand, domestic cats that experienced and survived acute infections were protected against challenge with virulent C. felis schizonts. No domestic cats have recrudesced with clinical cytauxzoonosis after recovery from the acute infection (98). Collectively, these data suggest that the immune response to initial infection by C. felis schizonts is important for the domestic cat to develop sufficient immunity to protect against C. felis schizonts.
In order to further characterize immune responses in the domestic cat, Frontera-Acevedo et al. (99) studied cats that died of cytauxzoonosis, acutely ill C. felis-infected cats, healthy survivors of C. felis infection, and healthy uninfected cats. They showed that the concentrations of tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β) were higher and the serum albumin concentrations significantly lower in cats that died of cytauxzoonosis than in the cats of the other three groups. The concentration of serum albumin, a negative acute-phase protein, decreased during the acute inflammatory response. CD18, an important leukocyte adhesion molecule, was upregulated during inflammation and stimulated the release of some proinflammatory cytokines and chemokines, such as TNF-α, IL-1β, and IL-6. Compared to the healthy uninfected cats, cats that succumbed to infection had the highest CD18 level, with an 11.6-fold increase, acutely ill cats had a moderate CD18 level, with a 9.0-fold increase, and healthy survivors had a 1.3-fold increase. These data collectively indicate that C. felis infection causes a robust systemic inflammatory response and that the immune response plays an important role in the pathogenesis of this disease (99).
In addition to a systemic inflammatory response, C. felis infections also cause local inflammatory responses in the affected organs, such as the spleen and the lungs. Interstitial pneumonia was common in the domestic cats with fatal cytauxzoonosis, suggesting that inflammation was likely a consequence of reaction to the proinflammatory mediators that were released by the schizont-laden macrophages. Immunohistochemistry analysis of C. felis-infected cat lungs confirmed that the expression of proinflammatory mediators, such as TNF-α, IL-1β, IL-6, inducible nitric oxide synthase, and major histocompatibility complex class II, was significantly increased compared to that in uninfected lungs. Furthermore, these proinflammatory mediators were distributed in almost all the infected lung tissues, particularly in the cytoplasm of schizont-laden macrophages (100). Taken together, the data show that C. felis infections activate the M1 macrophage response in infected macrophages, with secretion and expression of various proinflammatory cytokines and other molecules, which plays a pivotal role in the morbidity and mortality of cytauxzoonosis.
DIAGNOSIS
Microscopic Diagnosis
Diagnosis of C. felis infection is made by various methods (2–6). Microscopic detection of parasites in thin blood smears and/or fine-needle aspirates, with Giemsa or H&E staining, is the most common method (2–6). In the acute disease phase in domestic cats in an area of endemicity, diagnosis is based on a combination of clinical signs and the finding of intraerythrocytic piroplasms in a blood smear or the enlarged schizont-filled macrophages in fine-needle tissue aspirates. The latter may be taken from the spleen, liver, lymph nodes, or lungs. It is worth keeping in mind that the sensitivity of blood smears is lower than that of tissue aspirates and that intraerythrocytic piroplasms may appear days after the onset of clinical signs (2–6).
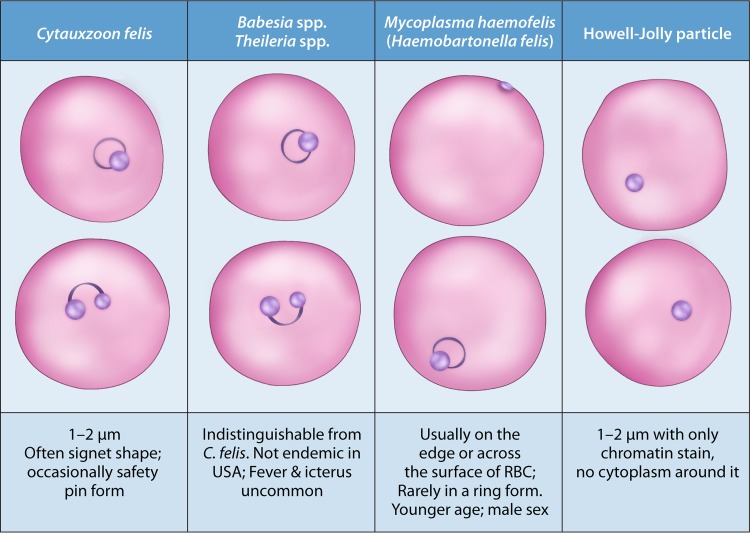
As just mentioned, intraerythrocytic piroplasms are widely used for diagnosis of cytauxzoonosis (2–6, 101, 102). C. felis piroplasms may present in several different shapes, with the characteristic one being the “signet ring” shape. This ring of 1 to 2 μm in diameter is characterized by a thick round nuclear chromatin at one point of the ring. Other forms include a bipolar oval “safety pin” form that has two nuclear areas on opposite sides and the anaplasmoid round “dots” form, which is less than 0.5 μm in diameter. Additional forms, such as small and linear, comma-shaped, and tetrad forms, may be seen (2–6). However, there are several biological and technical limitations to this method. First, levels of circulating piroplasms are usually low, and piroplasms may be absent in early infection, because clinical signs are caused by schizonts and often precede intraerythrocytic piroplasms detectable by microscopy. In general, the level of intraerythrocytic piroplasms continues to increase over the course of the disease. However, piroplasms are present in only about half of acute cases of feline cytauxzoonosis, from either natural or experimental infection (2–6). Therefore, a lack of piroplasms in a blood smear does not necessarily rule out the disease. In a suspected case, the preparation of daily blood smears is recommended. Second, these piroplasms are morphologically indistinguishable from those of Babesia spp. or Theileria spp. However, Babesia spp., such as Babesia felis and Babesia catis, are not endemic in the United States (2–6, 103). In addition, fever and icterus are uncommon in cats infected with Babesia spp. or Theileria spp. Third, the small and pleomorphic piroplasms may be mistaken as Howell-Jolly bodies, stain precipitate, water artifacts, or even Mycoplasma haemofelis (syn. Haemobartonella felis). Howell-Jolly bodies appear as dark round dots of various sizes. They do not have the typical characteristic ring forms of C. felis piroplasms, nor do they possess cytoplasm. Stain precipitate is usually observed between blood cells as well as overlying blood cells. M. haemofelis, a bacterium, is usually observed around the periphery of an infected erythrocyte and usually form dots, chains, and small rings, but it does not contain a nuclear area, and it is rarely located inside an erythrocyte in a ring form (104) (Fig. 5). A well-made thin blood smear should have blood cells spread out sufficiently to avoid overlying blood cells. Additionally, slides should be clean and thoroughly air-dried before starting the staining process to avoid the development of stain precipitate or water artifacts. Last but not least, intraerythrocytic piroplasms may occasionally be found in clinically healthy domestic cats, some of which are survivors of C. felis infection. The latter usually maintain low levels of parasitemia for years. Observation of intraerythrocytic piroplasms among these survivors does not necessarily confirm that clinical signs are due to cytauxzoonosis, because they may be infected with other disease-causing pathogens that may mistakenly be identified as fatal cytauxzoonosis in the presence of piroplasms (2–6).
FIG 5.
Microscopic differentiation of Cytauxzoon felis from other common intraerythrocytic pathogens or particles in a feline blood smear.
Clinical Diagnosis
Although there are no clinical signs specific for feline cytauxzoonosis, icterus, anorexia, and lethargy are the most common manifestations. In most cases, domestic cats died within less than 1 week of clinical sign onset, and these cats were usually in good nutritional condition (2–6). Domestic cats with outdoor exposure in areas of endemicity where tick infestation is common are more likely to be infected with C. felis. In addition, most cytauxzoonosis cases are reported in spring, summer, or early autumn, while the number of fatal cytauxzoonosis cases is significantly decreased in hot and dry periods of summer. Thus, an acute onset of icterus, anorexia, and lethargy in domestic cats in the epidemic areas should alert a veterinarian to possible cytauxzoonosis (2–6).
Complete blood counts and serum biochemical analyses, though nonspecific, are also useful for diagnosis of acute cytauxzoonosis. Abnormal hematologic parameters that are supportive of a diagnosis of cytauxzoonosis include the presence of disseminated intravascular coagulation (DIC) and pancytopenia, such as low white blood cell counts, thrombocytopenia, and lymphopenia (2–6, 92–94). Biochemical abnormalities in some fatal cytauxzoonosis cases include prolonged prothrombin time, low protein C activity, hyperglycemia, hyperbilirubinemia, hypoalbuminemia, hypocalcemia, and moderately increased alanine transaminase and aspartate aminotransferase levels (2–6, 92–94). In addition, hemolytic anemia is also a frequent finding in the acute phase. However, these abnormal hematologic parameters do not occur in all cases (2–6, 92–94).
Immunologic Diagnosis
Antibodies to Cytauxzoon spp. do exist in the sera from experimentally infected domestic cats. Due to the technical difficulty of generating purified antigens, no diagnostic kits for detection of antibodies to C. felis are commercially available (97, 105). On the other hand, an indirect fluorescent-antibody (IFA) test using serum isolated from a recovered domestic cat detects C. felis antigens in tissues of experimentally infected domestic cats, although its specificity has yet to be determined. However, this IFA test is not very useful for diagnosis of infected but clinically normal cats and the natural reservoir, bobcats, in which the titer of parasitic antigens is usually very low (105). Uilenberg et al. (54) used piroplasm antigens of C. felis to detect antibodies in infected domestic cats or bobcats. This method has limited usage in acute cases, because domestic cats have not yet developed antibodies or have only low titers of antibody before death.
Molecular Diagnosis
An in situ hybridization (ISH) assay was developed to examine various tissues of seven archived cases of feline cytauxzoonosis. The digoxigenin-labeled probe targeted the Babesia microti 16S rRNA-like gene, which is 91% identical to that of C. felis. ISH is 2 to 10 times more sensitive than H&E staining and has the advantage of application to archived tissue. However, ISH specificity is low, and there is variation among different tissues/cells (106). The low specificity in this case may be due to use of a nonspecific probe.
Another molecular technique is PCR, which has a high sensitivity and specificity (33, 68, 102, 107–111). So far, PCR target genes include the C. felis 18S rRNA gene (102), ITS-1 and ITS-2 (33, 68, 101, 108–110), and a multicopy mitochondrial gene (cox3) (111). PCR targeting the cox3 gene was more sensitive than that amplifying the 18S rRNA gene due to a higher copy number (111). PCR assays are generally 1,000-fold more sensitive than microscopic examination of a blood smear. PCR is especially applicable to confirm clinical suspicion of C. felis infection in the absence of microscopic piroplasms and to detect infections in domestic cat survivors of acute cytauxzoonosis. Further, PCR assay, especially along with DNA sequencing, can differentiate Cytauxzoon spp. from Theileria spp. and Babesia spp., even though they are morphologically indistinguishable (33, 43, 68, 102, 108–111). Drawbacks of PCR include being incapable of differentiating acute disease from chronic C. felis infection and requiring a time frame of several hours for onsite performance or days for samples remotely mailed out to a diagnostic laboratory. This delay of diagnosis is crucial, since veterinarians cannot afford to lose these crucial hours in the acute phase of the disease.
DISEASE TREATMENT AND PREVENTION
Historically, cytauxzoonosis has been considered a uniformly fatal infection in the domestic cat, with almost 100% mortality (2–6). However, recent studies have revealed domestic cat survivors of C. felis infection, although they are infrequent. Furthermore, the incidence of survival cases is increasing with recent advances in treatment and/or the possible existence of a less virulent strain of C. felis, as discussed below. There is currently no routine veterinary care or therapy for feline cytauxzoonosis. A variety of antibiotics, including clindamycin, enrofloxacin, and antiprotozoal agents, have been tested for treatment of domestic cats experimentally or naturally infected with C. felis, with various efficacies (2–6). The antiprotozoal agents parvaquone and buparvaquone, known to be effective in therapy of bovine theileriosis, are ineffective against experimental feline cytauxzoonosis (98). Diminazene aceturate has been used to treat babesiosis and other protozoal diseases in the United States, without approval of the U.S. Food and Drug Administration. Intramuscular (i.m.) injection of diminazene aceturate at a dose of 2 mg/kg successfully treated five of six naturally infected domestic cats in one trial (96). However, it failed to reduce parasite burdens in clinically healthy, chronically or naturally infected domestic cats in another trial, even at a higher dose of 4 mg/kg i.m. (112, 113). Moreover, these treated domestic cats experienced multiple adverse side effects, so this treatment is not recommended for chronic carrier domestic cats (112, 113).
Several cytauxzoonosis cases were successfully treated with imidocarb, an antiprotozoal drug used in the United States for treating canine babesiosis (14, 48, 114, 115). An open-label, randomized, prospective study was undertaken to evaluate the efficacy of treatment of feline cytauxzoonosis with imidocarb (3.5 mg/kg i.m.) or a combination of the antibiotics azithromycin (10 mg/kg p.o. q24h) and atovaquone (an antimalarial agent) (15 mg/kg p.o. q8h) (A&A). Thirty-two of 53 domestic cats (60.4%) treated with A&A survived. In contrast, only 25.9% of domestic cats (7/27 cats) receiving imidocarb survived. To date, A&A is considered the most effective treatment for acute infection (114). However, atovaquone is expensive and is difficult to access. Atovaquone targets C. felis cytochrome b, and its efficacy is highly variable (116, 117). A&A also fails to clear chronic parasitemia (114). Therefore, more studies are needed to verify the efficacy of A&A and to develop more effective and cheaper drugs.
In addition to antiprotozoal regimens, intensive supportive therapy is beneficial, although there is no obvious evidence to support such a claim. Because death appears soon after the onset of illness, immediate implementation of intensive supportive therapy is highly recommended. Infected domestic cats usually seem to be in pain, so an analgesic therapy, such as buprenorphine (0.01 mg/kg given intravenously [i.v.] or p.o. q8h), is recommended (2, 3). Antithrombotic therapies, such as heparin (200 U/kg given subcutaneously [s.c.] q8h), are administered to prevent DIC (2, 3). Other supportive therapies, including intravenous crystalloid fluid therapy, oxygen supplementation, plasma supplementation, blood cell transfusion, and antiemetic therapy (maropitant citrate; 1 mg/kg s.c. or p.o. q24h), may be needed based on the manifestations (dehydration, dyspnea, anemia, and vomiting) of the infected domestic cat (2–6).
Transmission of C. felis to domestic cats is usually by a tick vector and cannot occur from one domestic cat to another by direct contact, even when domestic cats are kept in a small cattery throughout the course of the disease, or via the placenta (57). Currently, no vaccines are commercially available, although a vaccine candidate, cf76, was recently identified by comparative apicomplexan genomics (52). Minimizing exposure to the tick vector is the only effective way to prevent transmission of C. felis to the domestic cat. Keeping cats indoors or implementing ectoparasite control on outdoor cats should be advocated in the regions of endemicity. As mentioned earlier, a collar containing 10.0% imidacloprid and 4.5% flumethrin (Seresto; Bayer Animal Health) was effective at preventing transmission of C. felis by preventing ticks from attaching to cats (73). Transmission of C. felis from a tick vector to a domestic cat requires more than 36 h (72). Therefore, it should be beneficial to develop an antiarachnid drug that kills ticks within a few hours of attachment.
In transfusion medicine, attention should be paid to detecting the presence of Cytauxzoon piroplasms in feline blood donors in the area of endemicity. Inoculation of piroplasms by blood transfusion may lead to recipient cats becoming chronic carriers and serving as a reservoir (118, 119).
MOLECULAR DIVERSITY OF THE PATHOGEN: CORRELATION BETWEEN PARASITES AND CLINICAL OUTCOMES
The increasing number of reports of domestic cat survival of C. felis infections suggests the existence of distinct genotypes of C. felis with different virulence potentials for domestic cats. To test this hypothesis, several genetic markers have been used to study the genetic variability among C. felis protozoan populations. The first marker is the 18S rRNA gene. However, C. felis 18S rRNA gene sequences from domestic cats that survived infections were almost identical to those from domestic cats that died of C. felis infections (14). The second one is ITS-1 and ITS-2. These genes evolve more quickly than the 18S rRNA gene and are more likely to reveal C. felis genotypic variability (120). Genetic analysis of the C. felis ITS regions showed that the ITS sequence variability is high, with many polymorphisms, and suggested that there is a diverse population of C. felis strains (33, 108–110). A combination of ITS-1 and ITS-2 was used to determine the genotypes. So far, more than 22 genotypes, named ITSa to ITSv, have been detected in C. felis strains isolated from various domestic cats and wild felids (Table 2). First, a study of the genetic variability of C. felis isolates from 88 infected domestic cats in Arkansas and Georgia showed that a strong association existed between the C. felis genotype and disease severity. Specifically, domestic cats infected with ITSa-type C. felis had a high survival rate. In contrast, genotype ITSb was associated with a high fatality rate. Moreover, 47 of 48 ITSa isolates were from Arkansas, and only one was from Georgia, whereas all ITSb isolates were from Georgia. These data demonstrated that ITS genotypes vary geographically (108). Another study of genetic variability of archived C. felis isolates from domestic cats in Georgia from 1995 to 2007 found 11 different genotypes among 48 C. felis samples, with the most common genotype, ITSo, detected in only one fatality sample (109). Collectively, these two studies support the existence of diverse C. felis populations and the coexistence of various genotypes in one area. Although ITSb was strongly associated with fatality, as mentioned earlier, an additional study including C. felis isolates from 25 infected but clinically normal domestic cats revealed three genotypes, i.e., ITSa, ITSb, and ITSc (33). Studies of the genetic variability of C. felis strains isolated from bobcats also yielded diverse genotypes, with some genotypes that were found only in bobcats (110) (Table 2). Taken together, the unique ITS phenotypes varied geographically and were suitable for studying genetic diversity among C. felis populations. An association might exist between ITS phenotypes and virulence potential, e.g., ITSb is associated with high mortality. However, extreme caution should be taken in interpretation of such data at this time, since this phenotype exists in clinically healthy infected domestic cats as well (Table 2) (33, 108–110, 114). Further studies with a bigger sample size are warranted. As far as markers other than ITS are concerned, very little is known. Whole-genome sequencing or identification of genetic variability in genes that encode key proteins playing roles in the pathogenicity of C. felis may be the best way to understand genetic variability and to investigate the pathogenicity and virulence of C. felis.
TABLE 2.
Information on Cytauxzoon felis genotypes and outcomes of infection
| Genotypei | GenBank accession no. |
No. of animals |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study 1a |
Study 2b |
Study 3c |
Study 4d |
Study 5e |
|||||||
| ITS-1 | ITS-2 | Domestic cats | Survivors | Archived domestic cats | Asymptomatic domestic cats | Bobcats | Bobcats | Pumas | Domestic cats | Survivors that received drug | |
| ITSa | EU450802 | EU450804 | 48 | 38 | 3 | 16 | 5 | 11 | 1 | 14 | 9f |
| ITSb | EU450802 | EU450805 | 21 | 4 | 8 | 8 | 0 | 1 | 0 | ||
| ITSc | EU450803 | EU450804 | 5 | 0 | 1 | 0 | 0 | 0 | 2 | 1g | |
| ITSd | GU581166 | EU450805 | 0 | 1 | 0 | 0 | |||||
| ITSe | GU581167 | GU581170 | 0 | 1 | 8 | 0 | |||||
| ITSf | EU450802 | FJ536421 | 2 | 0 | 1 | 0 | 1 | 0 | 0 | ||
| ITSg | GU581167 | GU581171 | 0 | 8 | 2 | 0 | |||||
| ITSh | EU450802 | GU581172 | 0 | 1 | 0 | 0 | |||||
| ITSi | EU450802 | FJ536419 | 4 | 2 | 3 | 0 | 3 | 2 | 0 | ||
| ITSj | FJ536425 | EU450804 | 0 | 2 | 0 | 0 | |||||
| ITSk | FJ536425 | GU581171 | 0 | 1 | 0 | 0 | |||||
| ITSl | GU581168 | GU581171 | 0 | 1 | 0 | 0 | |||||
| ITSm | GU581169 | GU581171 | 0 | 1 | 0 | 0 | |||||
| ITSn | EU450802 | EU450806 | 1 | 0 | 0 | 0 | |||||
| ITSo | EU450802 | FJ536418 | 1 | 0 | 27 | 0 | 0 | ||||
| ITSp | EU450802 | FJ536420 | 1 | 0 | 0 | ||||||
| ITSq | FJ536423 | FJ536418 | 1 | 0 | 0 | ||||||
| ITSr | FJ536424 | FJ536418 | 1 | 0 | 0 | ||||||
| ITSs | FJ536425 | FJ536418 | 1 | 0 | 0 | ||||||
| ITSt | FJ536426 | FJ536418 | 1 | 0 | 0 | 5 | 4h | ||||
| ITSu | EU450802 | FJ536422 | 1 | 0 | 0 | ||||||
| ISTv | FJ536425 | FJ536422 | 2 | 0 | 0 | 0 | |||||
Reference 108.
Reference 109.
Reference 33.
Reference 110.
Reference 114.
Seven of eight domestic cats that received atovaquone and azithromycin and two of six domestic cats that received imidocarb survived.
One of two domestic cats that received atovaquone and azithromycin survived.
All three domestic cats that received atovaquone and azithromycin and one of two domestic cats that received imidocarb survived.
Genotypes are designated based on the scheme from reference 110.
CONCLUDING REMARKS AND FUTURE PERSPECTIVES
Cytauxzoonosis is an emerging, hemoprotozoal disease of the domestic cat and wild felids and is caused by tick-borne Cytauxzoon spp. Over the past 40 years, knowledge about the disease and C. felis has grown considerably. The parasite is mainly endemic to North America and South America. Although infections by C. felis have historically been considered uniformly fatal in the domestic cat and clinically self-limited in wild felids, increasing evidence suggests that nonfatal infections occur in the domestic cat and that fatal cases occur in wild felids. It is plausible that the genetic differences among distinct C. felis populations may cause various pathogenicities. However, these warrant further investigations. Moreover, the mechanisms of disease pathogenesis, alternate tick vectors other than D. variabilis and A. americanum, and host immune responses are unclear. Currently, there are no commercially available diagnostic kits for C. felis infections, no vaccines against C. felis exist, and prevention is limited to tick control. In addition, C. felis has yet to be cultured continuously in vitro, which greatly hinders the study of the parasite's biology and vaccine development. Comprehensive and systematic research is urgently needed to illuminate the ecology, epidemiology, biology, and possible control of this devastating disease.
ACKNOWLEDGMENTS
This work was partially supported by the Fundamental Research Funds of the Chinese Academy of Agricultural Sciences (grant Y2016JC05) and the Agricultural Science and Technology Innovation Program (ASTIP) (grant CAAS-ASTIP-2014-LVRI-03).
We are very thankful to Mark Forman of the Ross University School of Veterinary Medicine for proofreading the manuscript.
C.Y. and X.-Q.Z. conceived the review. C.Y. and J.-L.W. wrote the first draft. T.-T.L. and G.-H.L. helped in the preparation of the manuscript. G.-H.L. performed phylogenetic tree analysis. All authors read and approved the final version.
Biographies

Jin-Lei Wang received a Bachelor of Veterinary Science (B.V.Sc.) degree from Shangdong Agricultural University, Taian, China, and a Ph.D. in parasitology from the Graduate School of Chinese Academy of Agricultural Sciences, Beijing, China. Dr. Wang is now a research scientist at the Department of Parasitology, Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences, China. He is interested in epidemiology, molecular phylogeny, and gene functions of parasitic protozoa as well as control strategies for parasitic infections in animals and humans.

Ting-Ting Li received a Bachelor of Veterinary Science (B.V.Sc.) degree from Shangdong Agricultural University, Taian, China, and a Master of Veterinary Science (M.V.Sc.) degree from Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Lanzhou, Gansu Province, China. She is most interested in epidemiology and control of parasitic infections and the innate immunity of hosts to parasitic infections.

Guo-Hua Liu received a Master of Veterinary Science (M.V.Sc.) degree and a Ph.D. in parasitology from Hunan Agricultural University, Changsha, China, and had his postdoctoral training in molecular parasitology at the Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences, China. Dr. Liu is currently a Professor of Parasitology at the Department of Parasitology, College of Veterinary Medicine, Hunan Agricultural University, China. His current research interests focus on the genetics, genomics, transcriptomics, systematics, epidemiology, and control of parasitic infections. He was awarded the Odile Bain Memorial Prize in 2016. He has published over 50 original papers in well-regarded international journals. His research has been well supported by research grants from the National Natural Science Foundation of China, the China Postdoctoral Science Foundation, and Hunan Agricultural University.

Xing-Quan Zhu obtained a Bachelor of Veterinary Science (B.V.Sc.) degree from the Sichuan Institute of Animal Sciences and Veterinary Medicine, a Master of Veterinary Science (M.V.Sc.) degree from the Graduate School of the Chinese Academy of Agricultural Sciences, China, and a Ph.D. in parasitology from the Department of Veterinary Science, The University of Melbourne, Australia. He acquired postdoctoral training in molecular parasitology at the Department of Veterinary Science, The University of Melbourne. He is currently Head and Professor at the Department of Parasitology, Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences, China. His current research interests focus on the genetics, genomics, transcriptomics, proteomics, and metabolomics of parasites; parasite-host interactions and molecular vaccines; and the epidemiology and control of parasitic infections. He serves as a subject editor for the journal Parasites & Vectors and on the editorial boards of several international parasitology journals. He has published more than 300 papers in well-regarded international journals.

Chaoqun Yao received Bachelor of Medicine and Master of Medicine degrees from the Huazhong University of Science and Technology in Wuhan, China, and a Ph.D. in veterinary parasitology from the University of Georgia in Athens, GA. He was a research faculty member at the University of Iowa, with a concomitant appointment as a Research Health Science Specialist at the Iowa City VA Medical Center, following postdoctoral fellowships at Washington State University and the University of Iowa. He held the position of tenure-track Assistant Professor at the University of Wyoming and was Head of the Parasitology Lab of the Wyoming State Veterinary Laboratory. He was an adjunct professor of the University of Washington at the same time. He is currently at the Ross University School of Veterinary Medicine. Dr. Yao's research interests since graduate school have been the molecular pathogenesis and host-parasite interactions of parasites of medical/veterinary importance.
REFERENCES
- 1.Wagner JE. 1976. A fatal cytauxzoonosis-like disease in cats. J Am Vet Med Assoc 168:585–588. [PubMed] [Google Scholar]
- 2.Sherrill MK, Cohn LA. 2015. Cytauxzoonosis: diagnosis and treatment of an emerging disease. J Feline Med Surg 17:940–948. doi: 10.1177/1098612X15610681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lloret A, Addie DD, Boucraut-Baralon C, Egberink H, Frymus T, Gruffydd-Jones T, Hartmann K, Horzinek MC, Hosie MJ, Lutz H, Marsilio F, Pennisi MG, Radford AD, Thiry E, Truyen U, Möstl K, European Advisory Board on Cat Diseases. 2015. Cytauxzoonosis in cats: ABCD guidelines on prevention and management. J Feline Med Surg 17:637–641. doi: 10.1177/1098612X15589878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greene CE, Meinkoth J, Kocan AA. 2006. Cytauxzoonosis, p 722–733. In Greene CE. (ed), Infectious diseases of the dog and cat, 3rd ed Saunders Elsevier, St. Louis, MO. [Google Scholar]
- 5.Meinkoth JH, Kocan AA. 2005. Feline cytauxzoonosis. Vet Clin North Am Small Anim Pract 35:89–101. doi: 10.1016/j.cvsm.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Holman PJ, Snowden KF. 2009. Canine hepatozoonosis and babesiosis, and feline cytauxzoonosis. Vet Clin North Am Small Anim Pract 39:1035–1053. doi: 10.1016/j.cvsm.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Allsopp MT, Cavalier-Smith T, De Waal DT, Allsopp BA. 1994. Phylogeny and evolution of the piroplasms. Parasitology 108:147–152. doi: 10.1017/S0031182000068232. [DOI] [PubMed] [Google Scholar]
- 8.Criado-Fornelio A, Gónzalez-del-Río MA, Buling-Saraña A, Barba-Carretero JC. 2004. The “expanding universe” of piroplasms. Vet Parasitol 119:337–345. doi: 10.1016/j.vetpar.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 9.Glenn BL, Rolley RE, Kocan AA. 1982. Cytauxzoon-like piroplasms in erythrocytes of wild-trapped bobcats in Oklahoma. J Am Vet Med Assoc 181:1251–1253. [PubMed] [Google Scholar]
- 10.Glenn BL, Kocan AA, Blouin EF. 1983. Cytauxzoonosis in bobcats. J Am Vet Med Assoc 183:1155–1158. [PubMed] [Google Scholar]
- 11.Hoover JP, Walker DB, Hedges JD. 1994. Cytauxzoonosis in cats: eight cases (1985–1992). J Am Vet Med Assoc 203:455–460. [PubMed] [Google Scholar]
- 12.Birkenheuer AJ, Le JA, Valenzisi AM, Tucker MD, Levy MG, Breitschwerdt EB. 2006. Cytauxzoon felis infection in cats in the mid-Atlantic states: 34 cases (1998–2004). J Am Vet Med Assoc 228:568–571. doi: 10.2460/javma.228.4.568. [DOI] [PubMed] [Google Scholar]
- 13.Hauck WN, Snider TG III, Lawrence JE. 1982. Cytauxzoonosis in a native Louisiana cat. J Am Vet Med Assoc 180:1472–1474. [PubMed] [Google Scholar]
- 14.Meinkoth J, Kocan AA, Whitworth L, Murphy G, Fox JC, Woods JP. 2000. Cats surviving natural infection with Cytauxzoon felis: 18 cases (1997–1998). J Vet Intern Med 14:521–525. [DOI] [PubMed] [Google Scholar]
- 15.Meier HT, Moore LE. 2000. Feline cytauxzoonosis: a case report and literature review. J Am Anim Hosp Assoc 36:493–496. doi: 10.5326/15473317-36-6-493. [DOI] [PubMed] [Google Scholar]
- 16.Miller J, Davis CD. 2013. Increasing frequency of feline cytauxzoonosis cases diagnosed in western Kentucky from 2001 to 2011. Vet Parasitol 198:205–208. doi: 10.1016/j.vetpar.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peixoto PV, Soares CO, Scofield A, Santiago CD, França TN, Barros SS. 2007. Fatal cytauxzoonosis in captive-reared lions in Brazil. Vet Parasitol 145:383–387. doi: 10.1016/j.vetpar.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 18.Maia LM, Cerqueira Ade M, de Barros Macieira D, de Souza AM, Moreira NS, da Silva AV, Messick JB, Ferreira RF, Almosny NR. 2013. Cytauxzoon felis and ‘Candidatus Mycoplasma haemominutum' coinfection in a Brazilian domestic cat (Felis catus). Rev Bras Parasitol Vet 22:289–291. doi: 10.1590/S1984-29612013000200049. [DOI] [PubMed] [Google Scholar]
- 19.Ketz-Riley CJ, Reichard MV, Van den Bussche RA, Hoover JP, Meinkoth J, Kocan AA. 2003. An intraerythrocytic small piroplasm in wild-caught Pallas's cats (Otocolobus manul) from Mongolia. J Wildl Dis 39:424–430. doi: 10.7589/0090-3558-39.2.424. [DOI] [PubMed] [Google Scholar]
- 20.Reichard MV, Van Den Bussche RA, Meinkoth JH, Hoover JP, Kocan AA. 2005. A new species of Cytauxzoon from Pallas' cats caught in Mongolia and comments on the systematics and taxonomy of piroplasmids. J Parasitol 9:420–426. doi: 10.1645/GE-384R. [DOI] [PubMed] [Google Scholar]
- 21.Luaces I, Aguirre E, García-Montijano M, Velarde J, Tesouro MA, Sánchez C, Galka M, Fernández P, Sainz A. 2005. First report of an intraerythrocytic small piroplasm in wild Iberian lynx (Lynx pardinus). J Wildl Dis 41:810–815. doi: 10.7589/0090-3558-41.4.810. [DOI] [PubMed] [Google Scholar]
- 22.Carli E, Trotta M, Chinelli R, Drigo M, Sinigoi L, Tosolini P, Furlanello T, Millotti A, Caldin M, Solano-Gallego L. 2012. Cytauxzoon sp. infection in the first endemic focus described in domestic cats in Europe. Vet Parasitol 183:343–352. doi: 10.1016/j.vetpar.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 23.Millán J, Naranjo V, Rodríguez A, de la Lastra JM, Mangold AJ, de la Fuente J. 2007. Prevalence of infection and 18S rRNA gene sequences of Cytauxzoon species in Iberian lynx (Lynx pardinus) in Spain. Parasitology 134:995–1001. doi: 10.1017/S003118200700248X. [DOI] [PubMed] [Google Scholar]
- 24.Alho AM, Silva J, Fonseca MJ, Santos F, Nunes C, de Carvalho LM, Rodrigues M, Cardoso L. 2016. First report of Cytauxzoon sp. infection in a domestic cat from Portugal. Parasit Vectors 9:220. doi: 10.1186/s13071-016-1506-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rassouli M, Sabouri S, Goudarzi A, Parsa M. 2015. Cytauxzoon felis in a stray cat in Iran. Comp Clin Pathol 24:75–77. doi: 10.1007/s00580-013-1858-6. [DOI] [Google Scholar]
- 26.Zaeemi M, Razmi GR, Khoshnegah J. 2015. The first detection of Cytauxzoon felis in a wild cat (Felis silvestris) in Iran. Comp Clin Pathol 24:181–184. doi: 10.1007/s00580-014-1898-6. [DOI] [Google Scholar]
- 27.Kelly P, Marabini L, Dutlow K, Zhang J, Loftis A, Wang C. 2014. Molecular detection of tick-borne pathogens in captive wild felids, Zimbabwe. Parasit Vectors 7:514. doi: 10.1186/s13071-014-0514-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leclaire S, Menard S, Berry A. 2015. Molecular characterization of Babesia and Cytauxzoon species in wild South-African meerkats. Parasitology 142:543–548. doi: 10.1017/S0031182014001504. [DOI] [PubMed] [Google Scholar]
- 29.Blouin EF, Kocan AA, Glenn BL, Kocan KM, Hair JA. 1984. Transmission of Cytauxzoon felis Kier, 1979 from bobcats, Felis rufus (Schreber), to domestic cats by Dermacentor variabilis (Say). J Wildl Dis 20:241–242. doi: 10.7589/0090-3558-20.3.241. [DOI] [PubMed] [Google Scholar]
- 30.Blouin EF, Kocan AA, Kocan KM, Hair J. 1987. Evidence of a limited schizogonous cycle for Cytauxzoon felis in bobcats following exposure to infected ticks. J Wildl Dis 23:499–501. doi: 10.7589/0090-3558-23.3.499. [DOI] [PubMed] [Google Scholar]
- 31.Glenn BL, Stair EL. 1984. Cytauxzoonosis in domestic cats: report of two cases in Oklahoma, with a review and discussion of the disease. J Am Vet Med Assoc 184:822–825. [PubMed] [Google Scholar]
- 32.Jackson CB, Fisher T. 2006. Fatal cytauxzoonosis in a Kentucky cat (Felis domesticus). Vet Parasitol 139:192–195. doi: 10.1016/j.vetpar.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 33.Brown HM, Lockhart JM, Latimer KS, Peterson DS. 2010. Identification and genetic characterization of Cytauxzoon felis in asymptomatic domestic cats and bobcats. Vet Parasitol 172:311–316. doi: 10.1016/j.vetpar.2010.04.041. [DOI] [PubMed] [Google Scholar]
- 34.Garner MM, Lung NP, Citino S, Greiner EC, Harvey JW, Homer BL. 1996. Fatal cytauxzoonosis in a captive-reared white tiger (Panthera tigris). Vet Pathol 33:82–86. doi: 10.1177/030098589603300111. [DOI] [PubMed] [Google Scholar]
- 35.Jakob W, Wesemeier HH. 1996. A fatal infection in a Bengal tiger resembling cytauxzoonosis in domestic cats. J Comp Pathol 114:439–444. doi: 10.1016/S0021-9975(96)80018-1. [DOI] [PubMed] [Google Scholar]
- 36.Nietfeld JC, Pollock C. 2002. Fatal cytauxzoonosis in a free-ranging bobcat (Lynx rufus). J Wildl Dis 38:607–610. doi: 10.7589/0090-3558-38.3.607. [DOI] [PubMed] [Google Scholar]
- 37.Rizzi TE, Reichard MV, Cohn LA, Birkenheuer AJ, Taylor JD, Meinkoth JH. 2015. Prevalence of Cytauxzoon felis infection in healthy cats from enzootic areas in Arkansas, Missouri, and Oklahoma. Parasit Vectors 8:13. doi: 10.1186/s13071-014-0618-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simpson CF, Harvey JW, Carlisle JW. 1985. Ultrastructure of the intraerythrocytic stage of Cytauxzoon felis. Am J Vet Res 46:1178–1180. [PubMed] [Google Scholar]
- 39.Criado-Fornelio A, Martinez-Marcos A, Buling-Saraña A, Barba-Carretero JC. 2003. Molecular studies on Babesia, Theileria and Hepatozoon in southern Europe. Part II. Phylogenetic analysis and evolutionary history. Vet Parasitol 114:173–194. [DOI] [PubMed] [Google Scholar]
- 40.Lack JB, Reichard MV, Van Den Bussche RA. 2012. Phylogeny and evolution of the Piroplasmida as inferred from 18S rRNA sequences. Int J Parasitol 42:353–363. doi: 10.1016/j.ijpara.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Sivakumar T, Hayashida K, Sugimoto C, Yokoyama N. 2014. Evolution and genetic diversity of Theileria. Infect Genet Evol 27:250–263. doi: 10.1016/j.meegid.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 42.Allsopp MT, Allsopp BA. 2006. Molecular sequence evidence for the reclassification of some Babesia species. Ann N Y Acad Sci 1081:509–517. doi: 10.1196/annals.1373.076. [DOI] [PubMed] [Google Scholar]
- 43.Schreeg ME, Marr HS, Tarigo JL, Cohn LA, Bird DM, Scholl EH, Levy MG, Wiegmann BM, Birkenheuer AJ. 2016. Mitochondrial genome sequences and structures aid in the resolution of Piroplasmida phylogeny. PLoS One 11:e0165702. doi: 10.1371/journal.pone.0165702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kocan AA, Kocan KM, Blouin EF, Mukolwe SW. 1992. A redescription of schizogony of Cytauxzoon felis in the domestic cat. Ann N Y Acad Sci 653:161–167. doi: 10.1111/j.1749-6632.1992.tb19639.x. [DOI] [PubMed] [Google Scholar]
- 45.Spooner RL, Innes EA, Glass EJ, Millar P, Brown CG. 1988. Bovine mononuclear cell lines transformed by Theileria parva or Theileria annulata express different subpopulation markers. Parasite Immunol 10:619–629. doi: 10.1111/j.1365-3024.1988.tb00249.x. [DOI] [PubMed] [Google Scholar]
- 46.Spooner RL, Innes EA, Glass EJ, Brown CG. 1989. Theileria annulata and T. parva infect and transform different bovine mononuclear cells. Immunology 66:284–288. [PMC free article] [PubMed] [Google Scholar]
- 47.Criado-Fornelio A, Buling A, Pingret JL, Etievant M, Boucraut-Baralon C, Alongi A, Agnone A, Torina A. 2009. Hemoprotozoa of domestic animals in France: prevalence and molecular characterization. Vet Parasitol 159:73–76. doi: 10.1016/j.vetpar.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 48.Carli E, Trotta M, Bianchi E, Furlanello T, Caldin M, Pietrobelli M, Solano-Gallego L. 2014. Cytauxzoon sp. infection in two free ranging young cats: clinicopathological findings, therapy and follow up. Turkiye Parazitol Derg 38:185–189. doi: 10.5152/tpd.2014.3540. [DOI] [PubMed] [Google Scholar]
- 49.Millán J, Candela MG, Palomares F, Cubero MJ, Rodríguez A, Barral M, de la Fuente J, Almería S, León-Vizcaíno L. 2009. Disease threats to the endangered Iberian lynx (Lynx pardinus). Vet J 182:114–124. doi: 10.1016/j.tvjl.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meli ML, Cattori V, Martínez F, López G, Vargas A, Simón MA, Zorrilla I, Muñoz A, Palomares F, López-Bao JV, Pastor J, Tandon R, Willi B, Hofmann-Lehmann R, Lutz H. 2009. Feline leukemia virus and other pathogens as important threats to the survival of the critically endangered Iberian lynx (Lynx pardinus). PLoS One 4:e4744. doi: 10.1371/journal.pone.0004744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gallusová M, Jirsová D, Mihalca AD, Gherman CM, D'Amico G, Qablan MA, Modrý D. 2016. Cytauxzoon infections in wild felids from Carpathian-Danubian-Pontic space: further evidence for a different Cytauxzoon species in European felids. J Parasitol 102:377–380. doi: 10.1645/15-881. [DOI] [PubMed] [Google Scholar]
- 52.Tarigo JL, Scholl EH, McK Bird D, Brown CC, Cohn LA, Dean GA, Levy MG, Doolan DL, Trieu A, Nordone SK, Felgner PL, Vigil A, Birkenheuer AJ. 2013. A novel candidate vaccine for cytauxzoonosis inferred from comparative apicomplexan genomics. PLoS One 8:e71233. doi: 10.1371/journal.pone.0071233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kier AB, Wightman SR, Wagner JE. 1982. Interspecies transmission of Cytauxzoon felis. Am J Vet Res 43:102–105. [PubMed] [Google Scholar]
- 54.Uilenberg G, Franssen FF, Perié NM. 1987. Relationships between Cytauxzoon felis and African piroplasmids. Vet Parasitol 26:21–28. doi: 10.1016/0304-4017(87)90073-2. [DOI] [PubMed] [Google Scholar]
- 55.Butt MT, Bowman D, Barr MC, Roelke ME. 1991. Iatrogenic transmission of Cytauxzoon felis from a Florida panther (Felix concolor coryi) to a domestic cat. J Wildl Dis 27:342–347. doi: 10.7589/0090-3558-27.2.342. [DOI] [PubMed] [Google Scholar]
- 56.Joyner PH, Reichard MV, Meinkoth JH, Milne VE, Confer AW, Kocan AA, Hoover JP. 2007. Experimental infection of domestic cats (Felis domesticus) with Cytauxzoon manul from Pallas' cats (Otocolobus manul). Vet Parasitol 146:302–306. doi: 10.1016/j.vetpar.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 57.Lewis KM, Cohn LA, Birkenheuer AJ. 2012. Lack of evidence for perinatal transmission of Cytauxzoon felis in domestic cats. Vet Parasitol 188:172–174. doi: 10.1016/j.vetpar.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 58.Rotstein DS, Taylor SK, Harvey JW, Bean J. 1999. Hematologic effects of cytauxzoonosis in Florida panthers and Texas cougars in Florida. J Wildl Dis 35:613–617. doi: 10.7589/0090-3558-35.3.613. [DOI] [PubMed] [Google Scholar]
- 59.Lewis KM, Cohn LA, Downey ME, Whitney MS, Birkenheuer AJ. 2012. Evaluation of Cytauxzoon felis infection status in captive-born wild felids housed in an area endemic for the pathogen. J Am Vet Med Assoc 241:1088–1092. doi: 10.2460/javma.241.8.1088. [DOI] [PubMed] [Google Scholar]
- 60.Yabsley MJ, Murphy SM, Cunningham MW. 2006. Molecular detection and characterization of Cytauxzoon felis and a Babesia species in cougars from Florida. J Wildl Dis 42:366–374. doi: 10.7589/0090-3558-42.2.366. [DOI] [PubMed] [Google Scholar]
- 61.Filoni C, Catão-Dias JL, Cattori V, Willi B, Meli ML, Corrêa SH, Marques MC, Adania CH, Silva JC, Marvulo MF, Ferreira Neto JS, Durigon EL, de Carvalho VM, Coutinho SD, Lutz H, Hofmann-Lehmann R. 2012. Surveillance using serological and molecular methods for the detection of infectious agents in captive Brazilian neotropic and exotic felids. J Vet Diagn Invest 24:166–173. doi: 10.1177/1040638711407684. [DOI] [PubMed] [Google Scholar]
- 62.André MR, Adania CH, Machado RZ, Allegretti SM, Felippe PA, Silva KF, Nakaghi AC, Dagnone AS. 2009. Molecular detection of Cytauxzoon spp. in asymptomatic Brazilian wild captive felids. J Wildl Dis 45:234–237. doi: 10.7589/0090-3558-45.1.234. [DOI] [PubMed] [Google Scholar]
- 63.Furtado MM, Taniwaki SA, Metzger B, Dos Santos Paduan K, O'Dwyer HL, de Almeida Jácomo AT, Porfírio GE, Silveira L, Sollmann R, Tôrres NM, Ferreira Neto JS. 2017. Is the free-ranging jaguar (Panthera onca) a reservoir for Cytauxzoon felis in Brazil? Ticks Tick Borne Dis 8:470–476. doi: 10.1016/j.ttbdis.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 64.Barandika JF, Espí A, Oporto B, del Cerro A, Barral M, Povedano I, García-Péreza L, Hurtado A. 2016. Occurrence and genetic diversity of piroplasms and other apicomplexa in wild carnivores. Parasitol Open 2:e6. doi: 10.1017/pao.2016.4. [DOI] [PubMed] [Google Scholar]
- 65.Veronesi F, Ravagnan S, Cerquetella M, Carli E, Olivieri E, Santoro A, Pesaro S, Berardi S, Rossi G, Ragni B, Beraldo P, Capelli G. 2016. First detection of Cytauxzoon spp. infection in European wildcats (Felis silvestris silvestris) of Italy. Ticks Tick Borne Dis 7:853–858. doi: 10.1016/j.ttbdis.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 66.Araya-Anchetta A, Busch JD, Scoles GA, Wagner DM. 2015. Thirty years of tick population genetics: a comprehensive review. Infect Genet Evol 29:164–179. doi: 10.1016/j.meegid.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 67.Shock BC, Moncayo A, Cohen S, Mitchell EA, Williamson PC, Lopez G, Garrison LE, Yabsley MJ. 2014. Diversity of piroplasms detected in blood-fed and questing ticks from several states in the United States. Ticks Tick Borne Dis 25:373–380. doi: 10.1016/j.ttbdis.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 68.Bondy PJ Jr, Cohn LA, Tyler JW, Marsh AE. 2005. Polymerase chain reaction detection of Cytauxzoon felis from field-collected ticks and sequence analysis of the small subunit and internal transcribed spacer 1 region of the ribosomal RNA gene. J Parasitol 91:458–461. doi: 10.1645/GE-374R. [DOI] [PubMed] [Google Scholar]
- 69.Reichard MV, Meinkoth JH, Edwards AC, Snider TA, Kocan KM, Blouin EF, Little SE. 2009. Transmission of Cytauxzoon felis to a domestic cat by Amblyomma americanum. Vet Parasitol 161:110–115. doi: 10.1016/j.vetpar.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 70.Reichard MV, Edwards AC, Meinkoth JH, Snider TA, Meinkoth KR, Heinz RE, Little SE. 2010. Confirmation of Amblyomma americanum (Acari: Ixodidae) as a vector for Cytauxzoon felis (Piroplasmorida: Theileriidae) to domestic cats. J Med Entomol 47:890–896. [DOI] [PubMed] [Google Scholar]
- 71.Shock BC, Murphy SM, Patton LL, Shock PM, Olfenbuttel C, Beringer J, Prange S, Grove DM, Peek M, Butfiloski JW, Hughes DW, Lockhart JM, Bevins SN, VandeWoude S, Crooks KR, Nettles VF, Brown HM, Peterson DS, Yabsley MJ. 2011. Distribution and prevalence of Cytauxzoon felis in bobcats (Lynx rufus), the natural reservoir, and other wild felids in thirteen states. Vet Parasitol 175:325–330. doi: 10.1016/j.vetpar.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 72.Thomas JE, Ohmes CM, Payton ME, Hostetler JA, Reichard MV. 2017. Minimum transmission time of Cytauxzoon felis by Amblyomma americanum to domestic cats in relation to duration of infestation, and investigation of ingestion of infected ticks as a potential route of transmission. J Feline Med Surg 1:1098612X17691172. doi: 10.1177/1098612X17691172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reichard MV, Thomas JE, Arther RG, Hostetler JA, Raetzel KL, Meinkoth JH, Little SE. 2013. Efficacy of an imidacloprid 10%/flumethrin 4.5% collar (Seresto®, Bayer) for preventing the transmission of Cytauxzoon felis to domestic cats by Amblyomma americanum. Parasitol Res 112(Suppl 1):S11–S20. doi: 10.1007/s00436-013-3277-7. [DOI] [PubMed] [Google Scholar]
- 74.Schuster FL. 2002. Cultivation of Babesia and Babesia-like blood parasites: agents of an emerging zoonotic disease. Clin Microbiol Rev 15:365–373. doi: 10.1128/CMR.15.3.365-373.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Homer MJ, Aguilar-Delfin I, Telford SR III, Krause PJ, Persing DH. 2000. Babesiosis. Clin Microbiol Rev 13:451–469. doi: 10.1128/CMR.13.3.451-469.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Uilenberg G. 2006. Babesia—a historical overview. Vet Parasitol 138:3–10. doi: 10.1016/j.vetpar.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 77.Schnittger L, Rodriguez AE, Florin-Christensen M, Morrison DA. 2012. Babesia: a world emerging. Infect Genet Evol 12:1788–1809. doi: 10.1016/j.meegid.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 78.Bishop R, Musoke A, Morzaria S, Gardner M, Nene V. 2004. Theileria: intracellular protozoan parasites of wild and domestic ruminants transmitted by ixodid ticks. Parasitology 129(Suppl):S271–S283. doi: 10.1017/S0031182003004748. [DOI] [PubMed] [Google Scholar]
- 79.Varshney JP, Deshmukh VV, Chaudhary PS. 2009. Fatal cytauxzoonosis in a kitten. Intas Polivet 10:392–393. [Google Scholar]
- 80.André MR, Herrera HM, Fernandes SJ, de Sousa KC, Gonçalves LR, Domingos IH, de Macedo GC, Machado RZ. 2015. Tick-borne agents in domesticated and stray cats from the city of Campo Grande, state of Mato Grosso do Sul, midwestern Brazil. Ticks Tick Borne Dis 6:779–786. doi: 10.1016/j.ttbdis.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 81.Reichard MV, Baum KA, Cadenhead SC, Snider TA. 2008. Temporal occurrence and environmental risk factors associated with cytauxzoonosis in domestic cats. Vet Parasitol 152:314–320. doi: 10.1016/j.vetpar.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 82.Raghavan RK, Almes K, Goodin DG, Harrington JA Jr, Stackhouse PW Jr. 2014. Spatially heterogeneous land cover/land use and climatic risk factors of tick-borne feline cytauxzoonosis. Vector Borne Zoonotic Dis 14:486–495. doi: 10.1089/vbz.2013.1496. [DOI] [PubMed] [Google Scholar]
- 83.Mueller EK, Baum KA, Papeş M, Cohn LA, Cowell AK, Reichard MV. 2013. Potential ecological distribution of Cytauxzoon felis in domestic cats in Oklahoma, Missouri, and Arkansas. Vet Parasitol 192:104–110. doi: 10.1016/j.vetpar.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 84.Walker DB, Cowell RL. 1995. Survival of a domestic cat with naturally acquired cytauxzoonosis. J Am Vet Med Assoc 206:1363–1365. [PubMed] [Google Scholar]
- 85.Haber MD, Tucker MD, Marr HS, Levy JK, Burgess J, Lappin MR, Birkenheuer AJ. 2007. The detection of Cytauxzoon felis in apparently healthy free-roaming cats in the USA. Vet Parasitol 146:316–320. doi: 10.1016/j.vetpar.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 86.Wightman SR, Kier AB, Wagner JE. 1977. Feline cytauxzoonosis: clinical features of a newly described blood parasite disease. Feline Pract 7:23–26. [Google Scholar]
- 87.Kier AB, Wagner JE, Kinden DA. 1987. The pathology of experimental cytauxzoonosis. J Comp Pathol 97:415–432. doi: 10.1016/0021-9975(87)90020-X. [DOI] [PubMed] [Google Scholar]
- 88.Harvey JW, Dunbar MR, Norton TM, Yabsley MJ. 2007. Laboratory findings in acute Cytauxzoon felis infection in cougars (Puma concolor cougar) in Florida. J Zoo Wildl Med 38:285–291. doi: 10.1638/1042-7260(2007)038[0285:LFIACF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 89.Snider TA, Confer AW, Payton ME. 2010. Pulmonary histopathology of Cytauxzoon felis infections in the cat. Vet Pathol 47:698–702. doi: 10.1177/0300985810364527. [DOI] [PubMed] [Google Scholar]
- 90.Clarke LL, Rissi DR. 2015. Neuropathology of natural Cytauxzoon felis infection in domestic cats. Vet Pathol 52:1167–1171. doi: 10.1177/0300985814564986. [DOI] [PubMed] [Google Scholar]
- 91.Ferris DH. 1979. A progress report on the status of a new disease of American cats: cytauxzoonosis. Comp Immunol Microbiol Infect Dis 1:269–276. doi: 10.1016/0147-9571(79)90028-6. [DOI] [PubMed] [Google Scholar]
- 92.Kocan AA, Blouin EF, Glenn BL. 1985. Hematologic and serum chemical values for free-ranging bobcats, Felis rufus (Schreber), with reference to animals with natural infections of Cytauxzoon felis Kier, 1979. J Wildl Dis 21:190–192. doi: 10.7589/0090-3558-21.2.190. [DOI] [PubMed] [Google Scholar]
- 93.Franks PT, Harvey JW, Shields RP, Lawman MJP. 1988. Hematologic findings in experimental feline cytauxzoonosis. J Am Anim Hosp Assoc 24:395–401. [Google Scholar]
- 94.Conner BJ, Hanel RM, Brooks MB, Cohn LA, Birkenheuer AJ. 2015. Coagulation abnormalities in 5 cats with naturally occurring cytauxzoonosis. J Vet Emerg Crit Care (San Antonio) 25:538–545. doi: 10.1111/vec.12326. [DOI] [PubMed] [Google Scholar]
- 95.Aschenbroich SA, Rech RR, Sousa RS, Carmichael KP, Sakamoto K. 2012. Pathology in practice. Cytauxzoon felis infection. J Am Vet Med Assoc 240:159–161. doi: 10.2460/javma.240.2.159. [DOI] [PubMed] [Google Scholar]
- 96.Greene CE, Latimer K, Hopper E, Shoeffler G, Lower K, Cullens F. 1999. Administration of diminazene aceturate or imidocarb dipropionate for treatment of cytauxzoonosis in cats. J Am Vet Med Assoc 215:497–500. [PubMed] [Google Scholar]
- 97.Cowell RL, Fox JC, Panciera RJ, Tyler RD. 1988. Detection of anticytauxzoon antibodies in cats infected with a Cytauxzoon organism from bobcats. Vet Parasitol 28:43–52. doi: 10.1016/0304-4017(88)90017-9. [DOI] [PubMed] [Google Scholar]
- 98.Motzel SL, Wagner JE. 1990. Treatment of experimentally induced cytauxzoonosis in cats with parvaquone and buparvaquone. Vet Parasitol 35:131–138. doi: 10.1016/0304-4017(90)90122-R. [DOI] [PubMed] [Google Scholar]
- 99.Frontera-Acevedo K, Balsone NM, Dugan MA, Makemson CR, Sellers LB, Brown HM, Peterson DS, Creevy KE, Garner BC, Sakamoto K. 2013. Systemic immune responses in Cytauxzoon felis-infected domestic cats. Am J Vet Res 74:901–909. doi: 10.2460/ajvr.74.6.901. [DOI] [PubMed] [Google Scholar]
- 100.Frontera-Acevedo K, Sakamoto K. 2015. Local pulmonary immune responses in domestic cats naturally infected with Cytauxzoon felis. Vet Immunol Immunopathol 163:1–7. doi: 10.1016/j.vetimm.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 101.Weisman JL, Woldemeskel M, Smith KD, Merrill A, Miller D. 2007. Blood smear from a pregnant cat that died shortly after partial abortion. Vet Clin Pathol 36:209–211. doi: 10.1111/j.1939-165X.2007.tb00212.x. [DOI] [PubMed] [Google Scholar]
- 102.Birkenheuer AJ, Marr H, Alleman AR, Levy MG, Breitschwerdt EB. 2006. Development and evaluation of a PCR assay for the detection of Cytauxzoon felis DNA in feline blood samples. Vet Parasitol 137:144–149. doi: 10.1016/j.vetpar.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 103.Ayoob AL, Prittie J, Hackner SG. 2010. Feline babesiosis. J Vet Emerg Crit Care (San Antonio) 20:90–97. doi: 10.1111/j.1476-4431.2009.00493.x. [DOI] [PubMed] [Google Scholar]
- 104.Tasker S, Lappin MR. 2002. Haemobartonella felis: recent developments in diagnosis and treatment. J Feline Med Surg 4:3–11. doi: 10.1053/jfms.2001.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shindel N, Dardiri AH, Ferris DH. 1978. An indirect fluorescent antibody test for the detection of Cytauxzoon-like organisms in experimentally infected cats. Can J Comp Med 42:460–465. [PMC free article] [PubMed] [Google Scholar]
- 106.Susta L, Torres-Velez F, Zhang J, Brown C. 2009. An in situ hybridization and immunohistochemical study of cytauxzoonosis in domestic cats. Vet Pathol 46:1197–1204. doi: 10.1354/vp.08-VP-0132-B-FL. [DOI] [PubMed] [Google Scholar]
- 107.Birkenheuer AJ, Marr HS, Warren C, Acton AE, Mucker EM, Humphreys JG, Tucker MD. 2008. Cytauxzoon felis infections are present in bobcats (Lynx rufus) in a region where cytauxzoonosis is not recognized in domestic cats. Vet Parasitol 153:126–130. doi: 10.1016/j.vetpar.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 108.Brown HM, Berghaus RD, Latimer KS, Britt JO, Rakich PM, Peterson DS. 2009. Genetic variability of Cytauxzoon felis from 88 infected domestic cats in Arkansas and Georgia. J Vet Diagn Invest 21:59–63. doi: 10.1177/104063870902100109. [DOI] [PubMed] [Google Scholar]
- 109.Brown HM, Modaresi SM, Cook JL, Latimer KS, Peterson DS. 2009. Genetic variability of archived Cytauxzoon felis histologic specimens from domestic cats in Georgia, 1995–2007. J Vet Diagn Invest 21:493–498. doi: 10.1177/104063870902100410. [DOI] [PubMed] [Google Scholar]
- 110.Shock BC, Birkenheuer AJ, Patton LL, Olfenbuttel C, Beringer J, Grove DM, Peek M, Butfiloski JW, Hughes DW, Lockhart JM, Cunningham MW, Brown HM, Peterson DS, Yabsley MJ. 2012. Variation in the ITS-1 and ITS-2 rRNA genomic regions of Cytauxzoon felis from bobcats and pumas in the eastern United States and comparison with sequences from domestic cats. Vet Parasitol 190:29–35. doi: 10.1016/j.vetpar.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 111.Schreeg ME, Marr HS, Griffith EH, Tarigo JL, Bird DM, Reichard MV, Cohn LA, Levy MG, Birkenheuer AJ. 2016. PCR amplification of a multi-copy mitochondrial gene (cox3) improves detection of Cytauxzoon felis infection as compared to a ribosomal gene (18S). Vet Parasitol 225:123–130. doi: 10.1016/j.vetpar.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 112.Lewis KM, Cohn LA, Marr HS, Birkenheuer AJ. 2012. Diminazene diaceturate for treatment of chronic Cytauxzoon felis parasitemia in naturally infected cats. J Vet Intern Med 26:1490–1493. doi: 10.1111/j.1939-1676.2012.01003.x. [DOI] [PubMed] [Google Scholar]
- 113.Lewis KM, Cohn LA, Marr HS, Birkenheuer AJ. 2014. Failure of efficacy and adverse events associated with dose-intense diminazene diaceturate treatment of chronic Cytauxzoon felis infection in five cats. J Feline Med Surg 16:157–163. doi: 10.1177/1098612X13502974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cohn LA, Birkenheuer AJ, Brunker JD, Ratcliff ER, Craig AW. 2011. Efficacy of atovaquone and azithromycin or imidocarb dipropionate in cats with acute cytauxzoonosis. J Vet Intern Med 25:55–60. doi: 10.1111/j.1939-1676.2010.0646.x. [DOI] [PubMed] [Google Scholar]
- 115.Brown HM, Latimer KS, Erikson LE, Cashwell ME, Britt JO, Peterson DS. 2008. Detection of persistent Cytauxzoon felis infection by polymerase chain reaction in three asymptomatic domestic cats. J Vet Diagn Invest 20:485–488. doi: 10.1177/104063870802000411. [DOI] [PubMed] [Google Scholar]
- 116.Schreeg ME, Marr HS, Tarigo J, Cohn LA, Levy MG, Birkenheuer AJ. 2013. Pharmacogenomics of Cytauxzoon felis cytochrome b: implications for atovaquone and azithromycin therapy in domestic cats with cytauxzoonosis. J Clin Microbiol 51:3066–3069. doi: 10.1128/JCM.01407-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schreeg ME, Marr HS, Tarigo JL, Cohn LA, Levy MG, Birkenheuer AJ. 2015. Rapid high-resolution melt analysis of Cytauxzoon felis cytochrome b to aid in the prognosis of cytauxzoonosis. J Clin Microbiol 53:2517–2524. doi: 10.1128/JCM.00635-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Reine NJ. 2004. Infection and blood transfusion: a guide to donor screening. Clin Tech Small Anim Pract 19:68–74. doi: 10.1053/j.ctsap.2004.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wardrop KJ, Reine N, Birkenheuer A, Hale A, Hohenhaus A, Crawford C, Lappin MR. 2005. Canine and feline blood donor screening for infectious disease. J Vet Intern Med 19:135–142. doi: 10.1111/j.1939-1676.2005.tb02672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hillis DM, Dixon MT. 1991. Ribosomal DNA: molecular evolution and phylogenetic inference. Q Rev Biol 66:411–453. doi: 10.1086/417338. [DOI] [PubMed] [Google Scholar]
- 121.Zieman EA, Jimenez FA, Nielsen CK. 29 March 2017. Concurrent examination of bobcats and ticks reveals high prevalence of Cytauxzoon felis in southern Illinois. J Parasitol doi: 10.1645/16-133. [DOI] [PubMed] [Google Scholar]
- 122.Díaz-Regañón D, Villaescusa A, Ayllón T, Rodríguez-Franco F, Baneth G, Calleja-Bueno L, García-Sancho M, Agulla B, Sainz Á. 2017. Molecular detection of Hepatozoon spp. and Cytauxzoon sp. in domestic and stray cats from Madrid, Spain. Parasit Vectors 10:112. doi: 10.1186/s13071-017-2056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]