SUMMARY
Staphylococcus aureus is often involved in severe infections, in which the effects of bacterial virulence factors have great importance. Antistaphylococcal regimens should take into account the different effects of antibacterial agents on the expression of virulence factors and on the host's immune response. A PubMed literature search was performed to select relevant articles on the effects of antibiotics on staphylococcal toxin production and on the host immune response. Information was sorted according to the methods used for data acquisition (bacterial strains, growth models, and antibiotic concentrations) and the assays used for readout generation. The reported mechanisms underlying S. aureus virulence modulation by antibiotics were reviewed. The relevance of in vitro observations is discussed in relation to animal model data and to clinical evidence extracted from case reports and recommendations on the management of toxin-related staphylococcal diseases. Most in vitro data point to a decreased level of virulence expression upon treatment with ribosomally active antibiotics (linezolid and clindamycin), while cell wall-active antibiotics (beta-lactams) mainly increase exotoxin production. In vivo studies confirmed the suppressive effect of clindamycin and linezolid on virulence expression, supporting their utilization as a valuable management strategy to improve patient outcomes in cases of toxin-associated staphylococcal disease.
KEYWORDS: Staphylococcus aureus, antimicrobial agents, virulence factors
INTRODUCTION
Since the introduction of effective antimicrobial drugs, the morbidity and mortality due to various bacterial pathogens still represent a significant burden. Notably, Staphylococcus aureus causes very diverse severe infections, such as pneumonia, bacteremia, scalded skin syndrome, and toxic shock syndrome, in which bacterial toxins are important mediators (1). During infection, impaired functions are caused by host tissue damage induced by various virulence factors released upon bacterial replication. Certain bacterial toxins specifically trigger the immune system, resulting in subsequent cytokine release and further tissue injury. Antibiotic treatment for most infectious diseases is currently based on the ability of antimicrobials to achieve prompt pathogen destruction. Though rapid bacterial eradication is often obtained, sometimes the elimination of the offending organism does not occur fast enough to prevent the deleterious effects of the bacterial virulence factors (2).
Different classes of antibacterial agents may have different effects on the production and release of bacterial toxins and on the subsequent immune response of the host. Antimicrobial agents that disrupt bacterial cell wall synthesis (beta-lactams) lead to bacterial death and the release of pathogen-associated molecular patterns, such as peptidoglycans, lipoproteins, or DNA (3).
More recently, it was shown that subinhibitory concentrations of beta-lactams actively enhance the expression of staphylococcal exotoxins and adhesion molecules (4, 5). In contrast, antimicrobial agents that inhibit the microbial ribosome system (e.g., protein synthesis inhibitors, such as lincosamides and oxazolidinones) suppress the synthesis of bacterial toxins and may have secondary effects that include the dampening of toxin-induced host inflammatory responses (3).
Based on several series of data obtained in vitro, the usage of antibiotics that inhibit the expression of virulence factors has been recommended for the treatment of toxin-mediated infections (e.g., toxic shock syndrome and necrotizing pneumonia) (6, 7). Nevertheless, it remains to be determined whether the effects of antibiotics on bacterial toxin production or immunomodulation have any effect on treatment outcomes (8).
Here we review the evidence in the published peer-reviewed literature for modulatory effects of antibiotics on the virulence of S. aureus and provide a hypothesis regarding the underlying mechanism of this effect as well as perspectives on the clinical relevance of these findings.
METHODS EMPLOYED TO STUDY EFFECTS OF ANTIBIOTICS ON S. AUREUS VIRULENCE
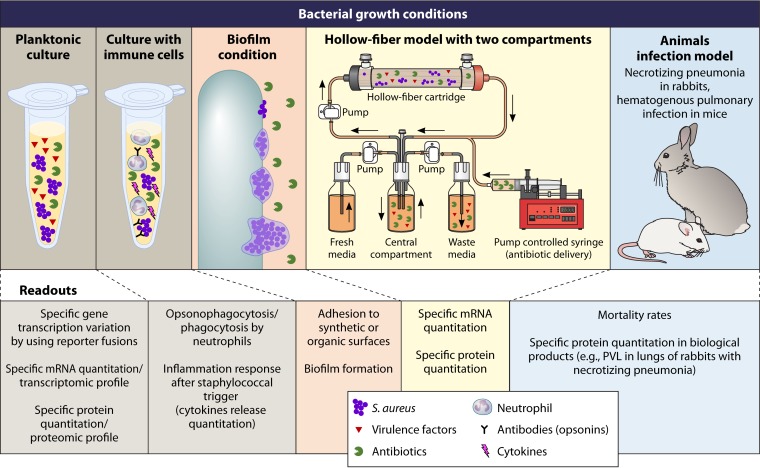
An exhaustive literature search of the PubMed database was performed from January through June 2016. The search included no year limitations and used keywords (antibiotics, beta-lactams, oxacillin, vancomycin, linezolid, clindamycin, protein synthesis inhibitor antibiotic, rifampin, trimethoprim, and fluoroquinolone) and each of the following terms: virulence, toxin, toxic shock staphylococcal toxin 1 (TSST-1), Panton-Valentine leukocidin (PVL), protein A, hemolysin, phenol-soluble modulins (PSM), Staphylococcus, and aureus. Reviews and non-English-language articles were excluded, and the remaining articles were screened manually for relevance (i.e., whether the article included data on antibiotic-mediated effects on the expression of staphylococcal virulence). A total of 107 selected references were categorized as in vitro, in vivo animal, or in vivo human studies. The key data were summarized and critically reviewed. Figure 1 illustrates the main experimental settings and assays used throughout the reviewed publications.
FIG 1.
Experimental conditions (top) and readouts (bottom) used in the different reviewed studies of the modulation of S. aureus virulence by antibiotics.
Bacterial Growth Conditions (Planktonic, Static or Dynamic, Biofilm, or Hollow-Fiber Infection Model)
Bacterial growth conditions have an important role in the assessment of bacterial virulence during normal cell growth and in the presence of antibiotic exposure. Secreted bacterial proteins, including enterotoxin B, TSST-1, PVL, PSM, and alpha-toxin, are produced during the late exponential and stationary growth phases, whereas bacterial surface proteins, such as fibronectin-binding protein (FnBP), protein A, clumping factor, and other surface-binding proteins, are produced during the early exponential growth phase (1). For a better understanding of these phenomena, in vitro assessments of virulence modification by antimicrobials should be performed under growth conditions that are optimal for the production of selected virulence factors. Planktonic cultures in the late exponential phase are optimal for the production of secreted proteins. In addition to use of a suitable growth phase, specific types of media should be used to optimize toxin production (9). The antibiotic susceptibility testing method recommended by the Clinical and Laboratory Standards Institute (CLSI), based on the utilization of cation-adjusted Mueller-Hinton medium, which yields insufficient PVL production, was modified. The broth was replaced by casein hydrolysate and yeast extract (CCY) medium, resulting in PVL levels that were approximately 50-fold higher and therefore easily detected by the dosage assay (10). Similarly, a modified CLSI method using tryptic soy broth (TSB) has also been used to determine the effects of antibiotics on PSM production, as PSM concentrations in TSB are consistent with the detection range of the quantitation assay commonly used (11). Other studies of PVL and alpha-toxin expression and/or quantification with antibiotic exposure were performed in CCY medium with agitation, with antibiotic added during the exponential phase, equivalent to 3 h of culture or a 2 McFarland standard (5, 12, 13). Indeed, growth with agitation allows better control of the growth phase than that with the CLSI-recommended static culture conditions. Other studies also initiated antibiotic treatment during mid-log-phase growth and measured TSST-1, alpha-toxin, and PVL in the supernatant following antibiotic exposure in brain heart infusion (BHI) medium supplemented with glucose, sodium bicarbonate, sodium chloride, disodium phosphate, l-glutamine, and magnesium sulfate. This medium permits maximum TSST-1 secretion, along with supporting alpha-toxin and PVL production (4). TSST-1 production levels were compared for two different growth phases. Overall, the stationary phase (overnight growth and adjustment to an inoculum of 1 × 109) allowed better discrimination of antibiotic effects on TSST-1 production than that with the early exponential phase (2 h of growth from 5 × 106 prior to the addition of antibiotics) (14), mainly because of the detection limitations of the dosing assays required for the assessment of antibiotic effects.
In addition to studies on planktonic bacteria, the effects of antibiotics have also been investigated in staphylococcal biofilms, as these remain a challenge in clinical treatment. When the biofilm phenotype is already achieved, the presence of sessile bacteria within an extracellular matrix of polysaccharide, proteins, and extracellular DNA reduces antibiotic effectiveness. Evaluation of virulence factors associated with bacterial attachment and biofilm development is performed in the early exponential growth phase, when these surface proteins are synthesized as a result of their upregulated expression; examples include protein A (spa), clumping factor B (clfB), collagen adhesion protein (cna), coagulase (coa), and fibronectin-binding protein (fnb) (15–17). Similarly, prevention of the biofilm phenotype is performed by adding antibiotics during the exponential phase of growth, followed by an 18- to 24-h incubation period (18). Cultures are grown in nutrient-rich medium, most commonly tryptic soy broth supplemented with <1% glucose, which has been proven to facilitate biofilm formation (19). Other, less utilized but nonetheless effective media (with or without glucose) for biofilm production include brain heart infusion medium and Luria-Bertani broth (20). However, given that antibiotic activity can be medium dependent (21), the effects of antibiotics on biofilm virulence modification should be interpreted carefully with regard to the growth medium.
In vitro pharmacokinetic and pharmacodynamic (PK/PD) models remain a valuable tool for assessing antimicrobial dose-response relationships both for existing antibiotics and for those in various stages of clinical development. These modeling systems have provided the basis for new PK/PD approaches in patients, including prolonged/continuous-infusion beta-lactam and extended-interval aminoglycoside therapies, among others (22). The use of these systems to investigate the in vitro effects of antimicrobials on the production of bacterial toxins remains very limited compared to their overall PK/PD application. Although the arrangement of PK/PD models can be variable due to investigator customization and preferences, the basic underlying principle of delivering human-simulated doses against a pathogen of interest is constant (23). Theoretically, the framework of these systems should permit evaluation of bacterial virulence effects throughout the dosing interval at concentrations above and below the organism's MIC. One-compartment models (those in which the antibiotic is delivered to the pathogen in a single chamber) have been used to provide basic assessments of antimicrobial effects on bacterial toxin production (24). The limitation of the single-compartment modeling system is the continuous flow of medium entering and exiting the system, which results in significant losses of organisms and/or extracellular protein. This loss hinders the assessment of virulence regulation or toxin protein production upon antibiotic exposure. The one-compartment modeling system has been used only to evaluate streptococcal exotoxin release upon pharmacodynamic antibiotic exposure (24), and no literature supports its validation for the assessment of S. aureus virulence.
Hollow-fiber modeling systems offer a more intricate type of pharmacodynamic model, but they follow the same principles outlined for PK/PD models to simulate human antibiotic exposure and duration. The exception with the hollow-fiber system is its two-compartment design, in which an artificial capillary membrane system is used to provide a separation between the central and peripheral compartments (25). The peripheral compartment is inoculated with the organism, and the fibers (available with either a 5-kDa or 20-kDa molecular mass cutoff) trap the bacteria and extracellular proteins, such as toxins and virulence factors. The central compartment remains sterile and delivers the medium and antimicrobial(s) to the peripheral compartment. Although the hollow-fiber system offers many built-in advantages, there has been very little exploration of the effects of antibiotic treatment on S. aureus virulence factor expression and toxin production by use of this model. The first and only such study, to date, used simulated human doses of clindamycin, linezolid, minocycline, trimethoprim-sulfamethoxazole, and vancomycin against methicillin-resistant S. aureus (MRSA) MW2 over a 72-h treatment period and assessed S. aureus virulence by the expression of PVL toxin and the enterotoxin genes sec4, sek, seq, and sel2 (26). The advantage of this system is that it allows dynamic monitoring of antibiotics, testing of antibiotics in different combinations, and characterization of toxin functionality in medium extracted at selected time points. Only one other study used the hollow-fiber model to study antivirulence effects, using linezolid and ciprofloxacin against Bacillus anthracis (27). There remains ample future research potential to identify antivirulence pharmacodynamics by using the hollow-fiber system.
Antibiotics (Molecules and Concentrations) Explored
A large number of in vitro studies focused on subinhibitory antibiotic concentrations (sub-MICs), as this setting allows decoupling of the virulence modulation effect from the antimicrobial effect. Moreover, though in clinical therapeutics antibiotics are usually used in high doses, sub-MICs of antistaphylococcal agents may occur, either due to antibiotic-resistant microorganisms or due to the pharmacokinetics of the antibiotic, in several ways.
First, the plasma concentration may fall below the MIC level at the end of the dosing interval for antibiotics with a short half-life and intermittent dosing administration. This is the case for gentamicin (9) and antistaphylococcal penicillins (oxacillin, cloxacillin, dicloxacillin, and nafcillin), whose half-life is only 30 to 60 min in patients with normal renal function (10, 11). In addition, the half-life of antistaphylococcal drugs may be reduced in special populations, such as burn patients (12), cystic fibrosis patients (13, 14), obese patients (15), critically ill patients with augmented renal clearance (16), or young children (17). Such patients have an increased risk of subinhibitory drug concentrations when treated with standard doses.
Second, the antibiotic concentration may be subinhibitory at the site of infection as a result of poor diffusion, especially since severe S. aureus infections are associated with intense necrosis. For example, linezolid concentrations of <4 mg/liter have been reported for epithelial lining fluid and alveolar macrophages (18). Another example is beta-lactam penetration into bone. Bone concentrations lower than MIC breakpoints and low plasma-to-bone-concentration ratios have been reported for several antistaphylococcal penicillins (19).
Third, low plasma and tissue exposure may occur in cases of drug-drug interactions. A relevant example among antistaphylococcal agents is rifampin, which is a strong inducer of cytochrome P450 enzymes and transporters (e.g., P-glycoprotein) involved in the metabolism and disposition of many drugs. It has been shown that rifampin can decrease exposure to sub-MIC levels for the companion antistaphylococcal drugs linezolid (20, 21) and clindamycin (22, 23).
Finally, sub-MICs are likely to occur in cases of poor drug adherence. This may be highly relevant for orally administered drugs in an ambulatory care setting (24, 25).
Early studies of antimicrobial effects on bacterial cell processes showed that sub-MICs of antibiotics can suppress or induce toxin production, and this is still largely the basis for investigating the alterations in bacterial virulence during antibiotic exposure. Because many of the studies share similarities regarding the type of antibiotic used and the antibiotic concentrations explored, this section highlights only a few studies that represent the field.
Clindamycin has widely displayed antivirulence properties due to the antibacterial effects of its binding to the 50S subunit of bacterial ribosomes. In a study of six toxic shock syndrome toxin-producing isolates, clindamycin concentrations ranging from 0.001 to 1.0 μg/ml were evaluated (28). Although clindamycin susceptibility was not reported in that study, it is assumed that the concentrations were sub-MICs because the bacterial growth was similar to that of the no-antibiotic control. The overall effect of toxin suppression by clindamycin observed in the early studies was consistent with most of the following reports, which varied the tested strains, the virulence factors, the antibiotic concentrations, and the inocula. Most often, clindamycin was used at 1/4 the MIC for evaluation of its effects on alpha-toxin and PVL expression (29), but higher antibiotic concentrations, such as 5 times the S. aureus MIC, have also been used for testing of higher bacterial inocula (4, 29).
Tetracyclines, including doxycycline and tigecycline (a tetracycline derivative in the glycylcycline subclass), inhibit bacterial growth by preventing the association of aminoacyl-tRNA with the bacterial ribosome via binding to the 30S ribosomal subunit (30). Tetracyclines retain activity against staphylococci, and they have been explored for virulence modification. Because it was the first in its class, tetracycline was initially evaluated at sub-MICs and was found to have inhibitory effects on coagulase and protein A production and to largely abolish alpha- and delta-hemolysin production (31). Tigecycline has been explored least in this regard, but it has been studied against community-acquired MRSA (CA-MRSA) due to its reliable activity. Sub-MICs of tigecycline (1/8, 1/4, and 1/2 MIC) were used to evaluate virulence factor expression in CA-MRSA (13), and concentrations of 1/8 and 1/4 MIC were studied for effects on the exoproteins, phenol-soluble modulins, alpha-hemolysin, and protein A (11).
More recently, a relatively new antibiotic, linezolid, has been studied for antivirulence activity in vitro. Linezolid is an oxazolidinone antibiotic that, similar to clindamycin, binds to the 50S subunit of the bacterial ribosome. It is often studied alongside clindamycin for comparative effects in vitro; the concentrations relative to the organism's MIC are similar to those discussed for clindamycin. These concentrations range from 1/4 to 5× MIC, which is commonly 2 mg/liter for most S. aureus strains (4, 12). This effect was confirmed in a hollow-fiber model using therapeutic exposures (26). A study of virulence factor expression in Gram-positive cocci, including S. aureus, determined that antivirulence properties of linezolid are apparent at 1/2 to as low as 1/8 MIC (32). A similar approach was used to test sub-MICs of linezolid (12.5, 25, 50, and 90% of the MIC) in another study of S. aureus virulence factor expression (33). Though the predominant effect observed for most of the studied toxins was a production decrease, it depended on the quantitation assay used, and the most effective concentrations were those close to the MIC.
The beta-lactam class of antibiotics is highly diverse, featuring multiple agents and subclasses (penicillins, cephalosporins, etc.). Antistaphylococcal beta-lactams are the main focus of studies on S. aureus virulence. Their mechanism of action of binding to penicillin-binding proteins (PBPs) and inhibiting transpeptidation and/or transglycosylation of the cell wall results in rapid cell lysis and death in susceptible strains. Sub-MICs of antistaphylococcal β-lactams consistently enhance toxin production by S. aureus. This was first noted with nafcillin concentrations slightly below the MIC for both methicillin-susceptible S. aureus (MSSA) and MRSA, which resulted in elevated alpha-toxin production (34). This was confirmed in a later study using a range of sub-MIC nafcillin concentrations, from 0.01 to 8 mg/liter (4). Another study evaluated the effects of multiple beta-lactam agents on PVL expression, using 1/2 MICs of oxacillin, imipenem, cefotaxime, cefaclor, and cefoxitin. After confirming that oxacillin induced PVL production at subinhibitory concentrations of 1/8 to 1/2 MIC (0.12 to 32 mg/liter) (10), the same group found that beta-lactam-induced expression of PVL was linked only to beta-lactams that bound penicillin-binding protein 1 (oxacillin and imipenem) (5). Another study with oxacillin confirmed that it increased the expression of secreted toxins at a single sub-MIC (0.5 mg/liter), albeit to a more moderate level than those noted in other studies (35).
Vancomycin and daptomycin have distinct mechanisms of action, and due to their use in severe MRSA infections, their individual effects on S. aureus virulence are important. Vancomycin binds to the cell wall precursors of peptidoglycan and inhibits transpeptidation of the cell wall. In several studies, vancomycin at concentrations of 1/8 to 1/2 MIC of 0.5 to 2 mg/liter had limited effects on S. aureus virulence as measured by gene expression or toxin production (10, 13). Daptomycin is a lipopeptide antibiotic with antibacterial activity on the cell membrane; it causes membrane depolarization and potassium efflux without cell lysis. One study of daptomycin at 1/2, 1/4, and 1/8 MIC in select MRSA strains (MIC range, 0.25 to 0.5 mg/liter) reported that these concentrations of the antibiotic had no major effects on virulence factor expression (13).
In vitro experiments mostly evaluated sub-MICs; for clindamycin and linezolid, an inhibitory effect on toxin production was often observed, while for beta-lactams an inducing effect was reported. In addition, miscellaneous antibiotics have also been evaluated over sub-MIC ranges (1/8 to 1/2 MIC): fusidic acid, which binds to elongation factor G, ultimately resulting in protein synthesis inhibition (36), was found to inhibit PVL production in a manner similar to that for the protein synthesis inhibitors clindamycin and linezolid (10), while the fluoroquinolone antibiotics enoxacin, lomefloxacin, and ciprofloxacin, which inhibit bacterial DNA synthesis by binding to gyrase and topoisomerase, were found to inhibit alpha-toxin production (37). Although some antibiotics have not been evaluated for virulence modification in S. aureus, data obtained using antibiotics within a similar class or with a similar mechanism of action can help in positing the potential effects of unstudied agents.
Readouts for Effects of Antibiotics on Virulence Expression
Specific gene transcription variation measured by reporter fusions.
The effects of antibiotics on virulence expression were explored by means of reporter fusions to examine specific gene transcription (10, 16, 29, 38–41). As a general pattern, S. aureus laboratory strains were transfected with a plasmid containing a fusion gene constructed using the promoter of a given gene (a staphylococcal virulence factor or a regulatory gene) and, as a reporter gene, an enzyme gene whose activity is easily measurable, such as lux (luciferase) (38, 40, 41), lacZ (galactosidase) (10, 16, 29), or blaZ (beta-lactamase) (39). Thus, the measured enzyme activity reflects the promoter activity of a specific gene, often hla (alpha-hemolysin) (16, 29, 39, 40) or spa (protein A) (16, 38, 39), but also the PVL gene (10). This method allows easy screening of the effects of several antibiotics on the transcription of a given gene, particularly if it is coupled with the use of antibiotic discs or Etest diffusion on agar plates (16, 38, 40). However, given that reporter gene experiment data do not take into account the importance of posttranscriptional and translational regulation, the yielded results are not expected to be fully concordant with the effective protein production level.
Specific mRNA quantitation.
Specific mRNA quantitation has frequently been used to study the impact of sub-MICs of antibiotics on the expression of a given gene (staphylococcal virulence factor or regulatory genes). Subsequent to RNA extraction and purification, two assays are used to measure specific mRNA levels: Northern blotting and quantitative reverse transcription-PCR (qRT-PCR). Virulence determinants whose expression was explored by specific mRNA quantitation were hla (4, 13, 29, 34, 35, 39, 42), pvl (4, 5, 12, 13, 26), and spa (13, 16, 38, 39, 43); mRNA levels were normalized with respect to the expression of housekeeping genes, mostly the 16S rRNA gene or gyrB. Variation in mRNA levels may occur either by transcriptional modulation or by posttranscriptional mechanisms involving mRNA turnover. Moreover, variations in the mRNA levels induced by sub-MICs of antibiotics do not always result in changes in protein synthesis, which should be taken into account before further conclusions are drawn.
Transcriptome profiles.
Several of the selected articles employed a transcriptomic approach using microarray data analysis to study the effects of sub-MICs of antibiotics on staphylococcal virulence (44, 45). This approach shares disadvantages with the quantitation of specific mRNA levels and may have decreased sensitivity for relatively small changes in gene expression. In short, it provides an overview of the transcriptomic modifications induced by sub-MICs of antibiotics, thus allowing the selection of specific candidate genes for further exploration thereafter. Nevertheless, this transcriptomic approach highlights some modifications in global metabolic pathways or regulatory systems but does not precisely measure any given virulence factor. With this method, Awad et al. showed that a sub-MIC of vancomycin (1/2 MIC) induced upregulation of 36 genes of an epidemic MRSA strain, including 15 loci involved in cell wall metabolism, capsule, and autolysis, and downregulation of 12 loci with still-unknown functions (44). Similarly, Kuroda et al. (45) showed that sub-MICs of cefoxitin induced the upregulation of several genes involved in the staphylococcal stress response and in the SaeRS regulatory system (discussed further in a later section).
Specific protein measurement.
Because of the above-mentioned misinterpretations linked to mRNA quantitation alone, most of the selected studies also determined whether specific protein expression levels were associated with mRNA quantitation. Different assays were used according to the staphylococcal virulence factor explored. For PVL, protein A (SpA), alpha-hemolysin (Hla), and FnBP, the authors used enzyme-linked immunosorbent assay (ELISA) (5, 10, 13, 43, 46), Western blotting, or immunoblotting (12, 29, 33, 35, 41, 42, 47, 48). Notably, confounding factors related to the technical limitations of these assays may hamper the results obtained. Indeed, Turner and Sriskandan (12) reported only slight impacts of flucloxacillin, clindamycin, or linezolid on the PVL production level, while a number of other authors agreed that sub-MICs of flucloxacillin and clindamycin or linezolid increased and decreased PVL production, respectively. These inconsistencies may be due partly to the signal saturation of Western blots for PVL quantitation when antibiotics are added during the exponential growth phase (29). For PSM, which are small peptides of only 20 to 40 amino acids that are not suitable for immune quantitation by ELISAs, the authors used a chromatography technique (generally high-pressure liquid chromatography [HPLC]) coupled with mass spectrometry (MS) (11, 35, 49, 50). For staphylococcal virulence factors with specific enzymatic or toxic functions, such as coagulase or hemolysin, the specific properties of the proteins rather than their antigenic levels were used to quantify their functional activity (45, 51). Given that biological activity is being assessed, more disparities in results may be expected, depending on the variability of the substrate.
Proteomic profiles.
Only a few articles (five in the selection) used proteomic profiles to observe the effects of sub-MICs of antibiotics on staphylococcal virulence. These studies used high-resolution semiquantitative assays, such as electrophoresis (SDS-PAGE or two-dimensional electrophoresis) followed by Coomassie blue or silver staining, to determine the effects of antibiotics on the staphylococcal protein profile (35, 39). Other authors complemented electrophoresis with identification of interesting bands by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF) to identify proteins that were specifically modulated by antibiotic treatment (33, 47). However, because this approach does not yield an accurate quantification of weakly expressed proteins, variations in the expression of these proteins may remain undetected. Consequently, the results obtained using these methods should be confirmed by specific protein measurements. Recently, Liu et al. described a label-free method based on liquid chromatography-tandem MS (LC-MS/MS) that can be used to obtain qualitative and quantitative proteomic profiles for comparison of MSSA and MRSA proteomic profile changes upon oxacillin exposure (52). This strategy provides an overview of the effects of sub-MICs of antibiotics on staphylococcal virulence, including the effects on metabolic pathways, thus permitting a global approach to virulence as a reflection of bacterial physiology. In summary, Liu et al. observed that, for MRSA strains, exposure at 1/8 MIC of oxacillin induced the downregulation of 16 genes involved in amino acid metabolism (alanine, aspartate, and glutamate) and the upregulation of 65 genes, including genes encoding PBP2a-mediated methicillin resistance, the beta-lactamase regulatory protein, the peptidoglycan synthesis network, and pantothenate and coenzyme A (CoA) biosynthesis proteins, with the last two also being upregulated in MSSA strains (52).
Ex vivo staphylococcal properties.
(i) Opsonophagocytosis and phagocytosis by neutrophils.
The first and probably most important mechanism in the host defense against S. aureus is the innate immunity that is mediated mainly through phagocytic cells, such as polymorphonuclear cells (PMNs) and macrophages. S. aureus has developed various mechanisms to escape the host immune system; these strategies include inhibition of PMN chemotaxis and PMN activation and antiphagocytosis strategies, such as inhibition of opsonophagocytosis. Therefore, an early interest was taken in the impact of antibiotics on S. aureus susceptibility to host defense mechanisms. Early studies, some dating from as far back as 30 years ago, explored various antibiotic families with respect to their impact on the host's antistaphylococcal immune response. Three different protocols were used in these studies. In the first protocol, S. aureus isolates were previously cultured with or without antibiotics at sub-MICs and subsequently incubated with PMNs or macrophages (32, 53–62), allowing measurement of the effects of antibiotics on the susceptibility of bacteria to opsonization and opsonophagocytosis or phagocytosis. In the second protocol, S. aureus and phagocytic cells were simultaneously cocultivated with antibiotics at sub-MICs (63), enabling study of the synergic effect of antibiotics and phagocytic cells on bacterial survival. In the third method, S. aureus and phagocytic cells were incubated together to allow phagocytosis; the remaining extracellular S. aureus cells were then lysed, and antibiotics at sub-MICs were added to the medium (64), providing data on the antibiotics' effects on intracellular bacteria. In addition to the assessment of the bactericidal effect of PMNs, several authors also investigated PMN chemotaxis by exploring the ability of the supernatant from antibiotic-treated S. aureus to differentially modulate PMN migration through a Boyden chamber (65).
(ii) In vitro hemolysis.
The hemolytic ability of S. aureus is linked to virulence factor production; therefore, in vitro hemolysis has long been studied as a surrogate for staphylococcal virulence assessment. Though a number of S. aureus toxins have the ability to lyse red blood cells, most of the hemolytic effect observed in vitro is due to Hla production. Two methods were used in the reviewed studies: (i) measurement of the hemolytic activity of S. aureus supernatants against rabbit erythrocytes after previous incubation of bacteria with or without antibiotics, with one hemolytic unit being defined as the amount of S. aureus supernatant required to liberate 50% of the total hemoglobin from the erythrocytes, expressed in units per milliliter or units per bacterial density (4, 32, 34, 42); and (ii) visual assessment of the hemolytic zones related to S. aureus plated on blood agar prior to the deposition of antibiotic disks or of strips containing a predefined gradient of antibiotic concentrations (Etest strips) (37, 45).
(iii) Adhesion to synthetic surfaces or cellular cultures and biofilm formation.
S. aureus possesses numerous surface proteins that facilitate its adhesion to synthetic and organic surfaces. The largest and most studied class of such proteins are cell wall-anchored proteins collectively termed microbial surface component-recognizing adhesive matrix molecules (MSCRAMMs), including SpA and serine-rich adhesin for platelets, which play a particular role (66). The expression of these cell wall-anchored proteins has been linked to a range of infection types, including endocarditis, pneumonia, prosthetic device infections, renal abscesses, mastitis, sepsis, and septic arthritis (67–70). Readouts for determining the production of adhesion virulence factors include either specific quantitation of several proteins involved in adhesion, such as SpA or FnBP (as already discussed), or overall measurement of the adhesion of bacterial cells to synthetic or organic surfaces, which represents the first step in biofilm formation. Bacterial biofilms are organized communities of bacteria embedded in a self-produced matrix of extracellular polymeric substances. Biofilms are increasingly being associated with human infections, especially due to the rise in the use of medical devices such as catheters or implants. The increased host immune system evasion as well as tolerance and resistance to antimicrobials displayed by biofilms leads to failure of conventional antimicrobial therapy. From this perspective, the ability of S. aureus strains to form biofilms may be regarded as a virulence factor, and antibiotics that interfere with biofilm formation can therefore modulate staphylococcal pathogenesis. Though biofilm development is complex and heterogeneous, phenotypic readouts have been accepted as standards to assess the impacts of antibiotics on biofilm formation. The assay that is probably most used for analysis of antibiotic prevention of biofilm formation is performed in a 96-well plate (MWP [i.e., multiwell plate]) after overnight incubation of bacteria added to medium in flat-bottomed wells. The newly developed biofilm is measured after staining with crystal violet and subsequent assessment by an absorbance measure (71). In the MWP, biofilms are formed either under static conditions or under low-shear conditions (when plates are placed on a shaker), but in both cases the amounts of available nutrients and aeration are limited. Other methods exist for bacterial quantification in biofilms following antibiotic exposure, including use of flow cells, dynamic biofilm PK/PD models, and other dynamic reactor systems characterized by a continuous flow of fresh nutrients. Biofilms obtained in these two settings display different functional characteristics and architectures, which may affect the results of antibiotic biofilm formation prevention experiments. Indeed, a recent study established that the ability of antibiotics to prevent biofilm formation in dynamic systems (measured via the log reduction in biofilm-embedded bacteria) was significantly lower than that found using the MWP (72). The main cause of discrepancy was the nutrient depletion in the static MWP model, as refreshment of the medium twice daily restored the antibiotic efficacy to levels similar to those observed in the dynamic model.
(iv) Ex vivo proinflammatory response assessment (cytokine profile).
Invasive S. aureus infection elicits a complex immune response in the host. Specific components of S. aureus are known to stimulate proinflammatory responses that lead to phagocytosis; however, certain staphylococcal proteins have a role in evading host recognition (73). Although no specific antigen has yet been identified as being essential for S. aureus pathogenicity, the secreted virulence factors have been associated with septic shock due to a dysregulated inflammatory response (74). Alpha-toxin, for example, increases the in vivo production of the cytokines interleukin-1β (IL-1β), IL-6, IL-8, and tumor necrosis factor alpha (TNF-α), in addition to its effect on cytokines involved in adaptive immunity, particularly IL-17 (75). Antibiotics may alter the proinflammatory response of the host to S. aureus, in part by preventing virulence factor production. The effects of antibiotics on cytokine production are explored by use of select ex vivo protocols. These methods use collected whole blood or cells, such as peripheral blood mononuclear cells (PBMCs), monocytes, or PMNs, or test the response of a standard cell type, such as macrophages (33, 76–78). S. aureus (live or heat killed) or bacterial components, such as peptidoglycans or purified toxins, are added to the ex vivo cell medium along with various concentrations of antibiotics. Following incubation, cytokine levels are measured using enzyme-linked immunoassays, with the readout interpreted as relative cytokine concentrations.
EFFECTS OF ANTIBIOTICS ON STAPHYLOCOCCAL VIRULENCE
Effects on Expression of Specific Virulence Factors
Alpha-toxin (Hla).
Alpha-toxin or alpha-hemolysin is a secreted, pore-forming cytotoxin that forms heptameric pores in host cell membranes, which result in the lysis of multiple host cell types, including epithelial cells, endothelial cells, monocytes, macrophages, and neutrophils (75, 79, 80). Its role in S. aureus pathogenesis is well established for multiple infection types, ranging from skin and skin structure infections to lethal invasive infections. It has recently garnered increasing interest as a potential target for vaccine development and passive immunity due to its being highly conserved in various S. aureus backgrounds and its contribution to disease (81). Because alpha-toxin was one of the first S. aureus toxins to be identified (82), the effects of antibiotics on its regulation and production have been well studied. In these analyses, antibiotics are often studied at sub-MIC levels, as described in the previous section. Initial observations noted that antibiotics that inhibit protein synthesis reduce the hemolytic activity of S. aureus. Several articles published since then have defined the roles of different antibiotic classes in altering alpha-toxin production.
Kernodle et al. studied the effect of nafcillin on alpha-toxin production in 37 S. aureus strains in vitro. Both nafcillin-susceptible (MSSA) and nonsusceptible (MRSA) strains displayed nafcillin-triggered increases in both alpha-toxin expression and hemolytic activity. Interestingly, the supernatants from nafcillin-exposed strains resulted in increased lethality compared to that of supernatants from unexposed strains when injected intraperitoneally into mice (34). Further effects of sub-MICs of antibiotics on alpha-toxin production were explored by Ohlsen et al., who confirmed increased alpha-toxin gene expression on beta-lactam exposure. They expanded on this finding, showing that beta-lactams induce more alpha-toxin production (up to 30-fold) in MRSA strains than in MSSA strains (29). Other investigations have confirmed alpha-toxin induction by beta-lactams (4, 42). In addition, alpha-toxin expression was completely abolished by clindamycin, reduced by erythromycin and aminoglycosides, unaffected by glycopeptides, and increased by fluoroquinolones (29). Multiple studies have identified linezolid as a potent inhibitor of alpha-toxin expression and secretion (32, 33). However, one study found a stronger concentration-dependent inhibition of alpha-toxin secretion with clindamycin than that with linezolid (13). It has more recently been accepted that protein synthesis inhibitors, especially clindamycin and linezolid, prevent the translation but not transcription of alpha-toxin (4, 26).
TSST-1.
Toxic shock syndrome toxin 1 (TSST-1), encoded by the tst gene, is considered the classic superantigen toxin in S. aureus. The contribution of TSST-1 to serious disease is well defined: it has been portrayed most prominently for its role in toxic shock syndrome in children (65) and in young women through the use of tampons (83). The latter has declined significantly due to public health preventative measures to curb toxic shock syndrome associated with tampon use during menstruation (84). However, it remains an important toxin in severe skin and wound infections, and antibiotics have demonstrated an important clinical role in altering the production of this virulence factor (8).
The in vitro data discriminate among antibiotic effects on tst expression and toxin secretion. An old study highlighted the inhibitory effect of sub-MICs of clindamycin (from 1/4 to 1/256 MIC) on TSST-1 production (85), and Herbert et al. showed a decrease of tst transcription and TSST-1 production with 0.02 μg/ml of clindamycin (39). A comparative study of flucloxacillin, gentamicin, and clindamycin found that clindamycin was most effective at suppressing TSST-1 production, reducing it by up to 95%, whereas the addition of gentamicin or flucloxacillin resulted in a 75% reduction in TSST-1 production (14). A case report of a patient successfully treated with linezolid for toxic shock syndrome correlated this with in vitro evidence of suppression of TSST-1 production with either linezolid or clindamycin, while TSST-1 production with nafcillin or vancomycin was no different from that of the control (86). In a study of the transcription and translation of toxins during antibiotic exposure, nafcillin increased and prolonged TSST-1 regulation and production, while clindamycin and linezolid suppressed translation but not transcription of TSST-1. Tigecycline was studied against S. aureus biofilm cultures, and it suppressed tst expression, with a 10-fold reduction in TSST-1 production compared to that in untreated cultures (15). Collectively, these studies show that protein synthesis inhibitors have mixed effects on tst expression but are effective in significantly reducing TSST-1 production.
Enterotoxins.
The family of S. aureus enterotoxins is highly diverse, with over 20 identified types, including but not limited to staphylococcal enterotoxins (SEA, SEB, SEC, SED, SEK, and SEE, among others). These toxins are widely recognized as causes of foodborne illnesses, but they have also been implicated for their role in colonization and infections resulting in skin and soft tissue inflammation and dermatitis (87). The toxicity and secretion of all enterotoxins have not been evaluated; rather, an abundance of literature exists on these properties for most prominent enterotoxins. Regardless, studies of antibiotic modulation of enterotoxins are limited compared to those on other toxins. In a study of sub-MICs of oxacillin and levofloxacin, enterotoxin sec expression levels were strain dependent, ranging from no change with either drug to a ±5-fold difference from the control level (17). A separate study found that linezolid effectively suppressed enterotoxin A and B secretion as much as 32% to 43%, in a concentration-dependent manner, at levels below the MIC (33). Moreover, enterotoxin gene regulation during therapeutic simulations was studied in the hollow-fiber model. This study evaluated sec4, sek, seq, and sel2 expression during treatment with clindamycin, linezolid, minocycline, trimethoprim-sulfamethoxazole, or vancomycin. Compared to the control, vancomycin and minocycline upregulated enterotoxin expression during the first 8 h, followed by downregulation thereafter. Both clindamycin and linezolid increased enterotoxin expression (26); however, given that both clindamycin and linezolid target protein translation, the observed mRNA increase may not be relevant with regard to the effective protein level, as already shown for PVL.
PVL.
PVL is a pore-forming toxin that possesses cytolytic properties and contributes to staphylococcal pathogenesis. PVL-producing S. aureus strains are involved in primary skin and soft tissue infections (SSTIs), high-mortality necrotizing pneumonia, and recurrent complicated osteomyelitis (88–90).
Along with alpha-hemolysin, PVL is probably one of the most explored toxins with regard to the effects of antibiotics on virulence expression. All published reports support an increase of PVL expression in strains cultured with beta-lactams. Both the antistaphylococcal penicillins oxacillin and nafcillin at sub-MICs (ranging from 1/8 to 1/2 MIC) induced increases in PVL production in different CA-MRSA backgrounds (ST1, ST8, ST80, and ST59) (4, 5, 27, 53, 64). For beta-lactams other than the antistaphylococcal penicillins, fewer data have been reported. A study by Dumitrescu et al. showed that a sub-MIC of imipenem (1/4 MIC) but not of cefoxitin, cefaclor, or cefotaxime induced significant increases in pvl mRNA and PVL production after 6 h of culture for four CA-MRSA strains and one laboratory strain (LUG855) (5). This observation provided insight into the mechanism underlying the oxacillin- and imipenem-induced PVL increase, as discussed in a later section. One study disagreed with others about the effect of beta-lactams on PVL production, not observing any effect of sub-MICs of flucloxacillin on the PVL mRNA expression level or protein production (12). Although Turner and Sriskandan used a protocol similar to a previously published one (4) by adding the antibiotics during the mid-exponential growth phase, prior to PVL quantitation by Western blotting, the results were discordant. This discrepancy may be explained by Western blot signal saturation before the antibiotics were added, as the PVL production level in the CCY medium used by Turner and Sriskandan was approximately 50 times higher than that in the BHI medium previously used by Stevens et al. (4).
Furthermore, Stevens et al., Dumitrescu et al., and Otto et al. highlighted the PVL antitoxin effects (inhibition of toxin expression) of sub-MICs of clindamycin and linezolid (4, 10, 13, 46). Clindamycin induced concentration-dependent decreases in pvl mRNA and PVL production for concentrations ranging from 1/8 to 1/2 MIC in the aforementioned 5 CA-MRSA strains (ST8, ST1, ST80, ST30, and ST59). Similarly, linezolid induced concentration-dependent decreases in pvl mRNA and PVL production, but to a lesser extent than those with clindamycin, and only for concentrations greater than 1/8 MIC. Moreover, a few studies showed an anti-PVL effect of sub-MICs of fusidic acid (1/4 and 1/2 MIC), rifampin (1/8 to 1/2 MIC), and tigecycline, pristinamycin, tetracycline, and ofloxacin (1/2 MIC) (10, 46). Finally, several authors reported no relevant impact of sub-MICs of vancomycin on PVL expression (10, 13, 46).
In summary, there is a strong body of evidence supporting the idea that sub-MICs of antistaphylococcal penicillins, notably oxacillin and nafcillin, lead to increased expression of PVL, while clindamycin, linezolid, and rifampin suppress PVL expression; finally, vancomycin does not affect the modulation of PVL expression. These phenotypes are controlled by a variety of mechanisms, such as two-component systems (TCS) and global virulence regulators, which are discussed in detail later. Other antibiotics should be tested further to confirm the limited previous reports.
PSM.
PSM are secreted virulence factors that elicit a proinflammatory immune response and mediate neutrophil lysis (91). A few recent articles reported the effects of sub-MICs or inhibitory concentrations of antibiotics on PSM expression. First, Joo et al. examined the effects of oxacillin, clindamycin, linezolid, erythromycin, tetracycline, and co-trimoxazole at subinhibitory or inhibitory concentrations on one CA-MRSA ST8 USA300 strain (LAC) and one hospital-acquired MRSA (HA-MRSA) strain (Sanger 252) (50). According to their protocol, oxacillin at a very low concentration (1/50 MIC) induced a significant decrease in PSMα1-4 production, with unmodified psmα mRNA levels, in the LAC strain. In contrast, clindamycin, linezolid, erythromycin, and tetracycline significantly increased PSMα1-4 production in both the LAC and Sanger 252 strains. Nevertheless, it is difficult to corroborate these data because the antibiotic concentrations tested in the different strains were very dissimilar. A second article compared the effects of sub-MICs of clindamycin and TR-700 (tedizolid), a new oxazolidinone, on PSM production (49). In contrast to Joo et al., Yamaki et al. found that clindamycin and TR-700 (1/2 MIC) induced decreased PSMα1-4 production in 7 clinical strains isolated from SSTIs (PVL-positive MSSA and MRSA isolates). These discrepancies may be due to differences in the protocols and strains used. However, thereafter, Yamaki et al. found an opposite effect of clindamycin (1/8 MIC); in their study, clindamycin at 1/8 MIC induced an increase in PSMα1-4 production in 7 of 13 clinical MRSA strains tested (11). This study also showed that 1/8 MIC of linezolid induced an increase in PSMα1-4 production in 3 isolates and an inhibitory effect in 5 isolates, whereas 1/4 and 1/8 MICs of tigecycline resulted in increased PSMα1-4 production by 11 isolates (11). These results tend to show that the effects of sub-MICs of clindamycin, linezolid, and tigecycline on PSMα1-4 production are antibiotic concentration and strain dependent. Finally, Rudkin et al. showed that oxacillin at 0.5 mg/liter induced a decrease in PSMα1-4 production by 2 CA-MRSA (ST8 and ST1) strains, but the exact effect relative to the MIC was not specified (35).
The expression of delta-hemolysin (Hld), also belonging to the PSMα family, was explored in a single study, which reported a significant increase in Hld mRNA levels upon vancomycin treatment at a concentration equal to the MIC (43). Nevertheless, no proteomic data were provided to confirm the observation.
Altogether, these data support the fact that the impacts of protein synthesis inhibitory agents on PSM production are strain and concentration dependent, while the suppressive effect of oxacillin is consistently found throughout the studies. Mechanisms underlying the modulatory effects of antibiotics on PSM expression may involve agr and AgrA, recently shown to influence PSM expression. However, this hypothesis failed to explain all the observed variations. Indeed, though in the first study Yamaki et al. observed significant decreases of AgrA transcripts after treatment with 1/4 and 1/8 MICs of TR-700, similar to the results for PSMα production, this did not apply for clindamycin. Likewise, Joo et al. showed increases of RNA III transcripts for the LAC strain treated with sub-MICs of tetracycline and clindamycin but no decrease in the level of RNA III after oxacillin treatment. Consequently, the mechanisms involved in the modulation of PSM production by antibiotics may be complex, also including the PSM-specific export system, Pmt (phenol-soluble modulin transporter) (92), and its recently described transcriptional regulator, PmtR (93).
Protein A (SpA).
Protein A is an adhesion molecule (MSCRAMM) and is one of the major virulence determinants of S. aureus. It promotes immune evasion by binding to the Fc region of antibodies and therefore blocking opsonophagocytosis. SpA is also a candidate for development of vaccines to prevent severe S. aureus infections. The effects of antibiotics on SpA expression have been studied for the past 30 years. A report published in 1986 examined the effect of clindamycin on SpA expression and found that 1/2 and 1/4 MICs of this antibiotic induced significant decreases in SpA production by a laboratory strain (Cowan I) and 3 clinical isolates (57). Subsequently, Herbert et al. and Otto et al. confirmed the inhibitory effects of clindamycin sub-MICs on spa transcription and SpA production for a different laboratory strain (NCTC 8325) and 4 CA-MRSA isolates (ST1, ST8, ST80, and ST30) (13, 39). For the other protein synthesis inhibitory agents, two authors reported that sub-MICs of linezolid (1/2 MIC) induced decreases in SpA production by the reference strain ATCC 29213 and 5 CA-MRSA clinical isolates (13, 33). One author reported that tigecycline at 1/2 MIC decreased SpA production in 4 CA-MRSA strains (ST1, ST80, ST30, and ST398) but not in a CA-MRSA ST8 isolate (13).
Subrt et al. and Nielsen et al. screened several beta-lactams by examining their impacts on spa promoter activity by use of a reporter fusion gene (16, 38). Both reported that sub-MICs of oxacillin, cephalothin, and penicillin induced spa promoter activity (consistent with the increase in spa mRNA levels). Furthermore, Subrt et al. also observed spa promoter upregulation upon methicillin and nafcillin treatment (consistent with qRT-PCR data) (38). In contrast, little or no effect was reported with imipenem, cloxacillin, and cefoperazone. Nielsen et al. also observed spa upregulation upon ampicillin, amoxicillin-clavulanic acid, ticarcillin, cefamandole, cefoxitin, ceftazidime, and cefixime triggering as well as spa downregulation upon cefotaxime, cefepime, and cefuroxime triggering (16).
Moreover, Nielsen et al. reported spa promoter upregulation upon treatment with sub-MICs of fluoroquinolones, whereas aminoglycosides were inhibitory with regard to spa promoter transcription (16). Finally, inconsistent vancomycin effects on SpA production were reported by three different teams: two studies found no relevant impact of vancomycin sub-MICs on SpA production (13, 38), whereas Cázares-Domínguez et al. found a stimulatory effect of vancomycin on SpA production at the MIC (43).
In summary, there is strong evidence to support an inhibitory effect of sub-MICs of clindamycin and linezolid on SpA expression. Moreover, several beta-lactams, such as oxacillin, cephalothin, and penicillin, lead to increased SpA expression, whereas vancomycin does not induce any relevant modification of SpA expression. For tigecycline and aminoglycosides, the available data are still too discrepant to conclude that they have a suppressive effect.
Other staphylococcal virulence factors.
Herbert et al. tested the effects of 0.02 mg/liter clindamycin on FnBP and coagulase production by a laboratory S. aureus strain (NCTC 8325) and a clinical isolate (WCUH29) (39). The aforementioned clindamycin concentration induced increased levels of fnb mRNA and coa mRNA, with a concomitant decrease in coagulase activity. Similar observations were reported by Blickwede et al. for the S. aureus Newman strain treated with clindamycin at 1/2 MIC (94). Moreover, Blickwede et al. showed that sub-MICs of florfenicol led to increased fnb mRNA and coa mRNA levels at mid-exponential growth phase and that a decreased cpa5 mRNA level correlated with reduced capsule production during post-exponential-phase growth (95, 96). Among other protein synthesis inhibitory agents, linezolid, azithromycin, clarithromycin, and telithromycin showed inhibitory effects on coagulase activity at 1/8 MIC (32, 51). Rasigade et al. reported that 1/4 MICs of oxacillin, moxifloxacin, and linezolid led to increased fnbA/B mRNA levels, consistent with the development of a hyperadhesive phenotype in a fibronectin adhesion assay (97). Finally, Bisognano et al. observed increased FnBP production after exposing bacteria to a sub-MIC of ciprofloxacin, but only in fluoroquinolone-resistant S. aureus strains (41, 48). In summary, protein synthesis inhibitory agents lead to decreased activity of staphylococcal coagulase, despite the increase in coa mRNA level, highlighting the differential effect of ribosome-acting antibiotics on transcription versus translation. Studies exploring FnBP have shown that sub-MICs of fluoroquinolones induce increased fnpB mRNA levels consistent with a hyperadhesive phenotype.
An overview of the reviewed data from the in vitro experiments is illustrated in Table 1 and further detailed in Table S1 in the supplemental material.
TABLE 1.
Overview of effects of sub-MIC antibiotic concentrations on S. aureus virulence expression from in vitro experiments
| Antibiotic(s) | Effect on expression of virulence factora |
||||
|---|---|---|---|---|---|
| PVL | TSST-1 | Alpha-hemolysin | Protein A | PSM | |
| Oxacillin, nafcillin, methicillin | ↑↑ | ↑ | ↑↑ | ↑↑ | ↓ |
| Vancomycin | — | — | — | — | — |
| Daptomycin | ↑ or — | ND | — | — | ND |
| Erythromycin | ↓ | ↓ | ↓ | ND | ↑ |
| Clindamycin | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↑↑ |
| Linezolid | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↑↑ |
| Streptogramins A and B | ↓ | ↓ | ↓ | ↓ or — | ND |
| Tigecycline | ↓ | ↓ | ↓ | ↓ | ↑↑ |
| Gentamicin | ND | ↓ | ↓ | ↓ | ND |
| Rifampin | ↓↓ | ↓ | ↓ | ↓ | ND |
| Fluoroquinolones | — | ND | ↑ | ↑ | ND |
↑, significant increase; ↑↑, high increase (>10-fold); ↓, significant decrease; ↓↓, abolished expression; —, no significant effect; ND, not determined.
Effects on Ex Vivo Staphylococcal Properties
Opsonophagocytosis and phagocytosis by neutrophils.
Most of the studies that explored the effects of antibiotics on the interaction of S. aureus with phagocytic cells were performed with lincosamides, such as clindamycin and lincomycin. Many of the results obtained were concordant: preincubation of S. aureus with sub-MICs of clindamycin or lincomycin led to increased susceptibility to opsonophagocytosis and faster PMN-induced killing in the presence of human serum or after phagocytosis (55–58, 62). Increased opsonophagocytosis of S. aureus after lincosamide treatment was based on the enhancement of opsonization through both the C3b complement fraction and antibody binding (55, 57, 58). Furthermore, Veringa and Verhoef showed that preincubation of S. aureus with sub-MICs of clindamycin induced a reduction of protein A synthesis, which is fully concordant with the immunoglobulin (Ig)-mediated increased opsonization hypothesis (57). Overall, Milatovic et al. and Veringa and Verhoef reported that subinhibitory concentrations of clindamycin alter the S. aureus morphology or cell wall, allowing better opsonization and subsequent enhancement of phagocytosis (55, 57, 58). Additionally, supernatants of S. aureus strains preincubated with 1/4 MIC of lincomycin resulted in a significant increase (approximately 2-fold) of PMN chemotaxis through a Boyden chamber (62), showing that antibiotics can also alter the production of soluble excreted staphylococcal factors with chemotactic activity for PMNs. Likewise, S. aureus treated with 1/2 MIC of linezolid displayed increased opsonophagocytosis by human PMNs, probably due to a decrease in SpA synthesis (32). To summarize, both lincosamides and oxazolidinones have the ability to enhance opsonophagocytosis and S. aureus killing by PMNs.
Furthermore, the effect of beta-lactams on S. aureus opsonophagocytosis varied according to the different classes and growth culture conditions. Using liquid broth cultures, Milatovic observed that S. aureus preincubation with 1/3 MIC of piperacillin and penicillin G did not alter bacterial opsonophagocytosis compared to that with S. aureus preincubated without antibiotics (56). Likewise, Root et al. showed that S. aureus uptake by PMNs was unchanged by penicillin G treatment, though the treatment resulted in a higher degree of susceptibility to PMNs (54). Similarly, Lorian and Atkinson (53) reported that oxacillin pretreatment of S. aureus grown on membranes did not significantly modify opsonophagocytosis. However, as for penicillin G, oxacillin pretreatment of broth-cultured S. aureus resulted in more susceptibility to PMN killing, while for membrane-cultured S. aureus, the same authors failed to observe an enhancement of PMN-induced killing. Moreover, the oxacillin-induced development of bacterial cell clusters on the membranes was deemed to be the cause of decreased susceptibility to PMNs' early bactericidal effect (after 30 and 60 min), even if the final killing effects (after 2 and 3 h) were similar with and without oxacillin treatment (53). On exploring the effects of cephalosporins, Labro et al. reported that S. aureus pretreated with 1/2 MIC of ceftriaxone, in either liquid broth or solid culture, were opsonophagocytosed and killed more efficiently by PMNs (98). The discrepancies between the effects observed with different beta-lactams could be explained by their affinities for PBPs and the subsequent impact on the staphylococcal cell wall. Finally, Elliot et al. showed that addition of both penicillin G and cephalothin to the culture medium led to a significant increase in the killing of already phagocytosed cells, probably by alteration of the bacterium by absorbed antibiotics (64). Altogether, these data support the fact that pretreatment of broth S. aureus cultures with sub-MICs of various beta-lactam classes does not modify S. aureus opsonophagocytosis but improves S. aureus killing by PMNs. These observed effects may differ from one beta-lactam to another, in connection with the targeted PBPs, thereby resulting in different changes in the structure of S. aureus and its susceptibility to PMN bactericidal mechanisms.
Given their ability to concentrate inside phagocytes and to promote the host's antibacterial responses, macrolides have also been brought into focus with regard to their effects on staphylococcal opsonophagocytosis. Hence, sub-MICs of erythromycin, but not azithromycin, resulted in an improvement of S. aureus opsonophagocytosis (59). The authors of that study explained this discrepancy by a reduction in azithromycin's antibacterial activity in the experimental setting, particularly in the acidic lysosome compartment. In addition, Herrera-Insúa et al. showed that the bactericidal activity of PMNs was enhanced by sub-MICs of azithromycin, thus contributing to a synergic effect on S. aureus killing (63).
Among the other classes of antibiotics studied, sub-MICs of doxycycline have been reported to improve S. aureus opsonophagocytosis, whereas fluoroquinolones (ofloxacin, ciprofloxacin, and gemifloxacin), gentamicin, and vancomycin had no significant impact on S. aureus opsonophagocytosis by PMNs (56, 60, 61). Nevertheless, preexposure of S. aureus to vancomycin and gemifloxacin resulted in increased PMN killing (54, 61).
In vitro hemolysis.
All of the selected studies that addressed in vitro hemolysis reported that sub-MICs of nafcillin induced significant increases in the hemolytic activity of S. aureus supernatants without regard to the methicillin susceptibility of the strain (34, 42). The increases in hemolytic activity ranged from 2- to 6-fold. Worlitzsch et al. reported similar observations with amoxicillin for one of three tested MSSA strains isolated from clinical samples (42). Moreover, they observed no impact of sub-MICs of moxifloxacin or gentamicin on the hemolytic activity of supernatants from antibiotic-treated S. aureus. Using a method of homogenous spreading of the staphylococcal inoculum on blood agar and subsequent deposition of cefoxitin Etest strips, Kuroda et al. confirmed that there was increased S. aureus hemolysis upon the diffusion of sub-MICs of cefoxitin into the medium (45). In contrast to the results for beta-lactams, Gemmell and Ford reported that sub-MICs of linezolid ranging from 1/2 to 1/8 MIC resulted in decreased hemolytic activity of the supernatants of two laboratory S. aureus strains (32). The similarity in the effects of the antibiotics on hemolysis and alpha-hemolysin, TSST-1, and PVL expression (i.e., increased expression upon beta-lactam treatment and decreased expression after protein synthesis inhibitory antibiotic treatment) supports the hypothesis of the involvement of global virulence regulators in staphylococcal virulence modulation (as developed below).
Adhesion to synthetic or organic surfaces and biofilm formation.
The variety of cell surface proteins associated with S. aureus adhesion exemplifies the complexity of studies in this field. In addition, other virulence factors are often included in these studies, because molecules such as protein A are used both to evade host immune recognition and to initiate adhesion and biofilm formation (99). Adhesion of S. aureus to synthetic or organic surfaces is the first step toward forming a biofilm, which is ultimately characterized by a highly complex architecture in its mature form (100). The genetic control of adhesion and biofilm formation is mostly correlative; therefore, the impacts of antibiotics on both phenomena follow similar patterns. Schilcher et al. reported upregulation of the expression of major adhesion genes, including fnbA/B, following exposure to 1/4 MIC of clindamycin (101). Fluoroquinolones, mainly ciprofloxacin and levofloxacin, have also been shown to increase bacterial production of FnBP(s) and attachment to artificial surfaces (59, 102). Cázares-Domínguez et al. reported that vancomycin (1/2 MIC) increased the expression of spa as well as the production of SpA >4-fold during post-exponential-phase growth, which correlated with vancomycin induction of biofilm formation (43). Nielsen and colleagues evaluated the effects of cell wall-active antibiotics on RNA III and spa transcription and on the biofilm phenotype. RNA III and spa expression was reduced on exposure to penicillins and to the compared non-cell-wall-active antibiotics, i.e., fluoroquinolones and aminoglycosides. The tested cephalosporins (cephalothin, cefamandole, cefoxitin, ceftazidime, cefixime, cefuroxime, cefotaxime, and cefepime) enhanced RNA III expression but had divergent effects on spa transcription (16). A separate study also found ceftaroline to have a strain-dependent effect on adhesion-associated genes (103). Moreover, studies have consistently shown that beta-lactam antibiotics stimulate biofilm production, more prominently noted for MRSA than for MSSA because beta-lactams induce MRSA extracellular DNA (eDNA) release that contributes to biofilm formation and adherence (91, 104, 105). Similarly, sub-MICs (1/4 MIC) of clindamycin altered the biofilm matrix composition by modifying eDNA release and the autolysis rate by increasing the expression of adhesion factors (SpA and FnBP) and secreted proteins (PSMβ), thus resulting in a more compact and stable biofilm (106). Limited studies exist on the effects of other protein synthesis inhibitors on S. aureus biofilm formation. In one study using both microarray and RT-PCR to evaluate gene expression, azithromycin at sub-MICs decreased biofilm formation by MRSA in a dose-dependent manner (107), while one of the recent anti-MRSA agents, tigecycline, was found to increase the expression of fnbA, clfB, and cna (15). In summary, low beta-lactam and clindamycin concentrations induce biofilm formation by increasing adhesion protein expression, by releasing eDNA, and by modifying the extracellular matrix composition. For the other antibiotics investigated, the observed effects were concentration and strain dependent and did not support a common pattern.
Proinflammatory response after staphylococcal stimulation.
Antibiotics may have the added benefit of attenuating the host's response to S. aureus toxins, in part due to inhibition of virulent toxin production but also due to their direct immunomodulatory properties. The role of antibiotics in this regard has been studied largely for antibiotics that inhibit protein synthesis. Several studies examined the effect of linezolid on the host inflammatory response in vitro by using concentrations ranging from sub-MICs to supratherapeutic concentrations. Although phagocytic cells, primarily PMNs, rapidly and extensively take up linezolid (108), this does not appear to affect PMN chemotaxis or phagocytosis of S. aureus (109, 110). In studies evaluating cytokine production following staphylococcal or toxin stimulation, Kushiya et al. found no effect of linezolid, vancomycin, teicoplanin, or arbekacin on cytokine production by TSST-1-stimulated PBMCs, whereas the macrolide antibiotic azithromycin slightly suppressed the production of proinflammatory cytokines (111). Pichereau et al., using antibiotic doses at the maximum concentrations found in serum, noted that clindamycin, daptomycin, vancomycin, and azithromycin had inconsistent effects on staphylococcal toxin-stimulated cytokine production by PBMCs. Linezolid inhibited TNF-α and IL-8 production and tigecycline inhibited IL-6 and gamma interferon (IFN-γ) production following PBMC toxin stimulation. Additionally, in that study, trimethoprim-sulfamethoxazole increased TNF-α and IL-8 production (112). Dey et al. reported that azithromycin was more effective than ciprofloxacin at regulating cytokine release from phagocytic cells (113). A study by van Langevelde et al. compared antibiotics in the beta-lactam class with protein synthesis inhibitors for the ability to alter endothelial cell secretion of selected chemokines following incubation with S. aureus. Endothelial cells treated with beta-lactam released higher concentrations of IL-8 and monocyte chemotactic protein-1 than those treated with the protein synthesis inhibitors erythromycin, clindamycin, and gentamicin (114). In a separate study, English et al. noted that daptomycin exposure reduced the inflammatory response of macrophages to S. aureus (reduced TNF-α release and reduced the accumulation of nitric oxide synthase compared to those with vancomycin or oxacillin) (78). To summarize these various studies, after staphylococcal stimulation of host cells, treatment with protein synthesis inhibitors (erythromycin, clindamycin, azithromycin, and linezolid) may lead to variable suppression of the production of proinflammatory cytokines. Among cell wall-active antibiotics, daptomycin rather than vancomycin or oxacillin reduced the inflammatory response of macrophages to S. aureus.
MECHANISMS INVOLVED IN S. AUREUS VIRULENCE MODULATION BY ANTIBIOTICS
The mechanisms underlying the modulation of staphylococcal virulence by antibiotics are still not well understood. Depending on the molecule, several modulatory pathways have been investigated to date. Linezolid and clindamycin act mainly by blocking ribosomal function and suppressing the protein synthesis of virulence factors as well as the synthesis of regulators of virulence expression. Other antibiotics, such as the beta-lactams, actively induce exoprotein gene transcription by triggering the SOS response in bacteria or by interfering with the complex regulatory network that governs virulence expression in S. aureus.
Protein Synthesis Inhibitory Agents
The most accepted explanation for the effect of clindamycin on S. aureus virulence is linked to the ribosome-blocking action. Thus, Herbert et al. investigated the effect of clindamycin on hla and tst expression in a strain harboring the ermB gene (S. aureus NCTC 8325 with a silent insertion of Tn551); they showed that the effect of clindamycin was eliminated by the standard macrolide-lincosamide-streptogramin B (MLSB) resistance mechanism, indicating that the basic biological activity of clindamycin was responsible for the observed effects (39). Using transcription fusion experiments, they showed that clindamycin also inhibits exoprotein transcription, an effect that is also abolished by the MLSB resistance mechanism. The suggested explanation for these observations is that clindamycin specifically interferes with the translation of one or more proteins that regulate transcription of the exoprotein genes. Moreover, some of the two-component signal transduction systems (TCS), which act as global regulators of S. aureus virulence expression, may be hampered by clindamycin. This hypothesis is consistent with more recent findings by Novick and Jiang showing that sub-MICs of clindamycin interfere with the regulation of the saeRS system, which is a strong positive regulator of exoprotein expression in S. aureus (115, 116). Therefore, antibiotic-induced dysfunction of the saeRS system may be partly responsible for the suppression of virulence expression in S. aureus upon clindamycin treatment.
Although linezolid also markedly suppressed exotoxin expression, its effects on transcription were highly variable. Upon linezolid treatment of five CA-MRSA strains, PVL and Hla release was constantly reduced, while the toxin-specific mRNA levels decreased for only two strains, suggesting that linezolid inhibits virulence expression mainly by blocking ribosomal translation (13). Whereas Stevens et al. reported no effect of linezolid on PVL gene translation (4), Otto et al. found that linezolid reduced mRNA levels of PVL (10, 13), though to a lesser extent than that with clindamycin, and only at concentrations close to the MIC. These discrepancies point to a possible clone-dependent specificity of virulence modulation by antibiotics. Indeed, Stevens et al. studied a clinical isolate of CA-MRSA belonging to the USA400 lineage (4), whereas Otto et al. explored five strains belonging to various CA-MRSA lineages. Moreover, Otto et al. observed an inhibitory effect of linezolid and clindamycin on spa transcription in ST30 and ST80 isolates, contrasting with the case for the USA300 lineage, for which repeated experiments conducted with two isolates failed to generate similar results (117). One explanation may be that antibiotics affect virulence expression by interfering with the complex S. aureus regulatory network, which responds to environmental triggers in a clone-specific manner (117).
However, linezolid-induced effects are more heterogeneous than those of clindamycin, possibly because different pathways underlie them. Thus, Otto et al. reported a dramatic increase of hla mRNA levels (9.74-fold) in linezolid-treated ST80 CA-MRSA strain cultures (13). This observation can be explained by the fact that 50S ribosome-inhibitory agents, such as linezolid, block several steps of the translational process, resulting in the accumulation of mRNA and other intermediate products of the translational complex (118). Consistently, using a hollow-fiber infection model, Pichereau et al. reported that linezolid may also increase the expression of the PVL and enterotoxin genes at 72 h (26). To summarize, linezolid triggers various modulatory pathways with potential effects on virulence expression (ribosome blockage, transcriptional upregulation, and interference with global regulatory networks), and the final outcome is forged by the complex balance of these responses, which are intimately linked to the genetic backgrounds of strains.
Cell Wall-Disrupting Antibiotics
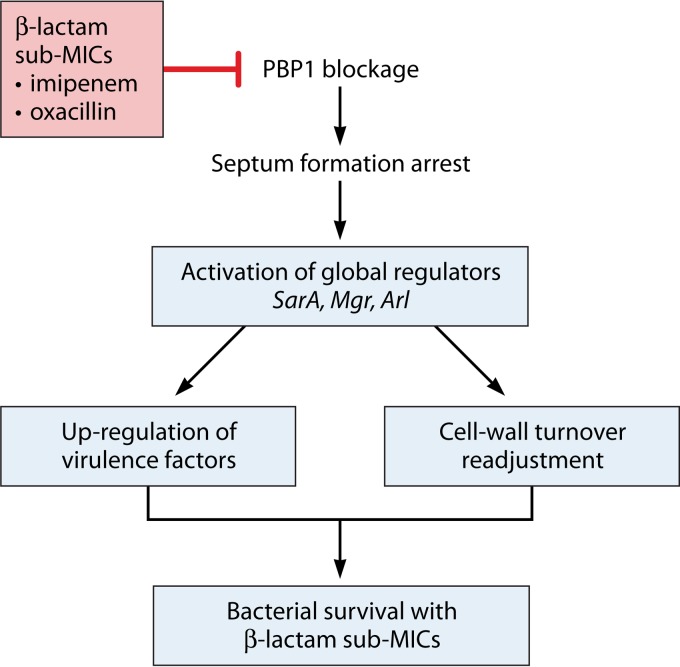
Numerous reports support the idea that beta-lactams lead to increased exotoxin levels (PVL, TSST-1, Hla, and enterotoxins) not only due to the release of intracellular toxin after bacterial lysis but also by actually increasing exotoxin gene expression (4, 10, 29). Active transcription from the PVL and Hla promoters after beta-lactam treatment has been established clearly by transcriptional fusion assays (10, 29, 40). Moreover, nafcillin was shown to upregulate PVL mRNA expression to a constantly elevated level for up to 34 h after antibiotic treatment, thus maintaining the increased toxin production (4). Meanwhile, vancomycin treatment did not affect PVL production, suggesting that the blockage of cell wall synthesis is not sufficient to increase toxin expression (4, 10). Moreover, not all beta-lactams increase PVL expression; only compounds such as oxacillin, nafcillin, and imipenem strongly induce PVL production, suggesting that these antibiotics trigger specific signaling mechanisms and the activation of regulatory pathways (5), which are discussed later.
CWSS, autolysis, and virulence modulation.
Beta-lactams, along with glycopeptides, bacitracin, and d-cycloserine, belong to the class of cell wall-disrupting antibiotic agents. Cell wall-active antibiotics inhibit peptidoglycan biosynthesis, thus resulting in bacterial growth arrest. S. aureus triggered with cell wall-active antibiotics displays a specific pattern of gene and protein expression. Using proteomic and transcriptomic approaches, it has been shown that a set of genes called the cell wall stress stimulon (CWSS) is triggered by cell wall-active antibiotics, possibly resulting in the modulation of virulence (119, 120). The CWSS also responds to peptidoglycan hydrolysis, to the inhibition of cell wall synthesis independent of antibiotics, and to other signals, such as the VraRS system activators that stimulate the intramembrane sensor VraS and, subsequently, the response regulator VraR (121). CWSS core genes include murZ, encoding one of the initiators of peptidoglycan biosynthesis (122); pbp2 and sgtB, which are involved in transglycosylation; fmtA, encoding a cell division septum-associated esterase that controls the d-Ala content of teichoic acids (123); and tca and vraR, which are involved in glycopeptide resistance (124). The CWSS is activated in various settings when S. aureus faces defective peptidoglycan synthesis; CWSS activation results in enhanced cell wall synthesis and a balanced decrease in autolysis (120). Perturbation of the CWSS by a nonantibiotic stimulus was investigated by Sobral et al. in an S. aureus murF conditional mutant with IPTG (isopropyl-β-d-thiogalactopyranoside)-controlled murF transcription (106). Microarray gene expression profiling of the murF mutant cultured under suboptimal conditions showed, in addition to the activation of genes belonging to the CWSS, some changes in the expression of virulence-related genes. These included several important virulence determinants, such as fibronectin-binding proteins A and B, clumping factor A, enterotoxin B, and gamma-hemolysin BC. Thus, in addition to modifications in CWSS-associated gene expression and in some metabolic pathways, cell wall stress induced upregulation of a surprisingly large number of virulence determinants, perhaps suggesting an enhanced “defensive” posture by the bacterium during a state of compromised synthetic capacity (106).
Concomitantly, cell wall stress in the murF suboptimal model resulted in low autolytic activity, which correlated with changes in the expression of the autolysis regulators LrgA and -B and the global regulator SarY. Peptidoglycan hydrolysis or autolysis in S. aureus is performed mainly by three nonessential enzymes: the major autolytic enzymes Atl and Sle1, which are murein hydrolases with an important role in the separation of daughter cells (125), and LytM, a glycylglycine endopeptidase involved in cell growth (100). To maintain cell wall integrity, the expression and activities of autolytic enzymes need to be finely tuned. Many environmental stimuli control the expression of autolysis-associated proteins at the posttranscriptional level. Nevertheless, the expression of some autolytic enzymes is also regulated at the transcriptional level. Several TCS systems and global regulators control the autolytic activity in S. aureus; these include MgrA (126), ArlRS (127), and SarA (102), all of which negatively modulate autolytic activity. Recently, Antignac et al. showed that a beta-lactam-associated disturbance of cell wall synthesis induced a strong repression of autolytic activity that was consistent with decreased transcription of the atl and sle-1 genes. These observations support the hypothesis that the S. aureus regulatory network maintains balanced control of autolytic activity in response to the perturbations of cell wall synthesis, and they provide evidence for coordinated transcriptional regulation of cell wall synthetic and hydrolytic enzymes (104). Although the regulator involved has not yet been identified, most of the above-mentioned TCS or transcriptional regulators that govern autolysis activity are also virulence regulators. Therefore, suppression of autolysis by cell wall-active antibiotics may be the critical link that connects the antibiotic trigger with regulatory modifications that result in enhanced S. aureus virulence.
Beta-lactams and PBP interference.
The bactericidal effect of beta-lactam antibiotics is based on their interaction with transmembrane penicillin-binding proteins (PBPs), which act as enzymes involved in peptidoglycan biosynthesis. Four PBPs have been detected (105) in wild-type S. aureus strains, among which PBPs 1 and 2 are essential (128). For different lineages of CA-MRSA strains, it was shown that PVL production is enhanced by sub-MICs of oxacillin (a nonselective beta-lactam) or imipenem (PBP1 selective [129]) but not cefotaxime (PBP2 selective [130]), cefaclor (PBP3 selective [131]), or cefoxitin (PBP4 selective [132]). This observation, which suggests that PBP1-specific blockage may be a stimulus for PVL induction upon beta-lactam treatment, was confirmed in a PBP1-specific inducible antisense RNA assay (5). The correlated kinetics of inducible pbp1 antisense RNA expression and the measured PVL mRNA level confirmed that a PBP1 defect might be the cause of increased PVL expression by S. aureus. PBP1 was recently shown to play a major role in the formation of the division septum involved in S. aureus daughter cell separation, by interacting with the autolytic system (133). Using an IPTG-inducible PBP1 conditional mutant, Pereira et al. demonstrated that suboptimal function of the transpeptidase domain of PBP1 induced transcriptional downregulation of the atl and sle-1 genes, which encode autolysins involved in the separation of daughter cells (134), consistent with the previous findings of Antignac et al. (104). It has been hypothesized that proper division in S. aureus is controlled by checkpoint-type mechanisms in which the transpeptidase activity of PBP1 takes an important part. In the case of a lack of PBP1 transpeptidase activity, as well as in the presence of beta-lactams, the newly synthetized deficient cell wall acts as a stimulus leading to repression of the autolytic system. By this means, only bacteria which have completed appropriate cell wall synthesis may proceed to the next division (134). At this point, it is interesting that control of autolysis in response to cell wall synthesis defects is governed by global regulators, such as MgrA, SarA, and ArlRS, which also modulate virulence expression in S. aureus; this scenario probably explains the PBP1-dependent beta-lactam induction of PVL expression reported previously (Fig. 2) (5).
FIG 2.
Mechanisms underlying the enhanced virulence expression by S. aureus triggered by beta-lactams. The presence of antibiotics is sensed by the bacterium as an environmental signal resulting in the arrest of septum formation; this then triggers the activation of global transcriptional regulators to adjust the cell wall turnover, eventually leading to enhanced toxin production, probably as an additional effect of bacterial adaptation to suboptimal growth conditions.
SOS response triggered by antibiotics and virulence expression modulation.
The SOS response is one of the possible stress responses developed by bacteria upon exposure to antibiotics (135). The classical SOS response is controlled by two major determinants: lexA and recA (136, 137). Though repressed under normal environmental conditions, the SOS response is initiated, by RecA activation, as a result of genotoxic damage. RecA then acts as a coprotease to initiate LexA repressor autocleavage, ultimately leading to the upregulation of an ensemble of DNA repair and recombination genes. LexA is a transcription factor able to bind to a large number of promoters with various affinities, which may increase consequent to severe or persistent DNA damage (138). Fluoroquinolones, which are DNA-damaging antibiotics, and beta-lactams, which promote defective cell wall synthesis, both initiate the SOS response at subinhibitory concentrations (139, 140). It has been shown that ciprofloxacin, a fluoroquinolone, induces LexA-dependent activation of the fnbB gene upon antibiotic stress (141). PBP1-specific blockage by beta-lactams was reported to mediate the SOS response through lexA/recA regulators (142). This may partially explain the increased transcription of exotoxin genes after cells were exposed to PBP1-selective beta-lactams, although no experimental data are available to support this hypothesis at this time.
Several virulence determinants modulated by antibiotics are encoded by transferable genetic elements, such as pathogenicity islands (PI) (TSST-1 and SaPIbov) (143) or prophages (PVL and phiPV83-pro, phiPVL, or phi11) (144). Although fully integrated into the regulatory mechanism of the host, the expression of transferable pathogenicity genes is tightly linked to the mobile element's genome, and these genes depend on the mobile element for horizontal transfer (145, 146). SOS-inducing antibiotics, such as fluoroquinolones and beta-lactams, may induce the transcription of genes carried by transferable elements both by activating their replication and by triggering phage internal promoters independently of the S. aureus regulatory network (145–147). Therefore, the SOS response-inducing antibiotics may activate virulence expression by two regulatory pathways: a lexA-dependent derepression of virulence-determining genes and an overexpression of virulence genes as a consequence of the activation of the internal regulatory mechanisms associated with transferable elements.
Involvement of global virulence regulators.
Upon exposure to an antibiotic trigger, similar patterns of expression have been observed for a large number of staphylococcal virulence factors, suggesting a pleiotropic effect of antibiotics that may involve global virulence regulators that fine-tune virulence factor production by S. aureus in response to the changing environment. These global regulatory systems include several TCS encoding a histidine kinase sensor and a response regulator. The core whole-genome sequence of S. aureus reveals that it contains 16 TCS, some of which have been well characterized as global regulatory systems. These are mainly represented by the accessory gene regulator Agr (148), the autolysis-related arlRS locus (127), the S. aureus exoprotein expression genes saeRS (116), and the staphylococcal respiratory response genes srrAB (149). More recently, another TCS involved in cell wall synthesis and turnover, the WalK/WalR system, was also shown to positively regulate some of the major virulence genes involved in host-matrix interactions (efb, emp, and fnbA/B) and cytolysis (hlgACB, hla, and hlb) through activation of saeRS (150). Similar to the WalK/WalR system, Agr, Sigma B, and SarA also affect the level of transcription of saeRS, thus placing saeRS downstream in the regulatory network as a key element that governs the expression of S. aureus exoproteins (115). This makes saeRS a good candidate for a TCS that may control S. aureus virulence expression upon exposure to an antibiotic trigger. Sub-MIC clindamycin concentrations delay the transcriptional shift normally observed in the saePQR promoters, which seems to be linked to the suppression of exotoxin expression in clindamycin-treated strains (115). SaeP promoter transcription is also blocked by linezolid at the mid-exponential growth phase, resulting in a lack of activation of the saeRS system, and consistent with the inhibitory effect of linezolid on exotoxin production by S. aureus (151). In contrast to protein synthesis inhibitory agents, the beta-lactam cefoxitin increased gamma-hemolysin expression by the S. aureus N315 strain in an SaeRS-dependent manner. Indeed, transcriptional studies showed a 2-fold induction of hlgC expression by cefoxitin, which was suppressed in the saeRS null mutant (45).
To explore the role of the S. aureus regulation network in PVL expression upon beta-lactam treatment, isogenic S. aureus phiPVL RN6390 strains individually deleted for the agr, saeRS, rot, and sarA loci were exposed to sub-MICs of imipenem. PVL production was assessed to determine the impact of each of these regulators on the modulator effect of imipenem on PVL expression. Experiments with agr and saeRS null mutants yielded increased PVL levels after antibiotic treatment; therefore, these regulators were presumed to be nonessential for PVL induction by beta-lactams. This inconsistency with the previous conclusions of Kuroda et al. (45) may be explained by strain variations (RN6390 versus N315), the molecules tested (imipenem versus cefoxitin), or the measured toxin production (PVL versus Hlg). With respect to antibiotic mechanisms, imipenem acts as a PBP1-selective blocker, while cefoxitin acts as a PBP4-selective blocker; therefore, one may hypothesize that different signaling pathways were triggered by these agents.
Upon imipenem treatment of sarA and rot null mutants, increased PVL expression could no longer been achieved. Restored phenotypes of the complemented strains confirmed the essential role of these regulators as mediators of PVL induction by beta-lactams (5). Kinetic measurement of the transcription levels of these regulators during S. aureus incubation with sub-MICs of imipenem showed increased SarA expression initiated by the antibiotic stimulus followed by Rot downregulation, resulting in subsequent enhanced PVL expression. The pathways leading to SarA trigger by beta-lactams remain unknown, while Rot downregulation may occur either as a direct effect of the antibiotic treatment or as a consequence of SarA overexpression (152).
Taken together, these observations suggest that the presence of antibiotics is sensed by the bacterium as an environmental signal; this then triggers TCS and the production of transcription factors, eventually leading to enhanced toxin production, probably as an additional effect of bacterial adaptation to suboptimal growth conditions.
THERAPEUTIC IMPACT OF S. AUREUS VIRULENCE MODULATION BY ANTIBIOTICS
Animal Infection Model Evidence
Based on initial in vitro data suggesting that protein synthesis inhibitory agents (clindamycin and linezolid) may abolish staphylococcal virulence factors and that cell wall-active antibiotics may increase toxin production at sub-MICs, animal studies have addressed the question of whether the administration of these antibiotics, in pharmacokinetic contexts that mimic therapeutic regimens in humans, would translate into differences in treatment efficacy and outcome.
Because rabbits are similar to humans in their susceptibility to PVL (153–155) and because PVL plays a major role in the pathogenesis of necrotizing pneumonia in this animal species (154, 156), linezolid and vancomycin were assessed in a model of CA-MRSA USA300-induced necrotizing pneumonia in rabbits to determine the efficacy of these drugs and whether protection is correlated with suppression of bacterial toxin production in the lungs. In this model, the overall mortality rate was 25% for animals treated with linezolid at a dosing regimen that yielded a peak serum concentration of 10.5 ± 2.3 μg/ml 1 h after dosing, 88% for animals treated with vancomycin at a dosing regimen that yielded a peak serum concentration of 36.1 ± 4.2 μg/ml 1 h after dosing, and 100% for untreated rabbits (157). Specific ELISA quantitation of PVL produced in the lungs of infected rabbits yielded significantly decreased levels of PVL in the linezolid-treated group (31%) compared to those in the vancomycin-treated group (57%) or the untreated group (100%).
A recent study confirmed that vancomycin is inferior to linezolid as well as to clindamycin in another rabbit model of CA-MRSA LAC USA300-induced necrotizing pneumonia, as the two protein synthesis inhibitors were more effective than vancomycin at suppressing bacterial production of PVL in rabbit lungs (158). In that study, a human-equivalent dosing regimen of ceftaroline, an anti-MRSA beta-lactam antibiotic, also demonstrated great protection against lethal pneumonia, likely due to its enhanced capacity to kill MRSA in the lungs.
In a mouse hematogenous pneumonia model, linezolid also demonstrated efficacy superior to that of vancomycin in protecting not only against death caused by PVL-negative vancomycin-insensitive S. aureus (VISA) but also against death caused by PVL-positive MRSA strains (159, 160). Because mice are insensitive to PVL leukotoxicity, the enhanced protective effects of linezolid compared to those of vancomycin may not be linked to decreased PVL production by S. aureus in mouse lungs. Linezolid may suppress in vivo expression of other bacterial toxins, e.g., alpha-toxin, that have proven roles in the mouse pneumonia model (161), although toxin levels in mouse lungs were not measured in these studies. However, linezolid was significantly better than vancomycin at limiting bacterial replication in mouse lungs, which may explain its enhanced protective efficacy (159, 160).
In piglet models of ventilator-associated pneumonia, linezolid showed better efficacy than that of vancomycin (162, 163). Piglets treated with linezolid showed less severe lung pathology, better clearance of MRSA, and longer survival times, but these effects could not be attributed to differences in PK/PD parameters, because all linezolid and vancomycin measured concentrations (plasma, epithelial lining fluid, and lung samples) were higher than the MICs (162, 163). In the absence of a clear reason for the better efficacy of linezolid (although toxin concentrations in the lungs of piglets treated with linezolid or vancomycin were not measured), Luna et al. speculated that the immune response may be modulated by linezolid (162). Nonetheless, linezolid did not seem to exhibit an advantage over vancomycin in modulating the pulmonary innate immune response in a mouse model of MRSA pneumonia (164).
To confirm the effects of sub-MICs of antibiotics on bacterial toxin gene expression in vivo, with potential therapeutic consequences, a bioluminescence reporter system in which the light-producing luxABCDE operon is fused to the alpha-toxin promoter was used in a mouse sepsis model. This bioluminescence reporter system aimed to show that in vitro hla promoter activity is enhanced by sub-MICs of teicoplanin, imipenem, and ciprofloxacin but not by sub-MICs of clindamycin and rifampin (40). Although it was possible to assess hla promoter activity by using this bioluminescence reporter system in a mouse infection model, this system may not be useful for evaluating the effects of antibiotic sub-MICs in vivo because of the difficulty of achieving specific antibiotic concentrations in the mouse.
Clinical Evidence
Compared to the solid body of in vitro experiment-based evidence, inpatient data supporting staphylococcal virulence modulation by antibiotics are rather frail. The main reason explaining the lack of clinical data is the pleiotropic presentation of severe staphylococcal diseases, which makes it very difficult to design the relevant clinical trials necessary to thoroughly investigate the role of virulence-modulating antibiotics in patient outcomes. Consequently, clinical guidelines offer conflicting recommendations on the use of protein synthesis inhibitors (e.g., linezolid and clindamycin) versus standard agents (e.g., vancomycin) to treat invasive MRSA infections. The United Kingdom's clinical guidelines, which seem to put a greater emphasis on in vitro and preclinical animal infection model data than the Infectious Diseases Society of America (IDSA) and Canadian clinical guidelines, recommend the use of clindamycin and linezolid because these agents suppress the production of PVL and other toxins and have been used successfully for treatment of severe sepsis and pneumonia (6, 7).
Several clinical case reports suggest that clindamycin or linezolid can be used for the treatment of severe sepsis or pneumonia caused by S. aureus, especially for toxin-producing strains. An early case report of four patients with pleuropulmonary complications of PVL-positive community-associated MRSA pneumonia noted not only inappropriate initial antimicrobial therapy but also that three of the patients failed to respond to subsequent treatment with vancomycin (165). These patients were treated subsequently with linezolid or vancomycin, with good clinical results. In a larger case series of 15 patients with community-associated MRSA pneumonia, 14 were treated with clindamycin or linezolid; only 2 (13%) of the patients died, both of whom were severely immunocompromised (166). Retrospective analysis of 92 cases of community-associated S. aureus necrotizing pneumonia, the vast majority of which were associated with PVL-positive strains, showed improved clinical outcomes in those who were treated with clindamycin or linezolid (167).
Another case series of three patients with PVL-positive S. aureus necrotizing pneumonia demonstrated successful treatment with multiple agents, some of which target bacterial toxin production (168). The rationale for the use of multiple agents, including (i) vancomycin plus clindamycin, (ii) clindamycin and linezolid plus intravenous immunoglobulin (IVIG), and (iii) clindamycin, linezolid, and ofloxacin plus IVIG, may be based on in vitro synergy but grounded in the fact that linezolid and clindamycin are known to inhibit in vitro bacterial toxin production. For one of the three patients, PVL was shown to be present at toxic concentrations in serial sputum samples, peaking at 3.9 mg/liter on day 2 postadmission (168). The addition of IVIG, which was previously shown to contain antibodies that neutralize PVL-mediated cytotoxicity of human PMNs (169) and to protect against lethal necrotizing pneumonia in a rabbit preclinical model (156), may improve clinical outcomes by neutralizing the toxic effects of these preformed toxins that are not targeted by antimicrobial treatment.
Another case report noted the successful treatment with linezolid of a patient with staphylococcal toxic shock syndrome caused by a MRSA strain producing toxic shock syndrome toxin type 1 (TSST-1) (86). Production of TSST-1 by the infecting MRSA strain was shown to be suppressed in vitro at concentrations of linezolid as low as 1 mg/liter (i.e., 1/4 MIC).
Though the issue of virulence modulation was not specifically addressed, randomized controlled clinical trials have provided evidence that linezolid has a higher clinical efficacy than that of vancomycin for the treatment of nosocomial MRSA infection (170). The two major factors explaining linezolid's efficacy were the greater penetration into the epithelial lining fluid and the significantly lower incidence of renal failure than that with vancomycin. Whether linezolid effectively contributes to decreased S. aureus virulence during infection remains to be proven in order to better understand the mechanisms underlying the clinical efficacy of antibiotics as an important factor in directing appropriate treatment decisions.
CONCLUSIONS
Antibiotics, mainly at subinhibitory concentrations, clearly modulate virulence expression in S. aureus. Most in vitro data point to a suppressive effect of ribosome-active antibiotics (linezolid and clindamycin) on virulence expression, whereas cell wall-active antibiotics (beta-lactams) mainly increase exotoxin production.
In vivo studies also support the suppressive effect of clindamycin and linezolid on virulence expression, supporting their utilization in the management of toxin-associated staphylococcal diseases and offering a potentially valuable strategy to improve patient outcomes.
The mechanisms underlying the effects of clindamycin and linezolid on S. aureus virulence are intimately linked to the ribosome-blocking effects of these agents, with subsequent impacts on the functions of global virulence regulators, such as SaeRS. With respect to the inductive effects of beta-lactam antibiotics on staphylococcal virulence, the main hypothesis is that the defect in cell wall synthesis caused by PBP1 blockage is sensed by the bacteria, triggering the regulatory network that controls both autolysis and virulence determinants. The balanced decrease of autolytic activity will then promote the survival of more virulent bacteria. Whether SarA plays a part in the complex regulation which links autolysis, peptidoglycan biosynthesis, and virulence remains to be proven. Deciphering these regulatory pathways may provide new insights into the connections relating the cell wall metabolism to virulence expression in S. aureus. A better understanding of these phenomena may supply a logical basis to explain the emergence of both virulent and resistant S. aureus strains.
Supplementary Material
ACKNOWLEDGMENTS
Although we did not receive any funding for this review, we have previously received research grants from Pfizer Inc. (G.L., O.D., and B.A.D.), Cubist (W.R., G.L., and O.D.), Basilea Pharmaceutica (E.H., O.D., and G.L.), Merck Inc. (W.R.), and Theravance Biopharma (W.R.).
Biographies

Elisabeth Hodille, Pharm.D., received her pharmacy degree from the University of Dijon, France. She did her medical microbiology residency at Université Lyon 1, where she completed her Ph.D. training. She has been an Assistant Professor in the Microbiology Department of the Faculty of Medicine Lyon Sud since 2011, and she is also a fellow of the staphylococcal pathogenesis research team at the Centre Internationale de Recherche en Infectiologie, INSERM U1111. Her research focuses on the pathogenic role of pore-forming toxin in S. aureus infections.

Warren Rose, Pharm.D., Ph.D., received his pharmacy degree from Butler University, Indianapolis, IN, and completed postdoctoral training at Rush University Medical Center (residency), Chicago, IL, and Wayne State University (fellowship), Detroit, MI, studying antimicrobial pharmacokinetics and pharmacodynamics. He joined the faculty at the University of Wisconsin-Madison in 2008, where he is currently an Associate Professor of Pharmacy. The focus of his research since 2008 has been the study of host and pathogen interactions in relation to antimicrobial treatment and resistance development in Gram-positive pathogens. This includes the effects of antibiotics on Staphylococcus aureus virulence. His interests in this area developed from the various patient outcomes observed in his clinical practice that cannot be attributed to antimicrobial susceptibility alone.

Binh An Diep, Ph.D., completed his doctorate in infectious diseases and immunity at the University of California, Berkeley, in 2005. He received postdoctoral training in microbial pathogenesis at the University of California, San Francisco, in 2008. He is currently an Associate Professor of Medicine at the University of California San Francisco School of Medicine. He has been the principal investigator for many works on the pathogenesis of Panton-Valentine leukocidin-producing S. aureus and community-acquired methicillin-resistant S. aureus. The goal of his research is to develop novel strategies (including immunotherapies and vaccines) against severe staphylococcal infections.

Sylvain Goutelle received Pharm.D. and Ph.D. degrees from Université Lyon, France. He has been a visiting researcher at the Laboratory of Applied Pharmacokinetics, USC School of Medicine (Los Angeles, CA), and at the Division of Clinical Pharmacology, University Hospital of Lausanne (Switzerland). He is currently Senior Hospital Pharmacist and Associate Professor of Pharmacy at the University Hospitals of Lyon and the Lyon School of Pharmacy. His research focuses on quantitative pharmacokinetics/pharmacodynamics and their applications for optimization of anti-infective drug therapy.

Gerard Lina, M.D., Ph.D., is Professor of Clinical Microbiology at the School of Medicine and the Postgraduate School of Clinical Microbiology of Université Lyon 1. He received his medical degree from the School of Medicine of Université Lyon 1, where he also completed a Ph.D. in immunology and microbiology. He is Head of the Laboratory of Bacteriology of the Lyon Hospitals, Codirector of the Centre National de Reference des Legionelle, and a fellow of the French National Reference Laboratory for Staphylococci, Lyon, France. He is Chair of the French Society of Microbiology (SFM), Secretary of the French Committee of Antimicrobial Testing (CA-SFM), and a member of the steering committee of EUCAST. He has been the principal investigator for many works on staphylococcal pathogenesis and antimicrobial agents at the Centre Internationale de Recherche en Infectiologie, INSERM U1111, and has coauthored over 200 papers published in peer-reviewed journals.

Oana Dumitrescu, M.D., Ph.D., received her medical degree and microbiology doctorate from Université Lyon 1, France, where she also did her medical microbiology residency. She is Associate Professor in the Microbiology Department of the Faculty of Medicine Lyon Sud, Lyon, France. She has been a fellow of the French National Reference Laboratory for Staphylococci since 2007 and is currently working in the field of S. aureus virulence and resistance to antibiotics, with a particular focus on the effects of antibiotics on staphylococcal virulence.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/CMR.00120-16.
REFERENCES
- 1.Lowy FD. 1998. Staphylococcus aureus infections. N Engl J Med 339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Arditi M, Kabat W, Yogev R. 1993. Antibiotic-induced bacterial killing stimulates tumor necrosis factor-alpha release in whole blood. J Infect Dis 167:240–244. doi: 10.1093/infdis/167.1.240. [DOI] [PubMed] [Google Scholar]
- 3.Nau R, Eiffert H. 2002. Modulation of release of proinflammatory bacterial compounds by antibacterials: potential impact on course of inflammation and outcome in sepsis and meningitis. Clin Microbiol Rev 15:95–110. doi: 10.1128/CMR.15.1.95-110.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens DL, Ma Y, Salmi DB, McIndoo E, Wallace RJ, Bryant AE. 2007. Impact of antibiotics on expression of virulence-associated exotoxin genes in methicillin-sensitive and methicillin-resistant Staphylococcus aureus. J Infect Dis 195:202–211. doi: 10.1086/510396. [DOI] [PubMed] [Google Scholar]
- 5.Dumitrescu O, Choudhury P, Boisset S, Badiou C, Bes M, Benito Y, Wolz C, Vandenesch F, Etienne J, Cheung AL, Bowden MG, Lina G. 2011. Beta-lactams interfering with PBP1 induce Panton-Valentine leukocidin expression by triggering sarA and rot global regulators of Staphylococcus aureus. Antimicrob Agents Chemother 55:3261–3271. doi: 10.1128/AAC.01401-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Health Protection Agency. 7 November 2008. Guidance on the diagnosis and management of PVL-associated Staphylococcus aureus infections (PVL-SA) in England, 2nd ed http://www.sbp.com.br/pdfs/Guidance_diagnosis_management_PVL-associatedStaphylococcus_aureus_infections_PVL-SA_inEngland-HPA.pdf.
- 7.Nathwani D, Morgan M, Masterton RG, Dryden M, Cookson BD, French G, Lewis D, British Society for Antimicrobial Chemotherapy Working Party on Community-Onset MRSA Infections. 2008. Guidelines for UK practice for the diagnosis and management of methicillin-resistant Staphylococcus aureus (MRSA) infections presenting in the community. J Antimicrob Chemother 61:976–994. doi: 10.1093/jac/dkn096. [DOI] [PubMed] [Google Scholar]
- 8.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Rybak MJ, Talan DA, Chambers HF, Infectious Diseases Society of America. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 52:e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 9.Woodin AM. 1959. Fractionation of a leucocidin from Staphylococcus aureus. Biochem J 73:225–237. doi: 10.1042/bj0730225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumitrescu O, Boisset S, Badiou C, Bes M, Benito Y, Reverdy M-E, Vandenesch F, Etienne J, Lina G. 2007. Effect of antibiotics on Staphylococcus aureus producing Panton-Valentine leukocidin. Antimicrob Agents Chemother 51:1515–1519. doi: 10.1128/AAC.01201-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamaki J, Synold T, Wong-Beringer A. 2013. Tigecycline induction of phenol-soluble modulins by invasive methicillin-resistant Staphylococcus aureus strains. Antimicrob Agents Chemother 57:4562–4565. doi: 10.1128/AAC.00470-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner CE, Sriskandan S. 2015. Panton-Valentine leucocidin expression by Staphylococcus aureus exposed to common antibiotics. J Infect 71:338–346. doi: 10.1016/j.jinf.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otto MP, Martin E, Badiou C, Lebrun S, Bes M, Vandenesch F, Etienne J, Lina G, Dumitrescu O. 2013. Effects of subinhibitory concentrations of antibiotics on virulence factor expression by community-acquired methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother 68:1524–1532. doi: 10.1093/jac/dkt073. [DOI] [PubMed] [Google Scholar]
- 14.van Langevelde P, van Dissel JT, Meurs CJ, Renz J, Groeneveld PH. 1997. Combination of flucloxacillin and gentamicin inhibits toxic shock syndrome toxin 1 production by Staphylococcus aureus in both logarithmic and stationary phases of growth. Antimicrob Agents Chemother 41:1682–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith K, Gould KA, Ramage G, Gemmell CG, Hinds J, Lang S. 2010. Influence of tigecycline on expression of virulence factors in biofilm-associated cells of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 54:380–387. doi: 10.1128/AAC.00155-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nielsen LN, Roggenbuck M, Haaber J, Ifrah D, Ingmer H. 2012. Diverse modulation of spa transcription by cell wall active antibiotics in Staphylococcus aureus. BMC Res Notes 5:457. doi: 10.1186/1756-0500-5-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jo A, Ahn J. 2016. Phenotypic and genotypic characterisation of multiple antibiotic-resistant Staphylococcus aureus exposed to subinhibitory levels of oxacillin and levofloxacin. BMC Microbiol 16:170. doi: 10.1186/s12866-016-0791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang H-J, Chen C-C, Cheng K-C, Wu K-Y, Lin Y-C, Zhang C-C, Weng T-C, Yu W-L, Chiu Y-H, Toh H-S, Chiang S-R, Su BA, Ko W-C, Chuang Y-C. 2013. In vitro efficacies and resistance profiles of rifampin-based combination regimens for biofilm-embedded methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 57:5717–5720. doi: 10.1128/AAC.01236-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boles BR, Horswill AR. 2008. Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog 4:e1000052. doi: 10.1371/journal.ppat.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seidl K, Goerke C, Wolz C, Mack D, Berger-Bächi B, Bischoff M. 2008. Staphylococcus aureus CcpA affects biofilm formation. Infect Immun 76:2044–2050. doi: 10.1128/IAI.00035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clinical and Laboratory Standards Institute. 2012. M100-S25. Performance standards for antimicrobial susceptibility testing; 25th informational supplement. CLSI, Wayne, PA. [Google Scholar]
- 22.Gloede J, Scheerans C, Derendorf H, Kloft C. 2010. In vitro pharmacodynamic models to determine the effect of antibacterial drugs. J Antimicrob Chemother 65:186–201. doi: 10.1093/jac/dkp434. [DOI] [PubMed] [Google Scholar]
- 23.Nightingale CH. (ed). 2007. Antimicrobial pharmacodynamics in theory and clinical practice, 2nd ed CRC Press, Boca Raton, FL. [Google Scholar]
- 24.Coyle EA, Cha R, Rybak MJ. 2003. Influences of linezolid, penicillin, and clindamycin, alone and in combination, on streptococcal pyrogenic exotoxin A release. Antimicrob Agents Chemother 47:1752–1755. doi: 10.1128/AAC.47.5.1752-1755.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blaser J, Stone BB, Zinner SH. 1985. Two compartment kinetic model with multiple artificial capillary units. J Antimicrob Chemother 15(Suppl A):131–137. [DOI] [PubMed] [Google Scholar]
- 26.Pichereau S, Pantrangi M, Couet W, Badiou C, Lina G, Shukla SK, Rose WE. 2012. Simulated antibiotic exposures in an in vitro hollow-fiber infection model influence toxin gene expression and production in community-associated methicillin-resistant Staphylococcus aureus strain MW2. Antimicrob Agents Chemother 56:140–147. doi: 10.1128/AAC.05113-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Louie A, Vanscoy BD, Heine HS, Liu W, Abshire T, Holman K, Kulawy R, Brown DL, Drusano GL. 2012. Differential effects of linezolid and ciprofloxacin on toxin production by Bacillus anthracis in an in vitro pharmacodynamic system. Antimicrob Agents Chemother 56:513–517. doi: 10.1128/AAC.05724-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlievert PM, Kelly JA. 1984. Clindamycin-induced suppression of toxic-shock syndrome-associated exotoxin production. J Infect Dis 149:471. doi: 10.1093/infdis/149.3.471. [DOI] [PubMed] [Google Scholar]
- 29.Ohlsen K, Ziebuhr W, Koller KP, Hell W, Wichelhaus TA, Hacker J. 1998. Effects of subinhibitory concentrations of antibiotics on alpha-toxin (hla) gene expression of methicillin-sensitive and methicillin-resistant Staphylococcus aureus isolates. Antimicrob Agents Chemother 42:2817–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chopra I, Roberts M. 2001. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev 65:232–260. doi: 10.1128/MMBR.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doss SA, Tillotson GS, Amyes SG. 1993. Effect of sub-inhibitory concentrations of antibiotics on the virulence of Staphylococcus aureus. J Appl Bacteriol 75:123–128. doi: 10.1111/j.1365-2672.1993.tb02756.x. [DOI] [PubMed] [Google Scholar]
- 32.Gemmell CG, Ford CW. 2002. Virulence factor expression by Gram-positive cocci exposed to subinhibitory concentrations of linezolid. J Antimicrob Chemother 50:665–672. doi: 10.1093/jac/dkf192. [DOI] [PubMed] [Google Scholar]
- 33.Bernardo K, Pakulat N, Fleer S, Schnaith A, Utermöhlen O, Krut O, Müller S, Krönke M. 2004. Subinhibitory concentrations of linezolid reduce Staphylococcus aureus virulence factor expression. Antimicrob Agents Chemother 48:546–555. doi: 10.1128/AAC.48.2.546-555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kernodle DS, McGraw PA, Barg NL, Menzies BE, Voladri RK, Harshman S. 1995. Growth of Staphylococcus aureus with nafcillin in vitro induces alpha-toxin production and increases the lethal activity of sterile broth filtrates in a murine model. J Infect Dis 172:410–419. doi: 10.1093/infdis/172.2.410. [DOI] [PubMed] [Google Scholar]
- 35.Rudkin JK, Laabei M, Edwards AM, Joo H-S, Otto M, Lennon KL, O'Gara JP, Waterfield NR, Massey RC. 2014. Oxacillin alters the toxin expression profile of community-associated methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 58:1100–1107. doi: 10.1128/AAC.01618-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moellering RC, Corey GR, Grayson ML. 2011. Introduction: fusidic acid enters the United States. Clin Infect Dis 52(Suppl 7):S467–S468. doi: 10.1093/cid/cir171. [DOI] [PubMed] [Google Scholar]
- 37.Sonstein SA, Burnham JC. 1993. Effect of low concentrations of quinolone antibiotics on bacterial virulence mechanisms. Diagn Microbiol Infect Dis 16:277–289. doi: 10.1016/0732-8893(93)90078-L. [DOI] [PubMed] [Google Scholar]
- 38.Subrt N, Mesak LR, Davies J. 2011. Modulation of virulence gene expression by cell wall active antibiotics in Staphylococcus aureus. J Antimicrob Chemother 66:979–984. doi: 10.1093/jac/dkr043. [DOI] [PubMed] [Google Scholar]
- 39.Herbert S, Barry P, Novick RP. 2001. Subinhibitory clindamycin differentially inhibits transcription of exoprotein genes in Staphylococcus aureus. Infect Immun 69:2996–3003. doi: 10.1128/IAI.69.5.2996-3003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steinhuber A, Landmann R, Goerke C, Wolz C, Flückiger U. 2008. Bioluminescence imaging to study the promoter activity of hla of Staphylococcus aureus in vitro and in vivo. Int J Med Microbiol 298:599–605. doi: 10.1016/j.ijmm.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 41.Bisognano C, Vaudaux P, Rohner P, Lew DP, Hooper DC. 2000. Induction of fibronectin-binding proteins and increased adhesion of quinolone-resistant Staphylococcus aureus by subinhibitory levels of ciprofloxacin. Antimicrob Agents Chemother 44:1428–1437. doi: 10.1128/AAC.44.6.1428-1437.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Worlitzsch D, Kaygin H, Steinhuber A, Dalhoff A, Botzenhart K, Döring G. 2001. Effects of amoxicillin, gentamicin, and moxifloxacin on the hemolytic activity of Staphylococcus aureus in vitro and in vivo. Antimicrob Agents Chemother 45:196–202. doi: 10.1128/AAC.45.1.196-202.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cázares-Domínguez V, Ochoa SA, Cruz-Córdova A, Rodea GE, Escalona G, Olivares AL, Olivares-Trejo JJ, Velázquez-Guadarrama N, Xicohtencatl-Cortes J. 2015. Vancomycin modifies the expression of the agr system in multidrug-resistant Staphylococcus aureus clinical isolates. Front Microbiol 6:369. doi: 10.3389/fmicb.2015.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Awad S, Alharbi AE, Alshami I. 2013. Exposure of vancomycin-sensitive Staphylococcus aureus to subinhibitory levels of vancomycin leads to upregulated capsular gene expression. Br J Biomed Sci 70:58–61. doi: 10.1080/09674845.2013.11669936. [DOI] [PubMed] [Google Scholar]
- 45.Kuroda H, Kuroda M, Cui L, Hiramatsu K. 2007. Subinhibitory concentrations of beta-lactam induce haemolytic activity in Staphylococcus aureus through the SaeRS two-component system. FEMS Microbiol Lett 268:98–105. doi: 10.1111/j.1574-6968.2006.00568.x. [DOI] [PubMed] [Google Scholar]
- 46.Dumitrescu O, Badiou C, Bes M, Reverdy M-E, Vandenesch F, Etienne J, Lina G. 2008. Effect of antibiotics, alone and in combination, on Panton-Valentine leukocidin production by a Staphylococcus aureus reference strain. Clin Microbiol Infect 14:384–388. doi: 10.1111/j.1469-0691.2007.01947.x. [DOI] [PubMed] [Google Scholar]
- 47.Koszczol C, Bernardo K, Krönke M, Krut O. 2006. Subinhibitory quinupristin/dalfopristin attenuates virulence of Staphylococcus aureus. J Antimicrob Chemother 58:564–574. doi: 10.1093/jac/dkl291. [DOI] [PubMed] [Google Scholar]
- 48.Bisognano C, Vaudaux PE, Lew DP, Ng EY, Hooper DC. 1997. Increased expression of fibronectin-binding proteins by fluoroquinolone-resistant Staphylococcus aureus exposed to subinhibitory levels of ciprofloxacin. Antimicrob Agents Chemother 41:906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamaki J, Synold T, Wong-Beringer A. 2011. Antivirulence potential of TR-700 and clindamycin on clinical isolates of Staphylococcus aureus producing phenol-soluble modulins. Antimicrob Agents Chemother 55:4432–4435. doi: 10.1128/AAC.00122-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joo H-S, Chan JL, Cheung GYC, Otto M. 2010. Subinhibitory concentrations of protein synthesis-inhibiting antibiotics promote increased expression of the agr virulence regulator and production of phenol-soluble modulin cytolysins in community-associated methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 54:4942–4944. doi: 10.1128/AAC.00064-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yanagihara K, Morinaga Y, Nakamura S, Seki M, Izumikawa K, Kakeya H, Yamamoto Y, Yamada Y, Kamihira S, Kohno S. 2008. Subinhibitory concentrations of telithromycin, clarithromycin and azithromycin reduce methicillin-resistant Staphylococcus aureus coagulase in vitro and in vivo. J Antimicrob Chemother 61:647–650. doi: 10.1093/jac/dkm507. [DOI] [PubMed] [Google Scholar]
- 52.Liu X, Hu Y, Pai P-J, Chen D, Lam H. 2014. Label-free quantitative proteomics analysis of antibiotic response in Staphylococcus aureus to oxacillin. J Proteome Res 13:1223–1233. doi: 10.1021/pr400669d. [DOI] [PubMed] [Google Scholar]
- 53.Lorian V, Atkinson B. 1980. Killing of oxacillin-exposed staphylococci in human polymorphonuclear leukocytes. Antimicrob Agents Chemother 18:807–813. doi: 10.1128/AAC.18.5.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Root RK, Isturiz R, Molavi A, Metcalf JA, Malech HL. 1981. Interactions between antibiotics and human neutrophils in the killing of staphylococci. J Clin Invest 67:247–259. doi: 10.1172/JCI110020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Milatovic D, Braveny I, Verhoef J. 1983. Clindamycin enhances opsonization of Staphylococcus aureus. Antimicrob Agents Chemother 24:413–417. doi: 10.1128/AAC.24.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Milatović D. 1982. Effect of subinhibitory antibiotic concentrations on the phagocytosis of Staphylococcus aureus. Eur J Clin Microbiol 1:97–101. doi: 10.1007/BF02014199. [DOI] [PubMed] [Google Scholar]
- 57.Veringa EM, Verhoef J. 1986. Influence of subinhibitory concentrations of clindamycin on opsonophagocytosis of Staphylococcus aureus, a protein-A-dependent process. Antimicrob Agents Chemother 30:796–797. doi: 10.1128/AAC.30.5.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Veringa EM, Verhoef J. 1987. Clindamycin at subinhibitory concentrations enhances antibody- and complement-dependent phagocytosis by human polymorphonuclear leukocytes of Staphylococcus aureus. Chemotherapy 33:243–249. doi: 10.1159/000238502. [DOI] [PubMed] [Google Scholar]
- 59.Pascual A, López-López G, Aragón J, Perea EJ. 1990. Effect of azithromycin, roxithromycin and erythromycin on human polymorphonuclear leukocyte function against Staphylococcus aureus. Chemotherapy 36:422–427. doi: 10.1159/000238799. [DOI] [PubMed] [Google Scholar]
- 60.Pascual A, Martínez-Martínez L, Perea EJ. 1989. Effect of ciprofloxacin and ofloxacin on human polymorphonuclear leukocyte activity against staphylococci. Chemotherapy 35:17–22. doi: 10.1159/000238631. [DOI] [PubMed] [Google Scholar]
- 61.Dal Sasso M, Culici M, Bovio C, Braga PC. 2003. Gemifloxacin: effects of sub-inhibitory concentrations on various factors affecting bacterial virulence. Int J Antimicrob Agents 21:325–333. doi: 10.1016/S0924-8579(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 62.Bassaris HP, Lianou PE, Votta EG, Skoutelis T, Papavassiliou T. 1984. Enhancement of polymorphonuclear leukocyte function against gram-positive aerobic organisms grown in the presence of lincomycin. Infection 12:369–371. doi: 10.1007/BF01645216. [DOI] [PubMed] [Google Scholar]
- 63.Herrera-Insúa I, Pérez P, Ramos C, Martínez P, Gómez-Lus ML, Prieto J. 1997. Synergistic effect of azithromycin on the phagocytic killing of Staphylococcus aureus by human polymorphonuclear leukocytes. Eur J Clin Microbiol Infect Dis 16:13–16. doi: 10.1007/BF01575113. [DOI] [PubMed] [Google Scholar]
- 64.Elliott GR, Peterson PK, Verbrugh HA, Freiberg MR, Hoidal JR, Quie PG. 1982. Influence of subinhibitory concentrations of penicillin, cephalothin, and clindamycin on Staphylococcus aureus growth in human phagocytic cells. Antimicrob Agents Chemother 22:781–784. doi: 10.1128/AAC.22.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Todd J, Fishaut M, Kapral F, Welch T. 1978. Toxic-shock syndrome associated with phage-group-I staphylococci. Lancet ii:1116–1118. [DOI] [PubMed] [Google Scholar]
- 66.Foster TJ, Geoghegan JA, Ganesh VK, Höök M. 2014. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol 12:49–62. doi: 10.1038/nrmicro3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walsh EJ, O'Brien LM, Liang X, Hook M, Foster TJ. 2004. Clumping factor B, a fibrinogen-binding MSCRAMM (microbial surface components recognizing adhesive matrix molecules) adhesin of Staphylococcus aureus, also binds to the tail region of type I cytokeratin 10. J Biol Chem 279:50691–50699. doi: 10.1074/jbc.M408713200. [DOI] [PubMed] [Google Scholar]
- 68.Moreillon P, Entenza JM, Francioli P, McDevitt D, Foster TJ, François P, Vaudaux P. 1995. Role of Staphylococcus aureus coagulase and clumping factor in pathogenesis of experimental endocarditis. Infect Immun 63:4738–4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arrecubieta C, Asai T, Bayern M, Loughman A, Fitzgerald JR, Shelton CE, Baron HM, Dang NC, Deng MC, Naka Y, Foster TJ, Lowy FD. 2006. The role of Staphylococcus aureus adhesins in the pathogenesis of ventricular assist device-related infections. J Infect Dis 193:1109–1119. doi: 10.1086/501366. [DOI] [PubMed] [Google Scholar]
- 70.Vergara-Irigaray M, Valle J, Merino N, Latasa C, García B, Ruiz de Los Mozos I, Solano C, Toledo-Arana A, Penadés JR, Lasa I. 2009. Relevant role of fibronectin-binding proteins in Staphylococcus aureus biofilm-associated foreign-body infections. Infect Immun 77:3978–3991. doi: 10.1128/IAI.00616-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M. 2000. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods 40:175–179. doi: 10.1016/S0167-7012(00)00122-6. [DOI] [PubMed] [Google Scholar]
- 72.Manner S, Goeres DM, Skogman M, Vuorela P, Fallarero A. 2017. Prevention of Staphylococcus aureus biofilm formation by antibiotics in 96-microtiter well plates and drip flow reactors: critical factors influencing outcomes. Sci Rep 7:43854. doi: 10.1038/srep43854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rooijakkers SHM, van Kessel KPM, van Strijp JAG. 2005. Staphylococcal innate immune evasion. Trends Microbiol 13:596–601. doi: 10.1016/j.tim.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 74.Thomer L, Schneewind O, Missiakas D. 2016. Pathogenesis of Staphylococcus aureus bloodstream infections. Annu Rev Pathol 11:343–364. doi: 10.1146/annurev-pathol-012615-044351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Berube BJ, Bubeck Wardenburg J. 2013. Staphylococcus aureus α-toxin: nearly a century of intrigue. Toxins 5:1140–1166. doi: 10.3390/toxins5061140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schmitz FJ, Verhoef J, Hadding U, Heinz HP, Jones ME. 1998. Reduction in cytokine release from human monocytes by modifications in cell wall structure of Staphylococcus aureus induced by subinhibitory concentrations of oxacillin. J Med Microbiol 47:533–541. doi: 10.1099/00222615-47-6-533. [DOI] [PubMed] [Google Scholar]
- 77.Navarro J. 1987. Modulation of [3H]dihydropyridine receptors by activation of protein kinase C in chick muscle cells. J Biol Chem 262:4649–4652. [PubMed] [Google Scholar]
- 78.English BK, Maryniw EM, Talati AJ, Meals EA. 2006. Diminished macrophage inflammatory response to Staphylococcus aureus isolates exposed to daptomycin versus vancomycin or oxacillin. Antimicrob Agents Chemother 50:2225–2227. doi: 10.1128/AAC.01559-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bhakdi S, Tranum-Jensen J. 1991. Alpha-toxin of Staphylococcus aureus. Microbiol Rev 55:733–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nygaard TK, Pallister KB, DuMont AL, DeWald M, Watkins RL, Pallister EQ, Malone C, Griffith S, Horswill AR, Torres VJ, Voyich JM. 2012. Alpha-toxin induces programmed cell death of human T cells, B cells, and monocytes during USA300 infection. PLoS One 7:e36532. doi: 10.1371/journal.pone.0036532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tabor DE, Yu L, Mok H, Tkaczyk C, Sellman BR, Wu Y, Oganesyan V, Slidel T, Jafri H, McCarthy M, Bradford P, Esser MT. 2016. Staphylococcus aureus alpha-toxin is conserved among diverse hospital respiratory isolates collected from a global surveillance study and is neutralized by monoclonal antibody MEDI4893. Antimicrob Agents Chemother 60:5312–5321. doi: 10.1128/AAC.00357-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Glenny AT, Stevens MF. 1935. Staphylococcus toxins and antitoxins. J Pathol Bacteriol 40:201–210. doi: 10.1002/path.1700400202. [DOI] [Google Scholar]
- 83.Davis JP, Chesney PJ, Wand PJ, LaVenture M. 1980. Toxic-shock syndrome: epidemiologic features, recurrence, risk factors, and prevention. N Engl J Med 303:1429–1435. doi: 10.1056/NEJM198012183032501. [DOI] [PubMed] [Google Scholar]
- 84.Dinges MM, Orwin PM, Schlievert PM. 2000. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev 13:16–34. doi: 10.1128/CMR.13.1.16-34.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dickgiesser N, Wallach U. 1987. Toxic shock syndrome toxin-1 (TSST-1): influence of its production by subinhibitory antibiotic concentrations. Infection 15:351–353. doi: 10.1007/BF01647737. [DOI] [PubMed] [Google Scholar]
- 86.Stevens DL, Wallace RJ, Hamilton SM, Bryant AE. 2006. Successful treatment of staphylococcal toxic shock syndrome with linezolid: a case report and in vitro evaluation of the production of toxic shock syndrome toxin type 1 in the presence of antibiotics. Clin Infect Dis 42:729–730. doi: 10.1086/500265. [DOI] [PubMed] [Google Scholar]
- 87.Bunikowski R, Mielke M, Skarabis H, Herz U, Bergmann RL, Wahn U, Renz H. 1999. Prevalence and role of serum IgE antibodies to the Staphylococcus aureus-derived superantigens SEA and SEB in children with atopic dermatitis. J Allergy Clin Immunol 103:119–124. doi: 10.1016/S0091-6749(99)70535-X. [DOI] [PubMed] [Google Scholar]
- 88.Dohin B, Gillet Y, Kohler R, Lina G, Vandenesch F, Vanhems P, Floret D, Etienne J. 2007. Pediatric bone and joint infections caused by Panton-Valentine leukocidin-positive Staphylococcus aureus. Pediatr Infect Dis J 26:1042–1048. doi: 10.1097/INF.0b013e318133a85e. [DOI] [PubMed] [Google Scholar]
- 89.Gillet Y, Issartel B, Vanhems P, Fournet J-C, Lina G, Bes M, Vandenesch F, Piémont Y, Brousse N, Floret D, Etienne J. 2002. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 359:753–759. doi: 10.1016/S0140-6736(02)07877-7. [DOI] [PubMed] [Google Scholar]
- 90.Lina G, Piémont Y, Godail-Gamot F, Bes M, Peter MO, Gauduchon V, Vandenesch F, Etienne J. 1999. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis 29:1128–1132. doi: 10.1086/313461. [DOI] [PubMed] [Google Scholar]
- 91.Wang R, Braughton KR, Kretschmer D, Bach T-HL, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, DeLeo FR, Otto M. 2007. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med 13:1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 92.Chatterjee SS, Joo H-S, Duong AC, Dieringer TD, Tan VY, Song Y, Fischer ER, Cheung GYC, Li M, Otto M. 2013. Essential Staphylococcus aureus toxin export system. Nat Med 19:364–367. doi: 10.1038/nm.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Joo H-S, Chatterjee SS, Villaruz AE, Dickey SW, Tan VY, Chen Y, Sturdevant DE, Ricklefs SM, Otto M. 2016. Mechanism of gene regulation by a Staphylococcus aureus toxin. mBio 7:e01579-16. doi: 10.1128/mBio.01579-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Blickwede M, Wolz C, Valentin-Weigand P, Schwarz S. 2005. Influence of clindamycin on the stability of coa and fnbB transcripts and adherence properties of Staphylococcus aureus Newman. FEMS Microbiol Lett 252:73–78. doi: 10.1016/j.femsle.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 95.Blickwede M, Goethe R, Wolz C, Valentin-Weigand P, Schwarz S. 2005. Molecular basis of florfenicol-induced increase in adherence of Staphylococcus aureus strain Newman. J Antimicrob Chemother 56:315–323. doi: 10.1093/jac/dki233. [DOI] [PubMed] [Google Scholar]
- 96.Blickwede M, Valentin-Weigand P, Schwarz S. 2004. Subinhibitory concentrations of florfenicol enhance the adherence of florfenicol-susceptible and florfenicol-resistant Staphylococcus aureus. J Antimicrob Chemother 54:286–288. doi: 10.1093/jac/dkh273. [DOI] [PubMed] [Google Scholar]
- 97.Rasigade JP, Moulay A, Lhoste Y, Tristan A, Bes M, Vandenesch F, Etienne J, Lina G, Laurent F, Dumitrescu O. 2011. Impact of sub-inhibitory antibiotics on fibronectin-mediated host cell adhesion and invasion by Staphylococcus aureus. BMC Microbiol 11:263. doi: 10.1186/1471-2180-11-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Labro MT, Pochet I, Babin-Chevaye C, Hakim J. 1987. Effect of ceftriaxone-induced alterations of bacteria on neutrophil bactericidal function. J Antimicrob Chemother 20:857–869. doi: 10.1093/jac/20.6.857. [DOI] [PubMed] [Google Scholar]
- 99.Burke FM, Di Poto A, Speziale P, Foster TJ. 2011. The A domain of fibronectin-binding protein B of Staphylococcus aureus contains a novel fibronectin binding site. FEBS J 278:2359–2371. doi: 10.1111/j.1742-4658.2011.08159.x. [DOI] [PubMed] [Google Scholar]
- 100.Ramadurai L, Jayaswal RK. 1997. Molecular cloning, sequencing, and expression of lytM, a unique autolytic gene of Staphylococcus aureus. J Bacteriol 179:3625–3631. doi: 10.1128/jb.179.11.3625-3631.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schilcher K, Andreoni F, Dengler Haunreiter V, Seidl K, Hasse B, Zinkernagel AS. 2016. Modulation of Staphylococcus aureus biofilm matrix by subinhibitory concentrations of clindamycin. Antimicrob Agents Chemother 60:5957–5967. doi: 10.1128/AAC.00463-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fujimoto DF, Bayles KW. 1998. Opposing roles of the Staphylococcus aureus virulence regulators, Agr and Sar, in Triton X-100- and penicillin-induced autolysis. J Bacteriol 180:3724–3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lázaro-Díez M, Remuzgo-Martínez S, Rodríguez-Mirones C, Acosta F, Icardo JM, Martínez-Martínez L, Ramos-Vivas J. 2016. Effects of subinhibitory concentrations of ceftaroline on methicillin-resistant Staphylococcus aureus (MRSA) biofilms. PLoS One 11:e0147569. doi: 10.1371/journal.pone.0147569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Antignac A, Sieradzki K, Tomasz A. 2007. Perturbation of cell wall synthesis suppresses autolysis in Staphylococcus aureus: evidence for coregulation of cell wall synthetic and hydrolytic enzymes. J Bacteriol 189:7573–7580. doi: 10.1128/JB.01048-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Suginaka H, Blumberg PM, Strominger JL. 1972. Multiple penicillin-binding components in Bacillus subtilis, Bacillus cereus, Staphylococcus aureus, and Escherichia coli. J Biol Chem 247:5279–5288. [PubMed] [Google Scholar]
- 106.Sobral RG, Jones AE, Des Etages SG, Dougherty TJ, Peitzsch RM, Gaasterland T, Ludovice AM, de Lencastre H, Tomasz A. 2007. Extensive and genome-wide changes in the transcription profile of Staphylococcus aureus induced by modulating the transcription of the cell wall synthesis gene murF. J Bacteriol 189:2376–2391. doi: 10.1128/JB.01439-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gui Z, Wang H, Ding T, Zhu W, Zhuang X, Chu W. 2014. Azithromycin reduces the production of α-hemolysin and biofilm formation in Staphylococcus aureus. Indian J Microbiol 54:114–117. doi: 10.1007/s12088-013-0438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pascual A, Ballesta S, García I, Perea EJ. 2002. Uptake and intracellular activity of linezolid in human phagocytes and nonphagocytic cells. Antimicrob Agents Chemother 46:4013–4015. doi: 10.1128/AAC.46.12.4013-4015.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ballesta S, Pascual A, García I, Perea EJ. 2003. Effect of linezolid on the phagocytic functions of human polymorphonuclear leukocytes. Chemotherapy 49:163–166. doi: 10.1159/000071139. [DOI] [PubMed] [Google Scholar]
- 110.Naess A, Stenhaug Kilhus K, Nystad TW, Sørnes S. 2006. Linezolid and human polymorphonuclear leukocyte function. Chemotherapy 52:122–124. doi: 10.1159/000092539. [DOI] [PubMed] [Google Scholar]
- 111.Kushiya K, Nakagawa S, Taneike I, Iwakura N, Imanishi K, Uchiyama T, Tsukada H, Gejyo F, Yamamoto T. 2005. Inhibitory effect of antimicrobial agents and anisodamine on the staphylococcal superantigenic toxin-induced overproduction of proinflammatory cytokines by human peripheral blood mononuclear cells. J Infect Chemother 11:192–195. doi: 10.1007/s10156-005-0389-8. [DOI] [PubMed] [Google Scholar]
- 112.Pichereau S, Moran JJM, Hayney MS, Shukla SK, Sakoulas G, Rose WE. 2012. Concentration-dependent effects of antimicrobials on Staphylococcus aureus toxin-mediated cytokine production from peripheral blood mononuclear cells. J Antimicrob Chemother 67:123–129. doi: 10.1093/jac/dkr417. [DOI] [PubMed] [Google Scholar]
- 113.Dey S, Majhi A, Mahanti S, Dey I, Bishayi B. 2015. In vitro anti-inflammatory and immunomodulatory effects of ciprofloxacin or azithromycin in Staphylococcus aureus-stimulated murine macrophages are beneficial in the presence of cytochalasin D. Inflammation 38:1050–1069. doi: 10.1007/s10753-014-0070-4. [DOI] [PubMed] [Google Scholar]
- 114.van Langevelde P, Ravensbergen E, Grashoff P, Beekhuizen H, Groeneveld PH, van Dissel JT. 1999. Antibiotic-induced cell wall fragments of Staphylococcus aureus increase endothelial chemokine secretion and adhesiveness for granulocytes. Antimicrob Agents Chemother 43:2984–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Novick RP, Jiang D. 2003. The staphylococcal saeRS system coordinates environmental signals with agr quorum sensing. Microbiology 149:2709–2717. doi: 10.1099/mic.0.26575-0. [DOI] [PubMed] [Google Scholar]
- 116.Giraudo AT, Cheung AL, Nagel R. 1997. The sae locus of Staphylococcus aureus controls exoprotein synthesis at the transcriptional level. Arch Microbiol 168:53–58. doi: 10.1007/s002030050469. [DOI] [PubMed] [Google Scholar]
- 117.Cardot Martin E, Michel A, Raynal B, Badiou C, Laurent F, Vandenesch F, Etienne J, Lina G, Dumitrescu O. 2015. Community-acquired meticillin-resistant Staphylococcus aureus strain USA300 resists staphylococcal protein A modulation by antibiotics and antimicrobial peptides. Int J Antimicrob Agents 45:19–24. doi: 10.1016/j.ijantimicag.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 118.Maguire BA. 2009. Inhibition of bacterial ribosome assembly: a suitable drug target? Microbiol Mol Biol Rev 73:22–35. doi: 10.1128/MMBR.00030-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Singh VK, Jayaswal RK, Wilkinson BJ. 2001. Cell wall-active antibiotic induced proteins of Staphylococcus aureus identified using a proteomic approach. FEMS Microbiol Lett 199:79–84. [DOI] [PubMed] [Google Scholar]
- 120.Utaida S, Dunman PM, Macapagal D, Murphy E, Projan SJ, Singh VK, Jayaswal RK, Wilkinson BJ. 2003. Genome-wide transcriptional profiling of the response of Staphylococcus aureus to cell-wall-active antibiotics reveals a cell-wall-stress stimulon. Microbiology 149:2719–2732. doi: 10.1099/mic.0.26426-0. [DOI] [PubMed] [Google Scholar]
- 121.Gardete S, Wu SW, Gill S, Tomasz A. 2006. Role of VraSR in antibiotic resistance and antibiotic-induced stress response in Staphylococcus aureus. Antimicrob Agents Chemother 50:3424–3434. doi: 10.1128/AAC.00356-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Blake KL, O'Neill AJ, Mengin-Lecreulx D, Henderson PJF, Bostock JM, Dunsmore CJ, Simmons KJ, Fishwick CWG, Leeds JA, Chopra I. 2009. The nature of Staphylococcus aureus MurA and MurZ and approaches for detection of peptidoglycan biosynthesis inhibitors. Mol Microbiol 72:335–343. doi: 10.1111/j.1365-2958.2009.06648.x. [DOI] [PubMed] [Google Scholar]
- 123.Rahman MM, Hunter HN, Prova S, Verma V, Qamar A, Golemi-Kotra D. 2016. The Staphylococcus aureus methicillin resistance factor FmtA is a d-amino esterase that acts on teichoic acids. mBio 7:e02070-15. doi: 10.1128/mBio.02070-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McAleese F, Wu SW, Sieradzki K, Dunman P, Murphy E, Projan S, Tomasz A. 2006. Overexpression of genes of the cell wall stimulon in clinical isolates of Staphylococcus aureus exhibiting vancomycin-intermediate-S. aureus-type resistance to vancomycin. J Bacteriol 188:1120–1133. doi: 10.1128/JB.188.3.1120-1133.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Komatsuzawa H, Sugai M, Nakashima S, Yamada S, Matsumoto A, Oshida T, Suginaka H. 1997. Subcellular localization of the major autolysin, ATL and its processed proteins in Staphylococcus aureus. Microbiol Immunol 41:469–479. doi: 10.1111/j.1348-0421.1997.tb01880.x. [DOI] [PubMed] [Google Scholar]
- 126.Ingavale S, van Wamel W, Luong TT, Lee CY, Cheung AL. 2005. Rat/MgrA, a regulator of autolysis, is a regulator of virulence genes in Staphylococcus aureus. Infect Immun 73:1423–1431. doi: 10.1128/IAI.73.3.1423-1431.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fournier B, Hooper DC. 2000. A new two-component regulatory system involved in adhesion, autolysis, and extracellular proteolytic activity of Staphylococcus aureus. J Bacteriol 182:3955–3964. doi: 10.1128/JB.182.14.3955-3964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Reed P, Atilano ML, Alves R, Hoiczyk E, Sher X, Reichmann NT, Pereira PM, Roemer T, Filipe SR, Pereira-Leal JB, Ligoxygakis P, Pinho MG. 2015. Staphylococcus aureus survives with a minimal peptidoglycan synthesis machine but sacrifices virulence and antibiotic resistance. PLoS Pathog 11:e1004891. doi: 10.1371/journal.ppat.1004891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yang Y, Bhachech N, Bush K. 1995. Biochemical comparison of imipenem, meropenem and biapenem: permeability, binding to penicillin-binding proteins, and stability to hydrolysis by beta-lactamases. J Antimicrob Chemother 35:75–84. doi: 10.1093/jac/35.1.75. [DOI] [PubMed] [Google Scholar]
- 130.Georgopapadakou NH, Dix BA, Mauriz YR. 1986. Possible physiological functions of penicillin-binding proteins in Staphylococcus aureus. Antimicrob Agents Chemother 29:333–336. doi: 10.1128/AAC.29.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Georgopapadakou NH, Smith SA, Bonner DP. 1982. Penicillin-binding proteins in a Staphylococcus aureus strain resistant to specific beta-lactam antibiotics. Antimicrob Agents Chemother 22:172–175. doi: 10.1128/AAC.22.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Curtis NAC, Hayes MV, Wyke AW, Ward JB. 1980. A mutant of Staphylococcus aureus H lacking penicillin-binding protein 4 and transpeptidase activity in vitro. FEMS Microbiol Lett 9:263–266. doi: 10.1111/j.1574-6968.1980.tb05649.x. [DOI] [Google Scholar]
- 133.Pereira SFF, Henriques AO, Pinho MG, de Lencastre H, Tomasz A. 2007. Role of PBP1 in cell division of Staphylococcus aureus. J Bacteriol 189:3525–3531. doi: 10.1128/JB.00044-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pereira SFF, Henriques AO, Pinho MG, de Lencastre H, Tomasz A. 2009. Evidence for a dual role of PBP1 in the cell division and cell separation of Staphylococcus aureus. Mol Microbiol 72:895–904. doi: 10.1111/j.1365-2958.2009.06687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.VanBogelen RA, Kelley PM, Neidhardt FC. 1987. Differential induction of heat shock, SOS, and oxidation stress regulons and accumulation of nucleotides in Escherichia coli. J Bacteriol 169:26–32. doi: 10.1128/jb.169.1.26-32.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Winterling KW, Chafin D, Hayes JJ, Sun J, Levine AS, Yasbin RE, Woodgate R. 1998. The Bacillus subtilis DinR binding site: redefinition of the consensus sequence. J Bacteriol 180:2201–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Courcelle J, Khodursky A, Peter B, Brown PO, Hanawalt PC. 2001. Comparative gene expression profiles following UV exposure in wild-type and SOS-deficient Escherichia coli. Genetics 158:41–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Anderson DG, Kowalczykowski SC. 1998. Reconstitution of an SOS response pathway: derepression of transcription in response to DNA breaks. Cell 95:975–979. doi: 10.1016/S0092-8674(00)81721-3. [DOI] [PubMed] [Google Scholar]
- 139.Phillips I, Culebras E, Moreno F, Baquero F. 1987. Induction of the SOS response by new 4-quinolones. J Antimicrob Chemother 20:631–638. doi: 10.1093/jac/20.5.631. [DOI] [PubMed] [Google Scholar]
- 140.Miller C, Thomsen LE, Gaggero C, Mosseri R, Ingmer H, Cohen SN. 2004. SOS response induction by beta-lactams and bacterial defense against antibiotic lethality. Science 305:1629–1631. doi: 10.1126/science.1101630. [DOI] [PubMed] [Google Scholar]
- 141.Bisognano C, Kelley WL, Estoppey T, Francois P, Schrenzel J, Li D, Lew DP, Hooper DC, Cheung AL, Vaudaux P. 2004. A recA-LexA-dependent pathway mediates ciprofloxacin-induced fibronectin binding in Staphylococcus aureus. J Biol Chem 279:9064–9071. doi: 10.1074/jbc.M309836200. [DOI] [PubMed] [Google Scholar]
- 142.Plata KB, Riosa S, Singh CR, Rosato RR, Rosato AE. 2013. Targeting of PBP1 by β-lactams determines recA/SOS response activation in heterogeneous MRSA clinical strains. PLoS One 8:e61083. doi: 10.1371/journal.pone.0061083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fitzgerald JR, Monday SR, Foster TJ, Bohach GA, Hartigan PJ, Meaney WJ, Smyth CJ. 2001. Characterization of a putative pathogenicity island from bovine Staphylococcus aureus encoding multiple superantigens. J Bacteriol 183:63–70. doi: 10.1128/JB.183.1.63-70.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zou D, Kaneko J, Narita S, Kamio Y. 2000. Prophage, phiPV83-pro, carrying Panton-Valentine leukocidin genes, on the Staphylococcus aureus P83 chromosome: comparative analysis of the genome structures of phiPV83-pro, phiPVL, phi11, and other phages. Biosci Biotechnol Biochem 64:2631–2643. doi: 10.1271/bbb.64.2631. [DOI] [PubMed] [Google Scholar]
- 145.Wirtz C, Witte W, Wolz C, Goerke C. 2009. Transcription of the phage-encoded Panton-Valentine leukocidin of Staphylococcus aureus is dependent on the phage life-cycle and on the host background. Microbiology 155:3491–3499. doi: 10.1099/mic.0.032466-0. [DOI] [PubMed] [Google Scholar]
- 146.Ubeda C, Maiques E, Knecht E, Lasa I, Novick RP, Penadés JR. 2005. Antibiotic-induced SOS response promotes horizontal dissemination of pathogenicity island-encoded virulence factors in staphylococci. Mol Microbiol 56:836–844. doi: 10.1111/j.1365-2958.2005.04584.x. [DOI] [PubMed] [Google Scholar]
- 147.Goerke C, Köller J, Wolz C. 2006. Ciprofloxacin and trimethoprim cause phage induction and virulence modulation in Staphylococcus aureus. Antimicrob Agents Chemother 50:171–177. doi: 10.1128/AAC.50.1.171-177.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Novick RP. 2003. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol 48:1429–1449. doi: 10.1046/j.1365-2958.2003.03526.x. [DOI] [PubMed] [Google Scholar]
- 149.Yarwood JM, McCormick JK, Schlievert PM. 2001. Identification of a novel two-component regulatory system that acts in global regulation of virulence factors of Staphylococcus aureus. J Bacteriol 183:1113–1123. doi: 10.1128/JB.183.4.1113-1123.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Delauné A, Dubrac S, Blanchet C, Poupel O, Mäder U, Hiron A, Leduc A, Fitting C, Nicolas P, Cavaillon J-M, Adib-Conquy M, Msadek T. 2012. The WalKR system controls major staphylococcal virulence genes and is involved in triggering the host inflammatory response. Infect Immun 80:3438–3453. doi: 10.1128/IAI.00195-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dumitrescu O, Forey F, Bes M, Vandenesch F, Etienne J, Lina G. 2011. Linezolid decreases exotoxin expression in Staphylococcus aureus by early repressing agr, sarA and sae regulators, abstr P1052. 21st Eur Congr Clin Microbiol Infect Dis, Milan, Italy. [Google Scholar]
- 152.Manna AC, Ray B. 2007. Regulation and characterization of rot transcription in Staphylococcus aureus. Microbiology 153:1538–1545. doi: 10.1099/mic.0.2006/004309-0. [DOI] [PubMed] [Google Scholar]
- 153.Löffler B, Hussain M, Grundmeier M, Brück M, Holzinger D, Varga G, Roth J, Kahl BC, Proctor RA, Peters G. 2010. Staphylococcus aureus Panton-Valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog 6:e1000715. doi: 10.1371/journal.ppat.1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Diep BA, Chan L, Tattevin P, Kajikawa O, Martin TR, Basuino L, Mai TT, Marbach H, Braughton KR, Whitney AR, Gardner DJ, Fan X, Tseng CW, Liu GY, Badiou C, Etienne J, Lina G, Matthay MA, DeLeo FR, Chambers HF. 2010. Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc Natl Acad Sci U S A 107:5587–5592. doi: 10.1073/pnas.0912403107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Diep BA, Le VTM, Visram ZC, Rouha H, Stulik L, Dip EC, Nagy G, Nagy E. 2016. Improved protection in a rabbit model of community-associated methicillin-resistant Staphylococcus aureus necrotizing pneumonia upon neutralization of leukocidins in addition to alpha-hemolysin. Antimicrob Agents Chemother 60:6333–6340. doi: 10.1128/AAC.01213-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Diep BA, Le VTM, Badiou C, Le HN, Pinheiro MG, Duong AH, Wang X, Dip EC, Aguiar-Alves F, Basuino L, Marbach H, Mai TT, Sarda MN, Kajikawa O, Matute-Bello G, Tkaczyk C, Rasigade J-P, Sellman BR, Chambers HF, Lina G. 2016. IVIG-mediated protection against necrotizing pneumonia caused by MRSA. Sci Transl Med 8:357ra124. doi: 10.1126/scitranslmed.aag1153. [DOI] [PubMed] [Google Scholar]
- 157.Diep BA, Afasizheva A, Le HN, Kajikawa O, Matute-Bello G, Tkaczyk C, Sellman B, Badiou C, Lina G, Chambers HF. 2013. Effects of linezolid on suppressing in vivo production of staphylococcal toxins and improving survival outcomes in a rabbit model of methicillin-resistant Staphylococcus aureus necrotizing pneumonia. J Infect Dis 208:75–82. doi: 10.1093/infdis/jit129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Croisier-Bertin D, Hayez D, Da Silva S, Labrousse D, Biek D, Badiou C, Dumitrescu O, Guerard P, Charles P-E, Piroth L, Lina G, Vandenesch F, Chavanet P. 2014. In vivo efficacy of ceftaroline fosamil in a methicillin-resistant Panton-Valentine leukocidin-producing Staphylococcus aureus rabbit pneumonia model. Antimicrob Agents Chemother 58:1855–1861. doi: 10.1128/AAC.01707-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yanagihara K, Kaneko Y, Sawai T, Miyazaki Y, Tsukamoto K, Hirakata Y, Tomono K, Kadota J-I, Tashiro T, Murata I, Kohno S. 2002. Efficacy of linezolid against methicillin-resistant or vancomycin-insensitive Staphylococcus aureus in a model of hematogenous pulmonary infection. Antimicrob Agents Chemother 46:3288–3291. doi: 10.1128/AAC.46.10.3288-3291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yanagihara K, Kihara R, Araki N, Morinaga Y, Seki M, Izumikawa K, Kakeya H, Yamamoto Y, Yamada Y, Kohno S, Tsukamoto K, Kamihira S. 2009. Efficacy of linezolid against Panton-Valentine leukocidin (PVL)-positive meticillin-resistant Staphylococcus aureus (MRSA) in a mouse model of haematogenous pulmonary infection. Int J Antimicrob Agents 34:477–481. doi: 10.1016/j.ijantimicag.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 161.Bubeck Wardenburg J, Bae T, Otto M, Deleo FR, Schneewind O. 2007. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med 13:1405–1406. doi: 10.1038/nm1207-1405. [DOI] [PubMed] [Google Scholar]
- 162.Luna CM, Bruno DA, García-Morato J, Mann KC, Risso Patrón J, Sagardía J, Absi R, García Bottino M, Marchetti D, Famiglietti A, Baleztena M, Biancolini C. 2009. Effect of linezolid compared with glycopeptides in methicillin-resistant Staphylococcus aureus severe pneumonia in piglets. Chest 135:1564–1571. doi: 10.1378/chest.08-2169. [DOI] [PubMed] [Google Scholar]
- 163.Martinez-Olondris P, Rigol M, Soy D, Guerrero L, Agusti C, Quera MA, Li Bassi G, Esperatti M, Luque N, Liapikou M, Filella X, Marco F, de la Bellacasa JP, Torres A. 2012. Efficacy of linezolid compared to vancomycin in an experimental model of pneumonia induced by methicillin-resistant Staphylococcus aureus in ventilated pigs. Crit Care Med 40:162–168. doi: 10.1097/CCM.0b013e31822d74a2. [DOI] [PubMed] [Google Scholar]
- 164.Akinnusi ME, Hattemer A, Gao W, El-Solh AA. 2011. Does linezolid modulate lung innate immunity in a murine model of methicillin-resistant Staphylococcus aureus pneumonia? Crit Care Med 39:1944–1952. doi: 10.1097/CCM.0b013e31821bd79e. [DOI] [PubMed] [Google Scholar]
- 165.Micek ST, Dunne M, Kollef MH. 2005. Pleuropulmonary complications of Panton-Valentine leukocidin-positive community-acquired methicillin-resistant Staphylococcus aureus: importance of treatment with antimicrobials inhibiting exotoxin production. Chest 128:2732–2738. doi: 10.1378/chest.128.4.2732. [DOI] [PubMed] [Google Scholar]
- 166.Lobo LJ, Reed KD, Wunderink RG. 2010. Expanded clinical presentation of community-acquired methicillin-resistant Staphylococcus aureus pneumonia. Chest 138:130–136. doi: 10.1378/chest.09-1562. [DOI] [PubMed] [Google Scholar]
- 167.Li H-T, Zhang T-T, Huang J, Zhou Y-Q, Zhu J-X, Wu B-Q. 2011. Factors associated with the outcome of life-threatening necrotizing pneumonia due to community-acquired Staphylococcus aureus in adult and adolescent patients. Respir Int Rev Thorac Dis 81:448–460. doi: 10.1159/000319557. [DOI] [PubMed] [Google Scholar]
- 168.Rouzic N, Janvier F, Libert N, Javouhey E, Lina G, Nizou J-Y, Pasquier P, Stamm D, Brinquin L, Pelletier C, Vandenesch F, Floret D, Etienne J, Gillet Y. 2010. Prompt and successful toxin-targeting treatment of three patients with necrotizing pneumonia due to Staphylococcus aureus strains carrying the Panton-Valentine leukocidin genes. J Clin Microbiol 48:1952–1955. doi: 10.1128/JCM.01892-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gauduchon V, Cozon G, Vandenesch F, Genestier A-L, Eyssade N, Peyrol S, Etienne J, Lina G. 2004. Neutralization of Staphylococcus aureus Panton Valentine leukocidin by intravenous immunoglobulin in vitro. J Infect Dis 189:346–353. doi: 10.1086/380909. [DOI] [PubMed] [Google Scholar]
- 170.Wunderink RG, Niederman MS, Kollef MH, Shorr AF, Kunkel MJ, Baruch A, McGee WT, Reisman A, Chastre J. 2012. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis 54:621–629. doi: 10.1093/cid/cir895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.