SUMMARY
The two ligands B cell-activating factor of the tumor necrosis factor family (BAFF) and a proliferation-inducing ligand (APRIL) and the three receptors BAFF receptor (BAFF-R), transmembrane activator and calcium-modulating cyclophilin ligand interactor (TACI), and B cell maturation antigen (BCMA) are members of the “BAFF system molecules.” BAFF system molecules are primarily involved in B cell homeostasis. The relevance of BAFF system molecules in host responses to microbial assaults has been investigated in clinical studies and in mice deficient for each of these molecules. Many microbial products modulate the expression of these molecules. Data from clinical studies suggest a correlation between increased expression levels of BAFF system molecules and elevated B cell responses. Depending on the pathogen, heightened B cell responses may strengthen the host response or promote susceptibility. Whereas pathogen-mediated increases in the expression levels of the ligands and/or the receptors appear to promote microbial clearance, certain pathogens have evolved to ablate B cell responses by suppressing the expression of TACI and/or BAFF-R on B cells. Other than its well-established role in B cell responses, the TACI-mediated activation of macrophages is also implicated in resistance to intracellular pathogens. An improved understanding of the role that BAFF system molecules play in infection may assist in devising novel strategies for vaccine development.
KEYWORDS: APRIL, BAFF, BAFF-R, bacteria, DNA vaccines, parasite, TACI, virus, host response
INTRODUCTION
The so-called “BAFF system molecules,” which consist of two ligands and three receptors, are critical for B cell homeostasis (Fig. 1). The ligand B cell-activating factor belonging to the tumor necrosis factor (TNF) family (BAFF) (1), also called B lymphocyte activator (BlyS) (2), TNF- and apoptosis ligand-related leukocyte-expressed ligand 1 (TALL-1) (3), or TNF homologue that activates apoptosis, nuclear factor kappa light-chain enhancer of activated B cells (NF-κB), and c-Jun N-terminal kinase (JNK) (THANK) (4), maintains the survival of mature naive B cells (5, 6) by increasing the levels of the prosurvival molecules B cell lymphoma 2 (Bcl-2) and Bcl-x and by decreasing the levels of the proapoptotic molecule Bcl-2-homologous antagonist/killer (Bak) (7). BAFF is synthesized as a membrane-bound form, which can be further processed to a soluble form by furin convertase cleavage (1), a posttranslational modification process shared by all members of the BAFF system molecules (Fig. 1A). Soluble BAFF can form homotrimers (3-mers) or multimers as large as 60-mers (8). The ablation of BAFF activity by genetic deletion or by using blocking anti-BAFF antibodies results in the depletion of transitional type 2 (T2) B cells, while the T1 B cell population is preserved (Fig. 1B). Also, peritoneal B1 cell numbers are not affected by BAFF deficiency, but the numbers of the splenic subsets follicular (FO) B cells, marginal zone (MZ) B cells, and B1 cells are severely reduced (6, 9, 10). In the absence of BAFF, the decrease in B2 cell numbers is accompanied by a mild reduction in serum IgA levels and a severe decrease in IgG levels. In addition to the dramatic reduction in B cell numbers, BAFF is important for the maintenance of T cells because CD44+ CD62L− effector/memory T cell numbers are decreased in spleens of BAFF-deficient mice (9). While BAFF is required for B cell homeostasis, the excessive production of BAFF is detrimental to the host. Transgenic mice overexpressing BAFF (BAFF Tg mice) suffer from increased production of autoantibodies, proteinuria, salivary gland destruction, immunoglobulin deposits in the kidney, and splenomegaly, all of which are symptoms of autoimmune diseases reminiscent of systemic lupus erythematosus (SLE) and Sjögren's syndrome (11–13). The development of these autoimmune manifestations in BAFF Tg mice is largely attributed to the dysregulated expansion of B cell compartments, especially MZ and T2 MZ cells (11–14). The proportion of effector T cells is also increased in BAFF Tg mice (11, 12, 14), but the contribution of effector T cells to these disorders is likely to be limited because BAFF Tg mice lacking T cells develop SLE-like symptoms comparable to those of BAFF Tg mice with T cells (15). Like BAFF, a proliferation-inducing ligand (APRIL), the other ligand of the BAFF system molecules, also has membrane-bound and secreted forms (8, 16, 17). It can be intracellularly processed in the Golgi apparatus or on the cell membrane by furin convertase (8, 18) (Fig. 1A). Compared to BAFF, APRIL deficiency or APRIL overexpression (APRIL Tg mice) does not lead to significant abnormalities in the spleen, mesenteric lymph nodes, Peyer's patches, and B cell development (19–21). APRIL deficiency also does not change serum IgG levels but results in lower levels of IgA. In contrast, the only alteration in serum immunoglobulins in APRIL Tg mice is an elevation of IgM levels. The numbers of T cells are not different between APRIL-deficient and wild-type mice except for a slight increase in the percentage of splenic CD44high CD62Llow CD4+ T cells. The T cell population in peripheral lymph nodes of APRIL Tg mice is small, whereas T cell viability is high compared to that of littermate controls. Finally, while BAFF and APRIL are equally potent in inducing bone marrow plasma cell survival, the survival of memory B cells is independent of either ligand (22).
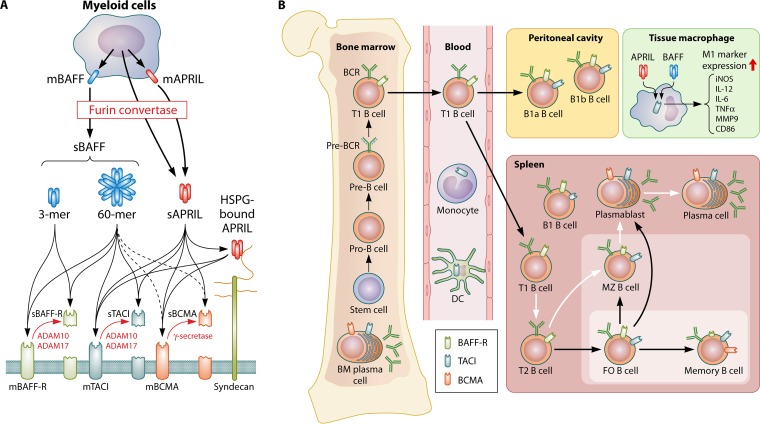
FIG 1.
BAFF system molecules. (A) BAFF system molecules are composed of two ligands (BAFF and APRIL) and three receptors (BAFF-R, TACI, and BCMA). BAFF and APRIL are primarily expressed by myeloid cells. BAFF is initially synthesized as a membrane-bound form (mBAFF). BAFF also has a soluble form (sBAFF). The soluble form is produced by the cleavage of mBAFF with furin convertase. sBAFF exists in a homotrimeric (3-mer) or oligomeric (60-mer) form. The cleavage of APRIL by furin convertase occurs in the Golgi apparatus or on the cell membrane. Similar to BAFF, soluble APRIL forms a homotrimer or partners with heparan sulfate proteoglycan (HSPG) to form multimers. Membrane-bound BAFF-R (mBAFF-R) is shed by ADAM10 and ADAM17 following BAFF stimulation. TACI can also be found in a soluble form (sTACI) after the cleavage of mTACI by ADAM10 and ADAM17. The activity of ADAM17 requires the stimulation of B cells by 60-mer BAFF. mBCMA is processed by γ-secretase. The cleaved ectodomains of the three receptors may function as soluble decoy receptors (sBAFF-R, sTACI, and sBCMA) for BAFF and APRIL. (B) BAFF engagement of BAFF-R is essential for the survival and development of B cells beyond the immature T1 B cell stage. TACI is mostly expressed on marginal zone (MZ) B cells, follicular (FO) B cells, and plasma cells. BCMA expression is increased on plasmablasts and plasma cells. BAFF and APRIL also act on macrophages to stimulate the expression of molecules associated with the inflammatory M1 phenotype. BM, bone marrow; BCR, B cell receptor; DC, dendritic cell; iNOS, inducible nitric oxide synthase; TNFα, tumor necrosis factor alpha.
The receptor that is responsible for mediating the B cell survival function of BAFF is “BAFF receptor” (BAFF-R) (Fig. 1A and B) (23–25). APRIL engages the two receptors transmembrane activator and calcium-modulating cyclophilin ligand interactor (TACI) and B cell maturation antigen (BCMA), both of which also bind to BAFF (26, 27). In humans, BAFF-R is expressed on most mature B cells beyond pre-B cells, except for plasma cells (28, 29) (Fig. 1B). In contrast, human plasma cells and memory B cells express both TACI and BCMA (29). In mice, BAFF-R expression is more pronounced on FO B cells and germinal center (GC) B cells, while TACI is primarily expressed on MZ B cells, followed by FO B cells, memory B cells, and plasma cells (22, 30). BCMA is found on murine splenic and bone marrow plasma cells (22, 31, 32). Resembling the phenotype of BAFF-deficient mice, BAFF-R ablation strategies demonstrated that BAFF-R is crucial for the survival of mature naive B cells, but it is not needed for the maintenance of memory B cells and long-lived plasma cells in the bone marrow (10). Of the three BAFF receptors, TACI and BCMA are likely to be responsible for the survival of splenic B1a and B1b cells because BAFF-R deficiency does not influence splenic B1 cell numbers (33). TACI mediates the BAFF- and APRIL-induced generation of plasma cells and T cell-independent (TI) immunoglobulin isotype switching and secretion (22, 30, 34–36), whereas the function of BCMA is restricted to the maintenance of plasma cells and antigen presentation by B cells (31, 37–39) (Fig. 1B). Finally, APRIL has been shown to bind heparin sulfate proteoglycans (HSPGs) (8, 40, 41). It is not clear whether APRIL-bound HSPG transduces signals, but the binding of APRIL to HSPG has been suggested to potentiate TACI and BCMA activation through its multimerization, akin to the 60-mer form of BAFF (42).
Membrane-bound receptors of BAFF system molecules are also shed as soluble decoy receptors. TACI and BCMA are constitutively processed by a disintegrin and metalloproteinase 10 (ADAM10) (43) and γ-secretase (44), respectively (Fig. 1A). Soluble TACI antagonizes both BAFF and APRIL, whereas soluble BCMA binds only to APRIL. The processing of BAFF-R is more elaborately regulated than TACI and BCMA processing (Fig. 1A). Smulski et al. recently reported that unlike TACI and BCMA, BAFF-R is not constitutively shed, and processing requires BAFF stimulation and TACI coexpression (45). Stimulation with a homotrimeric BAFF 3-mer, which triggers BAFF-R but not TACI (46), activates ADAM10 to cleave and release BAFF-R from the membrane in the presence of TACI. In response to the oligomeric BAFF 60-mer that can bind to BAFF-R and TACI, ADAM10 and ADAM17 process BAFF-R and TACI. As APRIL does not induce the cleavage of BAFF-R, the activation of these proteases requires BAFF-R signaling with TACI coexpression. Although not formally shown, cleaved BAFF-R may also function as a decoy receptor, as part of a negative-feedback mechanism, to inhibit B cell responses to BAFF.
The expression of TACI, and, to some degree, BAFF-R, on B cells is regulated by various physiological and microbial signals (30, 47–50). Signals mediated by B cell receptor engagement along with Toll-like receptor (TLR) activation or CD40 ligation induce TACI expression on B cells, render B cells more sensitive to BAFF and APRIL, and promote plasma cell generation and immunoglobulin secretion (30, 36, 47–50). Moreover, the expression of TACI on B cells has been shown to be age dependent. Both human and mouse neonatal B cells express severely reduced levels of TACI (51–53), which appears to be due to weak B cell receptor signaling during the newborn period (50, 52). In human B cells, interleukin-2 (IL-2) and IL-10 can augment B cell receptor- and CD40-mediated TACI expression, although these cytokines do not alter TACI expression by themselves (29). The detection of diminished receptor levels in antibody binding assays such as flow cytometry assays should be cautiously interpreted and requires verification with gene expression analysis and microscopy studies because the occupancy of receptors by bound ligands can also result in a low level of receptor detection (54, 55).
In addition to its well-defined involvement in B cell homeostasis, we recently discovered a role for TACI in promoting the inflammatory (M1) macrophage phenotype. Despite its intracellular location in macrophages, TACI mediates BAFF- and APRIL-induced signals to suppress the expression of molecules (FIZZ1, Arg1, IL-10, IL-1RN, CCL22, CD206, and IL-4Rα) associated with the alternatively activated (M2) macrophage phenotype and induces the expression of M1 markers (MMP9, CD80, and IL-6) (56). A similar observation was made by Chang and colleagues, who reported BAFF-induced inflammatory cytokine secretion from human monocytes and dendritic cells (DCs) through intracellular TACI (57, 58).
The recognition of a central role for BAFF and APRIL in B cell homeostasis catalyzed research in diseases where exaggerated B cell responses are pivotal for pathogenesis. Autoimmune diseases such as SLE, rheumatoid arthritis, and Sjögren's syndrome and hematological malignancies such as multiple myeloma, lymphoma, and lymphocytic leukemia are areas that have benefitted most from research on BAFF system molecules. These studies led to approaches to ablate BAFF/APRIL activity as a treatment strategy, and belimumab, an anti-BAFF antibody, was approved in 2011 by the FDA for the treatment of active SLE. Reviews summarizing the advancements in these areas are available for more information (59–62). In this review, we focus on studies reporting the involvement of BAFF system molecules in response to infections. We divide the review into five sections based on studies covering immunization and infections by viruses, parasites, bacteria, and fungi.
IMMUNIZATION
We begin our review by discussing the immunization studies involving BAFF system molecules because host responses to introduced antigens are directly associated with responses to microbial challenges. Most successful vaccines elicit protection against infectious diseases by activating B cells specific for microbial antigens (63–66). Since the discovery of the BAFF system molecules almost 2 decades ago, primary immunobiological findings related to these molecules have been generated by immunizing mice deficient in each of the five members of the BAFF system molecules. The most dramatic phenotype was observed in BAFF- or BAFF-R-deficient mice immunized with T cell-dependent (TD) and TI antigens (6, 9, 67–70) (Table 1). Both strains of mice exhibited severely impaired antibody responses to TD and TI antigens. The inability of these strains to respond to antigens was attributed to the low number of mature B cells since BAFF-induced BAFF-R activation is essential for the survival and activation of B cell subsets beyond the immature stage (6, 9, 68, 69). A diminished response to TD antigens may also implicate suboptimal T cell responses because BAFF-deficient mice manifest decreased numbers of CD44+ CD62L− effector/memory T cells (9).
TABLE 1.
Roles for BAFF system molecules in immunizationa
| Molecule | Role(s) | Method(s) of proof | Reference(s) |
|---|---|---|---|
| BAFF | Mediating antibody response to TD and TI antigens | Immunization of BAFF−/− mice with TD and TI antigens | 6, 9, 68, 69 |
| Augmenting antibody responses against vaccines as an adjuvant | Immunization of exptl animals with BAFF-containing vaccines | 7, 90–94 | |
| APRIL | Inducing IgA isotype switch and production in response to TD and TI-I antigens | Systemic and mucosal immunization of normal, APRIL−/−, and APRIL-transgenic mice with TD and TI antigens | 19, 71, 72 |
| Augmenting antibody responses against vaccines as an adjuvant | Immunization of exptl animals with APRIL-containing vaccines | 91, 92 | |
| Immunization of APRIL Tg mice with TD and TI-II antigens | 20 | ||
| BAFF-R | Mediating antibody response to TD and TI antigens | Immunization of BAFF-R−/− mice with TD and TI antigens | 6, 9, 68, 69 |
| TACI | Mediating antibody response to TI-II antigens | Immunization of TACI−/− mice with TI-II antigens or CVID patients with polysaccharide vaccines | |
| TACI expression levels determine antibody responses to TI-II antigens | Responses of newborn mice and XID mice to TI-II antigens | 35, 50, 52, 73–75, 79, 80 | |
| Mediating TD antigen-specific plasma cell survival | Immunization of TACI−/− mice with TD antigens | 85, 89 | |
| Controlling expansion of Tfh and GC cells | Immunization of HIV-infected patients with influenza vaccine | ||
| BCMA | Contributing to long-term survival of antigen-specific bone marrow plasma cells | Immunization of BCMA−/− mice with a TD antigen | 31 |
TD, T cell dependent; TI, T cell independent; TI-II, TI type II; APRIL Tg, APRIL transgenic; CVID, combined variable immunodeficiency; XID, X-linked immunodeficient; Tfh, T follicular helper; GC, germinal center; HIV, human immunodeficiency virus.
By analyzing immune responses in immunized APRIL-deficient mice, Varfolomeev et al. suggested that APRIL is redundant with BAFF in T cell activation and humoral responses to TI type II (TI-II) and TD antigens (21). Others, however, reported that APRIL-deficient mice have lower levels of serum IgA than do wild-type mice, and mucosal but not systemic immunization of APRIL-deficient mice with a TD antigen results in diminished antigen-specific IgA antibodies in serum (19) (Table 1). Mucosally immunized APRIL-deficient mice also manifest a selective deficiency in IgA-positive (IgA+) plasma cells in the lamina propria of the small intestine. In contrast to the preserved immune response to TD antigens, defective serum IgA antibody responses are detected in APRIL-deficient mice immunized with TI type I (TI-I) and TI-II antigens (19, 71). Further highlighting the role of APRIL in driving IgA responses to TI antigens, immunization of APRIL-transgenic mice results in significantly elevated IgA antibody production compared to that in wild-type mice (71). Although the level of IgA produced in normal mice intranasally immunized with pneumococcal surface protein A correlates with the levels of BAFF and APRIL expressed by mucosal DCs (72), in vitro assessments demonstrated that APRIL induces IgA class switching in naive B cells more efficiently than does BAFF (19, 71). Thus, there seems to be an overall redundancy between BAFF and APRIL in regulating humoral responses, but APRIL is more potent than BAFF in inducing IgA isotype switch signals.
Despite its critical role in the long-term survival of antigen-specific bone marrow plasma cells, immunization of BCMA-deficient mice indicated that BCMA is dispensable for antibody development against TD antigens (31) (Table 1).
The most prominent phenotype associated with TACI deficiency has been observed in TACI-deficient mice immunized with TI-II antigens (73–75) (Table 1). Data from these studies indicated that TACI is needed for the generation of antibody responses against TI-II antigens (35) (Fig. 2A). Also, its expression levels have been shown to be a determining factor in the magnitude of antibody responses. The upregulation of TACI expression by TLR agonists results in augmented antibody responses against TI-II antigens through enhanced B cell sensitivity to BAFF and APRIL (30, 36, 49), whereas low levels of TACI expression in newborns or downregulated TACI expression by bacterial polysaccharides leads to unresponsiveness to TI-II antigens (52, 76) (Table 1 and Fig. 2B). In addition, our laboratory recently reported that the well-known unresponsiveness of X-linked immunodeficient mice to TI-II antigens (77) is a result of low TACI expression levels on B cells (50) (Table 1). Other evidences for the importance of TACI in the response to TI-II antigens emerge from combined variable immunodeficiency (CVID) patients immunized with polysaccharide vaccines. A subset of CVID patients has been shown to express various levels of TACI on B cells due to a heterozygous mutation in TNFRSF13B, the gene encoding TACI (78). By analyzing the responses of CVID patients to pneumococcal polysaccharide vaccines (TI-II antigens), Chinen et al. discovered that patients with the lowest density of B cell TACI expression also have the lowest levels of serum antibodies compared to normal individuals (79) (Table 1). Further supporting the involvement of TACI in host responses to TI-II antigens, elevated levels of TACI expression on antigen-specific plasma cells in individuals immunized with pneumococcal polysaccharide vaccines have been observed (80). Marginal zone B cells are the B cell subset that is primarily responsible for the development of antibody responses to TI-II antigens (81). In secondary lymph organs, these TACIhigh MZ B cells recognize soluble antigens and become activated by BAFF and APRIL, which are produced primarily by neutrophils (82, 83) and innate lymphoid cells (84).
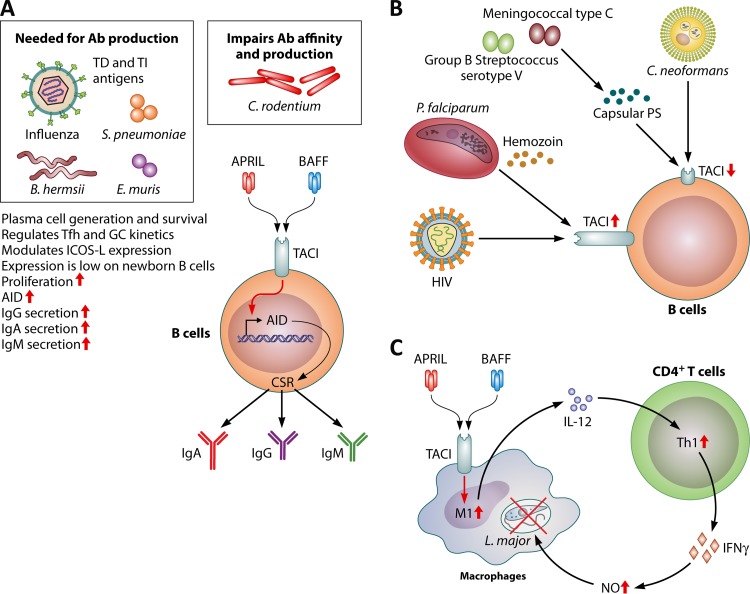
FIG 2.
Role of TACI during infection. (A) TACI is mostly expressed on mature B cells and plasma cells. Its elevated expression levels on MZ B cells and B1 cells lead to augmented antibody (Ab) responses against TI-II antigens. Together with the multivalent cross-linking of B cell receptor by polysaccharides, the engagement of TACI by BAFF or APRIL increases activation-induced cytidine deaminase (AID) transcript levels, leading to a polysaccharide-specific antibody isotype switch to IgA and IgG. TACI activation also induces the generation of plasma cells. The requirement for TACI expression to maintain plasma cells also extends to responses against T cell-dependent (TD) antigens. TACI has been shown to regulate the magnitude and kinetics of T follicular helper (Tfh) cell development and germinal center (GC) formation. The consequence of TACI deficiency is mostly detrimental to the survival of plasma cells after immunizations or infections. Citrobacter rodentium infection of TACI-deficient mice, however, leads to an enhanced clearance of the bacteria and higher levels of antibacterial antibodies with increased affinity. (B) TACI expression can be modulated by pathogens. Whereas C. neoformans and capsular polysaccharides (PS) from a meningococcal type C strain and a group B streptococcus serotype V strain blunt B cell responses to BAFF and APRIL by strongly suppressing TACI expression, B cells from HIV- or P. falciparum-infected patients manifest elevated levels of TACI. (C) Expression of TACI in macrophages has been shown to be required for M1 polarization. IL-12 secreted from M1 macrophages induces Th1 responses and IFN-γ production. IFN-γ promotes macrophage inflammatory responses, including nitric oxide (NO) generation, to control intracellular L. major infection. TI, T cell independent; ICOS-L, inducible costimulator ligand; CSR, class switch recombination.
Whereas the role for TACI in the response to TI-II antigens is well described, its contribution to the development of antibody responses against TD antigens has only recently been appreciated. Early studies suggested that TACI is dispensable for TD antibody responses (73, 75). However, by immunizing TACI-deficient mice with the TD antigen nitrophenyl-chicken gamma globulin (NP-CGG), Ou et al. recently reported that TACI is required for the survival of plasma cells specific for TD antigens (85). This study also showed that TACI controls the expansion of T follicular helper (Tfh) cells and GC cells by regulating inducible costimulator (ICOS) ligand (ICOSL) (also known as B7H2) expression on B cells, as the interaction of ICOSL with ICOS on Tfh cells stimulates Tfh cell generation (86) (Table 1 and Fig. 2A). Conceivably, TACI may support the fitness of plasma cells by regulating the kinetics of Tfh cell development and GC reactions. Interestingly, while the global deletion of TACI appears to enhance Tfh cell development and GC reactions, with detrimental consequences for plasma cell survival (86, 87), under physiological conditions, the CD40- and IL-21-mediated downregulation of TACI on GC B cells appears to be required for the generation of high-affinity antibody-secreting cells in NP-CGG-immunized mice (88). An increase in TACI expression on plasma cells alone can be relevant for efficient antibody development because B cells of human immunodeficiency virus (HIV)-infected patients who are able to respond to an influenza virus vaccine have been shown to express elevated levels of TACI (89). These patients also present with elevated serum BAFF, APRIL, and IL-2 levels. In contrast, TACI levels remain unaltered compared to prevaccination levels in nonresponder patients (Table 1).
The recognition of a central role for BAFF and APRIL in orchestrating B cell survival and activation led to strategies to incorporate BAFF and APRIL as adjuvants into vaccines. For example, in a mouse study, the coadministration of BAFF with the TI vaccine Pneumovax23 resulted in significantly higher IgM and IgA antibody responses without any enhancement of IgG antibody levels, while BAFF boosted both IgM and IgG responses against TD antigens (7). In parallel, a series of DNA vaccines containing HIV envelope proteins together with BAFF and/or APRIL trimers or in combination with other molecules such as CD40L and IL-12 has been tested in mice and rabbits (90–92). These studies showed that either BAFF- or ARPIL-containing DNA vaccines can augment antibody responses with neutralizing capabilities, but vaccines containing APRIL are consistently superior to those containing BAFF (92). Similarly, the systemic coadministration of BAFF with heat-killed Pseudomonas aeruginosa or the veterinary vaccine formalin-inactivated infectious bursal disease virus significantly improves antibody responses (93, 94). How BAFF and APRIL achieve strong antibody responses in these vaccines has not been investigated. One possibility is the targeting of the fused vaccine to B cells since BAFF and APRIL activate B cells by engaging their receptors. Additional targeting of vaccines to other antigen-presenting cells may also contribute to the improved host response since BAFF and APRIL activate macrophages and DCs through TACI (56–58). Further research is needed to elucidate the mechanisms of adjuvant activity afforded by systemically administered BAFF and APRIL with vaccines as well as to assess potentially undesired side effects, since elevated BAFF levels are associated with autoimmune diseases (8).
VIRUSES
HIV
Soon after the discovery of BAFF system molecules, several studies reported the involvement of these molecules in HIV infection and AIDS. These studies suggest an association between elevated plasma BAFF and APRIL levels and hypergammaglobulinemia in HIV-infected individuals (95–99) (Table 2). Hypergammaglobulinemia appears to be a result of a dysregulated blood B cell compartment characterized by the BAFF-induced expansion of MZ B cells (97, 100–102). Dendritic cells, monocytes, and macrophages are the sources of increased BAFF and APRIL levels during HIV infection (97, 99, 101, 103) (Fig. 3). BAFF production in innate cells is triggered directly by viral products, including double-stranded RNA (104), negative factor (Nef) (105), and the exterior envelope glycoprotein gp120 (106), and indirectly by type I interferon (IFN) secreted from plasmacytoid DCs in response to viral particles (107, 108). Among the HIV molecules, Nef has been reported to induce BAFF expression in human DCs and murine macrophages, but the induction of BAFF expression by Nef is negated if the macrophages are M1 polarized (105, 109). The role for gp120 in HIV infection is especially interesting because this glycoprotein not only stimulates BAFF and APRIL expression in monocytes but also engages human tonsillar IgD+ B cells through interactions with mannose C-type lectin receptors (MCLRs). The exposure of tonsillar IgD+ B cells to gp120 leads to B cell proliferation, increases in the levels of activation-induced cytidine deaminase (AID) transcripts, and immunoglobulin isotype switching from IgM to IgG and IgA. BAFF further sensitizes B cells by increasing the expression levels of MCLRs on B cells, which augments gp120-induced B cell activation. The increased sensitivity of B cells to gp120 in HIV infection may also be linked to changes in expression levels of BAFF and APRIL receptors because Moir et al. showed that the expression level of BAFF-R is decreased, while the expression levels of TACI and BCMA are increased, on CD21low B cells in HIV-infected viremic patients (110) (Table 2 and Fig. 2B and 4B). Since the TACI expression level determines the sensitivity of B cells to BAFF and APRIL (30, 52), elevated TACI levels on B cells are a likely cause of augmented hypergammaglobulinemia, the most prominent B cell defect associated with HIV infection. Also, the BAFF-induced preferential expansion of CD21hi B cells over CD21low B cells may be the reason for the persistent activation of MZ B cells in HIV-infected individuals, since CD21 is one of the markers defining MZ B cells as CD21hi (101).
TABLE 2.
Role of BAFF system molecules in viral infectiona
| Molecule | Infection(s) | Phenotype(s) | Reference(s) |
|---|---|---|---|
| BAFF | HIV | Level is increased in serum | 95, 97, 98, 101–103 |
| Viral products trigger BAFF expression | 104–106 | ||
| HCV | Level is increased in serum; higher BAFF expression level is correlated with poor prognosis | 113, 118 | |
| HBV | Level is increased in serum | 120, 121 | |
| Influenza virus | Systemic level is increased | 128 | |
| RSV | Expression is increased in epithelium and upper airway | 133 | |
| Cytomegalovirus and coronavirus | Production by astrocytes and glial cells is increased | 134–136 | |
| Production by tonsil B cells is increased | 137 | ||
| Human metapneumovirus, bocavirus, and rhinovirus | Level is increased in upper airway secretions | 133 | |
| APRIL | HIV | Level is increased in serum | 97 |
| Influenza virus | Expression is increased in lung | 129 | |
| RSV | Expression is increased in epithelia of infected infants | 131 | |
| Cytomegalovirus and coronavirus | Production by astrocytes and glial cells is increased | 134–136 | |
| RABV | Production by splenic B cells is increased | 138 | |
| BAFF-R | HIV | Expression is decreased on B cells | 110 |
| VSV and LCMV | Higher susceptibility in BAFF-R−/− mice due to loss of CD169+ macrophages | 124, 126, 127 | |
| HCMV | Expression is increased on tonsil B cells | 137 | |
| TACI | HIV | Expression is increased on B cells | 110 |
| Influenza virus | Short-lived plasma cell and diminished antibody responses in TACI−/− mice | 87 | |
| BCMA | HIV | Expression is increased on B cells | 110 |
HCV, hepatitis C virus; HBV, hepatitis B virus; RSV, respiratory syncytial virus; RABV, rabies virus; VSV, vesicular stomatitis virus; LCMV, lymphocytic choriomeningitis virus; HCMV, human cytomegalovirus.
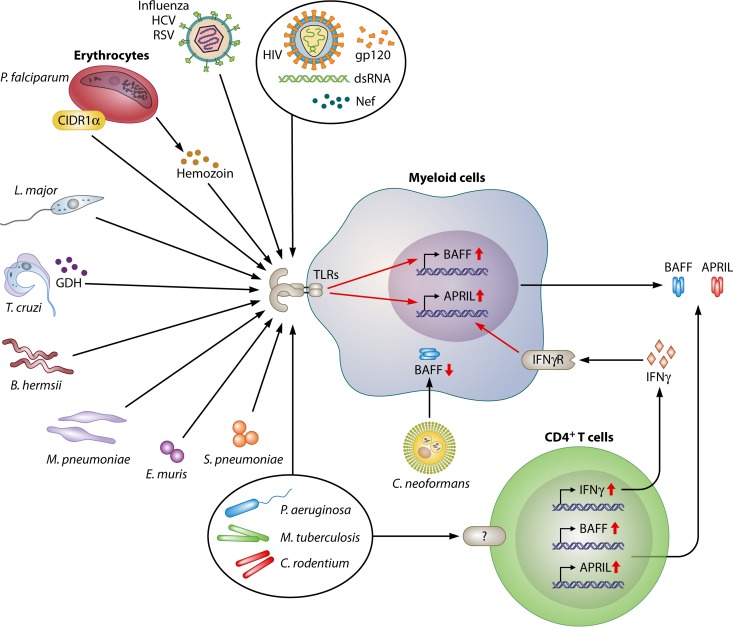
FIG 3.
Roles of BAFF and APRIL during infection. Many pathogens induce the expression of BAFF and APRIL in myeloid cells or epithelial cells through the engagement of pattern recognition receptors by pathogen-associated molecular patterns. In addition, pathogen-induced type I and II IFNs can elicit BAFF and APRIL expression. The main consequence of elevated BAFF and APRIL expression levels is the activation of B cells and the induction of pathogen-specific antibody production. Unlike most pathogens, C. neoformans has been shown to suppress the expression of BAFF in human PBMCs. Diminished BAFF expression in C. neoformans infection is likely a virulence mechanism developed by the fungus because C. neoformans-infected patients have low levels of circulating immunoglobulins. TLRs, Toll-like receptors; GDH, glutamate dehydrogenase; CIDR1α, cysteine-rich interdomain region 1α; HCV, hepatitis C virus; RSV, respiratory syncytial virus; Nef, negative factor; IFNγR, interferon gamma receptor; dsRNA, double-stranded RNA.
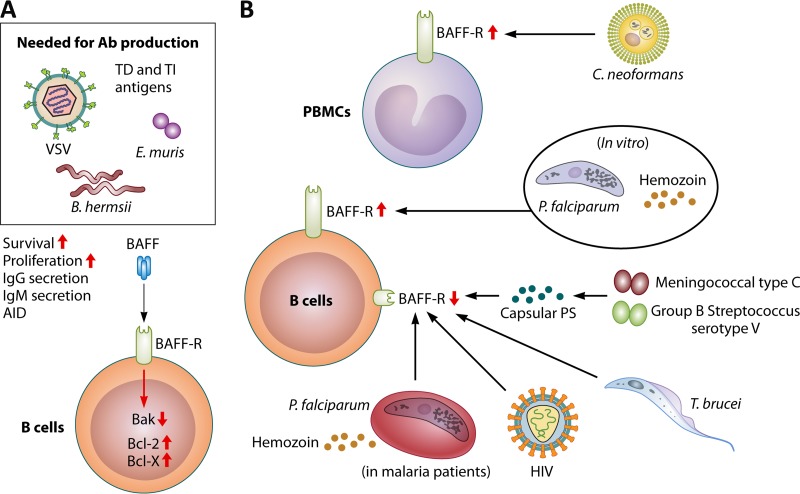
FIG 4.
Role of BAFF-R during infection. BAFF-R is detected on B cells as early as the immature B cell stage (Fig. 1), and its expression is diminished with the generation of plasma cells. (A) Engagement of BAFF by BAFF-R simultaneously with B cell receptor stimulation promotes the survival and proliferation of pathogen-specific B cells by decreasing the level of the proapoptotic molecule B cell lymphoma 2 (Bcl-2)-homologous antagonist/killer (Bak) and increasing the levels of the prosurvival molecules Bcl-2 and Bcl-x, leading to the enhanced secretion of isotype-switched and unswitched antibodies. (B) Although the mechanism is not elucidated, bacterial polysaccharides from group C meningococcus and group B streptococcus type V have been shown to downregulate the expression of BAFF-R on B cells. BAFF-R levels on B cells are reduced in HIV-infected patients. A decrease in the BAFF-R level is also observed during the acute phase of P. falciparum infection in children as well as experimentally infected adults. The in vivo factors responsible for diminished BAFF-R expression in P. falciparum-infected patients are unknown. Paradoxically, in vitro stimulation of human B cells with the P. falciparum schizont fraction or hemozoin results in increased BAFF-R expression. BAFF-R expression is also downregulated on MZ B cells in T. brucei-infected mice, leading to a decrease in the number of antiparasite IgM+ B cells. Unlike malaria and African trypanosomiasis, C. neoformans infection upregulates BAFF-R expression in PBMCs.
Hepatitis Viruses
Similar to HIV infection, hepatitis C virus (HCV) infection manifests as pathogenic B cell lymphoproliferation and elevated serum autoantibody levels (111, 112). The discovery of increased circulating BAFF levels in HCV-infected patients (113) provided a possible explanation for the Sjögren's syndrome-like autoimmune phenomenon in HCV infection, especially because elevated BAFF-mediated B cell activation is implicated in autoantibody production in autoimmune diseases such as systemic lupus erythematosus (8) (Table 2 and Fig. 3). The expansion of BAFF-sensitive and apoptosis-resistant CD5+ B cells is likely responsible for the appearance of autoantibodies in these patients (113, 114). Further supporting this hypothesis, patients undergoing IFN-α therapy who typically experience an increase in autoimmune manifestations (115, 116) also have increased circulating BAFF levels (117). Paradoxically, BAFF-induced B cell activation appears to be detrimental for the host because Tarantino et al. reported a correlation between higher BAFF levels during the acute phase of HCV infection and the subsequent persistence of HCV infection (118) (Table 2 and Fig. 4B). The underlying reasons for this unusual outcome have not been investigated. However, a possibility is the promotion of TI B cell responses to HCV antigens by BAFF, a process known to elicit low-affinity antibody production (119). These antibodies may be poor in viral neutralization, or they may target antigens that are not important for virus neutralization.
Serum BAFF levels are also increased in chronic hepatitis B virus (HBV) infection. Similar to HCV, elevated BAFF levels appear to correlate with disease severity in HBV infection since asymptomatic HBV carriers have the lowest and hepatocellular carcinoma patients have the highest levels of serum BAFF (120). The increased expression of BAFF in HBV patients with more severe disease is likely associated with higher viral loads because HBV e (HBe) antigen induces BAFF production in monocytes, and patients positive for HBe antigen have been shown to have higher serum BAFF levels than HBe-negative HBV patients (121). Although the increase in HBV-induced BAFF levels appears not to aid in host resistance, perhaps paradoxically, higher BAFF expression levels in liver are associated with better IFN-α treatment outcomes in chronic hepatitis B patients (122). Interestingly, a recent study reported an association between polymorphisms in the BAFF gene (TNFSF13B) and susceptibility to HBV infection, but these polymorphisms did not lead to increased levels of circulating BAFF (123). Further research is needed to assess the functional consequences of TNFSF13B polymorphisms.
Zoonotic Viruses
The two zoonotic viruses vesicular stomatitis virus (VSV) and lymphocytic choriomeningitis virus (LCMV) are frequently used as models to study host responses to viral infections. By using BAFF-R-deficient mice, Xu et al. recently reported that BAFF-R is critical for host resistance to VSV and LCMV infections (124) (Table 2 and Fig. 4A). Interestingly, the susceptibility of BAFF-R-deficient mice to viral infection appears to be an indirect consequence of mature B cell loss. Lymphotoxin expressed by B cells is known to be required for the maintenance of CD169+ macrophages located in the subcapsular sinus and in the medulla of lymph nodes (125). In the absence of mature B cells, and, thus, lymphotoxin, BAFF-R-deficient mice cannot maintain CD169+ macrophages, which are essential for controlling neurotropic viral infections by secreting type I IFN (125). As CD169+ macrophages also activate B cells located in the underlying follicles, host susceptibility is amplified with the loss of BAFF-R (126, 127). Thus, a consequence of an arrest in mature B cell survival in BAFF-R-deficient mice is the loss of a link between innate and adaptive immune systems during VSV and LCMV infections (124).
Respiratory Viruses
The expression and function of BAFF system molecules in mice infected by viruses with tropism for the respiratory tract have also been reported. For example, BAFF (128) and APRIL (129) expression levels are increased in influenza virus-challenged mouse lungs (Table 2), yet the significance of elevated BAFF and APRIL expression levels in controlling influenza virus infection is not clear, especially because neither APRIL overexpression nor APRIL deficiency impacts host resistance to influenza virus challenge in mice (129). However, BAFF and APRIL may be important in determining the quality of plasma cells generated during a TD response in influenza virus infection. Supporting this hypothesis, Wolf et al. discovered that infection of TACI-deficient mice with influenza virus results in short-lived plasma cells, which appears to be responsible for the increased susceptibility to rechallenge infection with influenza virus (87) (Table 2 and Fig. 2A). The compromised fitness of plasma cells in TACI-deficient mice is possibly due to a suboptimal TD response, as shown by Ou et al., who also detected a reduction in the number of plasma cells in NP-CGG-immunized TACI-deficient mice despite expanded Tfh and GC responses (85).
Respiratory syncytial virus (RSV) is the most common cause of bronchiolitis and pneumonia in infants (130). Both BAFF and APRIL have been implicated in RSV infection because epithelial cells isolated from infants with fatal RSV infection express BAFF and APRIL, which are colocalized with cells expressing type I IFN (131) (Table 2). Furthermore, a positive correlation between BAFF and APRIL levels in nasopharyngeal secretions of RSV-infected infants and levels of antiviral IgA and IgM antibodies in these infants has been established. RSV-induced type I IFN is likely responsible for elevated BAFF and APRIL levels because RSV induces the potent BAFF- and APRIL-stimulating cytokine IFN-β through TLR3 (132). Also, the type I IFN-mediated induction of BAFF from RSV-infected epithelial cells was confirmed by McNamara et al. using cultured airway epithelial cells (133). Those authors also measured elevated BAFF levels in the bronchoalveolar lavage fluid of infants with severe RSV infection as well as in upper airway secretions from children with human metapneumovirus, influenza virus (H1N1), bocavirus, rhinovirus, RSV, and Mycoplasma pneumoniae infections (Table 2). Interestingly, despite measuring increased BAFF levels in infected airways, McNamara et al. did not detect elevated APRIL levels following RSV infection (133). This is in contrast to the findings of Reed et al., who emphasized a role for APRIL in better outcomes of RSV infection (131). Further studies are needed to clarify the role of APRIL in the host response to RSV infection.
Viral Infections of the Central Nervous System
Viruses with tropism for the central nervous system induce BAFF and APRIL expression in astrocytes and glial cells (134–136). Interestingly, cytomegalovirus augments BAFF expression by astrocytes through IFN-γ, while APRIL expression is a direct result of virus recognition by astrocytes (136). Cytomegalovirus- and coronavirus-induced BAFF and APRIL are implicated in host resistance because they help maintain virus-specific plasma cells through TACI and BCMA in the central nervous system (136). Furthermore, cytomegalovirus also increases human tonsillar B cell survival by stimulating the expression of BAFF and BAFF-R on these cells (137). In contrast to cytomegalovirus, the involvement of the APRIL-TACI axis in host resistance appears to be questionable because while rabies virus induces APRIL expression in murine B cells, immunization with a recombinant rabies vaccine expressing APRIL does not improve antibody responses, and virus-specific plasma cell generation is not diminished in immunized TACI-deficient mice (138).
BACTERIA
Bacterial Infections of the Respiratory System
Elevated BAFF and APRIL levels have been reported to accompany bacterial infections of the respiratory system. In Mycobacterium tuberculosis-infected patients, increased levels of serum BAFF and APRIL appear to correlate with disease severity (Table 3 and Fig. 3). Liu et al. focused on the expression of BAFF and APRIL in CD4+ T cells in M. tuberculosis-infected patients and found elevated expression levels of both cytokines in CD4+ cells of patients with active M. tuberculosis infection (139). Moreover, there was a correlation between increased BAFF and APRIL mRNA expression levels and elevated IFN-γ levels. Those authors also reported increased BAFF expression levels in pleural effusions of M. tuberculosis-infected patients with pleurisy. In another study, APRIL expression was shown to be increased in B cells of M. tuberculosis-infected patients after 6 months of anti-M. tuberculosis treatment (140). Finally, a recent study confirmed the BAFF- and APRIL-inducing effect of M. tuberculosis by measuring serum BAFF and APRIL levels in patients with latent M. tuberculosis infection (141). Although the levels of both cytokines were increased in these patients, a significant correlation was present only between BAFF and IgG levels. The importance of elevated BAFF and APRIL levels in M. tuberculosis pathogenesis has not been investigated. Increased BAFF and APRIL levels may promote antibody development in M. tuberculosis-infected hosts, but the significance of anti-M. tuberculosis antibodies remains controversial (142, 143). Another target for BAFF and APRIL is macrophages (56). Further research is needed to unveil whether BAFF- and APRIL-induced M1 polarization of macrophages impacts disease outcomes of M. tuberculosis infection.
TABLE 3.
Role of BAFF system molecules in bacterial infectiona
| Molecule | Infection(s) | Phenotype(s) | Reference(s) |
|---|---|---|---|
| BAFF | M. pneumoniae | Level is elevated in upper airway secretions | 133 |
| P. aeruginosa | Level is increased in bronchoalveolar lavage fluids and lung homogenates | 145 | |
| Coadministration of BAFF with heat-killed P. aeruginosa increases Th1 response | 93 | ||
| M. tuberculosis | Increased serum levels in M. tuberculosis-infected patients correlate with disease severity; BAFF is also found in peripheral CD4+ T cells and in pleural effusions of M. tuberculosis-infected patients with pleurisy | 139 | |
| A. pleuropneumoniae | Adhesin Apa2H1 induces BAFF and APRIL expression on DCs | 144 | |
| E. muris | Systemic ablation of BAFF with a neutralizing antibody increases susceptibility of mice | 150 | |
| APRIL | M. tuberculosis | Increased serum levels in M. tuberculosis-infected patients correlate with disease severity; APRIL is also found in peripheral CD4+ T cells | 139 |
| BAFF-R | B. hermsii | BAFF-R−/− mice manifest increased susceptibility | 154–156 |
| TACI | C. rodentium | Infected TACI−/− mice show enhanced GC reaction and bacterial clearance | 149 |
| S. pneumoniae | TACI−/− mice immunized with heat-killed S. pneumoniae do not develop anticapsular antibodies | 157 | |
| Meningococcal type C, group B Streptococcus serotype V | The capsular polysaccharides downregulate TACI expression on B cells, causing B cell unresponsiveness to BAFF and APRIL | 76 |
Th1, type 1 T helper; DCs, dendritic cells.
Actinobacillus pleuropneumoniae is the causative pathogen of porcine pleuropneumonia. An enhancement of pathogen-specific antibody development by elevated BAFF and APRIL levels was demonstrated for A. pleuropneumoniae infection (144). The protective antigen Apa2h1 appears to contribute to BAFF and APRIL production in A. pleuropneumoniae infection because incubation of mouse DCs with Apa2h1 upregulates the expressions of BAFF and APRIL. Thus, the increase in BAFF and APRIL levels may be instrumental in controlling A. pleuropneumoniae infection by boosting the humoral immune response.
Pseudomonas aeruginosa is a respiratory pathogen frequently isolated from the lungs of cystic fibrosis patients. Bronchoalveolar lavage fluids of children with cystic fibrosis contain high levels of BAFF, and increased BAFF expression levels have been measured in lung homogenates of mice infected intranasally with P. aeruginosa (145) (Table 3 and Fig. 3). Although this observational study did not provide insight into its function in P. aeruginosa infection, an elevated BAFF level appears to drive B cell responses to P. aeruginosa, because when heat-killed P. aeruginosa is coadministered with an adenovirus expressing full-length BAFF, mice are better protected against lethal respiratory P. aeruginosa challenge and mount higher levels of antibody responses in sera and lungs than animals immunized with heat-killed P. aeruginosa alone (93). Supporting a role for BAFF in host responses involving T cells, improved protection and higher antibody levels were accompanied by elevated type 1 T helper (Th1) cell responses (88, 146–148). Nevertheless, the contribution of individual receptors to BAFF-mediated T cell responses is less clear and needs to be investigated.
Bacterial Infections of the Gut
Citrobacter rodentium is a rodent pathogen that is frequently used as a model for severe human enteritis. In contrast to all other pathogens tested in TACI-deficient mice, C. rodentium challenge of TACI-deficient mice results in improved resistance to infection (149) (Table 3 and Fig. 2B). Citrobacter rodentium infection of TACI-deficient mice is accompanied by an enhanced GC reaction, and accelerated bacterial clearance appears to be due to higher-affinity antibodies elicited against bacterial proteins. The development of higher-affinity antibodies is attributed to enhanced BAFF-R-mediated antiapoptotic signals on GC B cells in the absence of TACI-mediated inhibitory signals. How and why enhanced Tfh and GC reactions in TACI-deficient mice result in improved protection against C. rodentium infection with increased levels of high-affinity antibodies while promoting poor plasma cell survival in response to NP-CGG immunization (85) and increased susceptibility to influenza virus challenge, mostly because of impaired plasma cell responses (87), remain unknown and warrant further study.
Tick-Borne Bacterial Pathogens
A role for BAFF in the development of TI responses to the tick-borne intracellular pathogen Ehrlichia muris has been shown in a murine infection model (Table 3 and Fig. 3). Jones et al. investigated the contribution of an elevated level of BAFF in a fatal E. muris infection model (150), which is known to be controlled by IgM-producing CD138high IgMhigh plasma cells (151, 152). This study showed that the systemic ablation of BAFF with a neutralizing antibody halts the terminal differentiation of bacterium-specific IgM-secreting plasmablasts and renders mice more susceptible to infection, indicating a direct role for BAFF in host resistance. Interestingly, gene expression analysis with anti-BAFF-injected mice suggested that the regulation of CD138 expression and IgM secretion by BAFF involves a posttranslational mechanism. However, the receptor responsible for BAFF action in E. muris infection remains unknown.
Lyme neuroborreliosis is a serious complication of tick-borne Borrelia burgdorferi infection. Analysis of cerebrospinal fluids of patients with a variety of inflammatory diseases, including Lyme disease, indicated that samples from patients with Lyme neuroborreliosis contain significantly higher BAFF and APRIL levels than do samples from patients with most other diseases (153). Elevated BAFF and APRIL levels are likely responsible for the increased numbers of B cells and plasmablasts as well as elevated IgA and IgM levels in the cerebrospinal fluids of these patients.
Another tick-borne pathogen, Borrelia hermsii, the causative agent of relapsing fever, is also controlled by TI IgM responses (154–156). Challenge of BAFF-R-deficient mice, but not TACI-deficient mice, results in an impairment of B. hermsii clearance, highlighting the importance of BAFF-R in splenic B1b cell-mediated resistance to B. hermsii infection (Table 3 and Fig. 4A).
Infections by Encapsulated Bacteria
Similar to findings with purified polysaccharides (52, 73), TACI expression is required for the development of antibodies against the capsular polysaccharide after injection with heat-killed Streptococcus pneumoniae (157) (Table 3 and Fig. 2A). This diminished antibody response in TACI-deficient mice injected with heat-killed S. pneumoniae results in greater susceptibility to subsequent pneumococcal challenge than in the S. pneumoniae-immunized BAFF-R-deficient or wild-type mice (157). Encapsulated bacteria appear to also modulate the expression of BAFF system molecules. We reported that polysaccharides of meningococcal type C and group B streptococcus type V downregulate the expression of TACI on B cells (76) (Fig. 2B). Downregulation of TACI has biological consequences because after exposure to these polysaccharides, B cells are not able to respond to the TACI ligands BAFF and APRIL. Thus, the suppression of TACI by bacterial polysaccharides appears to be a virulence mechanism adopted by encapsulated bacteria since TACI is essential for the development of antibodies against polysaccharides to efficiently kill encapsulated bacteria. We also showed that meningococcal type C polysaccharide specifically inhibits lipooligosaccharide-induced innate cell activation by competing for CD14 and lipopolysaccharide (LPS) binding protein, two molecules known to enhance TLR4 activation (158). Since LPS is a potent inducer of BAFF and APRIL (159, 160), the inhibitory effect of meningococcal polysaccharides on LPS is likely another example of their virulent properties.
PARASITES
Leishmaniasis and Chagas' Disease
BAFF and B cell responses are important for resisting diseases caused by protozoan parasites (161). However, increased expression levels of BAFF and APRIL can also lead to dysregulated B cell responses and susceptibility to parasites. In visceral leishmaniasis, polyclonal B cell activation and hypergammaglobulinemia are associated with APRIL and BAFF expression in the spleens of infected dogs and in sera of infected mice, which may be responsible for the inefficient humoral immune response and disease progression seen in leishmaniasis (162, 163). Similarly, in the chronic phase of Chagas' disease, hypergammaglobulinemia caused by excessive polyclonal B cell activation is recognized as the source of circulating autoantibodies (164), and BAFF secreted from myeloid cells seems to be responsible for polyclonal B cell activation (165) (Table 4 and Fig. 3). The significance of BAFF in infected humans is not as clear as animal models suggest, because patients with visceral leishmaniasis also manifest elevated serum BAFF levels, but a correlation with serum IgG levels was not found (166). In the same study, a second group of patients with Chagas' disease did not have elevated serum BAFF or increased IgG levels. This observation is interesting, because mice infected with Trypanosoma cruzi, the causative agent of Chagas' disease, exhibit elevated levels of serum BAFF, increased numbers of splenic and lymph node B220+ cells, as well as high levels of IgM and IgG production from splenic cells (165). Montes et al. demonstrated that a major component of T. cruzi, glutamate dehydrogenase (GDH), induces BAFF secretion from CD11b+ cells (164). Systemic BAFF ablation experiments verified the role of BAFF as the cause of polyclonal B cell activation, because injection of the soluble BAFF receptor BR3:Fc in T. cruzi-infected mice decreases total mature B cell numbers in the spleen, serum IgM antibodies against parasite antigens, as well as IgG antibodies against host nuclear antigens (165). Interestingly, BAFF ablation does not change the systemic parasite burden but results in elevated parasite counts in cardiac tissue. The authors of that study suggested that the reduction in B cell numbers is a plausible cause of localized parasite replication in the heart. An alternative explanation may be related to the polarizing effect of BAFF on macrophages, since both BAFF and APRIL drive M1 polarization of macrophages through TACI signaling (56). Given that M2 polarization of macrophages promotes parasite persistence in the myocardium (167), a shift in the phenotype of macrophages as a result of decreased BAFF activity in BR3:Fc-injected mice may be responsible for elevated parasite counts in the heart.
TABLE 4.
Role of BAFF system molecules in parasitic infectiona
| Molecule | Infection | Phenotype(s) | Reference(s) |
|---|---|---|---|
| BAFF | T. cruzi | Level in serum is increased through activation of myeloid cells by T. cruzi GDH | 164, 165 |
| Visceral leishmaniasis | Level is increased in serum and spleen | 162, 163 | |
| P. falciparum | Expression by macrophages and monocytes is upregulated by P. falciparum metabolites and antigens | 170, 175, 177 | |
| Level in serum is increased in children with acute malarial infection | 179 | ||
| Level is increased in placental samples of patients with placental malaria | 178 | ||
| Expression is increased in human blood myeloid cells during the acute phase of exptl P. falciparum challenge | 180 | ||
| P. yoelii | Expression is reduced in murine lymphoid DCs | 185 | |
| APRIL | P. falciparum | Level is increased in placental samples of cases of placental malaria | 178 |
| BAFF-R | P. falciparum | Expression on B cells is upregulated in in vitro cocultures with monocytes in the presence of P. falciparum metabolites and antigens | 177 |
| Expression is reduced on peripheral B cells of children during acute malarial infection | 179 | ||
| T. brucei | Expression is reduced, leading to loss of parasite-specific IgM+ B cells | 169 | |
| TACI | L. major | TACI−/− mice are more susceptible to cutaneous Leishmania because of M2-skewed phenotype of macrophages | 56 |
| P. falciparum | Expression is elevated on peripheral B cells of children during acute malarial infection | 179 | |
| BCMA | P. falciparum | Expression is elevated on peripheral B cells of children during acute malarial infection | 179 |
GDH, glutamate dehydrogenase.
A more direct proof of the consequences of blocking BAFF- and APRIL-mediated macrophage polarization was documented for Leishmania-infected TACI-deficient mice (Table 4 and Fig. 2). The so-called “Leishmania-resistant” C57BL/6 mouse strain controls cutaneous Leishmania major infection by mounting a Th1 response and by the M1 polarization of skin macrophages (168). We observed an increase in the parasite load and more severe skin pathology in L. major-challenged TACI-deficient C57BL/6 mice (56). The susceptibility of TACI-deficient mice is primarily due to the M2-skewed macrophage phenotype, because the transfer of macrophages from wild-type C57BL/6 mice is sufficient to render TACI-deficient mice resistant to Leishmania infection.
Susceptibility to parasitic infections can also be a result of the parasite-orchestrated suppression of BAFF system molecules. The causative agent of sleeping sickness, Trypanosoma brucei, induces a rapid TI antibody response that suppresses the parasite load during the early phase of infection. However, experimentally infected mice succumb to infection by a second wave of infection partially because of the parasite-driven apoptosis of MZ B cells (169). Apoptosis is likely mediated by a reduction in BAFF-R expression and the subsequent downregulation of the antiapoptotic Bcl-2 protein in infected murine MZ B cells (Table 4 and Fig. 4B). These mice not only lose parasite-specific IgM+ B cells but also are impaired in mounting a humoral immune response to unrelated pathogens and vaccines.
Malaria
Plasmodium falciparum is the causative agent of human malaria, and like T. cruzi, P. falciparum infection is accompanied by hypergammaglobulinemia, a consequence of parasite-induced polyclonal B cell activation (170–174). During malaria infection, the host's immune system is activated by the P. falciparum metabolite hemozoin (malarial pigment) (175) and cysteine-rich interdomain region 1α (CIDR1α), a specific cell surface antigen of P. falciparum-infected erythrocytes (176). Macrophages stimulated with hemozoin produce reactive oxygen species that can potentially mediate BAFF expression (170, 175). Indeed, using cocultures of monocytes and naive B cells, Kumsiri et al. demonstrated BAFF secretion from monocytes stimulated with the schizont fraction of P. falciparum and hemozoin (177) (Table 4 and Fig. 3). Moreover, both the schizont fraction and hemozoin stimulate BAFF-R expression on B cells. Thus, it appears that parasite-induced BAFF activity on B cell proliferation and P. falciparum-specific IgG production is augmented by the upregulation of BAFF-R expression (Fig. 4B).
Altered expression levels of BAFF system molecules in clinical samples from malaria-infected patients have also been reported. For example, the expression levels of both TNFSF13B (BAFF) and TNFSF13 (APRIL) mRNAs are significantly increased in placental malaria (PM)-positive placenta samples, and increased levels of BAFF, IgG, and IgM are positively correlated with increased placental malarial pigment deposition (a pathological feature of chronic PM) (178) (Table 4). During the acute phase of malaria infection, children have high plasma BAFF levels and elevated B cell TACI and BCMA expression levels, while the BAFF-R expression level is decreased (Fig. 2B and 4B) (179). High plasma BAFF and low BAFF-R expression levels on B cells are also observed in human subjects experimentally challenged with cryopreserved P. falciparum (180). A well-established fact about malaria is the slow development of clinical immunity against the parasite due to a defect in the long-term maintenance of malaria-specific memory B cells (170, 181–184). Low BAFF-R expression levels may be an underlying factor for insufficient memory B cell formation in children after natural infection, since BAFF-R provides B cell survival signals. Supporting this hypothesis, BAFF-R expression was shown to correlate with schizont-specific IgM and IgG production (179). Whereas the survival of naive B cells is dependent on BAFF-R signaling, the survival of antibody-secreting cells (ASCs) requires TACI and BCMA signaling (46). However, despite the presence of circulating BAFF in malaria, ASCs may not be receiving sufficient survival signals, because unlike BAFF-R, which responds to both homotrimeric BAFF (3-mer) and oligomeric BAFF (60-mer), TACI signaling is activated only by oligomeric BAFF and membrane-bound BAFF (46). Since the majority of BAFF in circulation is in the trimeric form, the form unable to engage TACI, ASCs may not receive sufficient survival signals (46). Interestingly, experimentally infected individuals exhibit increased BAFF expression levels on inflammatory monocytes as well as on blood DCs during the acute phase of Plasmodium challenge (180). The increased BAFF levels on myeloid cells during acute human malaria are in contrast to findings for murine malaria, in which the expression of surface BAFF on DCs is reduced following Plasmodium yoelii infection (185). The differences in membrane-bound BAFF expression between human and murine studies can be partly explained by the fact that human BAFF expression was measured on blood myeloid cells, while murine BAFF expression was measured on lymphoid DCs. Nevertheless, it is not clear why membrane-bound BAFF on blood myeloid cells does not provide sufficient survival signals for TACI-expressing ASCs during acute human malaria. It remains to be investigated whether BAFF expression on myeloid cells changes with anatomical location and how the changes in membrane-bound BAFF expression on DCs impact the survival of ASCs.
FUNGI
Cryptococcal meningitis (CM) is caused by the fungi Cryptococcus neoformans and Cryptococcus gattii. Cryptococcus neoformans does not cause any serious disease in healthy individuals, but its infection can lead to life-threatening complications in patients with a compromised immune system, such as those with HIV/AIDS (186). Examination of peripheral blood mononuclear cells (PBMCs) from HIV-negative CM patients showed that the levels of BAFF, TACI, and BCMA were decreased, whereas the APRIL level remained unchanged and the BAFF-R expression level was increased compared to those in healthy individuals (187) (Fig. 4B). A likely outcome of decreased BAFF levels is that these patients have lower serum IgG levels than do healthy immunocompetent individuals (187). The same study also showed that in vitro stimulation of PBMCs with BAFF inhibits C. neoformans growth (187). This study did not investigate the mechanism of the BAFF-mediated inhibition of C. neoformans growth, but the induction of anti-C. neoformans antibodies from BAFF-activated B cells may play a role. Another possibility is the killing of C. neoformans by macrophages (188) polarized toward the M1 phenotype in response to BAFF stimulation (56). In addition to peripheral blood, BAFF and APRIL levels are increased in the cerebrospinal fluid of CM patients (189). Exactly how BAFF expression levels determine host susceptibility to C. neoformans infection and the significance of increased BAFF and APRIL levels in the cerebrospinal fluid of CM patients remain to be elucidated.
CONCLUSIONS AND PERSPECTIVES
Most microbes stimulate the expression of BAFF and APRIL in a range of cells through their pathogen-associated molecular patterns by directly engaging molecules such as TLRs or inducing type I and II interferons. Similarly, microbial products can modulate the expression of BAFF and APRIL receptors. The pattern of engagement of BAFF and APRIL receptors on B cells by their ligands determines the type of B cell responses required to control infection by a given pathogen. Both BAFF and APRIL are required when a TI host response is imperative for the control of infection. During these infections, BAFF-R is essential for B cell survival, and TACI expression is critical for the generation of plasma cells. Also, BAFF-R and TACI can mediate signals for immunoglobulin isotype switching and secretion. Since the expression levels of these receptors dictate their function, TLR-mediated increases in the expression of ligands and/or receptors during infection primarily promote B cell activation. Interestingly, pathogens may have evolved to exploit BAFF system molecules and skew the humoral immune response away from its antimicrobial function because the increase in the expression levels of BAFF system molecules does not necessarily benefit the host. The correlation between elevated BAFF levels during the acute phase of HCV infection and poor disease prognosis is a good example of this phenomenon. Furthermore, the breakage of tolerance as a consequence of chronic exposure to BAFF-inducing viruses (HIV and HCV) or parasites (T. cruzi) may be related to the preferential activation of polyreactive MZ B cells (14, 141, 142, 168, 169). Pathogens susceptible to antibody-mediated microbicidal host responses appear to prevent the development of antibody responses by downregulating the expression of BAFF and APRIL receptors. In the case of T. brucei infection, the suppression of BAFF-R expression benefits the protozoan by driving MZ B cell apoptosis and limiting IgM antibody production. A central role for TACI in mediating the essential second signal for B cell responses to TI-II antigens makes it an attractive target for encapsulated bacteria. To this point, capsular polysaccharides of encapsulated bacteria blunt B cell responses to BAFF and APRIL by downregulating TACI expression. Pathogens may also utilize BAFF-induced and TACI-mediated stimulation of IL-10-producing regulatory B (Breg) cells as a strategy to prevent antibody production (190). The expansion of BAFF-induced non-IL-10-producing B cells in autoimmune diseases (191, 192) and the activation of Breg cells in chronic lymphocytic leukemia appear to be critical for disease pathogenesis (190). The contribution of these IL-10-producing B cells to suppressing humoral and cellular responses during parasitic, bacterial, and viral infections is well recognized (193). Whether pathogen-induced BAFF plays a role in inhibiting host immune responses by activating Breg cells is yet to be explored.
The soluble forms of TACI and BCMA receptors appear to function as decoy receptors for BAFF and APRIL (43, 44). A decoy function has not been shown for BAFF-R, but the shedding of BAFF-R deprives B cells of survival signals (45). The biological significance of these recently described soluble receptors in infections has not been demonstrated. It is conceivable to think that the immune-dampening properties of soluble receptors present an attractive target for the pathogens to exploit them. Possible mechanisms of immune evasion may include the production of molecules with enzymatic properties by the pathogens themselves or the induction of the expression of host proteolytic enzymes to shed membrane receptors.
BAFF and APRIL are also involved in the development of antibodies against pathogen protein (TD) antigens during infection. In TD responses, the activity of ligands varies according to the time and location in which they engage their receptors during infections. For instance, TACI expression on GC B cells determines the magnitude and quality of ASCs exiting the GC. With the exception of C. rodentium infection, TACI deficiency results in an impaired host response and susceptibility to infection. Apart from its well-recognized role in plasma cell maintenance, TACI is also important for macrophage activation, as both BAFF and APRIL can drive the polarization of macrophages toward the M1 phenotype. TACI-mediated M1 polarization of macrophages appears to be critical for controlling the growth of intracellular pathogens such as Leishmania. It is conceivable to assume that TACI-mediated macrophage activation may play similar roles in host resistance to other intracellular pathogens. Another unexplored prospect is the influence of TACI-mediated macrophage activation on the development of adoptive immunity to pathogens. Since macrophage responses directly impact the magnitude and quality of T cell responses, modulation of TACI-mediated signals may have implications for macrophage antigen presentation functions. Taken together, an improved understanding of the role of BAFF system molecules in host resistance and susceptibility to infections can catalyze the development of more effective vaccines by devising molecules that can modulate the expression of BAFF system molecules.
ACKNOWLEDGMENTS
The work in our laboratory was supported by intramural funds from the U.S. Food and Drug Administration. Jiro Sakai was supported by a postdoctoral fellowship from the Oak Ridge Institute for Science and Education (Oak Ridge, TN).
We declare no conflict of interest.
Biographies

Jiro Sakai received his B.A. and M.S. from Hokkaido University, Japan. He spent one year at the Massachusetts Institute of Technology and three years at Harvard Medical School, studying immunology and molecular cell biology before starting his Ph.D. training. He obtained a Ph.D. in Veterinary Medicine from the University of Cambridge, UK. His thesis work focused on the NF-κB signaling pathway in response to intracellular Salmonella enterica serovar Typhimurium infection in macrophages. He is currently pursuing a postdoctoral fellowship at the U.S. Food and Drug Administration (Silver Spring, MD), investigating B cell receptor (BCR) signaling in newborns.

Mustafa Akkoyunlu earned his M.D. from Ankara University (Ankara, Turkey), and he received his Ph.D. training in Microbiology/Immunology at Lund University (Malmo, Sweden). He completed a postdoctoral fellowship at Yale University (New Haven, CT) before joining the FDA. He is currently a Senior Investigator at the Laboratory of Bacterial Polysaccharides, Center for Biologics Evaluation and Research, FDA (Silver Spring, MD), and he leads the Cellular Immunology section of the Laboratory of Bacterial Polysaccharides, where he investigates the mechanisms of antibody development against bacterial polysaccharide vaccines for pediatric pathogens such as Neisseria meningitidis and Streptococcus pneumoniae.
REFERENCES
- 1.Schneider P, MacKay F, Steiner V, Hofmann K, Bodmer JL, Holler N, Ambrose C, Lawton P, Bixler S, Acha-Orbea H, Valmori D, Romero P, Werner-Favre C, Zubler RH, Browning JL, Tschopp J. 1999. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med 189:1747–1756. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore PA, Belvedere O, Orr A, Pieri K, LaFleur DW, Feng P, Soppet D, Charters M, Gentz R, Parmelee D, Li Y, Galperina O, Giri J, Roschke V, Nardelli B, Carrell J, Sosnovtseva S, Greenfield W, Ruben SM, Olsen HS, Fikes J, Hilbert DM. 1999. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science 285:260–263. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- 3.Khare SD, Sarosi I, Xia XZ, McCabe S, Miner K, Solovyev I, Hawkins N, Kelley M, Chang D, Van G, Ross L, Delaney J, Wang L, Lacey D, Boyle WJ, Hsu H. 2000. Severe B cell hyperplasia and autoimmune disease in TALL-1 transgenic mice. Proc Natl Acad Sci U S A 97:3370–3375. doi: 10.1073/pnas.97.7.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mukhopadhyay A, Ni J, Zhai Y, Yu GL, Aggarwal BB. 1999. Identification and characterization of a novel cytokine, THANK, a TNF homologue that activates apoptosis, nuclear factor-kappaB, and c-Jun NH2-terminal kinase. J Biol Chem 274:15978–15981. doi: 10.1074/jbc.274.23.15978. [DOI] [PubMed] [Google Scholar]
- 5.Batten M, Groom J, Cachero TG, Qian F, Schneider P, Tschopp J, Browning JL, Mackay F. 2000. BAFF mediates survival of peripheral immature B lymphocytes. J Exp Med 192:1453–1466. doi: 10.1084/jem.192.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, Frew E, Scott ML. 2001. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science 293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 7.Do RK, Hatada E, Lee H, Tourigny MR, Hilbert D, Chen-Kiang S. 2000. Attenuation of apoptosis underlies B lymphocyte stimulator enhancement of humoral immune response. J Exp Med 192:953–964. doi: 10.1084/jem.192.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackay F, Schneider P. 2009. Cracking the BAFF code. Nat Rev Immunol 9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 9.Gross JA, Dillon SR, Mudri S, Johnston J, Littau A, Roque R, Rixon M, Schou O, Foley KP, Haugen H, McMillen S, Waggie K, Schreckhise RW, Shoemaker K, Vu T, Moore M, Grossman A, Clegg CH. 2001. TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease: impaired B cell maturation in mice lacking BLyS. Immunity 15:289–302. doi: 10.1016/S1074-7613(01)00183-2. [DOI] [PubMed] [Google Scholar]
- 10.Scholz JL, Crowley JE, Tomayko MM, Steinel N, O'Neill PJ, Quinn WJ III, Goenka R, Miller JP, Cho YH, Long V, Ward C, Migone TS, Shlomchik MJ, Cancro MP. 2008. BLyS inhibition eliminates primary B cells but leaves natural and acquired humoral immunity intact. Proc Natl Acad Sci U S A 105:15517–15522. doi: 10.1073/pnas.0807841105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groom J, Kalled SL, Cutler AH, Olson C, Woodcock SA, Schneider P, Tschopp J, Cachero TG, Batten M, Wheway J, Mauri D, Cavill D, Gordon TP, Mackay CR, Mackay F. 2002. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjogren's syndrome. J Clin Invest 109:59–68. doi: 10.1172/JCI0214121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gross JA, Johnston J, Mudri S, Enselman R, Dillon SR, Madden K, Xu W, Parrish-Novak J, Foster D, Lofton-Day C, Moore M, Littau A, Grossman A, Haugen H, Foley K, Blumberg H, Harrison K, Kindsvogel W, Clegg CH. 2000. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature 404:995–999. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- 13.Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, Tschopp J, Browning JL. 1999. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med 190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thien M, Phan TG, Gardam S, Amesbury M, Basten A, Mackay F, Brink R. 2004. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity 20:785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Groom JR, Fletcher CA, Walters SN, Grey ST, Watt SV, Sweet MJ, Smyth MJ, Mackay CR, Mackay F. 2007. BAFF and MyD88 signals promote a lupuslike disease independent of T cells. J Exp Med 204:1959–1971. doi: 10.1084/jem.20062567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hahne M, Kataoka T, Schroter M, Hofmann K, Irmler M, Bodmer JL, Schneider P, Bornand T, Holler N, French LE, Sordat B, Rimoldi D, Tschopp J. 1998. APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. J Exp Med 188:1185–1190. doi: 10.1084/jem.188.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SM, Kim WJ, Suk K, Lee WH. 2010. Cell to cell interaction can activate membrane-bound APRIL which are expressed on inflammatory macrophages. Immune Netw 10:173–180. doi: 10.4110/in.2010.10.5.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez-Fraga M, Fernandez R, Albar JP, Hahne M. 2001. Biologically active APRIL is secreted following intracellular processing in the Golgi apparatus by furin convertase. EMBO Rep 2:945–951. doi: 10.1093/embo-reports/kve198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castigli E, Scott S, Dedeoglu F, Bryce P, Jabara H, Bhan AK, Mizoguchi E, Geha RS. 2004. Impaired IgA class switching in APRIL-deficient mice. Proc Natl Acad Sci U S A 101:3903–3908. doi: 10.1073/pnas.0307348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stein JV, Lopez-Fraga M, Elustondo FA, Carvalho-Pinto CE, Rodriguez D, Gomez-Caro R, De Jong J, Martinez AC, Medema JP, Hahne M. 2002. APRIL modulates B and T cell immunity. J Clin Invest 109:1587–1598. doi: 10.1172/JCI0215034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varfolomeev E, Kischkel F, Martin F, Seshasayee D, Wang H, Lawrence D, Olsson C, Tom L, Erickson S, French D, Schow P, Grewal IS, Ashkenazi A. 2004. APRIL-deficient mice have normal immune system development. Mol Cell Biol 24:997–1006. doi: 10.1128/MCB.24.3.997-1006.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benson MJ, Dillon SR, Castigli E, Geha RS, Xu S, Lam KP, Noelle RJ. 2008. Cutting edge: the dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J Immunol 180:3655–3659. doi: 10.4049/jimmunol.180.6.3655. [DOI] [PubMed] [Google Scholar]
- 23.Yan M, Marsters SA, Grewal IS, Wang H, Ashkenazi A, Dixit VM. 2000. Identification of a receptor for BLyS demonstrates a crucial role in humoral immunity. Nat Immunol 1:37–41. doi: 10.1038/76889. [DOI] [PubMed] [Google Scholar]
- 24.Thompson JS, Bixler SA, Qian F, Vora K, Scott ML, Cachero TG, Hession C, Schneider P, Sizing ID, Mullen C, Strauch K, Zafari M, Benjamin CD, Tschopp J, Browning JL, Ambrose C. 2001. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science 293:2108–2111. doi: 10.1126/science.1061965. [DOI] [PubMed] [Google Scholar]
- 25.Harless SM, Lentz VM, Sah AP, Hsu BL, Clise-Dwyer K, Hilbert DM, Hayes CE, Cancro MP. 2001. Competition for BLyS-mediated signaling through Bcmd/BR3 regulates peripheral B lymphocyte numbers. Curr Biol 11:1986–1989. doi: 10.1016/S0960-9822(01)00598-X. [DOI] [PubMed] [Google Scholar]
- 26.Rennert P, Schneider P, Cachero TG, Thompson J, Trabach L, Hertig S, Holler N, Qian F, Mullen C, Strauch K, Browning JL, Ambrose C, Tschopp J. 2000. A soluble form of B cell maturation antigen, a receptor for the tumor necrosis factor family member APRIL, inhibits tumor cell growth. J Exp Med 192:1677–1684. doi: 10.1084/jem.192.11.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu G, Boone T, Delaney J, Hawkins N, Kelley M, Ramakrishnan M, McCabe S, Qiu WR, Kornuc M, Xia XZ, Guo J, Stolina M, Boyle WJ, Sarosi I, Hsu H, Senaldi G, Theill LE. 2000. APRIL and TALL-I and receptors BCMA and TACI: system for regulating humoral immunity. Nat Immunol 1:252–256. doi: 10.1038/79802. [DOI] [PubMed] [Google Scholar]
- 28.Rodig SJ, Shahsafaei A, Li B, Mackay CR, Dorfman DM. 2005. BAFF-R, the major B cell-activating factor receptor, is expressed on most mature B cells and B-cell lymphoproliferative disorders. Hum Pathol 36:1113–1119. doi: 10.1016/j.humpath.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Darce JR, Arendt BK, Wu X, Jelinek DF. 2007. Regulated expression of BAFF-binding receptors during human B cell differentiation. J Immunol 179:7276–7286. doi: 10.4049/jimmunol.179.11.7276. [DOI] [PubMed] [Google Scholar]
- 30.Katsenelson N, Kanswal S, Puig M, Mostowski H, Verthelyi D, Akkoyunlu M. 2007. Synthetic CpG oligodeoxynucleotides augment BAFF- and APRIL-mediated immunoglobulin secretion. Eur J Immunol 37:1785–1795. doi: 10.1002/eji.200636800. [DOI] [PubMed] [Google Scholar]
- 31.O'Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, Lin LL, Mantchev GT, Bram RJ, Noelle RJ. 2004. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med 199:91–98. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stadanlick JE, Kaileh M, Karnell FG, Scholz JL, Miller JP, Quinn WJ III, Brezski RJ, Treml LS, Jordan KA, Monroe JG, Sen R, Cancro MP. 2008. Tonic B cell antigen receptor signals supply an NF-kappaB substrate for prosurvival BLyS signaling. Nat Immunol 9:1379–1387. doi: 10.1038/ni.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lentz VM, Hayes CE, Cancro MP. 1998. Bcmd decreases the life span of B-2 but not B-1 cells in A/WySnJ mice. J Immunol 160:3743–3747. [PubMed] [Google Scholar]
- 34.Castigli E, Wilson SA, Scott S, Dedeoglu F, Xu S, Lam KP, Bram RJ, Jabara H, Geha RS. 2005. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med 201:35–39. doi: 10.1084/jem.20032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mantchev GT, Cortesao CS, Rebrovich M, Cascalho M, Bram RJ. 2007. TACI is required for efficient plasma cell differentiation in response to T-independent type 2 antigens. J Immunol 179:2282–2288. doi: 10.4049/jimmunol.179.4.2282. [DOI] [PubMed] [Google Scholar]
- 36.Ozcan E, Garibyan L, Lee JJ, Bram RJ, Lam KP, Geha RS. 2009. Transmembrane activator, calcium modulator, and cyclophilin ligand interactor drives plasma cell differentiation in LPS-activated B cells. J Allergy Clin Immunol 123:1277.e5–1286.e5. doi: 10.1016/j.jaci.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Underhill GH, George D, Bremer EG, Kansas GS. 2003. Gene expression profiling reveals a highly specialized genetic program of plasma cells. Blood 101:4013–4021. doi: 10.1182/blood-2002-08-2673. [DOI] [PubMed] [Google Scholar]
- 38.Tarte K, Zhan F, De Vos J, Klein B, Shaughnessy J Jr. 2003. Gene expression profiling of plasma cells and plasmablasts: toward a better understanding of the late stages of B-cell differentiation. Blood 102:592–600. doi: 10.1182/blood-2002-10-3161. [DOI] [PubMed] [Google Scholar]
- 39.Peperzak V, Vikstrom I, Walker J, Glaser SP, LePage M, Coquery CM, Erickson LD, Fairfax K, Mackay F, Strasser A, Nutt SL, Tarlinton DM. 2013. Mcl-1 is essential for the survival of plasma cells. Nat Immunol 14:290–297. doi: 10.1038/ni.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huard B, McKee T, Bosshard C, Durual S, Matthes T, Myit S, Donze O, Frossard C, Chizzolini C, Favre C, Zubler R, Guyot JP, Schneider P, Roosnek E. 2008. APRIL secreted by neutrophils binds to heparan sulfate proteoglycans to create plasma cell niches in human mucosa. J Clin Invest 118:2887–2895. doi: 10.1172/JCI33760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ingold K, Zumsteg A, Tardivel A, Huard B, Steiner QG, Cachero TG, Qiang F, Gorelik L, Kalled SL, Acha-Orbea H, Rennert PD, Tschopp J, Schneider P. 2005. Identification of proteoglycans as the APRIL-specific binding partners. J Exp Med 201:1375–1383. doi: 10.1084/jem.20042309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kimberley FC, van Bostelen L, Cameron K, Hardenberg G, Marquart JA, Hahne M, Medema JP. 2009. The proteoglycan (heparan sulfate proteoglycan) binding domain of APRIL serves as a platform for ligand multimerization and cross-linking. FASEB J 23:1584–1595. doi: 10.1096/fj.08-124669. [DOI] [PubMed] [Google Scholar]
- 43.Hoffmann FS, Kuhn PH, Laurent SA, Hauck SM, Berer K, Wendlinger SA, Krumbholz M, Khademi M, Olsson T, Dreyling M, Pfister HW, Alexander T, Hiepe F, Kumpfel T, Crawford HC, Wekerle H, Hohlfeld R, Lichtenthaler SF, Meinl E. 2015. The immunoregulator soluble TACI is released by ADAM10 and reflects B cell activation in autoimmunity. J Immunol 194:542–552. doi: 10.4049/jimmunol.1402070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laurent SA, Hoffmann FS, Kuhn PH, Cheng Q, Chu Y, Schmidt-Supprian M, Hauck SM, Schuh E, Krumbholz M, Rubsamen H, Wanngren J, Khademi M, Olsson T, Alexander T, Hiepe F, Pfister HW, Weber F, Jenne D, Wekerle H, Hohlfeld R, Lichtenthaler SF, Meinl E. 2015. Gamma-secretase directly sheds the survival receptor BCMA from plasma cells. Nat Commun 6:7333. doi: 10.1038/ncomms8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smulski CR, Kury P, Seidel LM, Staiger HS, Edinger AK, Willen L, Seidl M, Hess H, Salzer U, Rolink AG, Rizzi M, Schneider P, Eibel H. 2017. BAFF- and TACI-dependent processing of BAFFR by ADAM proteases regulates the survival of B cells. Cell Rep 18:2189–2202. doi: 10.1016/j.celrep.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Bossen C, Cachero TG, Tardivel A, Ingold K, Willen L, Dobles M, Scott ML, Maquelin A, Belnoue E, Siegrist CA, Chevrier S, Acha-Orbea H, Leung H, Mackay F, Tschopp J, Schneider P. 2008. TACI, unlike BAFF-R, is solely activated by oligomeric BAFF and APRIL to support survival of activated B cells and plasmablasts. Blood 111:1004–1012. doi: 10.1182/blood-2007-09-110874. [DOI] [PubMed] [Google Scholar]
- 47.Ng LG, Ng CH, Woehl B, Sutherland AP, Huo J, Xu S, Mackay F, Lam KP. 2006. BAFF costimulation of Toll-like receptor-activated B-1 cells. Eur J Immunol 36:1837–1846. doi: 10.1002/eji.200635956. [DOI] [PubMed] [Google Scholar]
- 48.Rowland SL, Leahy KF, Halverson R, Torres RM, Pelanda R. 2010. BAFF receptor signaling aids the differentiation of immature B cells into transitional B cells following tonic BCR signaling. J Immunol 185:4570–4581. doi: 10.4049/jimmunol.1001708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Treml LS, Carlesso G, Hoek KL, Stadanlick JE, Kambayashi T, Bram RJ, Cancro MP, Khan WN. 2007. TLR stimulation modifies BLyS receptor expression in follicular and marginal zone B cells. J Immunol 178:7531–7539. doi: 10.4049/jimmunol.178.12.7531. [DOI] [PubMed] [Google Scholar]
- 50.Uslu K, Coleman AS, Allman WR, Katsenelson N, Bram RJ, Alugupalli KR, Akkoyunlu M. 2014. Impaired B cell receptor signaling is responsible for reduced TACI expression and function in X-linked immunodeficient mice. J Immunol 192:3582–3595. doi: 10.4049/jimmunol.1203468. [DOI] [PubMed] [Google Scholar]
- 51.Akkoyunlu M. 2012. TACI expression is low both in human and mouse newborns. Scand J Immunol 75:368. doi: 10.1111/j.1365-3083.2011.02651.x. [DOI] [PubMed] [Google Scholar]
- 52.Kanswal S, Katsenelson N, Selvapandiyan A, Bram RJ, Akkoyunlu M. 2008. Deficient TACI expression on B lymphocytes of newborn mice leads to defective Ig secretion in response to BAFF or APRIL. J Immunol 181:976–990. doi: 10.4049/jimmunol.181.2.976. [DOI] [PubMed] [Google Scholar]
- 53.Kaur K, Chowdhury S, Greenspan NS, Schreiber JR. 2007. Decreased expression of tumor necrosis factor family receptors involved in humoral immune responses in preterm neonates. Blood 110:2948–2954. doi: 10.1182/blood-2007-01-069245. [DOI] [PubMed] [Google Scholar]
- 54.Carter RH, Zhao H, Liu X, Pelletier M, Chatham W, Kimberly R, Zhou T. 2005. Expression and occupancy of BAFF-R on B cells in systemic lupus erythematosus. Arthritis Rheum 52:3943–3954. doi: 10.1002/art.21489. [DOI] [PubMed] [Google Scholar]
- 55.Yeramilli VA, Knight KL. 2010. Requirement for BAFF and APRIL during B cell development in GALT. J Immunol 184:5527–5536. doi: 10.4049/jimmunol.1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allman WR, Dey R, Liu L, Siddiqui S, Coleman AS, Bhattacharya P, Yano M, Uslu K, Takeda K, Nakhasi HL, Akkoyunlu M. 2015. TACI deficiency leads to alternatively activated macrophage phenotype and susceptibility to Leishmania infection. Proc Natl Acad Sci U S A 112:E4094–E4103. doi: 10.1073/pnas.1421580112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang SK, Arendt BK, Darce JR, Wu X, Jelinek DF. 2006. A role for BLyS in the activation of innate immune cells. Blood 108:2687–2694. doi: 10.1182/blood-2005-12-017319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang SK, Mihalcik SA, Jelinek DF. 2008. B lymphocyte stimulator regulates adaptive immune responses by directly promoting dendritic cell maturation. J Immunol 180:7394–7403. doi: 10.4049/jimmunol.180.11.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hengeveld PJ, Kersten MJ. 2015. B-cell activating factor in the pathophysiology of multiple myeloma: a target for therapy? Blood Cancer J 5:e282. doi: 10.1038/bcj.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu Z, Davidson A. 2011. BAFF inhibition: a new class of drugs for the treatment of autoimmunity. Exp Cell Res 317:1270–1277. doi: 10.1016/j.yexcr.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vincent FB, Morand EF, Schneider P, Mackay F. 2014. The BAFF/APRIL system in SLE pathogenesis. Nat Rev Rheumatol 10:365–373. doi: 10.1038/nrrheum.2014.33. [DOI] [PubMed] [Google Scholar]
- 62.Yang S, Li JY, Xu W. 2014. Role of BAFF/BAFF-R axis in B-cell non-Hodgkin lymphoma. Crit Rev Oncol Hematol 91:113–122. doi: 10.1016/j.critrevonc.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 63.Igietseme JU, Eko FO, He Q, Black CM. 2004. Antibody regulation of Tcell immunity: implications for vaccine strategies against intracellular pathogens. Expert Rev Vaccines 3:23–34. doi: 10.1586/14760584.3.1.23. [DOI] [PubMed] [Google Scholar]
- 64.Weintraub A. 2003. Immunology of bacterial polysaccharide antigens. Carbohydr Res 338:2539–2547. doi: 10.1016/j.carres.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 65.Krammer F, Palese P. 2015. Advances in the development of influenza virus vaccines. Nat Rev Drug Discov 14:167–182. doi: 10.1038/nrd4529. [DOI] [PubMed] [Google Scholar]
- 66.Hajj Hussein I, Chams N, Chams S, El Sayegh S, Badran R, Raad M, Gerges-Geagea A, Leone A, Jurjus A. 2015. Vaccines through centuries: major cornerstones of global health. Front Public Health 3:269. doi: 10.3389/fpubh.2015.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miller DJ, Hanson KD, Carman JA, Hayes CE. 1992. A single autosomal gene defect severely limits IgG but not IgM responses in B lymphocyte-deficient A/WySnJ mice. Eur J Immunol 22:373–379. doi: 10.1002/eji.1830220213. [DOI] [PubMed] [Google Scholar]
- 68.Rahman ZS, Rao SP, Kalled SL, Manser T. 2003. Normal induction but attenuated progression of germinal center responses in BAFF and BAFF-R signaling-deficient mice. J Exp Med 198:1157–1169. doi: 10.1084/jem.20030495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sasaki Y, Casola S, Kutok JL, Rajewsky K, Schmidt-Supprian M. 2004. TNF family member B cell-activating factor (BAFF) receptor-dependent and -independent roles for BAFF in B cell physiology. J Immunol 173:2245–2252. doi: 10.4049/jimmunol.173.4.2245. [DOI] [PubMed] [Google Scholar]
- 70.Gorelik L, Cutler AH, Thill G, Miklasz SD, Shea DE, Ambrose C, Bixler SA, Su L, Scott ML, Kalled SL. 2004. Cutting edge: BAFF regulates CD21/35 and CD23 expression independent of its B cell survival function. J Immunol 172:762–766. doi: 10.4049/jimmunol.172.2.762. [DOI] [PubMed] [Google Scholar]
- 71.Hardenberg G, van Bostelen L, Hahne M, Medema JP. 2008. Thymus-independent class switch recombination is affected by APRIL. Immunol Cell Biol 86:530–534. doi: 10.1038/icb.2008.17. [DOI] [PubMed] [Google Scholar]
- 72.Joo S, Fukuyama Y, Park EJ, Yuki Y, Kurashima Y, Ouchida R, Ziegler SF, Kiyono H. 7 December 2016. Critical role of TSLP-responsive mucosal dendritic cells in the induction of nasal antigen-specific IgA response. Mucosal Immunol doi: 10.1038/mi.2016.103. [DOI] [PubMed] [Google Scholar]
- 73.von Bulow GU, van Deursen JM, Bram RJ. 2001. Regulation of the T-independent humoral response by TACI. Immunity 14:573–582. doi: 10.1016/S1074-7613(01)00130-3. [DOI] [PubMed] [Google Scholar]
- 74.Seshasayee D, Valdez P, Yan M, Dixit VM, Tumas D, Grewal IS. 2003. Loss of TACI causes fatal lymphoproliferation and autoimmunity, establishing TACI as an inhibitory BLyS receptor. Immunity 18:279–288. doi: 10.1016/S1074-7613(03)00025-6. [DOI] [PubMed] [Google Scholar]
- 75.Yan M, Wang H, Chan B, Roose-Girma M, Erickson S, Baker T, Tumas D, Grewal IS, Dixit VM. 2001. Activation and accumulation of B cells in TACI-deficient mice. Nat Immunol 2:638–643. doi: 10.1038/89790. [DOI] [PubMed] [Google Scholar]
- 76.Kanswal S, Katsenelson N, Allman W, Uslu K, Blake MS, Akkoyunlu M. 2011. Suppressive effect of bacterial polysaccharides on BAFF system is responsible for their poor immunogenicity. J Immunol 186:2430–2443. doi: 10.4049/jimmunol.1002976. [DOI] [PubMed] [Google Scholar]
- 77.Rawlings DJ, Saffran DC, Tsukada S, Largaespada DA, Grimaldi JC, Cohen L, Mohr RN, Bazan JF, Howard M, Copeland NG, Jenkins NA, Witte ON. 1993. Mutation of unique region of Bruton's tyrosine kinase in immunodeficient XID mice. Science 261:358–361. doi: 10.1126/science.8332901. [DOI] [PubMed] [Google Scholar]
- 78.Salzer U, Chapel HM, Webster AD, Pan-Hammarstrom Q, Schmitt-Graeff A, Schlesier M, Peter HH, Rockstroh JK, Schneider P, Schaffer AA, Hammarstrom L, Grimbacher B. 2005. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nat Genet 37:820–828. doi: 10.1038/ng1600. [DOI] [PubMed] [Google Scholar]
- 79.Chinen J, Martinez-Gallo M, Gu W, Cols M, Cerutti A, Radigan L, Zhang L, Potocki L, Withers M, Lupski JR, Cunningham-Rundles C. 2011. Transmembrane activator and CAML interactor (TACI) haploinsufficiency results in B-cell dysfunction in patients with Smith-Magenis syndrome. J Allergy Clin Immunol 127:1579–1586. doi: 10.1016/j.jaci.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Iyer AS, Khaskhely NM, Leggat DJ, Ohtola JA, Saul-McBeth JL, Khuder SA, Westerink MA. 2016. Inflammatory markers and immune response to pneumococcal vaccination in HIV-positive and -negative adults. PLoS One 11:e0150261. doi: 10.1371/journal.pone.0150261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cerutti A, Cols M, Puga I. 2013. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol 13:118–132. doi: 10.1038/nri3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Puga I, Cols M, Barra CM, He B, Cassis L, Gentile M, Comerma L, Chorny A, Shan M, Xu W, Magri G, Knowles DM, Tam W, Chiu A, Bussel JB, Serrano S, Lorente JA, Bellosillo B, Lloreta J, Juanpere N, Alameda F, Baro T, de Heredia CD, Toran N, Catala A, Torrebadell M, Fortuny C, Cusi V, Carreras C, Diaz GA, Blander JM, Farber CM, Silvestri G, Cunningham-Rundles C, Calvillo M, Dufour C, Notarangelo LD, Lougaris V, Plebani A, Casanova JL, Ganal SC, Diefenbach A, Arostegui JI, Juan M, Yague J, Mahlaoui N, Donadieu J, Chen K, Cerutti A. 2012. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol 13:170–180. doi: 10.1038/ni.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Parsa R, Lund H, Georgoudaki AM, Zhang XM, Ortlieb Guerreiro-Cacais A, Grommisch D, Warnecke A, Croxford AL, Jagodic M, Becher B, Karlsson MC, Harris RA. 2016. BAFF-secreting neutrophils drive plasma cell responses during emergency granulopoiesis. J Exp Med 213:1537–1553. doi: 10.1084/jem.20150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Magri G, Miyajima M, Bascones S, Mortha A, Puga I, Cassis L, Barra CM, Comerma L, Chudnovskiy A, Gentile M, Llige D, Cols M, Serrano S, Arostegui JI, Juan M, Yague J, Merad M, Fagarasan S, Cerutti A. 2014. Innate lymphoid cells integrate stromal and immunological signals to enhance antibody production by splenic marginal zone B cells. Nat Immunol 15:354–364. doi: 10.1038/ni.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ou X, Xu S, Lam KP. 2012. Deficiency in TNFRSF13B (TACI) expands T-follicular helper and germinal center B cells via increased ICOS-ligand expression but impairs plasma cell survival. Proc Natl Acad Sci U S A 109:15401–15406. doi: 10.1073/pnas.1200386109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, Dong C. 2008. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wolf AI, Mozdzanowska K, Quinn WJ III, Metzgar M, Williams KL, Caton AJ, Meffre E, Bram RJ, Erickson LD, Allman D, Cancro MP, Erikson J. 2011. Protective antiviral antibody responses in a mouse model of influenza virus infection require TACI. J Clin Invest 121:3954–3964. doi: 10.1172/JCI57362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goenka R, Matthews AH, Zhang B, O'Neill PJ, Scholz JL, Migone TS, Leonard WJ, Stohl W, Hershberg U, Cancro MP. 2014. Local BLyS production by T follicular cells mediates retention of high affinity B cells during affinity maturation. J Exp Med 211:45–56. doi: 10.1084/jem.20130505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pallikkuth S, Kanthikeel SP, Silva SY, Fischl M, Pahwa R, Pahwa S. 2011. Innate immune defects correlate with failure of antibody responses to H1N1/09 vaccine in HIV-infected patients. J Allergy Clin Immunol 128:1279–1285. doi: 10.1016/j.jaci.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kanagavelu SK, Snarsky V, Termini JM, Gupta S, Barzee S, Wright JA, Khan WN, Kornbluth RS, Stone GW. 2012. Soluble multi-trimeric TNF superfamily ligand adjuvants enhance immune responses to a HIV-1 Gag DNA vaccine. Vaccine 30:691–702. doi: 10.1016/j.vaccine.2011.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gupta S, Clark ES, Termini JM, Boucher J, Kanagavelu S, LeBranche CC, Abraham S, Montefiori DC, Khan WN, Stone GW. 2015. DNA vaccine molecular adjuvants SP-D-BAFF and SP-D-APRIL enhance anti-gp120 immune response and increase HIV-1 neutralizing antibody titers. J Virol 89:4158–4169. doi: 10.1128/JVI.02904-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Melchers M, Bontjer I, Tong T, Chung NP, Klasse PJ, Eggink D, Montefiori DC, Gentile M, Cerutti A, Olson WC, Berkhout B, Binley JM, Moore JP, Sanders RW. 2012. Targeting HIV-1 envelope glycoprotein trimers to B cells by using APRIL improves antibody responses. J Virol 86:2488–2500. doi: 10.1128/JVI.06259-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tertilt C, Joh J, Krause A, Chou P, Schneeweiss K, Crystal RG, Worgall S. 2009. Expression of B-cell activating factor enhances protective immunity of a vaccine against Pseudomonas aeruginosa. Infect Immun 77:3044–3055. doi: 10.1128/IAI.00927-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen L, Ran MJ, Shan XX, Cao M, Cao P, Yang XM, Zhang SQ. 2009. BAFF enhances B-cell-mediated immune response and vaccine-protection against a very virulent IBDV in chickens. Vaccine 27:1393–1399. doi: 10.1016/j.vaccine.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 95.Rodriguez B, Valdez H, Freimuth W, Butler T, Asaad R, Lederman MM. 2003. Plasma levels of B-lymphocyte stimulator increase with HIV disease progression. AIDS 17:1983–1985. doi: 10.1097/00002030-200309050-00018. [DOI] [PubMed] [Google Scholar]
- 96.Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O'Shea MA, Roby G, Kottilil S, Arthos J, Proschan MA, Chun TW, Fauci AS. 2008. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med 205:1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fontaine J, Chagnon-Choquet J, Valcke HS, Poudrier J, Roger M, Montreal Primary HIV Infection and Long-Term Non-Progressor Study Groups. 2011. High expression levels of B lymphocyte stimulator (BLyS) by dendritic cells correlate with HIV-related B-cell disease progression in humans. Blood 117:145–155. doi: 10.1182/blood-2010-08-301887. [DOI] [PubMed] [Google Scholar]
- 98.Stohl W, Cheema GS, Briggs WS, Xu D, Sosnovtseva S, Roschke V, Ferrara DE, Labat K, Sattler FR, Pierangeli SS, Hilbert DM. 2002. B lymphocyte stimulator protein-associated increase in circulating autoantibody levels may require CD4+ T cells: lessons from HIV-infected patients. Clin Immunol 104:115–122. doi: 10.1006/clim.2002.5238. [DOI] [PubMed] [Google Scholar]
- 99.Xu W, Santini PA, Sullivan JS, He B, Shan M, Ball SC, Dyer WB, Ketas TJ, Chadburn A, Cohen-Gould L, Knowles DM, Chiu A, Sanders RW, Chen K, Cerutti A. 2009. HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nat Immunol 10:1008–1017. doi: 10.1038/ni.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gauvin J, Chagnon-Choquet J, Poudrier J, Roger M, Montreal Primary HIV Infection and Slow Progressor Cohorts. 2016. Fluctuations in blood marginal zone B-cell frequencies may reflect migratory patterns associated with HIV-1 disease progression status. PLoS One 11:e0155868. doi: 10.1371/journal.pone.0155868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Poudrier J, Soulas C, Chagnon-Choquet J, Burdo T, Autissier P, Oskar K, Williams KC, Roger M. 2015. High expression levels of BLyS/BAFF by blood dendritic cells and granulocytes are associated with B-cell dysregulation in SIV-infected rhesus macaques. PLoS One 10:e0131513. doi: 10.1371/journal.pone.0131513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sabourin-Poirier C, Fourcade L, Chagnon-Choquet J, Labbe AC, Alary M, Guedou F, Poudrier J, Roger M. 2016. Blood B lymphocyte stimulator (BLyS)/BAFF levels may reflect natural immunity to HIV in highly exposed uninfected Beninese commercial sex workers. Sci Rep 6:32318. doi: 10.1038/srep32318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Borhis G, Burelout C, Chaoul N, Smith N, Goujard C, Meyer L, Paul S, Saoudin H, Hosmalin A, Gilbert C, Herbeuval JP, Richard Y. 2016. Plasmacytoid dendritic cells and myeloid cells differently contribute to B-cell-activating factor belonging to the tumor necrosis factor superfamily overexpression during primary HIV infection. AIDS 30:365–376. doi: 10.1097/QAD.0000000000000965. [DOI] [PubMed] [Google Scholar]
- 104.Xu W, Santini PA, Matthews AJ, Chiu A, Plebani A, He B, Chen K, Cerutti A. 2008. Viral double-stranded RNA triggers Ig class switching by activating upper respiratory mucosa B cells through an innate TLR3 pathway involving BAFF. J Immunol 181:276–287. doi: 10.4049/jimmunol.181.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chagnon-Choquet J, Gauvin J, Roger J, Fontaine J, Poudrier J, Roger M, Montreal Primary HIV Infection and Slow Progressor Study Groups. 2015. HIV Nef promotes expression of B-lymphocyte stimulator by blood dendritic cells during HIV infection in humans. J Infect Dis 211:1229–1240. doi: 10.1093/infdis/jiu611. [DOI] [PubMed] [Google Scholar]
- 106.He B, Qiao X, Klasse PJ, Chiu A, Chadburn A, Knowles DM, Moore JP, Cerutti A. 2006. HIV-1 envelope triggers polyclonal Ig class switch recombination through a CD40-independent mechanism involving BAFF and C-type lectin receptors. J Immunol 176:3931–3941. doi: 10.4049/jimmunol.176.7.3931. [DOI] [PubMed] [Google Scholar]
- 107.Adalid-Peralta L, Godot V, Colin C, Krzysiek R, Tran T, Poignard P, Venet A, Hosmalin A, Lebon P, Rouzioux C, Chene G, Emilie D, Interprim ANRS 112 Study Group. 2008. Stimulation of the primary anti-HIV antibody response by IFN-alpha in patients with acute HIV-1 infection. J Leukoc Biol 83:1060–1067. doi: 10.1189/jlb.1007675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gomez AM, Ouellet M, Tremblay MJ. 2015. HIV-1-triggered release of type I IFN by plasmacytoid dendritic cells induces BAFF production in monocytes. J Immunol 194:2300–2308. doi: 10.4049/jimmunol.1402147. [DOI] [PubMed] [Google Scholar]
- 109.Gomez AM, Ouellet M, Deshiere A, Breton Y, Tremblay MJ. 2016. HIV-1-mediated BAFF secretion in macrophages does not require endosomal TLRs, type-I IFN, and Nef, but depends on the cellular phenotype status. J Immunol 196:3806–3817. doi: 10.4049/jimmunol.1501249. [DOI] [PubMed] [Google Scholar]
- 110.Moir S, Malaspina A, Pickeral OK, Donoghue ET, Vasquez J, Miller NJ, Krishnan SR, Planta MA, Turney JF, Justement JS, Kottilil S, Dybul M, Mican JM, Kovacs C, Chun TW, Birse CE, Fauci AS. 2004. Decreased survival of B cells of HIV-viremic patients mediated by altered expression of receptors of the TNF superfamily. J Exp Med 200:587–599. doi: 10.1084/jem.20032236. [DOI] [PubMed] [Google Scholar]
- 111.Pawlotsky JM, Roudot-Thoraval F, Simmonds P, Mellor J, Ben Yahia MB, Andre C, Voisin MC, Intrator L, Zafrani ES, Duval J, Dhumeaux D. 1995. Extrahepatic immunologic manifestations in chronic hepatitis C and hepatitis C virus serotypes. Ann Intern Med 122:169–173. doi: 10.7326/0003-4819-122-3-199502010-00002. [DOI] [PubMed] [Google Scholar]
- 112.Clifford BD, Donahue D, Smith L, Cable E, Luttig B, Manns M, Bonkovsky HL. 1995. High prevalence of serological markers of autoimmunity in patients with chronic hepatitis C. Hepatology 21:613–619. doi: 10.1016/0270-9139(95)90507-3. [DOI] [PubMed] [Google Scholar]
- 113.Toubi E, Gordon S, Kessel A, Rosner I, Rozenbaum M, Shoenfeld Y, Zuckerman E. 2006. Elevated serum B-lymphocyte activating factor (BAFF) in chronic hepatitis C virus infection: association with autoimmunity. J Autoimmun 27:134–139. doi: 10.1016/j.jaut.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 114.Zuckerman E, Slobodin G, Kessel A, Sabo E, Yeshurun D, Halas K, Toubi E. 2002. Peripheral B-cell CD5 expansion and CD81 overexpression and their association with disease severity and autoimmune markers in chronic hepatitis C virus infection. Clin Exp Immunol 128:353–358. doi: 10.1046/j.1365-2249.2002.01844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Russo MW, Fried MW. 2003. Side effects of therapy for chronic hepatitis C. Gastroenterology 124:1711–1719. doi: 10.1016/S0016-5085(03)00394-9. [DOI] [PubMed] [Google Scholar]
- 116.Prummel MF, Laurberg P. 2003. Interferon-alpha and autoimmune thyroid disease. Thyroid 13:547–551. doi: 10.1089/105072503322238809. [DOI] [PubMed] [Google Scholar]
- 117.Kajiyama Y, Kikuchi K, Takai A, Hosoya N, Hoshino H, Hino K, Miyakawa H. 2012. B-cell-activating factor affects the occurrence of thyroid autoimmunity in chronic hepatitis C patients treated with interferon alpha. Clin Dev Immunol 2012:247973. doi: 10.1155/2012/247973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tarantino G, Marco VD, Petta S, Almasio PL, Barbaria F, Licata A, Bosco GL, Tripodo C, Stefano RD, Craxi A. 2009. Serum BLyS/BAFF predicts the outcome of acute hepatitis C virus infection. J Viral Hepat 16:397–405. doi: 10.1111/j.1365-2893.2009.01093.x. [DOI] [PubMed] [Google Scholar]
- 119.Mond JJ, Lees A, Snapper CM. 1995. T cell-independent antigens type 2. Annu Rev Immunol 13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 120.Yang C, Li N, Wang Y, Zhang P, Zhu Q, Li F, Han Q, Lv Y, Yu L, Wei P, Liu Z. 2014. Serum levels of B-cell activating factor in chronic hepatitis B virus infection: association with clinical diseases. J Interferon Cytokine Res 34:787–794. doi: 10.1089/jir.2014.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lu B, Zhang B, Wang L, Ma C, Liu X, Zhao Y, Jiao Y. 2017. Hepatitis B virus e antigen regulates monocyte function and promotes B lymphocyte activation. Viral Immunol 30:35–44. doi: 10.1089/vim.2016.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wu HL, Hsiao TH, Chen PJ, Wong SH, Kao JH, Chen DS, Lu JY, Lu TP, Chen Y, Chuang EY, Tu HC, Liu CJ. 2016. Liver gene expression profiles correlate with virus infection and response to interferon therapy in chronic hepatitis B patients. Sci Rep 6:31349. doi: 10.1038/srep31349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Han Q, Yang C, Li N, Li F, Sang J, Lv Y, Zhao W, Li C, Liu Z. 2017. Association of genetic variation in B-cell activating factor with chronic hepatitis B virus infection. Immunol Lett 188:53–58. doi: 10.1016/j.imlet.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 124.Xu HC, Huang J, Khairnar V, Duhan V, Pandyra AA, Grusdat M, Shinde P, McIlwain DR, Maney SK, Gommerman J, Lohning M, Ohashi PS, Mak TW, Pieper K, Sic H, Speletas M, Eibel H, Ware CF, Tumanov AV, Kruglov AA, Nedospasov SA, Haussinger D, Recher M, Lang KS, Lang PA. 2015. Deficiency of the B cell-activating factor receptor results in limited CD169+ macrophage function during viral infection. J Virol 89:4748–4759. doi: 10.1128/JVI.02976-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Moseman EA, Iannacone M, Bosurgi L, Tonti E, Chevrier N, Tumanov A, Fu YX, Hacohen N, von Andrian UH. 2012. B cell maintenance of subcapsular sinus macrophages protects against a fatal viral infection independent of adaptive immunity. Immunity 36:415–426. doi: 10.1016/j.immuni.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Honke N, Shaabani N, Cadeddu G, Sorg UR, Zhang DE, Trilling M, Klingel K, Sauter M, Kandolf R, Gailus N, van Rooijen N, Burkart C, Baldus SE, Grusdat M, Lohning M, Hengel H, Pfeffer K, Tanaka M, Haussinger D, Recher M, Lang PA, Lang KS. 2012. Enforced viral replication activates adaptive immunity and is essential for the control of a cytopathic virus. Nat Immunol 13:51–57. doi: 10.1038/ni.2169. [DOI] [PubMed] [Google Scholar]
- 127.Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, Fink K, Henrickson SE, Shayakhmetov DM, Di Paolo NC, van Rooijen N, Mempel TR, Whelan SP, von Andrian UH. 2007. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature 450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- 128.Wang J, Li Q, Xie J, Xu Y. 2015. Cigarette smoke inhibits BAFF expression and mucosal immunoglobulin A responses in the lung during influenza virus infection. Respir Res 16:37. doi: 10.1186/s12931-015-0201-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hardenberg G, van der Sluijs K, van der Poll T, Medema JP. 2008. APRIL affects antibody responses and early leukocyte infiltration, but not influenza A viral control. Mol Immunol 45:3050–3058. doi: 10.1016/j.molimm.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 130.Greenough A, Cox S, Alexander J, Lenney W, Turnbull F, Burgess S, Chetcuti PA, Shaw NJ, Woods A, Boorman J, Coles S, Turner J. 2001. Health care utilisation of infants with chronic lung disease, related to hospitalisation for RSV infection. Arch Dis Child 85:463–468. doi: 10.1136/adc.85.6.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Reed JL, Welliver TP, Sims GP, McKinney L, Velozo L, Avendano L, Hintz K, Luma J, Coyle AJ, Welliver RC Sr. 2009. Innate immune signals modulate antiviral and polyreactive antibody responses during severe respiratory syncytial virus infection. J Infect Dis 199:1128–1138. doi: 10.1086/597386. [DOI] [PubMed] [Google Scholar]
- 132.Kato A, Truong-Tran AQ, Scott AL, Matsumoto K, Schleimer RP. 2006. Airway epithelial cells produce B cell-activating factor of TNF family by an IFN-beta-dependent mechanism. J Immunol 177:7164–7172. doi: 10.4049/jimmunol.177.10.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McNamara PS, Fonceca AM, Howarth D, Correia JB, Slupsky JR, Trinick RE, Al Turaiki W, Smyth RL, Flanagan BF. 2013. Respiratory syncytial virus infection of airway epithelial cells, in vivo and in vitro, supports pulmonary antibody responses by inducing expression of the B cell differentiation factor BAFF. Thorax 68:76–81. doi: 10.1136/thoraxjnl-2012-202288. [DOI] [PubMed] [Google Scholar]
- 134.Lokensgard JR, Mutnal MB, Prasad S, Sheng W, Hu S. 2016. Glial cell activation, recruitment, and survival of B-lineage cells following MCMV brain infection. J Neuroinflammation 13:114. doi: 10.1186/s12974-016-0582-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mutnal MB, Hu S, Lokensgard JR. 2012. Persistent humoral immune responses in the CNS limit recovery of reactivated murine cytomegalovirus. PLoS One 7:e33143. doi: 10.1371/journal.pone.0033143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Phares TW, Marques CP, Stohlman SA, Hinton DR, Bergmann CC. 2011. Factors supporting intrathecal humoral responses following viral encephalomyelitis. J Virol 85:2589–2598. doi: 10.1128/JVI.02260-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xu H, Dong P, Ma X, Song D, Xue D, Xu R, Lu H, He X. 2017. B cell-activating factor regulates the survival of B lymphocytes infected with human cytomegalovirus. Immunol Lett 187:1–6. doi: 10.1016/j.imlet.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 138.Haley SL, Tzvetkov EP, Lytle AG, Alugupalli KR, Plummer JR, McGettigan JP. 2017. APRIL:TACI axis is dispensable for the immune response to rabies vaccination. Antiviral Res 144:130–137. doi: 10.1016/j.antiviral.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu K, Zhang Y, Hu S, Yu Y, Yang Q, Jin D, Chen X, Jin Q, Liu H. 2012. Increased levels of BAFF and APRIL related to human active pulmonary tuberculosis. PLoS One 7:e38429. doi: 10.1371/journal.pone.0038429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.van Rensburg IC, Wagman C, Stanley K, Beltran C, Ronacher K, Walzl G, Loxton AG. 22 September 2016. Successful TB treatment induces B-cells expressing FASL and IL5RA mRNA. Oncotarget doi: 10.18632/oncotarget.12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Anuradha R, Munisankar S, Bhootra Y, Dolla C, Kumaran P, Nutman TB, Babu S. 2017. Modulation of Mycobacterium tuberculosis-specific humoral immune responses is associated with Strongyloides stercoralis co-infection. PLoS Negl Trop Dis 11:e0005569. doi: 10.1371/journal.pntd.0005569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lu LL, Chung AW, Rosebrock TR, Ghebremichael M, Yu WH, Grace PS, Schoen MK, Tafesse F, Martin C, Leung V, Mahan AE, Sips M, Kumar MP, Tedesco J, Robinson H, Tkachenko E, Draghi M, Freedberg KJ, Streeck H, Suscovich TJ, Lauffenburger DA, Restrepo BI, Day C, Fortune SM, Alter G. 2016. A functional role for antibodies in tuberculosis. Cell 167:433.e14–443.e14. doi: 10.1016/j.cell.2016.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zimmermann N, Thormann V, Hu B, Kohler AB, Imai-Matsushima A, Locht C, Arnett E, Schlesinger LS, Zoller T, Schurmann M, Kaufmann SH, Wardemann H. 2016. Human isotype-dependent inhibitory antibody responses against Mycobacterium tuberculosis. EMBO Mol Med 8:1325–1339. doi: 10.15252/emmm.201606330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Qin W, Wang L, Zhai R, Ma Q, Liu J, Bao C, Sun D, Zhang H, Sun C, Feng X, Gu J, Du C, Han W, Langford PR, Lei L. 2017. Apa2H1, the first head domain of Apa2 trimeric autotransporter adhesin, activates mouse bone marrow-derived dendritic cells and immunization with Apa2H1 protects against Actinobacillus pleuropneumoniae infection. Mol Immunol 81:108–117. doi: 10.1016/j.molimm.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 145.Neill DR, Saint GL, Bricio-Moreno L, Fothergill JL, Southern KW, Winstanley C, Christmas SE, Slupsky JR, McNamara PS, Kadioglu A, Flanagan BF. 2014. The B lymphocyte differentiation factor (BAFF) is expressed in the airways of children with CF and in lungs of mice infected with Pseudomonas aeruginosa. PLoS One 9:e95892. doi: 10.1371/journal.pone.0095892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Huard B, Schneider P, Mauri D, Tschopp J, French LE. 2001. T cell costimulation by the TNF ligand BAFF. J Immunol 167:6225–6231. doi: 10.4049/jimmunol.167.11.6225. [DOI] [PubMed] [Google Scholar]
- 147.Ng LG, Sutherland AP, Newton R, Qian F, Cachero TG, Scott ML, Thompson JS, Wheway J, Chtanova T, Groom J, Sutton IJ, Xin C, Tangye SG, Kalled SL, Mackay F, Mackay CR. 2004. B cell-activating factor belonging to the TNF family (BAFF)-R is the principal BAFF receptor facilitating BAFF costimulation of circulating T and B cells. J Immunol 173:807–817. doi: 10.4049/jimmunol.173.2.807. [DOI] [PubMed] [Google Scholar]
- 148.Sutherland AP, Ng LG, Fletcher CA, Shum B, Newton RA, Grey ST, Rolph MS, Mackay F, Mackay CR. 2005. BAFF augments certain Th1-associated inflammatory responses. J Immunol 174:5537–5544. doi: 10.4049/jimmunol.174.9.5537. [DOI] [PubMed] [Google Scholar]
- 149.Tsuji S, Stein L, Kamada N, Nunez G, Bram R, Vallance BA, Sousa AE, Platt JL, Cascalho M. 2014. TACI deficiency enhances antibody avidity and clearance of an intestinal pathogen. J Clin Invest 124:4857–4866. doi: 10.1172/JCI74428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jones DD, Jones M, DeIulio GA, Racine R, MacNamara KC, Winslow GM. 2013. B cell activating factor inhibition impairs bacterial immunity by reducing T cell-independent IgM secretion. Infect Immun 81:4490–4497. doi: 10.1128/IAI.00998-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Racine R, Chatterjee M, Winslow GM. 2008. CD11c expression identifies a population of extrafollicular antigen-specific splenic plasmablasts responsible for CD4 T-independent antibody responses during intracellular bacterial infection. J Immunol 181:1375–1385. doi: 10.4049/jimmunol.181.2.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bitsaktsis C, Nandi B, Racine R, MacNamara KC, Winslow G. 2007. T-cell-independent humoral immunity is sufficient for protection against fatal intracellular ehrlichia infection. Infect Immun 75:4933–4941. doi: 10.1128/IAI.00705-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kowarik MC, Cepok S, Sellner J, Grummel V, Weber MS, Korn T, Berthele A, Hemmer B. 2012. CXCL13 is the major determinant for B cell recruitment to the CSF during neuroinflammation. J Neuroinflammation 9:93. doi: 10.1186/1742-2094-9-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. 2004. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity 21:379–390. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 155.Alugupalli KR, Gerstein RM, Chen J, Szomolanyi-Tsuda E, Woodland RT, Leong JM. 2003. The resolution of relapsing fever borreliosis requires IgM and is concurrent with expansion of B1b lymphocytes. J Immunol 170:3819–3827. doi: 10.4049/jimmunol.170.7.3819. [DOI] [PubMed] [Google Scholar]
- 156.Colombo MJ, Alugupalli KR. 2008. Complement factor H-binding protein, a putative virulence determinant of Borrelia hermsii, is an antigenic target for protective B1b lymphocytes. J Immunol 180:4858–4864. doi: 10.4049/jimmunol.180.7.4858. [DOI] [PubMed] [Google Scholar]
- 157.Dickinson GS, Sun G, Bram RJ, Alugupalli KR. 2014. Efficient B cell responses to Borrelia hermsii infection depend on BAFF and BAFFR but not TACI. Infect Immun 82:453–459. doi: 10.1128/IAI.01147-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kocabas C, Katsenelson N, Kanswal S, Kennedy MN, Cui X, Blake MS, Segal DM, Akkoyunlu M. 2007. Neisseria meningitidis type C capsular polysaccharide inhibits lipooligosaccharide-induced cell activation by binding to CD14. Cell Microbiol 9:1297–1310. doi: 10.1111/j.1462-5822.2006.00872.x. [DOI] [PubMed] [Google Scholar]
- 159.Nardelli B, Belvedere O, Roschke V, Moore PA, Olsen HS, Migone TS, Sosnovtseva S, Carrell JA, Feng P, Giri JG, Hilbert DM. 2001. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood 97:198–204. doi: 10.1182/blood.V97.1.198. [DOI] [PubMed] [Google Scholar]
- 160.Huard B, Arlettaz L, Ambrose C, Kindler V, Mauri D, Roosnek E, Tschopp J, Schneider P, French LE. 2004. BAFF production by antigen-presenting cells provides T cell co-stimulation. Int Immunol 16:467–475. doi: 10.1093/intimm/dxh043. [DOI] [PubMed] [Google Scholar]
- 161.Amezcua Vesely MC, Bermejo DA, Montes CL, Acosta-Rodriguez EV, Gruppi A. 2012. B-cell response during protozoan parasite infections. J Parasitol Res 2012:362131. doi: 10.1155/2012/362131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Omachi S, Fujii W, Azuma N, Morimoto A, Sanjoba C, Matsumoto Y, Goto Y. 2017. B-cell activating factor deficiency suppresses splenomegaly during Leishmania donovani infection. Biochem Biophys Res Commun 489:528–533. doi: 10.1016/j.bbrc.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 163.Silva-O'Hare J, de Oliveira IS, Klevorn T, Almeida VA, Oliveira GG, Atta AM, de Freitas LA, Dos-Santos WL. 2016. Disruption of splenic lymphoid tissue and plasmacytosis in canine visceral leishmaniasis: changes in homing and survival of plasma cells. PLoS One 11:e0156733. doi: 10.1371/journal.pone.0156733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Montes CL, Acosta-Rodriguez EV, Mucci J, Zuniga EI, Campetella O, Gruppi A. 2006. A Trypanosoma cruzi antigen signals CD11b+ cells to secrete cytokines that promote polyclonal B cell proliferation and differentiation into antibody-secreting cells. Eur J Immunol 36:1474–1485. doi: 10.1002/eji.200535537. [DOI] [PubMed] [Google Scholar]
- 165.Bermejo DA, Amezcua-Vesely MC, Montes CL, Merino MC, Gehrau RC, Cejas H, Acosta-Rodriguez EV, Gruppi A. 2010. BAFF mediates splenic B cell response and antibody production in experimental Chagas disease. PLoS Negl Trop Dis 4:e679. doi: 10.1371/journal.pntd.0000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Goto Y, Omachi S, Sanjoba C, Matsumoto Y. 2014. Elevation of serum B-cell activating factor levels during visceral leishmaniasis. Am J Trop Med Hyg 91:912–914. doi: 10.4269/ajtmh.14-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ponce NE, Sanmarco LM, Eberhardt N, Garcia MC, Rivarola HW, Cano RC, Aoki MP. 2016. CD73 inhibition shifts cardiac macrophage polarization toward a microbicidal phenotype and ameliorates the outcome of experimental Chagas cardiomyopathy. J Immunol 197:814–823. doi: 10.4049/jimmunol.1600371. [DOI] [PubMed] [Google Scholar]
- 168.Liu D, Uzonna JE. 2012. The early interaction of Leishmania with macrophages and dendritic cells and its influence on the host immune response. Front Cell Infect Microbiol 2:83. doi: 10.3389/fcimb.2012.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Radwanska M, Guirnalda P, De Trez C, Ryffel B, Black S, Magez S. 2008. Trypanosomiasis-induced B cell apoptosis results in loss of protective anti-parasite antibody responses and abolishment of vaccine-induced memory responses. PLoS Pathog 4:e1000078. doi: 10.1371/journal.ppat.1000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Scholzen A, Sauerwein RW. 2013. How malaria modulates memory: activation and dysregulation of B cells in Plasmodium infection. Trends Parasitol 29:252–262. doi: 10.1016/j.pt.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 171.Donati D, Zhang LP, Chene A, Chen Q, Flick K, Nystrom M, Wahlgren M, Bejarano MT. 2004. Identification of a polyclonal B-cell activator in Plasmodium falciparum. Infect Immun 72:5412–5418. doi: 10.1128/IAI.72.9.5412-5418.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Banic DM, Viana-Martins FS, De Souza JM, Peixoto TD, Daniel-Ribeiro C. 1991. Polyclonal B-lymphocyte stimulation in human malaria and its association with ongoing parasitemia. Am J Trop Med Hyg 44:571–577. doi: 10.4269/ajtmh.1991.44.571. [DOI] [PubMed] [Google Scholar]
- 173.Greenwood BM, Vick RM. 1975. Evidence for a malaria mitogen in human malaria. Nature 257:592–594. doi: 10.1038/257592a0. [DOI] [PubMed] [Google Scholar]
- 174.Tobie JE, Abele DC, Wolff SM, Contacos PG, Evans CB. 1966. Serum immunoglobulin levels in human malaria and their relationship to antibody production. J Immunol 97:498–505. [PubMed] [Google Scholar]
- 175.Jaramillo M, Godbout M, Olivier M. 2005. Hemozoin induces macrophage chemokine expression through oxidative stress-dependent and -independent mechanisms. J Immunol 174:475–484. doi: 10.4049/jimmunol.174.1.475. [DOI] [PubMed] [Google Scholar]
- 176.Donati D, Mok B, Chene A, Xu H, Thangarajh M, Glas R, Chen Q, Wahlgren M, Bejarano MT. 2006. Increased B cell survival and preferential activation of the memory compartment by a malaria polyclonal B cell activator. J Immunol 177:3035–3044. doi: 10.4049/jimmunol.177.5.3035. [DOI] [PubMed] [Google Scholar]
- 177.Kumsiri R, Potup P, Chotivanich K, Petmitr S, Kalambaheti T, Maneerat Y. 2010. Blood stage Plasmodium falciparum antigens induce T cell independent immunoglobulin production via B cell activation factor of the TNF family (BAFF) pathway. Acta Trop 116:217–226. doi: 10.1016/j.actatropica.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 178.Muehlenbachs A, Fried M, Lachowitzer J, Mutabingwa TK, Duffy PE. 2007. Genome-wide expression analysis of placental malaria reveals features of lymphoid neogenesis during chronic infection. J Immunol 179:557–565. doi: 10.4049/jimmunol.179.1.557. [DOI] [PubMed] [Google Scholar]
- 179.Nduati E, Gwela A, Karanja H, Mugyenyi C, Langhorne J, Marsh K, Urban BC. 2011. The plasma concentration of the B cell activating factor is increased in children with acute malaria. J Infect Dis 204:962–970. doi: 10.1093/infdis/jir438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Scholzen A, Teirlinck AC, Bijker EM, Roestenberg M, Hermsen CC, Hoffman SL, Sauerwein RW. 2014. BAFF and BAFF receptor levels correlate with B cell subset activation and redistribution in controlled human malaria infection. J Immunol 192:3719–3729. doi: 10.4049/jimmunol.1302960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Doolan DL, Dobano C, Baird JK. 2009. Acquired immunity to malaria. Clin Microbiol Rev 22:13–36. doi: 10.1128/CMR.00025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Abele DC, Tobie JE, Hill GJ, Contacos PG, Evans CB. 1965. Alterations in serum proteins and 19s antibody production during the course of induced malarial infections in man. Am J Trop Med Hyg 14:191–197. doi: 10.4269/ajtmh.1965.14.191. [DOI] [PubMed] [Google Scholar]
- 183.McGregor IA, Gilles HM, Walters JH, Davies AH, Pearson FA. 1956. Effects of heavy and repeated malarial infections on Gambian infants and children; effects of erythrocytic parasitization. Br Med J 2:686–692. doi: 10.1136/bmj.2.4994.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Struik SS, Riley EM. 2004. Does malaria suffer from lack of memory? Immunol Rev 201:268–290. doi: 10.1111/j.0105-2896.2004.00181.x. [DOI] [PubMed] [Google Scholar]
- 185.Liu XQ, Stacey KJ, Horne-Debets JM, Cridland JA, Fischer K, Narum D, Mackay F, Pierce SK, Wykes MN. 2012. Malaria infection alters the expression of B-cell activating factor resulting in diminished memory antibody responses and survival. Eur J Immunol 42:3291–3301. doi: 10.1002/eji.201242689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. 2009. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 187.Xia R, Han Z, Zhou Y, Chen S, Chen B, Gu M, Deng A, Zhong R, Wen H. 2010. BLyS and APRIL expression in peripheral blood mononuclear cells of cryptococcal meningitis patients and their clinical significance. Clin Biochem 43:397–400. doi: 10.1016/j.clinbiochem.2009.10.059. [DOI] [PubMed] [Google Scholar]
- 188.Mansour MK, Reedy JL, Tam JM, Vyas JM. 2014. Macrophage Cryptococcus interactions: an update. Curr Fungal Infect Rep 8:109–115. doi: 10.1007/s12281-013-0165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kimura A, Yoshikura N, Koumura A, Hayashi Y, Inuzuka T. 2015. B-cell-activating factor belonging to the tumor necrosis factor family (BAFF) and a proliferation-inducing ligand (APRIL) levels in cerebrospinal fluid of patients with meningoencephalitis. J Neurol Sci 352:79–83. doi: 10.1016/j.jns.2015.03.036. [DOI] [PubMed] [Google Scholar]
- 190.Saulep-Easton D, Vincent FB, Quah PS, Wei A, Ting SB, Croce CM, Tam C, Mackay F. 2016. The BAFF receptor TACI controls IL-10 production by regulatory B cells and CLL B cells. Leukemia 30:163–172. doi: 10.1038/leu.2015.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ma N, Zhang Y, Liu Q, Wang Z, Liu X, Zhu G, Yu D, Han G, Chen G, Hou C, Wang T, Ma Y, Shen B, Li Y, Xiao H, Wang R. 2017. B cell activating factor (BAFF) selects IL-10− B cells over IL-10+ B cells during inflammatory responses. Mol Immunol 85:18–26. doi: 10.1016/j.molimm.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 192.Yang M, Sun L, Wang S, Ko KH, Xu H, Zheng BJ, Cao X, Lu L. 2010. Novel function of B cell-activating factor in the induction of IL-10-producing regulatory B cells. J Immunol 184:3321–3325. doi: 10.4049/jimmunol.0902551. [DOI] [PubMed] [Google Scholar]
- 193.Zhang X. 2013. Regulatory functions of innate-like B cells. Cell Mol Immunol 10:113–121. doi: 10.1038/cmi.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]